There has been increasing recognition that genetic factors play important roles in both sporadic and familial cases of idiopathic pulmonary fibrosis (IPF) (1). Current data indicate that at least one-third of the risk to develop sporadic or familial IPF can be explained by common genetic variants identified through large genome-wide association studies (2). Interestingly, several of these loci appear to have prognostic implications for patients with IPF (3, 4). In addition to common variants, a growing number of genes carrying rare genetic variants are being identified that influence susceptibility to disease in familial interstitial pneumonia (FIP), the familial form of IPF (1). As of yet, there is no guidance for clinicians regarding when to pursue genetic testing in patients with IPF or how to use test results in patient care. Below, we offer our perspective on the current usefulness of genetic evaluation and testing in familial and sporadic IPF, as well as for individuals at risk for development of IPF. Continued research investigating the genetic underpinnings of IPF is needed to support future development and validation of more comprehensive, evidence-based guidelines for genetic testing and screening for IPF.
Familial Interstitial Pneumonia
FIP is defined by the diagnosis of an idiopathic interstitial pneumonia (IIP), predominantly IPF, in two or more relatives who share common ancestry (5, 6). Deleterious rare genetic variants (mutations) in 9 genes (telomerase reverse transcriptase [TERT], telomerase RNA component [hTR], dyskerin [DKC1], telomere repeat binding factor 1–interacting nuclear factor 2 [TINF2], regulator of telomere elongation helicase [RTEL1], poly(A)-specific ribonuclease [PARN], surfactant protein C [SFTPC], surfactant protein A2 [SFTPA2], and adenosine triphosphate–binding cassette subfamily A member 3 [ABCA3]) (7–20) and common variants at 10 loci (3q26, 4q22, 5p15, 6p24, 7q22, 10q24, 11p15, 13q34, 15q14–15, and 19q13) (2) have been implicated in FIP, including 6 genes related to telomere biology, and 3 genes related to surfactant production. ABCA3 has a recessive mode and DKC1 is X-linked, but the other disease-associated rare genetic variants are inherited in an autosomal dominant manner such that affected individuals carry a single (heterozygous) mutation. Current evidence indicates that disease penetrance in carriers of these rare genetic variants is incomplete; however, further studies are needed to define disease penetrance in relationship to individual disease-associated genes. Mutations in currently identified FIP-causing genes are found in approximately 20% of affected families; thus rare genetic variants are yet to be identified in 80% of FIP families. In addition to FIP, pulmonary fibrosis is also a feature of some multisystem genetic disorders, including Hermansky–Pudlak syndrome and dyskeratosis congenita. As opposed to FIP, the role of genetic testing for Hermansky–Pudlak syndrome and dyskeratosis congenita is well described (21, 22).
In all subjects with IPF, a thorough family history should be performed regardless of patient age and should be updated at each visit. In our experience, up to 10% of patients presenting as “sporadic IPF” will subsequently have a bloodline relative diagnosed with an IIP during follow-up, sometimes many years later. Because most patients are not aware of the possibility of a familial basis at the initial referral visit, we frequently find that the second case in the family is reported at the second visit, after the patient has been educated to seek out IIP in relatives. In addition to family history of interstitial lung disease, particular emphasis should be placed on determining whether there is a family history of cryptogenic cirrhosis, aplastic anemia, and/or premature graying, suggesting that a short telomere syndrome could be present (Table 1). A family history of neonatal respiratory distress or childhood interstitial lung disease is relevant for suggesting the possibility of a surfactant-related disorder, which can present across a wide age spectrum.
Table 1.
Key Personal and Family History Elements
| Short Telomere Syndromes | |
|---|---|
| Adult ILD | ++ |
| Premature graying* | ++ |
| Cryptogenic cirrhosis | ++ |
| Aplastic anemia | ++ |
| Myelodysplasia/leukemia | + |
Definition of abbreviation: ILD = interstitial lung disease.
The presence of two or more features in an individual or family suggests a short telomere syndrome. Other features including osteoporosis, enterocolitis, and nonmelanoma skin cancer are also observed in some individuals and families with short telomere syndromes.
Significant graying before age 30 years.
We believe that genetic testing for rare variations (mutations) in disease-associated genes, with appropriate counseling on potential risks and benefits of such testing, should be offered to all patients with FIP and recommended in circumstances when a genetic diagnosis is likely to be achieved. A positive genetic diagnosis in FIP can aid in disease course prognostication and risk stratification when considering lung transplantation. In addition, a genetic diagnosis can have implications for estimating the risk for relatives and determining the need for testing of other family members.
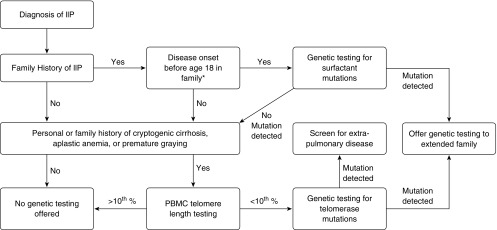
To maximize the positive predictive value of genetic tests, our current practice is to use a combination of personal medical and family history in combination with biomarkers to identify families in which there is high likelihood (pretest probability) of identifying a genetic diagnosis (Figure 1). For example, in families or patients with a personal or family history suggestive of a short telomere syndrome, we recommend peripheral blood mononuclear cell (PBMC) telomere length testing performed on a clinical basis through a CLIA (Clinical Laboratory Improvement Amendments)–certified laboratory. Many different laboratory approaches are available to assess telomere length; however, we recommend flow cytometric measurements because they are precise, well validated, and controlled (23, 24). If the PBMC telomere length is short (<10% for age), our experience suggests that the likelihood of identifying a pathogenic mutation in a known telomerase-related gene is high. Of note, telomere length can be inherited independently from a culprit rare genetic variant, and thus it is possible to inherit short telomeres (and disease risk) without inheriting a mutation; this has been termed “occult genetic disease” (25, 26). Alternatively, when the family history is notable for early-onset lung disease (especially in childhood), or if there is a family history of disease onset before age 45 years and a history of lung cancer (27), a mutation in a surfactant-related gene is more likely. Thus, the only genes that we currently recommend sequencing for rare variants are telomerase genes (TERT, hTR, DKC1, TINF2, RTEL1, and PARN) or surfactant protein genes (SFTPC, SFTPA2, and ABCA3); we anticipate that this list will grow in the future. However, our limited understanding of disease penetrance in individuals with disease-associated rare variants constrains our ability to risk-stratify unaffected FIP family members based only on the presence of rare variants.
Figure 1.
Recommendations for diagnostic genetic testing for familial interstitial pneumonia (FIP)–associated genetic variants. *Age 18 is included because at this age patients will have reached majority and can provide personal consent for genetic testing and/or sharing of protected health information; however, consideration should be given to testing for surfactant mutations in patients with FIP with a family history of disease onset before age 30. IIP = idiopathic interstitial pneumonia; PBMC = peripheral blood mononuclear cell.
Approaches to interpretation of genetic variation continue to evolve, and the current guidelines from the American College of Medical Genetics and Genomics provide a framework for reporting and interpreting pathogenicity of rare genetic variants (pathogenic, likely pathogenic, uncertain significance, likely benign, and benign) (28). Nonsense and splice variants of FIP-causing genes typically lead to loss of function and are generally pathogenic, whereas missense genetic variants that result in amino acid substitution present greater challenges and are frequently classified as genetic variants of uncertain significance (VOUS). The usefulness of these VOUSs in guiding clinical care and informing at-risk individuals presents a particular challenge for clinicians and genetic counselors. Currently employed algorithms to predict the pathogenicity of missense VOUSs have only fair accuracy (65–80%); therefore, assigning significance to these genetic variants can be problematic.
Identification of a disease-associated rare genetic variant in a patient with FIP may suggest the need for additional screening and/or testing for extrapulmonary disease. For example, subjects with telomerase pathway mutations may undergo periodic monitoring of blood counts and liver tests (29). In addition, the presence of an FIP-associated mutation in an affected family member should prompt consideration of genetic testing for at-risk asymptomatic family members in concert with genetic counseling. The American Board of Genetic Counseling (www.abgc.net) and the National Society of Genetic Counselors (www.nsgc.org) provide resources for identifying a local genetic counselor. Decisions by individuals about whether to pursue genetic testing in this setting are highly personal, and some family members choose not to learn their mutation carrier status. Issues related to the personal knowledge of genetic risk for disease are particularly relevant in diseases such as FIP, where disease onset typically occurs late in life and age- and sex-specific penetrance cannot be predicted with high confidence. In addition, because environmental influences in FIP other than cigarette smoking are not well understood, opportunities for risk modification are modest. However, as knowledge of mutation carrier status may impact a variety of personal and health decisions, including decisions regarding the desire and approach to bearing children, there can be benefits for asymptomatic relatives of patients with FIP with known FIP-causing mutations to learn their mutation status. In particular, in families with telomerase mutations, earlier-onset and more severe extrapulmonary disease have been observed in successively younger generations, suggesting that closer monitoring of asymptomatic mutation carriers may be beneficial (30). Consideration of genetic testing in asymptomatic children within families with FIP may be appropriate in the context of available information about the natural history of the specific disease-causing gene. Our approach is to defer recommendations for genetic testing until adult age unless manifestations of disease in childhood are likely. Information about genetics and testing for professionals and the general public is available online at Genetics Home Reference at https://ghr.nlm.nih.gov/.
The issue of radiologic screening for at-risk relatives of patients with FIP is complex, and there are insufficient data to support a strong recommendation for or against screening at this time. If radiographic screening is performed, we favor using high-resolution computed tomography (HRCT) as symptom-based screening and pulmonary function testing have limited sensitivity for early disease (31). Some providers may recommend periodic screening for individuals who carry known familial mutations to clarify disease status because normal findings can be reassuring. However, we and others have reported that radiologic evidence of mild interstitial abnormalities is common in asymptomatic relatives of patients with FIP (6, 31, 32), and the frequency and timing of progression from mild radiologic abnormalities to clinical FIP are unknown, although this important question is the subject of an ongoing longitudinal study by our group. In our cohort of more than 200 asymptomatic first-degree relatives of patients with FIP (median age, 50 yr), 24% had evidence of mild interstitial disease on HRCT. The availability of U.S. Food and Drug Administration–approved drugs to treat IPF (33, 34) provides another potential rationale for HRCT screening of at-risk relatives of patients with FIP. Although it has not been shown that treating individuals with “presymptomatic” IPF affects long-term outcomes, analyses of patients with mild disease in trials of both pirfenidone (35) and nintedanib (36) seem to suggest these medications can be efficacious in patients with mild symptoms and near-normal pulmonary function. Thus, screening for presymptomatic disease in high-risk individuals followed by initiating early treatment seems to be a promising but unproven strategy. Further studies of early disease treatments are needed to clarify this critical question, and pose challenging issues regarding their design and end points.
Sporadic IPF
Patients with IPF who do not have a known family history of IIP are classified as having sporadic IPF. Although genetic association studies have indicated that common genetic variants contribute similarly to disease risk in sporadic and familial IPF (2), large studies evaluating genetic screening for disease-associated rare genetic variants in these patients with sporadic IPF have not been performed. Available studies testing for TERT and SFTPC mutations have suggested that the prevalence of mutations in telomerase and surfactant protein genes is low in patients with sporadic IPF (37–39). Because current evidence indicates a low pretest probability for finding disease-causing rare genetic variants, we do not recommend genetic testing in individuals with sporadic IPF or other IIPs unless their family history suggests a short telomere syndrome as described previously. If the personal and/or family history does suggest a short telomere syndrome, we favor clinical telomere length testing as the next step, similar to patients with FIP. If telomere length is short (<10th percentile), then we suggest that genetic testing for mutations in telomerase-related genes be considered to detect de novo or low-penetrance heterozygous mutations.
It is recognized that approximately one-third of patients with IPF have short telomeres (less than the 10th percentile, adjusted for age) in peripheral blood (39, 40), and telomere length is shorter in patients with IPF compared with that in patients with other interstitial lung diseases (41). In a significant proportion of patients with IPF, the cause of short telomeres is not yet known and may be acquired, rather than genetically determined in some cases. Regardless of etiology, it appears that PBMC telomere length provides prognostic information in IPF. In 2014, Stuart and colleagues reported that among patients with IPF (although not those with other interstitial lung diseases) from three academic centers in the United States, PBMC telomere length was independently associated with transplantation-free survival (42). Similar results have also been reported in a Chinese cohort (43). Extrapulmonary disease may be common in patients with IPF with short telomeres (29) and may be useful in anticipating complications after lung transplantation (44). Thus, we suggest that PBMC telomere length testing should be considered as a component of the pretransplantation evaluation of those patients with IPF in whom this treatment is being considered. Future studies are needed to more comprehensively define the lung transplantation outcomes of patients with short telomeres and to determine optimal approaches to minimize posttransplantation complications related to short telomeres. Although formal cost–benefit analyses have not yet been performed, the expense of telomere length testing from CLIA-certified laboratories is less than the cost of routinely performed pulmonary function tests in many centers. In the future, it may be possible to use PBMC telomere length to identify a subphenotype of patients with IPF who would benefit from telomere-directed therapy. Provocatively, in a small study of patients with dyskeratosis congenita, danazol treatment of subjects who had pulmonary fibrosis appeared to lengthen PBMC telomeres and was associated with stability of lung function (45). Further work will be needed to more comprehensively determine the safety and possible benefits of this approach.
Common genetic variants, including a polymorphism in the mucin 5B (MUC5B) promoter, have been linked to risk of developing familial and sporadic IPF (2, 46–51); however, because the positive and negative predictive values of risk allele carrier status are modest (i.e., disease penetrance in carriers of this polymorphism is low), we do not advocate testing for these common polymorphisms as a screening approach for IPF. In addition to correlations with disease risk, common polymorphisms in the MUC5B promoter (4) and in toll interacting protein (TOLLIP) (4) have been associated with outcomes in patients with IPF and offer promise as prognostic indicators. Carriers of the allele that confers risk for IPF have better outcomes than patients with IPF who do not have these alleles, suggesting that these variants identify a prognostic (and possibly biologically distinct) subtype of IPF. Carriers of at least one T allele of the MUC5B polymorphism appear to have at least 50% improved survival compared with GG carriers (3). Interestingly, T allele carriers have modestly better lung function than do G allele carriers at similar ages (3), consistent with a slower disease course. Carriers of the TOLLIP risk polymorphism rs5743890 A allele have approximately 35% reduced risk of mortality compared with G allele homozygotes (4). Although routine testing clinically for these two polymorphisms in patients with IPF seems premature, further prospective studies for risk stratification for disease progression and mortality are promising and warranted.
Another area of possible usefulness for testing of common genetic variants in IPF is stratification of patient populations in clinical intervention trials. It has been suggested that carriers of a TOLLIP polymorphism may benefit from treatment with N-acetylcysteine (52), whereas other single-nucleotide polymorphisms may predict harm from this intervention. Although these preliminary results require further validation and prospective clinical trials, the concept of studying genetically distinct disease subphenotypes and gene-by-drug interactions should be incorporated into future clinical studies of IPF. Peripheral blood gene expression (53) and biomarker signatures (54) also hold promise for identifying patients with IPF most likely to benefit from specific therapies, as well as those at high risk for rapid disease progression.
What Further Research Is Needed?
Numerous advances in the genetics and genomics of IPF have improved our understanding of this disease and offer the promise of new treatments and personalized information about disease risk and prognosis. Identifying additional disease-associated genes in FIP is a high priority, as is developing a better understanding of the mechanisms by which common genetic variants identified by genome-wide association studies affect risk and outcomes of familial and sporadic IPF. Defining the disease penetrance associated with rare and common genetic variants in individual genes will be important to aid in genetic counseling of patients. Importantly, the role of FIP- and IPF-associated genetic variants in other adult and pediatric interstitial lung diseases remains incompletely understood and requires further study. Given the shared rare and common genetic risks between familial and sporadic IPF, further characterization of the at-risk population is needed.
Related to the role of radiologic screening in familial and sporadic IPF, ongoing studies by our group are designed to clarify the natural history of radiographic abnormalities in individuals at risk for developing disease in FIP families. Further study is needed to determine whether relatives of patients with sporadic IPF are also at increased risk for developing disease themselves. If so, these individuals could represent another “high-risk” group to target for screening and/or early intervention. Although it seems likely that a combination of genetic information and biomarkers could be used to identify the highest risk individuals for screening and/or early intervention trials, this approach will require additional prospective studies. In addition to identifying individuals who might benefit from early treatment, these studies could have broader relevance because a substantial proportion of the population over age 50 years has subtle radiographic changes that could reflect early interstitial lung disease (55).
Further studies are required to understand if and how presently known genetic risk factors for disease progression can be used to personalize IPF treatment. For example, in the future it may be that reasonable treatment options for a 72-year-old patient with an FVC of 85%, normal-length telomeres, and low-risk TOLLIP/MUC5B genotypes could be N-acetylcysteine (an inexpensive, well-tolerated medication) (52) or continued observation. In contrast, a 56-year-old with similar pulmonary function but very short telomeres and high-risk TOLLIP/MUC5B genotypes would choose more aggressive (perhaps telomere-directed) treatment (45) alone or in combination with presently approved therapies (33, 34). In the future, we anticipate that treatment of IPF/FIP will be guided by personalized genetic and genomic information, and we believe the time has arrived to begin to use these data in the care of our patients and their families.
Footnotes
Supported by National Institutes of Health grants K08HL130595 (J.A.K.), P01HL92870 (T.S.B.), R01HL085317 (T.S.B.), R01HL097163 (D.A.S. and T.E.F.), UH2HL123442 (D.A.S.), R33HL120770 (D.A.S.), and U54HL127672 (L.R.Y. and J.A.K.); the Department of Veterans Affairs (T.S.B. and D.A.S.); the Francis Family Foundation (J.A.K.); the Pulmonary Fibrosis Foundation (J.A.K.); and the Vanderbilt Faculty Research Scholars (J.A.K.).
Author Contributions: Conceived manuscript: J.A.K., J.E.L.; wrote manuscript: J.A.K., L.R.Y., T.S.B., J.E.L.; manuscript revision: J.A.K., L.R.Y., J.D.C., D.B.M., L.H.L., W.R.M., J.A.W., C.M., N.L., T.E.F., D.A.S., W.E.L., T.S.B., J.A.P., and J.E.L.
Originally Published in Press as DOI: 10.1164/rccm.201609-1820PP on October 27, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kropski JA, Blackwell TS, Loyd JE. The genetic basis of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45:1717–1727. doi: 10.1183/09031936.00163814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, Loyd JE, Cosgrove GP, Lynch D, Groshong S, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45:613–620. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peljto AL, Zhang Y, Fingerlin TE, Ma SF, Garcia JG, Richards TJ, Silveira LJ, Lindell KO, Steele MP, Loyd JE, et al. Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA. 2013;309:2232–2239. doi: 10.1001/jama.2013.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noth I, Zhang Y, Ma SF, Flores C, Barber M, Huang Y, Broderick SM, Wade MS, Hysi P, Scuirba J, et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med. 2013;1:309–317. doi: 10.1016/S2213-2600(13)70045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastarache JA, Fremont RD, Kropski JA, Bossert FR, Ware LB. Procoagulant alveolar microparticles in the lungs of patients with acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1035–L1041. doi: 10.1152/ajplung.00214.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steele MP, Speer MC, Loyd JE, Brown KK, Herron A, Slifer SH, Burch LH, Wahidi MM, Phillips JA, III, Sporn TA, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med. 2005;172:1146–1152. doi: 10.1164/rccm.200408-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, III, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 8.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kropski JA, Mitchell DB, Markin C, Polosukhin VV, Choi L, Johnson JE, Lawson WE, Phillips JA, III, Cogan JD, Blackwell TS, et al. A novel dyskerin (DKC1) mutation is associated with familial interstitial pneumonia. Chest. 2014;146:e1–e7. doi: 10.1378/chest.13-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alder JK, Parry EM, Yegnasubramanian S, Wagner CL, Lieblich LM, Auerbach R, Auerbach AD, Wheelan SJ, Armanios M. Telomere phenotypes in females with heterozygous mutations in the dyskeratosis congenita 1 (DKC1) gene. Hum Mutat. 2013;34:1481–1485. doi: 10.1002/humu.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alder JK, Stanley SE, Wagner CL, Hamilton M, Hanumanthu VS, Armanios M. Exome sequencing identifies mutant TINF2 in a family with pulmonary fibrosis. Chest. 2015;147:1361–1368. doi: 10.1378/chest.14-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuart BD, Choi J, Zaidi S, Xing C, Holohan B, Chen R, Choi M, Dharwadkar P, Torres F, Girod CE, et al. Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening. Nat Genet. 2015;47:512–517. doi: 10.1038/ng.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cogan JD, Kropski JA, Zhao M, Mitchell DB, Rives L, Markin C, Garnett ET, Montgomery KH, Mason WR, McKean DF, et al. Rare variants in RTEL1 are associated with familial interstitial pneumonia. Am J Respir Crit Care Med. 2015;191:646–655. doi: 10.1164/rccm.201408-1510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kannengiesser C, Borie R, Ménard C, Réocreux M, Nitschké P, Gazal S, Mal H, Taillé C, Cadranel J, Nunes H, et al. Heterozygous RTEL1 mutations are associated with familial pulmonary fibrosis. Eur Respir J. 2015;46:474–485. doi: 10.1183/09031936.00040115. [DOI] [PubMed] [Google Scholar]
- 15.Thomas AQ, Lane K, Phillips J, III, Prince M, Markin C, Speer M, Schwartz DA, Gaddipati R, Marney A, Johnson J, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165:1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 16.van Moorsel CH, van Oosterhout MF, Barlo NP, de Jong PA, van der Vis JJ, Ruven HJ, van Es HW, van den Bosch JM, Grutters JC. Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a Dutch cohort. Am J Respir Crit Care Med. 2010;182:1419–1425. doi: 10.1164/rccm.200906-0953OC. [DOI] [PubMed] [Google Scholar]
- 17.Ono S, Tanaka T, Ishida M, Kinoshita A, Fukuoka J, Takaki M, Sakamoto N, Ishimatsu Y, Kohno S, Hayashi T, et al. Surfactant protein C G100S mutation causes familial pulmonary fibrosis in Japanese kindred. Eur Respir J. 2011;38:861–869. doi: 10.1183/09031936.00143610. [DOI] [PubMed] [Google Scholar]
- 18.Nogee LM, Dunbar AE, III, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 19.Campo I, Zorzetto M, Mariani F, Kadija Z, Morbini P, Dore R, Kaltenborn E, Frixel S, Zarbock R, Liebisch G, et al. A large kindred of pulmonary fibrosis associated with a novel ABCA3 gene variant. Respir Res. 2014;15:43. doi: 10.1186/1465-9921-15-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epaud R, Delestrain C, Louha M, Simon S, Fanen P, Tazi A. Combined pulmonary fibrosis and emphysema syndrome associated with ABCA3 mutations. Eur Respir J. 2014;43:638–641. doi: 10.1183/09031936.00145213. [DOI] [PubMed] [Google Scholar]
- 21.Dokal I, Vulliamy T, Mason P, Bessler M. Clinical utility gene card for: dyskeratosis congenita—update 2015. Eur J Hum Genet 2014 [accessed 2017 Apr 24];19. Available from: http://www.nature.com/ejhg/journal/v19/n11/full/ejhg201190a.html.
- 22.Gahl WA, Huizing M. Hermansky–Pudlak syndrome. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, et al., editors. GeneReviews. Seattle, WA: University of Washington; 1993. [Google Scholar]
- 23.Rufer N, Dragowska W, Thornbury G, Roosnek E, Lansdorp PM. Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry. Nat Biotechnol. 1998;16:743–747. doi: 10.1038/nbt0898-743. [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez-Rodrigues F, Santana-Lemos BA, Scheucher PS, Alves-Paiva RM, Calado RT. Direct comparison of flow-FISH and qPCR as diagnostic tests for telomere length measurement in humans. PLoS One. 2014;9:e113747. doi: 10.1371/journal.pone.0113747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz de Leon A, Cronkhite JT, Katzenstein AL, Godwin JD, Raghu G, Glazer CS, Rosenblatt RL, Girod CE, Garrity ER, Xing C, et al. Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PLoS One. 2010;5:e10680. doi: 10.1371/journal.pone.0010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hao LY, Armanios M, Strong MA, Karim B, Feldser DM, Huso D, Greider CW. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, DiMaio JM, Kinch LN, Grishin NV, Garcia CK. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84:52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George G, Rosas IO, Cui Y, McKane C, Hunninghake GM, Camp PC, Raby BA, Goldberg HJ, El-Chemaly S. Short telomeres, telomeropathy, and subclinical extrapulmonary organ damage in patients with interstitial lung disease. Chest. 2015;147:1549–1557. doi: 10.1378/chest.14-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armanios M, Chen JL, Chang YP, Brodsky RA, Hawkins A, Griffin CA, Eshleman JR, Cohen AR, Chakravarti A, Hamosh A, et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci USA. 2005;102:15960–15964. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosas IO, Ren P, Avila NA, Chow CK, Franks TJ, Travis WD, McCoy JP, Jr, May RM, Wu HP, Nguyen DM, et al. Early interstitial lung disease in familial pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:698–705. doi: 10.1164/rccm.200702-254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kropski JA, Pritchett JM, Zoz DF, Crossno PF, Markin C, Garnett ET, Degryse AL, Mitchell DB, Polosukhin VV, Rickman OB, et al. Extensive phenotyping of individuals at risk for familial interstitial pneumonia reveals clues to the pathogenesis of interstitial lung disease. Am J Respir Crit Care Med. 2015;191:417–426. doi: 10.1164/rccm.201406-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 34.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 35.Noble PW, Albera C, Bradford WZ, Costabel U, du Bois RM, Fagan EA, Fishman RS, Glaspole I, Glassberg MK, Lancaster L, et al. Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur Respir J. 2016;47:243–253. doi: 10.1183/13993003.00026-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costabel U, Inoue Y, Richeldi L, Collard HR, Tschoepe I, Stowasser S, Azuma A. Efficacy of nintedanib in idiopathic pulmonary fibrosis across prespecified subgroups in INPULSIS. Am J Respir Crit Care Med. 2016;193:178–185. doi: 10.1164/rccm.201503-0562OC. [DOI] [PubMed] [Google Scholar]
- 37.Coghlan MA, Shifren A, Huang HJ, Russell TD, Mitra RD, Zhang Q, Wegner DJ, Cole FS, Hamvas A. Sequencing of idiopathic pulmonary fibrosis–related genes reveals independent single gene associations. BMJ Open Respir Res. 2014;1:e000057. doi: 10.1136/bmjresp-2014-000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawson WE, Grant SW, Ambrosini V, Womble KE, Dawson EP, Lane KB, Markin C, Renzoni E, Lympany P, Thomas AQ, et al. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax. 2004;59:977–980. doi: 10.1136/thx.2004.026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cronkhite JT, Xing C, Raghu G, Chin KM, Torres F, Rosenblatt RL, Garcia CK. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:729–737. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snetselaar R, van Moorsel CH, Kazemier KM, van der Vis JJ, Zanen P, van Oosterhout MF, Grutters JC. Telomere length in interstitial lung diseases. Chest. 2015;148:1011–1018. doi: 10.1378/chest.14-3078. [DOI] [PubMed] [Google Scholar]
- 42.Stuart BD, Lee JS, Kozlitina J, Noth I, Devine MS, Glazer CS, Torres F, Kaza V, Girod CE, Jones KD, et al. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: an observational cohort study with independent validation. Lancet Respir Med. 2014;2:557–565. doi: 10.1016/S2213-2600(14)70124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai J, Cai H, Li H, Zhuang Y, Min H, Wen Y, Yang J, Gao Q, Shi Y, Yi L. Association between telomere length and survival in patients with idiopathic pulmonary fibrosis. Respirology. 2015;20:947–952. doi: 10.1111/resp.12566. [DOI] [PubMed] [Google Scholar]
- 44.Silhan LL, Shah PD, Chambers DC, Snyder LD, Riise GC, Wagner CL, Hellström-Lindberg E, Orens JB, Mewton JF, Danoff SK, Arcasoy MO, Armanios M. Lung transplantation in telomerase mutation carriers with pulmonary fibrosis. Eur Respir J. 2014;44:178–187. doi: 10.1183/09031936.00060014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Townsley DM, Dumitriu B, Liu D, Biancotto A, Weinstein B, Chen C, Hardy N, Mihalek AD, Lingala S, Kim YJ, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374:1922–1931. doi: 10.1056/NEJMoa1515319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borie R, Crestani B, Dieude P, Nunes H, Allanore Y, Kannengiesser C, Airo P, Matucci-Cerinic M, Wallaert B, Israel-Biet D, et al. The MUC5B variant is associated with idiopathic pulmonary fibrosis but not with systemic sclerosis interstitial lung disease in the European Caucasian population. PLoS One. 2013;8:e70621. doi: 10.1371/journal.pone.0070621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horimasu Y, Ohshimo S, Bonella F, Tanaka S, Ishikawa N, Hattori N, Kohno N, Guzman J, Costabel U. MUC5B promoter polymorphism in Japanese patients with idiopathic pulmonary fibrosis. Respirology. 2015;20:439–444. doi: 10.1111/resp.12466. [DOI] [PubMed] [Google Scholar]
- 48.Peljto AL, Selman M, Kim DS, Murphy E, Tucker L, Pardo A, Lee JS, Ji W, Schwarz MI, Yang IV, et al. The MUC5B promoter polymorphism is associated with idiopathic pulmonary fibrosis in a Mexican cohort but is rare among Asian ancestries. Chest. 2015;147:460–464. doi: 10.1378/chest.14-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stock CJ, Sato H, Fonseca C, Banya WA, Molyneaux PL, Adamali H, Russell AM, Denton CP, Abraham DJ, Hansell DM, et al. Mucin 5B promoter polymorphism is associated with idiopathic pulmonary fibrosis but not with development of lung fibrosis in systemic sclerosis or sarcoidosis. Thorax. 2013;68:436–441. doi: 10.1136/thoraxjnl-2012-201786. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Noth I, Garcia JG, Kaminski N. A variant in the promoter of MUC5B and idiopathic pulmonary fibrosis. N Engl J Med. 2011;364:1576–1577. doi: 10.1056/NEJMc1013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oldham JM, Ma SF, Martinez FJ, Anstrom KJ, Raghu G, Schwartz DA, Valenzi E, Witt L, Lee C, Vij R, et al. IPFnet Investigators. TOLLIP, MUC5B, and the response to N-acetylcysteine among Individuals with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2015;192:1475–1482. doi: 10.1164/rccm.201505-1010OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herazo-Maya JD, Noth I, Duncan SR, Kim S, Ma SF, Tseng GC, Feingold E, Juan-Guardela BM, Richards TJ, Lussier Y, et al. Peripheral blood mononuclear cell gene expression profiles predict poor outcome in idiopathic pulmonary fibrosis. Sci Transl Med. 2013;5:205ra136. doi: 10.1126/scitranslmed.3005964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, Li K, Choi J, Vuga LJ, Lindell KO, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185:67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, Nishino M, Zazueta OE, Kurugol S, Ross JC, et al. Development and progression of interstitial lung abnormalities in the Framingham Heart Study. Am J Respir Crit Care Med. 2016;194:1514–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]