Abstract
Context:
Gonadotropin-releasing hormone neurons originate outside the central nervous system in the olfactory placode and migrate into the central nervous system, becoming integral components of the hypothalamic-pituitary-gonadal axis. Failure of this migration can lead to idiopathic hypogonadotropic hypogonadism (IHH)/Kallmann syndrome (KS). We have previously shown that CCDC141 knockdown leads to impaired migration of GnRH neurons but not of olfactory receptor neurons.
Objective:
The aim of this study was to further describe the phenotype and prevalence of CCDC141 mutations in IHH/KS.
Design:
Using autozygosity mapping, candidate gene screening, whole-exome sequencing, and Sanger sequencing, those individuals carrying deleterious CDCD141 variants and their phenotypes were determined in a cohort of 120 IHH/KS families.
Patients and Interventions:
No interventions were made.
Results:
Our studies revealed nine affected individuals from four independent families in which IHH/KS is associated with inactivating CCDC141 variants, revealing a prevalence of 3.3%. Affected individuals (with the exception of those from family 1 who concomitantly have FEZF1 mutations) have normal olfactory function and anatomically normal olfactory bulbs. Four affected individuals show evidence of clinical reversibility. In three of the families, there was at least one more potentially deleterious variant in other known puberty genes with evidence of allelic heterogeneity within respective pedigrees.
Conclusions:
These studies confirm that inactivating CCDC141 variants cause normosmic IHH but not KS. This is consistent with our previous in vitro experiments showing exclusively impaired embryonic migration of GnRH neurons upon CCDC141 knockdown. These studies expand the clinical and genetic spectrum of IHH and also attest to the complexity of phenotype and genotype in IHH.
The aim of this study was to further describe CCDC141 mutations in IHH/KS. Our studies revealed nine affected individuals from four independent families who have KS but not IHH.
What controls the beginning of pubertal process in humans is an enduring question. Idiopathic hypogonadotropic hypogonadism (IHH) is characterized by failure initiation of puberty caused by deficient gonadotropin release for unknown reasons. In Kallmann syndrome (KS), there is an impairment of sense of smell in addition to IHH. This unique phenotype results from a defect in the shared development of gonadotropin-releasing hormone (GnRH) and olfactory neurons (1, 2). Both neurons originate from the olfactory placode, with the GnRH neurons migrating associated with olfactory axon bundles to the central nervous system. Upon entering the central nervous system, olfactory axons synapse with the olfactory bulb, while GnRH neurons further migrate to reach the mediobasal hypothalamus, where they form a functional network to initiate pulsatile GnRH secretion. Disruption of this migration has been known to result in KS. A growing list of genes have been implicated to be associated with IHH/KS (3). However, these genes account for less than one-half of all familial cases, and thus identification of new causative genes is highly likely, which may provide insight into the biology of GnRH neurons (4).
Here, we describe several independent families in which IHH, but not KS, is associated with loss-of-function mutations in the CCDC141 gene. These results support our previous findings that CCDC141 is required for successful migration of GnRH neurons but not of olfactory receptor neurons to reach their final destination in the central nervous system.
Case Reports
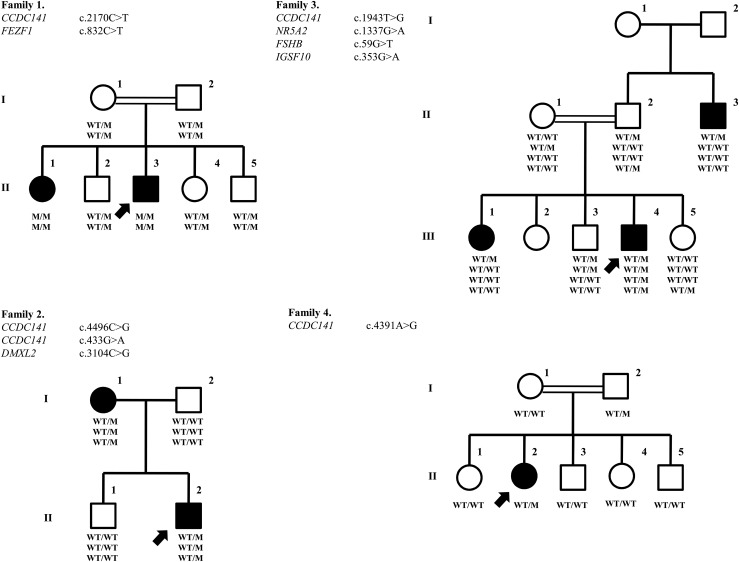
The pedigrees of the families are shown in Fig. 1.
Figure 1.
Pedigree and genotype sequencing of the families. Pedigrees of the families: Affected males are represented by black squares, affected females are represented by black circles, and index individuals are indicated by arrows. White square symbols indicate unaffected male family members, white circle symbols represent unaffected female family members, and the double line indicates consanguinity. Under each symbol are the genotypes, with WT and M denoting wild type and mutant, respectively.
Family 1
We previously reported this multiplex family (5). Briefly, the proband, a 19-year-old male (II-3), presented first with absent pubertal development at age 14 years. He received testosterone and human chorionic gonadotropin treatments and underwent surgery for undescended testicles. His penis developed to normal adult size only after a replacement testosterone treatment, but his testicles remained prepubertal. His 24-year-old sister (II-1) suffered from absent breast development and primary amenorrhea. Only after starting estrogen replacement at age 18 did her breast development and subsequent menstrual periods begin. They both have anosmia.
Family 2
A 13-year-old male (II-2) presented with a chief complaint of micropenis. His past medical history was remarkable for a small penis as an infant and undescended testicles, for which he received human chorionic gonadotropin treatment and subsequent orchiopexia at age 7. At presentation, he had 2 mL of testicles bilaterally and 4.5 cm of phallus with stage 1 axillary and stage 3 pubic hair. Although having a bone age of 13.5 years, his gonadotropin and testosterone levels were prepubertal (Table 1). He was started on a testosterone treatment course as 50 mg monthly intramuscular injections. One year into the treatment, he was noted to have testicular growth to 6 mL bilaterally. His gonadotropin and testosterone levels were early pubertal 2 months off treatment. Now, at age 15 years and 8 months, he has 20 mL of testicles bilaterally with testosterone and luteinizing hormone (LH) levels of 594 ng/dL and 6.0 mIU/mL, respectively. His 45-year-old mother (I-1) did not experience any spontaneous breast development as a teenager. She only had one episode of spotting. She was diagnosed with IHH elsewhere and given estrogen replacement therapy for a few months, followed by oral contraceptive pills. She could have regular menstruations only with oral contraceptive pills. Later in her early 20s, she self-discontinued oral contraceptive pills and subsequently had two unassisted pregnancies resulting in live term births. Since her second delivery she has not had any spontaneous menstrual cycles without using oral contraceptive pills.
Table 1.
Hormonal Results of the Probands Off Hormonal Treatment
| Family Member | Family 1II-3 | Family 2II-2 | Family 3II-2 | Family 4II-2 | Normal Range |
|---|---|---|---|---|---|
| Sex | M | M | M | F | |
| Age | 19 | 13 | 14 | 16 | |
| FSH (mIU/mL) | 0.1 | 1.31 | NA | 0.8 | M: 1.4–18.1 |
| F: 2.5–10.2 | |||||
| LH (mIU/mL) | 0.1 | 0.07 | 0.5 | 0.3 | M: 1.5–9.3 |
| F: 1.9–12.5 | |||||
| Estradiol (ng/dL) | NA | NA | NA | 1.3 | M: 0.8–3.5 |
| F: 6.3–16.5 | |||||
| Testosterone (ng/dL) | 69.0 | 9.8 | 0.1 | NA | 175–781 |
| Prolactin (ng/mL) | 6.6 | 6.5 | 4.9 | 4.7 | M: 2.1–17.7 F: 2.8–29.2 |
| TSH (mIU/mL) | 2.8 | 3.5 | 2.9 | 1.1 | 0.35–4.2 |
| Free T4 (ng/dL) | 1.1 | 1.0 | 1.1 | 0.9 | 0.89–1.8 |
| ACTH (pg/mL) | 9.1 | NA | 10.0 | NA | 5–55 |
| Cortisol (µg/dL) | 12.9 | 12.3 | 8.56 | 11.2 | 3–25 |
To convert the values for estradiol to picomoles per liter, multiply by 36.71. To convert the values for prolactin to picomoles per liter, multiply by 0.044. To convert the values for free T4 to picomoles per liter, multiply by 12.87. To convert the values for cortisol to nanomoles per liter, multiply by 27.58. To convert the values for dehydroepiandrosterone sulfate to nanomoles per liter, multiply by 27.21. The coefficients of variation within assays and between assays were less than 5%.
Abbreviations: ACTH, adrenocorticotropic hormone; F, female; M, male; NA, not available; TSH, thyroid-stimulating hormone.
Family 3
The proband (III-4) is a boy aged 14 years and 5 months, who first presented with micropenis. His pubic and axillary hair were at stage 4 and 1, respectively. His testes were 3 mL bilaterally in the scrotum. His stretched penile length was 5.5 cm. His bone age was consistent at 13 years and 6 months. Hormone assays showed prepubertal plasma testosterone and gonadotropin levels (Table 1). He was started on 3 months of testosterone treatment as 50 mg monthly intramuscular injections. Now, 5 months after the testosterone course, he has 8 mL of testicles bilaterally and 10 cm of penile length with testosterone and LH levels of 71 ng/dL and 1.2 mIU/mL, respectively. His older sister (II-1) was delayed in her pubertal development with her menarche at age 16. Now, at age 28, she has Tanner 5 breast and menstruates regularly. The affected uncle (II-3) is currently a 35-year-old man, who reportedly received testosterone treatment of delayed puberty and micropenis when he was around 20 years old. Later, he fully developed puberty and he fathered two children.
Family 4
A girl aged 16 years and 11 months (II-2) presented with a chief complaint of primary amenorrhea. Although she has a bone age of 13 years and 6 months, her gonadotropin and estradiol levels were prepubertal (Table 1). A GnRH stimulation test revealed maximal LH and follicle-stimulating hormone (FSH) levels of 2.1 and 5.1 mIU/mL, respectively. She was started on an estradiol replacement treatment. Currently her breasts and pubic and axillary hair are at stages 3, 4, and 2, respectively.
All patients in this study have otherwise been healthy. They have normal anthropometric measurements. They do not have any mental disorders, dysmorphic facial features (such as cleft lip, cleft palate, or hypodontia), deafness, renal agenesis, or bimanual synkinesia, which are sometimes associated with IHH/KS (4). Their other anterior pituitary hormone levels were within expected limits.
Except for the patients in family 1, all affected individuals report normal sense of smell.
Methods
Case report
The Ethics Committee of the Cukurova University Faculty of Medicine approved this study, and written informed consent was obtained for each participant. Plasma adrenocorticotropic hormone, serum FSH, LH, estradiol, dehydroepiandrosterone sulfate, cortisol, and testosterone levels were analyzed by commercial kits based on solid-phase, two-site sequential, or competitive chemiluminescent immunometric assay or electrochemiluminescence immunoassay.
The olfactory function of the proband in family 1 and his unaffected brother (II-3) was evaluated using the 40-item UPSIT smell identification test. The University of Pennsylvania Smell Identification Test (UPSIT) is a validated microencapsulated odor “scratch and sniff” test that correlates highly with other olfactory tests including odor detection thresholds (6). The UPSIT score was interpreted using the age- and sex-related normative classification system described in the UPSIT manual. To control for cross-cultural variation of smell identification, a culturally appropriate 20-item smell test was also administrated. The results of this test were found to be consistent with the UPSIT test. In other families, only a culturally appropriate 20-item smell test was administered.
Candidate gene screening
Genes known to be associated with or strong candidate for IHH/KS, including GNRHR, GNRH1, KISS1R, KISS1, TAC3, TACR3, FEZF1, KAL1, FGFR1, FGF8, PROK2, PROKR2, CHD7, WDR11, and HS6ST1 were screened by polymerase chain reaction amplifications of exons and splice junctions followed by sequencing on an ABI PRISM 3130 autosequencer (Applied Biosystems) (3).
Autozygosity mapping
Genome-wide single-nucleotide polymorphism (SNP) analysis used 250K NspI SNP microarrays (Affymetrix), and we analyzed the data using AutoSNPa software (http://autozygosity.org).
Exome sequencing
Briefly, samples were prepared as an Illumina sequencing library, and in the second step, the sequencing libraries were enriched for the desired target using the Illumina Exome Enrichment protocol. The captured libraries were sequenced using Illumina HiSEquation 2000 Sequencer (Macrogen, Seoul, Korea). The reads are mapped against UCSC hg19. Whole-exome sequencing data were filtered to find causative variants by wANNOVAR in both autosomal recessive and dominant modes (7).
Functional analysis
To confirm that disruption of CCDC141 alters GnRH neuronal migration and examine the mechanism of its action, CCDC141 deficiency was assessed in mouse nasal explants. Many in vivo aspects of GnRH neuronal development are recapitulated in this in vitro model (8, 9). Briefly, unilateral explants from E11.5 mouse embryos were cultured as previously described (9, 10). At 2 days in vitro, unilateral explants can be effectively electroporated. Cultures were transfected with 10 μL of 2% volume/volume control (general-purpose control small interfering RNA (siRNA)-A sc-37007; Santa Cruz Biotechnology, Inc., Dallas, TX) or Ccdc141 siRNA (sc-108804 for sequence 1; Santa Cruz Biotechnology, Inc.) (11). Explants were fixed and stained 3 days after electroporation for GnRH (primary antibody SW-1, 1:3000) or peripherin (peripheral intermediate filament marker, 1:2000; Chemicon, Temecula, CA). Procedures were conducted as previously described, and results from these functional experiments were also previously reported (9).
Results
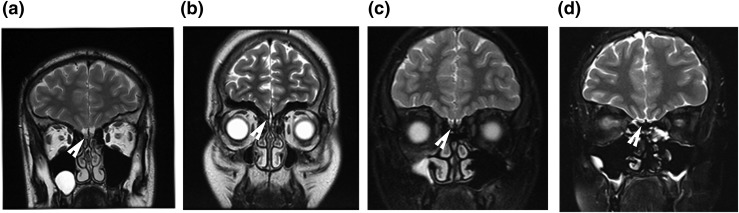
The proband from family 1 had complete anosmia upon UPSIT and culturally appropriate 20-item smell test as reported previously. The probands in the rest of the families had normosmia with the 20-item smell test administered. The brain magnetic resonance imaging (MRI) studies of the probands are shown in Fig. 2. Notably, only the proband from family 1 showed olfactory aplasia. The other two probands available for the imaging study had normal olfactory bulbs. Hormonal results of the probands, which are unequivocally consistent with hypogonadotropic hypogonadism, are shown in Table 1.
Figure 2.
Olfactory MRI of the probands. Brain MRIs at T2 coronal sections are shown. The arrowheads indicate olfactory bulbs. (a) A healthy young adult displaying anatomically normal olfactory bulbs. (b) The absence of olfactory bulbs (aplasia) in the proband from family 1. (c and d) Normal olfactory bulbs in the proband from family 2 and 4, respectively.
Genetic studies
Family 1
Autozygosity mapping identified two regions of homozygosity common to affected individuals but not found in the unaffected: a 5.8-Mb-long region on chromosome 1 from 165.7 Mb to 171.5 Mb and a 7.7-Mb-long region on chromosome 2 from 172.4 Mb to 180.1 Mb.
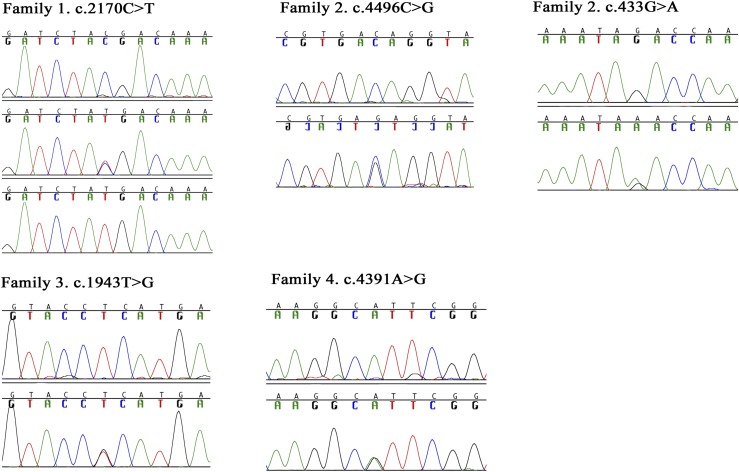
Analysis of the whole-exome sequencing data indicated a nonsense mutation (p. R724X) in the CCDC141 gene (HGNC: 26821), which is located in the homozygous region on chromosome 2. Subsequently, Sanger sequencing confirmed the presence of this mutation. Both affected individuals were homozygous for a C-to-T change at complementary DNA (cDNA) nucleotide 2270 (NM_173648.3:c.2170C>T). This mutation leads to the substitution of arginine at residue 724 for a stop codon (NP_775919.3:p.R724X). Parents and unaffected siblings were all heterozygous [Fig. 1(c)]. In addition, patients had homozygous missense mutations (p.H278Y) in the FEZF1 gene (5).
Family 2
Analysis of the whole-exome sequencing data in the proband indicated two missense mutations (p.T1499R and p.D145N) in the CCDC141 gene. Sanger sequencing confirmed the presence of these mutations. Proband and his affected mother were heterozygous for a C-to-G change at cDNA nucleotide 4496 (c.4496C>G) for the first mutation. They were also heterozygous for a G-to-A change in the cDNA nucleotide 433 (c.433G>A). These mutations lead to the substitution of threonine at residue 1499 for arginine (p.T1499R; rs780755107) and aspartic acid at residue 145 for asparagine (p.D145N), respectively. Unaffected individuals were all homozygous wild type.
These variants were neither found in 100 ethnically matched healthy adult controls nor in 110 in-house exomes. The minor allele frequencies of the p.T1499R and p.D145N variants were <0.0001 in the Exome Aggregation Consortium (ExAC) and 0.008 in the Turkish whole-exome database. PolyPhen-2 predicts that these variants are probably damaging, with a score of 0.98 and 1.00, respectively, and Mutation Taster predicts both of these variants are disease causing. The proband also has a p.P1035R (c.3104C>G) heterozygous mutation in the DMXL2 gene (HGNC: 2938). The minor allele frequency of this variant was 0.0004 in ExAC and <0.0001 in the Turkish whole-exome database. PolyPhen-2 and Mutation Taster predict this variant to be benign and polymorphic, respectively.
Family 3
Whole-exome sequencing in the proband revealed a heterozygous variant a T-to-G change at cDNA 1943 predicting substitution of Leucine at residue 648 for Arginine, p.L648R. The affected sister and the affected uncle were heterozygous. One of the unaffected sisters was homozygous wild type. The father and one unaffected brother were also heterozygous for this variant. These variants were not found in 100 ethnically matched healthy adult controls or 110 in-house exomes. The minor allele frequency of the p.L648R was <0.0001 in ExAC and 0.0004 in the Turkish whole-exome database. PolyPhen-2 predicts that this variant is probably damaging, with a score of 0.73. Mutation Taster predicts this variant to be a polymorphism.
The proband also has p.S20I (C.59G>T) in the FSHB gene (HGNC: 3964). The minor allele frequency of this variant was 0.0002 in both ExAC and the Turkish whole-exome database. PolyPhen-2 and Mutation Taster predict this variant to be benign and disease causing, respectively.
The proband also has p.R118Q (c.353G>A) in the IGSF10 gene (HGNC: 26384). The minor allele frequency of this variant was 0.0002 in ExAC and 0.0005 in the Turkish whole-exome database. PolyPhen-2 and Mutation Taster predict this variant to be benign and polymorphic, respectively.
The proband also has p.R446Q (c.1337G>A) in the NR5A2 gene (HGNC: 7984). The minor allele frequency of this variant was 0.0003 in ExAC and <0.0001 in the Turkish whole-exome database. PolyPhen-2 and Mutation Taster predict this variant to be benign and disease causing, respectively.
These additional variants in family 3 were heterozygous for the proband, but the affected sister and affected uncle were homozygous wild type.
Family 4
Analysis of the whole-exome sequencing data in the proband indicated a missense mutation (c.4391A>G) in CCDC141 gene and confirmed with Sanger sequencing (Fig. 3). An A-to-G change at cDNA nucleotide 4391 predicting the substitution of histidine at residue 1464 for arginine (p.H1464R) was found. The proband and her father were heterozygous, and her mother and unaffected siblings were all homozygous wild type (Fig. 3).
Figure 3.
Mutation in the CCDC141 gene. In family 1, the top line shows the homozygous wild-type genotype, the middle line shows the heterozygous genotype, and the bottom line shows the homozygous mutant genotype. In the other families, the top line shows the homozygous wild-type genotype and the bottom line shows the heterozygous genotype.
This variant was neither found in 100 ethnically matched healthy adult controls nor in 110 in-house exomes. p.H1464R was not seen in either ExAC or the Turkish whole-exome database. PolyPhen-2 predicts that this variant is probably damaging, with a score of 0.976, and Mutation Taster predicts this variant to be disease causing.
Functional studies
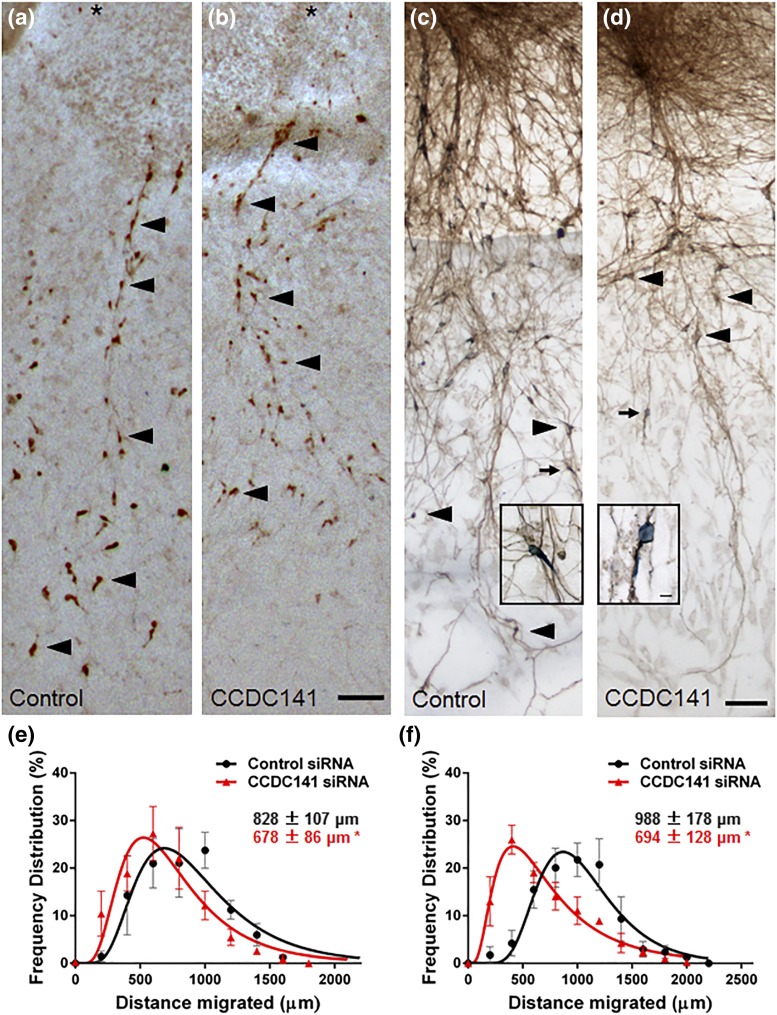
Ccdc141 is a cytoskeletal scaffolding protein that links cellular actin and microtubule components of the cytoskeleton, which underlie the shape and motility of a cell, with force-generating motor proteins (11). During migration of GnRH neurons from nasal regions to the hypothalamus, the actin cytoskeleton and myosin motor proteins generate force to move the nucleus forward (10). This force generates tension on the microtubule cytoskeleton, which forms a cage around the nucleus and thereby pulls the nucleus forward (12). To test the role of the cytoskeletal scaffolding protein Ccdc141 in GnRH neuronal migration, a series of explants was transfected with control or Ccdc141 siRNA and fixed/stained at division 5 for GnRH to assess overall GnRH cell migration, as previously reported (9). This treatment knocks down protein expression levels. GnRH neurons in Ccdc141 knockdown explants did not migrate as far from the main tissue mass as controls (treatment P < 0.001, two-way analysis of variance, n = 4 explants/group) (Fig. 4). Explants double-stained for GnRH and peripherin (to mark olfactory axons) showed no difference in axon extension [Fig. 4(c) and 4(d)]. This result demonstrated defective neuronal migration even when the olfactory axon pathway is intact. No changes were detected in olfactory axon outgrowth (10). The reduction of GnRH neuronal migration rates by approximately 25% may disrupt formation of normal GnRH neuronal circuit by altering the entrance/distribution of GnRH neurons in the brain.
Figure 4.
Ccdc141 knockdown reduces GnRH neuronal migration. (a and b) Bright field images of immune-positive GnRH neurons in explants transfected with (a) control or (b) Ccdc141 siRNA. The distance GnRH neurons (arrowheads highlight examples) migrated from the center of the nasal pit (asterisk) for the 3 days after electroporation was measured. (c and d) Double-staining of GnRH neurons (blue) and olfactory axon fibers (brown) in explants electroporated with (c) control or (d) Ccdc141 siRNA. Inset shows GnRH neurons (small arrows) closely apposed to axons, indicating that cell-cell adhesion was not disrupted. (e and f) Frequency distributions of the distances GnRH neurons migrated show a significant reduction in migration (shift to the left in frequency distribution curves) after knockdown using two different Ccdc141 siRNA sequences (*P < 0.001, two-way analysis of variance, n = 4 explants/condition). Inset measurement numbers show the mean ± standard deviation of migration distances. Scale is identical for all images (bar = 100 µm).
Discussion
We have previously reported inactivating CDCC141 mutations in two siblings with KS from a consanguineous family (5). More recently, we have demonstrated that migration of GnRH neurons was significantly reduced after knockdown of CCDC141, although no changes were detected in olfactory axon outgrowth after these manipulations (9). These data suggested that defects in GnRH neuronal migration per se, independent of olfactory receptor neuron migration, was responsible for the malformation of the GnRH network. This made CCDC141 the first IHH gene to be exclusively associated with cytoskeletal mechanics of GnRH neuronal migration (9).
In this study, we report several more independent IHH families with CCDC141 mutations. From these results, it appears that the CCDC141 mutations are relatively common (3.3%) among IHH/KS patients, which makes it potentially useful in laboratory diagnostics of IHH/KS.
Most notably, patients in this study (except for those in family 1) do not have impairment of sense of smell. This discordance could be explained by the fact that, in family 1, affected siblings have homozygous mutations also in FEZF1, which is required for the penetration of the olfactory sensory neurons into the brain (13) to synapse with the olfactory bulb. This is particularly relevant because our previousCCDC141 knockdown studies showed an unaffected olfactory axon outgrowth (9), suggesting that olfactory bulb development (thus sense of smell) should be normal in patients carrying CCDC141 mutations. Indeed, all patients in our newly reported families have normal sense of smell. This contention is further supported by their anatomically normal olfactory bulbs. Most recently, IGSF10 mutations were reported in patients with delayed puberty. Like in this study, IGSF10 knockdown caused a reduced migration of immature GnRH neurons in vitro, yet the affected individuals had normosmia. These two studies may highlight the disassociation of GnRH and olfactory neuron migration in the pathogenesis of IHH.
As in family 1, in other families in this study (except for family 4), there are additional potentially detrimental variants in one or more known IHH/KS genes. Even in family 4, there may be deleterious variants in yet-undiscovered puberty genes. Oligogenic inheritance is well established in IHH, accounting for about 10% of all cases (14, 15). With the increasing use of unbiased comprehensive genetic studies such as whole-exome sequencing, it is now further appreciated that oligogenic inheritance is more common than previously appreciated in Mendelian disorders (16). Recently, a dedicated database for digenic inheritance has been established in which IHH is listed along with other well-known oligogenic phenotypes such as nonsyndromic hearing impairment (17). This oligogenic inheritance may suggest that, to elicit an IHH/KS phenotype, a combination of several gene dysfunctions is required. It has been shown that intrafamilial and even intrasibship locus and allelic heterogeneity is not rare even in consanguineous families (16, 18, 19). Indeed, in this study, we have observed a complex genotype-phenotype relationship, which is challenging to explain in view of traditional Mendelian inheritance. For example, in family 3, the proband, who suffers from full-blown IHH, has variants in an additional three different puberty genes, although his older sister and paternal uncle, both having only pubertal delay, harbored only the CCDC141 variant. Yet, his brother and father appear to be unaffected despite carrying the same CCDC141 variant plus one more of the other three variants. It should be noted, however, that similar variability of phenotypes from IHH to delayed puberty to even normal timing in persons carrying the same genotype within the same family (phenotype heterogeneity) has been repeatedly observed in IHH/KS (20–23). Similar families with genotype phenotype inconsistencies were also reported with the most recent puberty gene, IGSF10 (24).
A certain proportion of the discordances in genotype-phenotype as elaborated earlier could be explained by spontaneous or induced gain of function in IHH. Regain of central gonadal function or reversibility is seen in about 10% of all IHH cases (25), even in the most severe cases of congenital IHH evidenced by cryptorchidism and micropenis in the newborn (26). Indeed, it is evident from the histories in this study that some of these individuals, for example, the proband and his mother in family 2, have gone through clinical reversibility after sex steroid replacement, and the older sister in family 3 apparently did so spontaneously. Furthermore, the mother in family 2 may have even reverted to full IHH after having a period of clinical recovery, which enabled her to have successful pregnancies. Such back-and-forth changes in central gonadal function have been previously reported (27).
The cases illustrated here also provide a conceptual framework for considering reversible vs irreversible IHH or KS. CCDC141 mutations in general do not appear to eliminate the olfactory axon pathway along which GnRH neurons migrate, instead preferentially affecting the migration of the neurons themselves (9). In half of these families, reversibility of symptoms was observed. These results suggest that mutations leave the GnRH circuit partially intact, by effecting a more modest reduction in GnRH motility alone, and open the possibility for reversal of symptoms through residual functionality of a malformed, but partially developed, GnRH neuronal circuit.
In sum, it appears that this complexity of genotype and phenotype in IHH in general and with CCDC141 in particular stems from the interplay of two well-established phenomena: oligogenic inheritance/locus heterogeneity and spontaneous or induced clinical reversibility. Allelic heterogeneity within the families (as seen in this study) is likely to be increasingly encountered with the advent of genotyping technologies and adds another layer of complexity. Taking these factors into account while analyzing pedigree data can increase the success rate in the identification of causal variants for IHH.
In conclusion, this study confirms the relevance of CCDC141 in the pathogenesis of IHH and expands the clinical and genetic spectrum. This study also attests to the complexity of phenotype and genotype in IHH. It is now increasingly clear that IHH is an oligogenic and phenotypically highly variable clinical condition. When IHH is considered in the context of a human disease model for studying puberty, it could be inferred from these data and the recent literature that puberty is likely to be a function of several if not many genes in association with other internal and external factors.
Acknowledgments
We thank the Advanced Genomics and Bioinformatics Research Center for checking the variant frequency in its in-house Turkish whole-exome database at the Scientific and Technological Research Council of Turkey-BILGEM.
Acknowledgments
This work was supported by the Scientific and Technological Research Council of Turkey project number 113S962, the Cukurova University scientific research project number 4579, and the Intramural Research Program of the National Institutes of Health, National Institute of Neurologic Disorders and Stroke Grant NS002824-25/26 (to B.I.H. and S.W.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- cDNA
- complementary DNA
- ExAC
- Exome Aggregation Consortium
- FSH
- follicle-stimulating hormone
- GnRH
- gonadotropin-releasing hormone
- IHH
- idiopathic hypogonadotropic hypogonadism
- KS
- Kallmann syndrome
- LH
- luteinizing hormone
- MRI
- magnetic resonance imaging
- siRNA
- small interfering RNA
- SNP
- single-nucleotide polymorphism
- UPSIT
- University of Pennsylvania Smell Identification Test.
References
- 1.Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338(6211):161–164. [DOI] [PubMed] [Google Scholar]
- 2.Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci USA. 1989;86(20):8132–8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topaloglu AK, Kotan LD. Genetics of hypogonadotropic hypogonadism. Endocr Dev. 2016;29:36–49. [DOI] [PubMed] [Google Scholar]
- 4.Topaloglu AK, Kotan LD. Molecular causes of hypogonadotropic hypogonadism. Curr Opin Obstet Gynecol. 2010;22(4):264–270. [DOI] [PubMed] [Google Scholar]
- 5.Kotan LD, Hutchins BI, Ozkan Y, Demirel F, Stoner H, Cheng PJ, Esen I, Gurbuz F, Bicakci YK, Mengen E, Yuksel B, Wray S, Topaloglu AK. Mutations in FEZF1 cause Kallmann syndrome. Am J Hum Genet. 2014;95(3):326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32(3):489–502. [DOI] [PubMed] [Google Scholar]
- 7.Chang X, Wang K. wANNOVAR: annotating genetic variants for personal genomes via the web. J Med Genet. 2012;49(7):433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wray S. From nose to brain: development of gonadotrophin-releasing hormone-1 neurones. J Neuroendocrinol. 2010;22(7):743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutchins BI, Kotan LD, Taylor-Burds C, Ozkan Y, Cheng PJ, Gurbuz F, Tiong JD, Mengen E, Yuksel B, Topaloglu AK, Wray S. CCDC141 mutation identified in anosmic hypogonadotropic hypogonadism (Kallmann syndrome) alters GnRH neuronal migration. Endocrinology. 2016;157(5):1956–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutchins BI, Klenke U, Wray S. Calcium release-dependent actin flow in the leading process mediates axophilic migration. J Neurosci. 2013;33(28):11361–11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuda T, Sugita S, Inatome R, Yanagi S. CAMDI, a novel disrupted in schizophrenia 1 (DISC1)-binding protein, is required for radial migration. J Biol Chem. 2010;285(52):40554–40561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutchins BI, Wray S. Capture of microtubule plus-ends at the actin cortex promotes axophilic neuronal migration by enhancing microtubule tension in the leading process. Front Cell Neurosci. 2014;8:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe Y, Inoue K, Okuyama-Yamamoto A, Nakai N, Nakatani J, Nibu K, Sato N, Iiboshi Y, Yusa K, Kondoh G, Takeda J, Terashima T, Takumi T. Fezf1 is required for penetration of the basal lamina by olfactory axons to promote olfactory development. J Comp Neurol. 2009;515(5):565–584. [DOI] [PubMed] [Google Scholar]
- 14.Quaynor SD, Kim HG, Cappello EM, Williams T, Chorich LP, Bick DP, Sherins RJ, Layman LC. The prevalence of digenic mutations in patients with normosmic hypogonadotropic hypogonadism and Kallmann syndrome. Fertil Steril. 2011;96(6):1424–1430.e6. [DOI] [PMC free article] [PubMed]
- 15.Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, Li WP, Maccoll G, Eliseenkova AV, Olsen SK, Ibrahimi OA, Hayes FJ, Boepple P, Hall JE, Bouloux P, Mohammadi M, Crowley W. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest. 2007;117(2):457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chong JX, Buckingham KJ, Jhangiani SN, Boehm C, Sobreira N, Smith JD, Harrell TM, McMillin MJ, Wiszniewski W, Gambin T, Coban Akdemir ZH, Doheny K, Scott AF, Avramopoulos D, Chakravarti A, Hoover-Fong J, Mathews D, Witmer PD, Ling H, Hetrick K, Watkins L, Patterson KE, Reinier F, Blue E, Muzny D, Kircher M, Bilguvar K, López-Giráldez F, Sutton VR, Tabor HK, Leal SM, Gunel M, Mane S, Gibbs RA, Boerwinkle E, Hamosh A, Shendure J, Lupski JR, Lifton RP, Valle D, Nickerson DA, Bamshad MJ; Centers for Mendelian Genomics . The genetic basis of mendelian phenotypes: discoveries, challenges, and opportunities. Am J Hum Genet. 2015;97(2):199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gazzo AM, Daneels D, Cilia E, Bonduelle M, Abramowicz M, Van Dooren S, Smits G, Lenaerts T. DIDA: a curated and annotated digenic diseases database. Nucleic Acids Res. 2015;44(D1):D900–D907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rehman AU, Santos-Cortez RL, Drummond MC, Shahzad M, Lee K, Morell RJ, Ansar M, Jan A, Wang X, Aziz A, Riazuddin S, Smith JD, Wang GT, Ahmed ZM, Gul K, Shearer AE, Smith RJ, Shendure J, Bamshad MJ, Nickerson DA, Hinnant J, Khan SN, Fisher RA, Ahmad W, Friderici KH, Riazuddin S, Friedman TB, Wilch ES, Leal SM; University of Washington Center for Mendelian Genomics . Challenges and solutions for gene identification in the presence of familial locus heterogeneity. Eur J Hum Genet. 2014;23(9):1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benayoun L, Spiegel R, Auslender N, Abbasi AH, Rizel L, Hujeirat Y, Salama I, Garzozi HJ, Allon-Shalev S, Ben-Yosef T. Genetic heterogeneity in two consanguineous families segregating early onset retinal degeneration: the pitfalls of homozygosity mapping. Am J Med Genet A. 2009;149A(4):650–656. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J, Choa RE, Guo MH, Plummer L, Buck C, Palmert MR, Hirschhorn JN, Seminara SB, Chan YM. A shared genetic basis for self-limited delayed puberty and idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2015;100(4):E646–E654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sykiotis GP, Plummer L, Hughes VA, Au M, Durrani S, Nayak-Young S, Dwyer AA, Quinton R, Hall JE, Gusella JF, Seminara SB, Crowley WF Jr, Pitteloud N. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc Natl Acad Sci USA. 2010;107(34):15140–15144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarfati J, Guiochon-Mantel A, Rondard P, Arnulf I, Garcia-Piñero A, Wolczynski S, Brailly-Tabard S, Bidet M, Ramos-Arroyo M, Mathieu M, Lienhardt-Roussie A, Morgan G, Turki Z, Bremont C, Lespinasse J, Du Boullay H, Chabbert-Buffet N, Jacquemont S, Reach G, De Talence N, Tonella P, Conrad B, Despert F, Delobel B, Brue T, Bouvattier C, Cabrol S, Pugeat M, Murat A, Bouchard P, Hardelin JP, Dodé C, Young J. A comparative phenotypic study of Kallmann syndrome patients carrying monoallelic and biallelic mutations in the prokineticin 2 or prokineticin receptor 2 genes. J Clin Endocrinol Metab. 2010;95(2):659–669. [DOI] [PubMed] [Google Scholar]
- 23.Boehm U, Bouloux PM, Dattani MT, de Roux N, Dodé C, Dunkel L, Dwyer AA, Giacobini P, Hardelin JP, Juul A, Maghnie M, Pitteloud N, Prevot V, Raivio T, Tena-Sempere M, Quinton R, Young J. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism—pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2015;11(9):547–564. [DOI] [PubMed] [Google Scholar]
- 24.Howard SR, Guasti L, Ruiz-Babot G, Mancini A, David A, Storr HL, Metherell LA, Sternberg MJ, Cabrera CP, Warren HR, Barnes MR, Quinton R, de Roux N, Young J, Guiochon-Mantel A, Wehkalampi K, André V, Gothilf Y, Cariboni A, Dunkel L. IGSF10 mutations dysregulate gonadotropin-releasing hormone neuronal migration resulting in delayed puberty. EMBO Mol Med. 2016;8(6):626–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raivio T, Falardeau J, Dwyer A, Quinton R, Hayes FJ, Hughes VA, Cole LW, Pearce SH, Lee H, Boepple P, Crowley WF Jr, Pitteloud N. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med. 2007;357(9):863–873. [DOI] [PubMed] [Google Scholar]
- 26.Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonça BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95(6):2857–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sidhoum VF, Chan YM, Lippincott MF, Balasubramanian R, Quinton R, Plummer L, Dwyer A, Pitteloud N, Hayes FJ, Hall JE, Martin KA, Boepple PA, Seminara SB. Reversal and relapse of hypogonadotropic hypogonadism: resilience and fragility of the reproductive neuroendocrine system. J Clin Endocrinol Metab. 2014;99(3):861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]