Abstract
Context:
It is hypothesized that obesity adversely affects the ovarian environment, which can disrupt oocyte maturation and embryonic development.
Objective:
This study aimed to compare oocyte gene expression profiles and follicular fluid (FF) content from overweight/obese (OW) women and normal-weight (NW) women who were undergoing fertility treatments.
Design:
Using single-cell transcriptomic analyses, we investigated oocyte gene expression using RNA sequencing.
Patients or Other Participants:
Eleven OW women and 13 NW women undergoing fertility treatments were enrolled.
Main Outcome Measures:
Oocyte messenger RNA profiles as well as serum and FF hormone and lipid levels were assessed.
Results:
OW women had significantly higher body mass index, body fat percentage, and serum homeostatic model assessment−insulin resistance index compared with NW women (P < 0.01). Serum leptin and C-reactive protein (CRP) levels as well as FF leptin, CRP, and triglyceride levels were increased (P < 0.05) in OW compared with NW women. Oocytes from OW women had increased expression of proinflammatory (CXCL2; P = 0.071) and oxidative stress–related (DUSP1; P = 0.051) genes but had decreased expression of GAS7 (fat metabolism; P = 0.065), TXNIP (oxidative stress; P = 0.055), and transcription factors ID3 (P = 0.075) and TWIST1 (P = 0.099) compared with NW women.
Conclusions:
These findings provide evidence for the significant influence of body composition on oocyte transcript abundance in women undergoing hormonal induction to retrieve oocytes. They further identify the potential for maternal diet to influence oocyte gene expression. The preconception period is, therefore, an important window of opportunity to consider for lifestyle interventions.
We studied oocyte mRNA profiles as well as serum and follicular fluid hormone and lipid levels in obese women compared with those in normal-weight women and found significant differences.
More than two-thirds of women of reproductive age are classified as overweight or obese [OW; body mass index (BMI) >25 kg/m2] and are predisposed to higher risk of infertility and pregnancy complications, leading to higher rates of fertility treatments (1, 2). During in vitro fertilization (IVF) procedures, OW women have significantly lower numbers of oocytes retrieved, decreased numbers of cleaved embryos, reduced high-grade embryos, and increased number of miscarriages compared with normal-weight (NW) women (2, 3). Studies have also demonstrated a reduction in pregnancy and live birth rates associated with higher oocyte donor BMI despite controlling for the recipients’ BMI, which underlines the importance of body weight and adiposity on oocyte integrity and competence (4).
A majority of clinical studies have focused on the effects of obesity on pregnancy and/or fertility outcomes (2, 3). Understanding the molecular changes present in oocytes from OW women compared with NW women before conception may provide insights into the causes of infertility, as well as the potential consequences of preconception female obesity on offspring development. Tools such as RNA sequencing (RNA-Seq) have provided the ability to study gene regulation at an individual cell level (5). In this study, we used global transcriptome sequencing of single human oocytes at various maturation stages—germinal vesicle (GV), metaphase I (MI), and metaphase II (MII)—to identify differentially expressed genes between NW and OW women. We hypothesized that oocytes from OW women would have differential expression of genes related to the inflammatory and lipid metabolism pathways compared with those from NW women. We further hypothesized that follicular fluid (FF) content of OW women would have greater levels of insulin, glucose, triglycerides, and inflammatory markers than FF from NW women.
Methods
Participants
Healthy women aged 18 to 38 years who were undergoing fertility treatment that included an oocyte retrieval procedure at a local fertility clinic were enrolled on the basis of BMI: NW (18.5 to 24.9 kg/m2; N = 13) and OW (≥25 kg/m2; N = 11). Participants’ infertility diagnosis included 25% male factor, 25% ovulatory disorder, 17% tubal factor, 12.5% endometriosis, and 21% unexplained (Table 1). Women diagnosed with polycystic ovary syndrome, diabetes, or cardiovascular diseases were excluded. The study protocol was approved by the institutional review board of the University of Arkansas for Medical Sciences, and informed consent was obtained (clinicaltrials.gov no. NCT01480024).
Table 1.
Participant Characteristics and Serum Concentrations
| BMI 18.5–24.9 kg/m2 (N = 13) | BMI >25 kg/m2 (N = 11) | P Value | |
|---|---|---|---|
| Age, y | 31.6 ± 0.9 | 32.1 ± 1.5 | 0.634 |
| Pregnancy, N | 8 | 6 | 0.999 |
| Live births, N | 7 | 3 | 0.245 |
| Fertility diagnosis, N | — | — | 0.971 |
| Male factor, N | 4 | 2 | — |
| Ovulatory disorder, N | 3 | 3 | — |
| Tubal factor, N | 2 | 2 | — |
| Endometriosis, N | 1 | 2 | — |
| Unexplained, N | 3 | 2 | — |
| BMI, kg/m2 | 22.3 ± 0.6 | 32.3 ± 1.8 | 0.0001 |
| Body fat, % | 32.1 ± 2.9 | 44.2 ± 2.1 | 0.0003 |
| REE, kcal/kg FFM0.89 | 21.8 ± 0.5 | 23.3 ± 0.7 | 0.093 |
| RER | 0.84 ± 0.02 | 0.83 ± 0.02 | 0.466 |
| Systolic BP, mm Hg | 106.3 ± 2.0 | 122.0 ± 3.3 | 0.001 |
| Diastolic BP, mm Hg | 65.7 ± 2.0 | 81.5 ± 3.2 | 0.0003 |
| LH, mIU/mL | 5.3 ± 1.2 | 4.5 ± 1.1 | 0.317 |
| FSH, mIU/mL | 8.0 ± 0.55 | 6.9 ± 0.7 | 0.155 |
| AMH, ng/mL | 7.1 ± 4.1 | 8.6 ± 2.8 | 0.077 |
| Estradiol, pg/mL | 53.0 ± 5.8 | 47.7 ± 9.1 | 0.403 |
| Glucose, mg/dL | 108.9 ± 3.5 | 111.5 ± 5.6 | 0.744 |
| Insulin, ng/mL | 5.4 ± 0.9 | 7.7 ± 0.8 | 0.113 |
| HOMA-IR | 0.7 ± 0.1 | 1.8 ± 0.3 | 0.002 |
| Total cholesterol, mg/dL | 232.9 ± 9.7 | 238.4 ± 15.5 | 0.909 |
| LDL, mg/dL | 75.3 ± 8.6 | 90.6 ± 8.8 | 0.212 |
| HDL, mg/dL | 49.3 ± 2.2 | 44.3 ± 2.6 | 0.150 |
| NEFA, μM | 569.1 ± 35.6 | 635.5 ± 68.3 | 0.687 |
| Omega-6/omega-3, % | 16.2 ± 2.2 | 17.8 ± 1.3 | 0.541 |
| Triglycerides, mg/dL | 64.3 ± 7.2 | 72.7 ± 8.8 | 0.287 |
| Leptin, ng/mL | 5.3 ± 1.0 | 27.2 ± 6.6 | 0.003 |
| IL-6, pg/mL | 3.1 ± 0.6 | 2.8 ± 0.4 | 0.613 |
| TNF-α, pg/mL | 2.4 ± 0.3 | 2.9 ± 0.4 | 0.299 |
| CCL2, pg/mL | 238.6 ± 22.4 | 403.6 ± 51.0 | 0.005 |
| C-reactive protein, μg/mL | 3.2 ± 0.7 | 5.7 ± 1.0 | 0.067 |
Data are expressed as mean ± standard error of the mean. Differences between groups were determined using Mann-Whitney U nonparametric tests with a statistical significance level of P ≤ 0.05.
Abbreviations: AMH, anti-Müllerian hormone; BP, blood pressure; CCL2, chemokine (C-C motif) ligand 2; FFM, fat-free mass; FSH, follicle-stimulating hormone; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment−insulin resistance index; IL-6, interleukin-6; LDL, low-density lipoprotein; LH, luteinizing hormone; NEFA, nonesterified fatty acid; REE, resting energy expenditure; RER, respiratory exchange ratio; TNF, tumor necrosis factor.
Oocyte and FF retrieval procedures
During fertility treatment, participants underwent a superovulation procedure that included an oocyte retrieval. (Details are provided in the Supplemental Methods (43.4KB, docx) ). Oocytes were separated from FF, and individual oocytes that were not used for IVF were staged (GV, MI, MII) and collected following standardized procedures. Oocytes were placed in 500 µL of Dynabeads messenger RNA (mRNA) lysis buffer (Life Technologies, Carlsbad, CA) and stored at −70°C. FF aspirates were centrifuged, and supernatants were collected for storage at −70°C.
Anthropometrics
Body mass was measured to the nearest 0.1 kg using a standing scale (BWB-800S digital scale; Tanita, Arlington Heights, IL), and stature was measured to the nearest 0.1 cm using a standard wall-mounted stadiometer. Blood pressure was measured using the HEM-907 (Omron, Lake Forest, IL).
Body composition
Measurements of fat mass, fat-free mass (FFM), and percent body fat of the overnight fasted participants were assessed by air displacement plethysmography (BodPod®; COSMED USA, Inc., Concord, CA) following manufacturer recommendations.
Dietary intake
Dietary intakes were assessed using 3-day food records analyzed with the Nutrient Data System for Research software (Nutrition Coordinating Center; University of Minnesota, MN). Daily dietary nutrient intakes were averaged over the 3 days. The percentage of calories from fat (% fat kcal), saturated fat (% SFA), and monounsaturated fat (% MUFA) was calculated.
Resting energy expenditure
Using indirect calorimetry (Moxus; AEI Technologies, Bastrio, TX), respiratory exchange ratio (RER) and resting energy expenditure (REE) were derived from oxygen consumption and carbon dioxide production during a 10-minute steady state in overnight fasted participants. To account for differences in body composition between the two groups, log-log regression models of REE and body FFM (kg) were used to assess their relationship, as previously described by Purcell et al. (6).
Serum and FF analyses
Serum samples were obtained from overnight fasted participants. Serum and FF glucose (Synermed, Westfield, IN), total cholesterol (Synermed), and triglyceride (Cayman Chemical, Ann Arbor, MI) levels were assessed using colorimetric assays. Serum and FF insulin, leptin, tumor necrosis factor (TNF)-α, C-reactive protein (CRP; EMD Millipore, Billerica, MA), and interleukin (IL)-6 (R&D Systems, Minneapolis, MN) were measured using enzyme-linked immunosorbent assay kits. Serum lipid profiles—nonesterified fatty acids, high-density lipoprotein, and low-density lipoprotein—were analyzed by enzymatic methods on an RX Daytona clinical analyzer (Randox Laboratories, Kearneysville, WV). All assays were performed in accordance with the manufacturers’ instructions. Omega fatty acid methyl esters were prepared from serum and FF samples using a direct in situ transesterification method (7). The individual fatty acid methyl esters were quantified using gas chromatography-mass spectrometry (8) with a Supelco SP-2330 GC column (30 m × 0.25 mm × 0.2 µm; Sigma-Aldrich, St. Louis, MO). The MS detector was operated in total ion chromatogram mode, and selected ions were extracted for quantification.
Oocyte RNA sequencing
Both mRNA and genomic DNA were isolated from individual oocytes using a Dynabeads mRNA Direct Kit (Life Technologies) as previously described (9). mRNA was amplified using a SMARTer Ultra Low Input RNA Kit for Sequencing-v3 (Clontech Laboratories, Mountain View, CA), and complementary DNA (cDNA) output was quantified using Qubit dsDNA High Sensitivity Assay Kit (Invitrogen, Carlsbad, CA). cDNA libraries were prepared using the Nextera XT DNA Kit (Illumina, San Diego, CA). Fragmented cDNA was evaluated using the Experion DNA 1K Chip (Bio-Rad, Hercules, CA) to determine size distribution of the libraries. Samples were sequenced (75-bp single reads) using the NextSEquation 500 System (Illumina).
Sequencing data analyses
Individual libraries were generated for each oocyte (N = 11 for GV [NW, N = 6; OW, N = 5]; N = 18 for MI [NW, N = 11; OW, N = 7]; and N = 10 for MII [NW, N = 5; OW, N = 5]), and RNA-Seq was conducted. Sequencing reads from each sample were trimmed and filtered. Using TopHat, reads were aligned to the human genome (hg19), and bam files were generated. Each biological replicate led to ∼14 to 20 million reads. All data were analyzed using SeqMonk and R software. Gene expression levels were expressed as raw read counts for differential expression analysis and as log-transformed normalized reads per kilobase per million mapped reads for visualization. Differentially expressed gene expression was identified using the DESeq2 package and was filtered at ±twofold change and statistical significance with a P value <0.05. Gene Ontology (GO), transcription factor (TF) target analysis, and pathway analyses were conducted with DAVID bioinformatics and/or WebGestalt, which included multiple testing corrections.
Real-time quantitative polymerase chain reaction
Isolated cDNA that was used for RNA-Seq was used for real-time quantitative polymerase chain reactions (RT-qPCRs) (ABI Prism 7500 Fast Sequence Detection System; Applied Biosystems, Foster City, CA), and gene-specific primers were designed using Primer Express Software (Applied Biosystems; Supplemental Table 1 (43.4KB, docx) ). Genes of interest were normalized to the geometric mean of β-actin and glyceraldehyde 3-phosphate dehydrogenase mRNA expression as they were previously used human cumulus cells (10–13). A total of nine genes were selected: CXCL2, CXCL3, CCL20, TWIST1, ID3, IL-34, DUSP1, GAS7, and TXNIP (additional gene information is presented in the Supplemental Materials (43.4KB, docx) ).
Statistical analysis
Continuous data are summarized as means ± standard error of the mean (SEM), and comparisons between NW and OW women were performed using the Mann-Whitney U test for nonparametric analyses. Categorical data are summarized as counts and percentages, and comparisons between NW and OW women were performed using Fisher’s exact test. Statistical significance was determined as P ≤ 0.05, and trending significance was defined as P < 0.10. RT-PCR results were expressed as mean fold change from NW women ± SEM. Correlation analyses were conducted using either Pearson correlations or nonparametric Spearman correlations. Statistical analyses were performed using GraphPad Prism 7.0 (La Jolla, CA). Multiple linear regression models were used to identify maternal factors (BMI, % body fat, REE, RER, % fat kcal, % SFA, and % MUFA) significantly associated with TWIST1 RNA-Seq expression using Stata version 14.1 (College Station, TX). The most parsimonious model was constructed by initially including all seven predictors in the model and then sequentially eliminating the predictor with the smallest and nonsignificant partial correlation. Similar results were also obtained when stepwise regression was used.
Results
Participant characteristics
Participants were 92% white, 4% Asian, and 4% African American (Table 1). By study design, OW participants had higher BMI and body fat percentage than NW women (P < 0.05; Table 1). There were no differences in RER or REE (kcal/kg FFM0.89) between the groups (Table 1). Dietary intake of OW women was ∼550 kcal/d greater than that of NW women. OW women ate fewer carbohydrates, more fat, and a greater proportion of energy from MUFAs than NW women did (P < 0.05; Supplemental Table 2 (43.4KB, docx) ).
Greater serum leptin, homeostatic model assessment−insulin resistance index, and inflammation in OW women
OW women had higher serum leptin level, homeostasis model assessment−insulin resistance (HOMA-IR) index, and levels of the inflammatory markers CRP and chemokine (C-C motif) ligand 2 (CCL2) than NW women (P < 0.05; Table 1). There were no significant group differences in levels of serum glucose, insulin, lipids (total cholesterol, triglycerides, high-density lipoprotein, low-density lipoprotein, omega-6/omega-3 ratio, or nonesterified fatty acid), inflammatory markers (TNF-α, IL-6), or reproductive markers (luteinizing hormone, follicle-stimulating hormone, anti-Müllerian hormone, estradiol) (Table 1).
Obesity was associated with altered FF metabolite contents
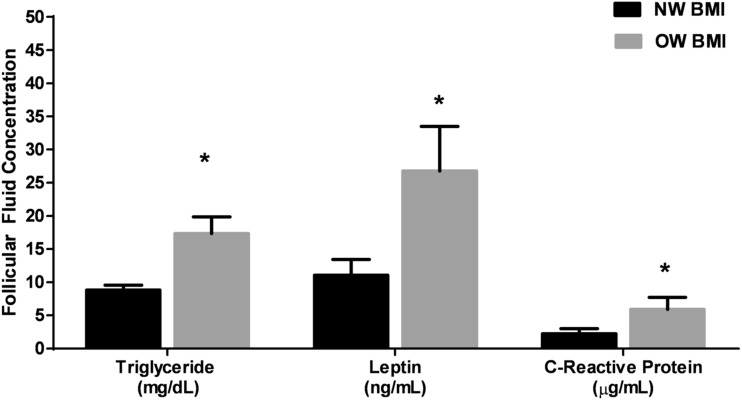
OW women had increased FF triglyceride, leptin, and CRP levels (P < 0.05; Fig. 1) compared with those of NW women. There were no significant differences in FF total cholesterol, glucose, insulin, CCL2, and TNF-α levels between groups (data not shown). However, FF TNF-α levels were significantly correlated with total body fat percentage (R = 0.74; P = 0.001). In addition, the serum omega-6/omega-3 ratio was significantly correlated with FF TNF-α levels (R = 0.570; P = 0.05) and FF CCL2 levels (R = 0.604; P = 0.02), suggesting that higher ratios of polyunsaturated fatty acids were associated with higher inflammation in the FF.
Figure 1.
FF levels of triglycerides, leptin, and CRP from NW (N = 13, black bars) and OW (N = 11, gray bars) women. Data are represented as mean ± SEM. A significant difference between groups was set at P < 0.05 (*) using the Mann-Whitney U nonparametric tests.
Oocytes of various maturation stages showed distinct gene expression profiles
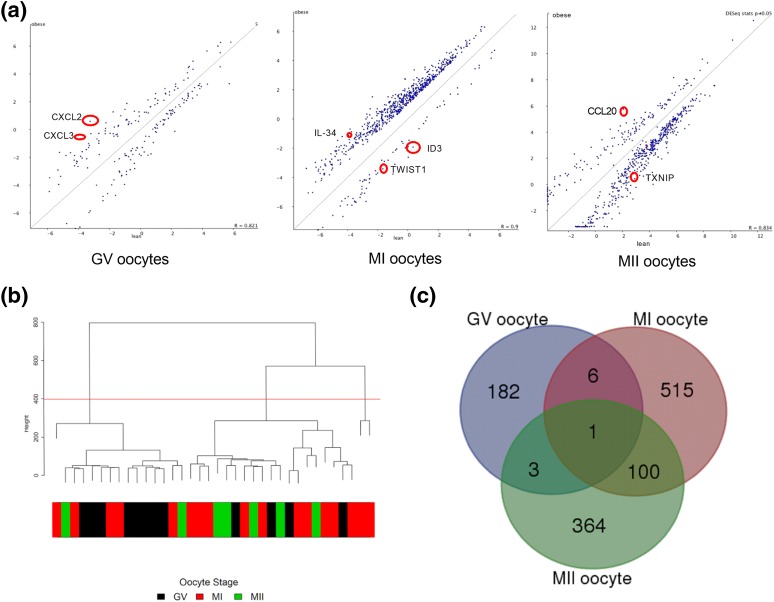
Results revealed dramatic changes in oocyte transcriptome at different maturation stages of OW women compared with those of NW women. OW women showed 192 genes to be differentially expressed in GV, 624 genes to be differentially expressed in MI, and 468 genes to be differentially expressed in MII oocytes compared with oocytes of NW women. Scatterplots were used to visualize differentially expressed genes between the three maturation stages of the oocytes from OW and NW women (Fig. 2). These results suggest that body composition may influence oocyte gene expression and/or mRNA stability in women undergoing fertility treatments. Although not a focus of this analysis, hierarchical clustering was used to observe potential differences between maturation stages of the oocytes. Results demonstrated that GV oocyte gene expression was more distinct than MI and MII oocyte gene expression regardless of body composition. Similarly, the Venn diagram analysis of differentially expressed genes between OW and NW women showed more overlap of genes between MI and MII oocytes compared with genes altered in GV samples. Supplemental Table 3 (43.4KB, docx) illustrates the similarities in gene expression due to maturation stages.
Figure 2.
(a) Scatterplots of differentially expressed genes between NW and OW groups identified on the basis of P value ≤0.05 and ±twofold change with SeqMonk. Selected genes for RT-qPCR confirmation are circled in red. (b) Hierarchical clustering of reads per kilobase per million mapped reads values using a function in the FactoMineR package of all genes showing grouping of individual samples that belong to different oocyte maturation stages. (c) Venn diagram for differentially expressed genes between maturation stages.
Oocytes from OW women showed distinct enrichment of GO terms, TFs, and KEGG pathways
GO term enrichments were used to understand the different functions associated with differentially expressed genes. The top 10 GO terms that were significantly enriched included cytokine receptor binding; cytokine activity; regulation of DNA damage response; regulation of transcription, DNA-dependent; regulation of RNA metabolic process and apoptosis; and regulation of gene expression, DNA binding, and nucleus [Supplemental Tables 4(a–c) (43.4KB, docx) ]. TF target analysis identified a variety of upstream regulators at each oocyte maturation stage. FOXO4 and AP4 were identified as upstream targets for TXNIP, DUSP1, ID3, TWIST1, IL-34, and GAS7 in MI and MII. SP1 was identified as a key TF in all maturation stages (Supplemental Table 5 (43.4KB, docx) ). A selection of the top 10 KEGG pathways that were differentially regulated in OW oocytes compared with NW oocytes included chemokine signaling pathway and cytokine-cytokine receptor interaction in the GV oocytes (adjusted P values of 0.007 and 0.01, respectively); metabolic pathways and MAPK signaling pathway in MI oocytes (adjusted P values of 0.02 and 0.07, respectively); and metabolic pathways and mTOR signaling pathway in MII oocytes (adjusted P value of 0.02; Table 2).
Table 2.
Top 10 Most Significantly Enriched KEGG Pathways for Each Oocyte Maturation Stage
| KEGG Pathways | Adjusted P Value | No. of Target Genes | Gene Name |
|---|---|---|---|
| GV oocytes | |||
| Chemokine signaling pathway | 0.007 | 5 | CXCL3, IL-8, CCL20, CXCL2, GNG7 |
| Cytokine-cytokine receptor interaction | 0.011 | 5 | CXCL3, IL-8, CCL20, CXCL2, ACVR1B |
| Pathways in cancer | 0.023 | 5 | PAPOLG, PPP2R3B, CPSF1, PPP2R2D, PPP2R5D |
| Metabolic pathways | 0.092 | 7 | NDUFA4L2, HKDC1, GALNT10, UGT2A1, PLCD1, PIGL, PLA2G12B |
| MI oocytes | |||
| Axon guidance | 0.004 | 8 | NFATC2, NTN1, SEMA4F, EPHA2, SEMA4A, NCK1, RGS3, SRGAP3 |
| Metabolic pathways | 0.004 | 30 | NDUFA4L2, PCCB, TK1, NDUFS1, AGPAT4, GGT6, HAL, EXTL3, GCNT3, CNDP1, BHMT, SDHA, PON1, DBH, FLAD1, SMPD3, PDXP, LCT, IPPK, AGPAT2, MAOA, DEGS2, POLR1B, POLR3B, NT5M, KHK, MGAT5, HDC, B4GALT2, PFKP |
| Neurotrophin signaling pathway | 0.020 | 6 | RPS6KA4, BRAF, ZNF274, SH2B2, FOXO3, MAPK12 |
| MAPK signaling pathway | 0.020 | 10 | HSPA1L, BRAF, TAOK2, TRAF2, FGF18, RPS6KA4, NFATC2, RASGRP1, MAPK8IP2, MAPK12 |
| Cell cycle | 0.020 | 6 | CDC25A, ORC1, CDC14A, RBL1, E2F2, MCM3 |
| Protein processing in endoplasmic reticulum | 0.020 | 7 | HSPA1L, UBE2J2, DNAJB1, HSPA4L, TRAF2, WFS1, PREB |
| mRNA surveillance pathway | 0.020 | 5 | PAPOLG, PPP2R3B, CPSF1, PPP2R2D, PPP2R5D |
| Tight junction | 0.021 | 6 | EXOC3, ACTN1, AMOTL1, MYH14, PPP2R2D, EPB41 |
| Pyrimidine metabolism | 0.023 | 5 | TK1, POLR1B, POLR3B, NT5M, TXNRD2 |
| Hepatitis C | 0.048 | 5 | BRAF, OAS3, TRAF2, PPP2R2D, MAPK12 |
| MII oocytes | |||
| ECM-receptor interaction | 0.005 | 6 | VWF, SPP1, SV2C, ITGA10, THBS2, COL6A6 |
| Metabolic pathways | 0.007 | 24 | POLA2, PCCB, ALG6, UPB1, IDH3A, UPP1, HAL, PPPAP2C, GCNT3, PMM2, GAL3ST1, PON1, ATP5D, FTCD, AGPAT2, HPSE, EXTL1, PLCG2, LPCAT4, AGMAT, ACOX3, KHK, POLR1E, HDC |
| Protein digestion and absorption | 0.009 | 5 | CELA3A, SLC16A10, COL15A1, SLC8A1, COL6A6 |
| mTOR signaling pathway | 0.009 | 4 | RPS6KA6, TSC1, STRADA, PDPK1 |
| Cell cycle | 0.033 | 5 | CDC25A, CDC6, MCM5, MCM3, SMC1A |
| Axon guidance | 0.033 | 5 | EPHA5, EFNA1, SEMA6A, FES, RND1 |
| Focal adhesion | 0.043 | 6 | VWF, PDPK1, SPP1, ITGA10, THBS2, COL6A6 |
| Pyrimidine metabolism | 0.043 | 4 | POLA2, UPB1, POLR1E, UPP1 |
| Purine metabolism | 0.053 | 5 | NPR1, POLA2, PDE6C, PDE9A, POLR1E |
| Neurotrophin signaling pathway | 0.076 | 4 | PSEN1, ZNF274, PLCG2, RPS6KA6 |
Validation of selected genes by RT-qPCR
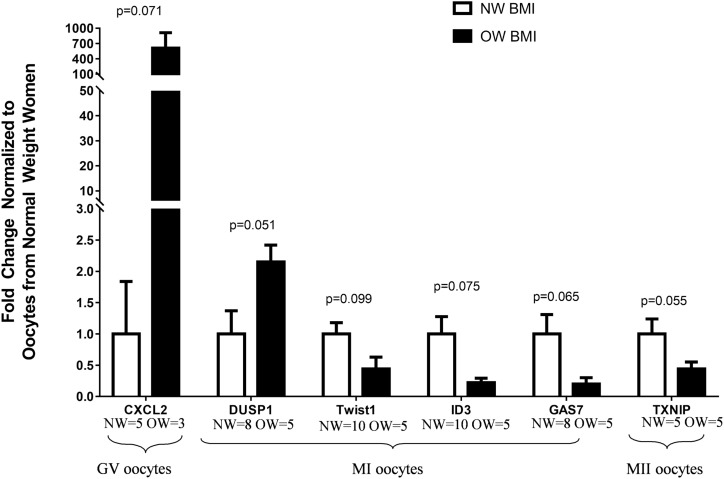
The chosen genes were identified from KEGG pathways or GO analyses with potential roles in obesity and/or reproduction. In GV oocytes, CXCL2 (P = 0.071) gene expression tended to be higher in OW women than in NW women (Fig. 3). In MI oocytes, DUSP1 (P = 0.051) tended to be upregulated, whereas GAS7 (P = 0.065), TWIST1 (P = 0.099), and ID3 (P = 0.075) tended to be downregulated in oocytes from OW women compared with NW women (Fig. 3). Lastly, in MII oocytes, TXNIP gene expression tended toward a downregulation (P = 0.055) in OW women compared with NW women (Fig. 3). Although results did not reach significance for all genes that were selected because of our small sample size, the RT-qPCR results were concordant with the sequencing results and, in most cases, showed a trend toward significance. Interestingly, CXCL3 (R = 0.63; P = 0.04) and IL-34 (R = 0.55; P = 0.041) gene expressions were significantly positively correlated with BMI, whereas TXNIP gene expression was significantly negatively correlated with body fat (R = −0.71; P = 0.03).
Figure 3.
Relative gene expression for selected genes using the isolated cDNA from the RNA-Seq analyses to conduct RT-qPCR for each oocyte maturation stage (NW women, white bars; OW women, black bars). All values were normalized using a geometric mean for glyceraldehyde 3-phosphate dehydrogenase and β-actin mRNA expression as mean fold change relative to NW women for GV, MI, and MII oocytes. Data are expressed as mean ± SEM. Significant differences between groups was set at P ≤ 0.05 using the Mann-Whitney U nonparametric test. Variation in sample size is due to undetectable expression by RT-qPCR. CXCL2, chemokine (C-X-C motif) ligand 2 (NW = 5, OW = 3); DUSP1, dual specificity protein phosphatase 1 (NW = 8, OW = 5); GAS7, growth arrest specific 7 (NW = 8, OW = 5); ID3, inhibitor of DNA binding 3, HLH protein (NW = 10, OW = 5); TXNIP, thioredoxin interacting protein (NW = 5, OW = 5); TWIST1, Twist family bHLH transcription factor 1 (NW = 10, OW = 5).
Serum and FF metabolites correlated with oocyte gene expression
Correlation analyses showed that serum and FF metabolites were associated with oocyte gene expression. Serum CRP levels were correlated with CXCL2 expression in GV oocytes (r = 0.65; P = 0.049), whereas FF CRP and cholesterol levels were correlated with TWIST1 expression in MI oocytes (r = −0.80; P = 0.014 and r = −0.57; P = 0.055, respectively).
Multiple linear regression models of TWIST1 RNA expression
TWIST1 is a TF that has been shown to upregulate inflammation and alter lipid metabolism (14, 15). Multiple regression analyses were used to examine maternal factors that could predict TWIST1 gene expression. The three most parsimonious models are presented in Table 3. In model 1, body fat (%), % SFA, and RER were retained, whereas in model 2, just % SFA and RER were retained. Neither body fat (%) nor RER explained a significant proportion of the variance seen in TWIST1 gene expression; thus, they were dropped from the final model. The most parsimonious model (i.e., model 3) retained only % SFA, accounting for 32% of the variance in TWIST1 gene expression (Table 3).
Table 3.
Three Most Parsimonious Multiple Linear Regression Models for Twist mRNA
| Body Fat (%) (r2, P Value) | Saturated Fat (% kcal) (r2, P Value) | RER (r2, P Value) | Adjusted R2 | |
|---|---|---|---|---|
| Model 1 | 0.036, 0.438 | 0.246, 0.058 | 0.040, 0.412 | 0.227 |
| Model 2 | — | 0.289, 0.039 | 0.033, 0.450 | 0.250 |
| Model 3 | — | 0.324, 0.027 | — | 0.272 |
Significance level was set at P ≤ 0.05.
Discussion
Obesity adversely affects the ovarian environment through reproductive hormone imbalance, lipid accumulation, inflammation, oxidative stress, insulin resistance, and mitochondrial dysfunction (16, 17). Together, these factors may disrupt oocyte development and competence and impact embryo development (18, 19). Clinical studies have focused on the effects of obesity on pregnancy and/or fertility outcomes rather than the molecular mechanisms underlying these effects. Thus, it is unclear how obesity impacts oocyte gene expression. This study examined the impact of obesity on oocyte gene expression from OW and NW women using a single-cell transcriptomic approach. Obesity led to differential oocyte gene expression at all maturation stages, with specific upregulation of CXCL2 and DUSP1 and downregulation of TWIST1, ID3, GAS7, and TXNIP gene expression. The FF milieu also differed, with greater levels of leptin, CRP, and triglycerides in OW women than in NW women. Interestingly, we not only observed changes in oocyte TWIST1 gene expression with increasing BMI, but maternal diet, specifically SFA intake, was also shown to be more predictive of changes in TWIST1 expression, suggesting that maternal diet may have a greater influence than body composition per se on changes in oocyte gene expression.
Inflammatory proteins play an important role in a variety of reproductive processes, including menstruation, ovulation, implantation, and parturition (20, 21). However, their role during oocyte maturation is not fully understood. In this study, we showed differential gene expression of inflammatory genes CXCL2 (14.3-fold) and CXCL3 (9.4-fold) in GV; IL-34 (7.9-fold) in MI; and CCL20 (10.5-fold) in MII oocytes between OW and NW women, although CXCL2 was the only one to be further confirmed by RT-qPCR analyses. These results are supported by functional annotation [Supplemental Tables 4(a–c (43.4KB, docx) )] and pathway analyses (Table 2) that identified both inflammatory pathways and processes as being enriched in oocytes from OW women compared with those from NW women. CXCL2 and CXCL3 are known to be expressed in the oocyte, granulosa cells, and FF (22–25). Our data are similar to previous findings that demonstrated an upregulation of inflammatory gene expression (FGF-12 and PPMIL) in cumulus cells from OW women compared with NW women going through IVF. These results are also consistent with an increased ovarian proinflammatory signaling associated with changes in oocyte gene expression in obese mice (12). Combined, these data demonstrate that obesity leads to an upregulation of proinflammatory-related transcripts in oocytes, which may lead to altered oocyte and embryo development. Further studies are needed to evaluate the mechanisms by which localized inflammation impacts oocyte development.
We identified DUSP1, GAS7, ID3, and TWIST1 in MI and TXNIP in MII oocytes as being differentially expressed in OW women compared with NW women. DUSP1 inhibits MAPK kinase activity and plays a role in oocyte maturation, developmental competence, spindle morphology, and chromosome alignment (26–29). In this study, although DUSP1 gene expression was upregulated by 2.2-fold in MII oocytes with RNA-Seq, we were unable to confirm it with RT-PCR because of high variability between participants. A greater number of participants per group would help to confirm that oocyte DUSP1 gene expression is affected by obesity in MII oocytes.
TXNIP is an oxidative stress–related gene with expression that was previously identified in mouse oocytes as well as in bovine and human cumulus cells and has been correlated with lower oocyte quality (30, 31). In this study, we showed a downregulation of TXNIP expression in MII oocytes from OW women compared with NW women, which is in accordance with previous results from animal models, suggesting that oocytes from OW women may have lower developmental competence than oocytes from NW women. Results also identified a downregulation of ID3 gene expression, a TF previously identified in bovine oocytes and porcine granulosa cells, in both MI and MII oocytes from OW women compared with NW women (32, 33). ID3 is regulated by FSH and is increased in cumulus oocyte complexes, suggesting an important role in oocyte development and maturation. GAS7 gene expression was also downregulated in MI and MII oocytes from OW women compared with NW women. GAS7 plays an important role in neuronal development and cell protection of embryonic stem cells as well as in the regulation of lipid metabolism and insulin signaling (12, 34). Thus far, it has been identified only in human cumulus cells (12). Although the role of GAS7 in the human oocyte needs further confirmation, these results suggest that obesity may alter key developmental pathways related to neuronal development as well as key metabolic pathways (lipid metabolism and insulin signaling), which are critical in the developing embryo. These results are in line with results in offspring from OW women that demonstrated differential brain development (35) as well as findings in offspring from obese dams that identified alterations in lipid metabolism (36). Furthermore, TF analyses suggest that FOX04, SP1, and AP4 could be potential upstream targets for the genes identified in this study. Future studies investigating these three targets are warranted to confirm their role in oocyte gene expression in obese women.
We identified a downregulation of TWIST1 gene expression in MI oocytes of OW women compared with NW women. TWIST1 is a TF highly expressed in the placenta and involved in embryonic development of tissues (37). TWIST1 expression upregulates inflammation and alters lipid metabolism (14, 15). In an in vitro model of human adipocytes, TWIST1 upregulated the expression of CCL2, IL-6, and TNF-α (15). In our hands, oocyte TWIST1 gene expression was not statistically linked to BMI or adiposity; however, it was significantly associated with maternal diet and metabolism. We also identified correlations between TWIST1 gene expression and FF CRP and cholesterol. Multiple linear regression analyses demonstrated that SFA intake was a significant predictor of TWIST1 oocyte gene expression. Thus, our results suggest that maternal dietary SFA may be of greater importance than maternal adiposity per se in regulating the inflammatory response of the oocyte. Future intervention studies focused on reducing SFA intake may elicit positive outcomes on oocyte gene expression and future offspring.
The ovarian follicular environment of OW women was also significantly altered, with elevated FF triglycerides, leptin, and CRP levels compared with those of NW women. These data are in line with previous results showing an elevation of FF CRP, insulin, and triglyceride levels in obese women compared with NW women (38, 39). In this study, serum and FF CRP levels were shown to be significantly correlated with CXCL2 and TWIST1 oocyte gene expression. Parallel to the inflammation presented in the oocyte and FF, we found higher serum CRP and CCL2 levels as well as increased leptin and HOMA-IR content in OW women compared with NW women. Lastly, we identified correlations between serum polyunsaturated fatty acids and FF cytokine expression and serum CRP and inflammatory gene expression of the oocyte, suggesting that higher systemic inflammation and dietary intake may lead to an inflammatory response identified in the FF and oocyte.
This study had some limitations. Participants had documented infertility and were hormonally superovulated before their oocyte retrieval, which may have altered the oocyte’s gene expression. However, because both NW and OW women were exposed to similar protocols, results should reflect the effect of overweight/obesity under these conditions. To our knowledge, superovulation is the most commonly used method to collect oocytes. Second, use of a single-cell isolation method limited cDNA availability for validation studies using RT-qPCR. However, the single cell−isolation method provided us with the ability to identify correlations between gene expression and clinical markers for each participant, which strengthened our findings. Third, the sample size was small for each maturation stage. However, compared with other methods, RNA-Seq is a robust method that creates high-throughput sequencing of an entire transcriptome with low background noise from low starting RNA amounts (40) and therefore provides a more comprehensive profile of the effects of adiposity on oocyte gene expression. Finally, the use of a single round of amplification should have diminished the potential for bias in over- or underrepresenting transcripts.
In conclusion, the single-cell transcriptomic approach helped us identify obesity-associated changes in the oocyte mRNA content related to neuronal development and key metabolic pathways, such as lipid metabolism and insulin signaling, which may be influenced by posttranscriptional mechanisms. Combined with findings of an upregulation of proinflammatory and oxidative stress−related genes in the oocytes, these results suggest that not only the integrity and competence of the oocytes but also the metabolism may be compromised by obesity. The preconception period may therefore be a critical window for lifestyle interventions aimed at preventing potential obesity-related effects on the oocyte, and maternal dietary SFA intake may be an important interventional target for these prevention efforts.
Acknowledgments
Acknowledgments
This work was funded by National Institute of Health (NIH) Translational Research Institute 1RR029884 and US Department of Agriculture–Agricultural Research Service Project 6026‐51000‐010‐05S. M.R. was partially supported by an NIH T32HD087166 grant.
Clinical trial registry: ClinicalTrials.gov no. NCT01480024 (registered 23 November 2011).
Disclosure summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CCL2
- chemokine (C-C motif) ligand 2
- cDNA
- complementary DNA
- CRP
- C-reactive protein
- FF
- follicular fluid
- FFM
- fat-free mass
- FSH
- follicle-stimulating hormone
- GO
- Gene Ontology
- GV
- germinal vesicle
- HDL
- high-density lipoprotein
- HOMA-IR
- homeostatic model assessment−insulin resistance
- IL
- interleukin
- IVF
- in vitro fertilization
- MI
- metaphase I
- MII
- metaphase II
- mRNA
- messenger RNA
- MUFA
- monounsaturated fat
- NW
- normal-weight
- OW
- overweight/obese
- REE
- resting energy expenditure
- RER
- respiratory exchange ratio
- RNA-Seq
- RNA sequencing
- RT-qPCR
- real-time quantitative polymerase chain reaction
- SEM
- standard error of the mean
- SFA
- saturated fat
- TF
- transcription factor
- TNF
- tumor necrosis factor.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dağ ZO, Dilbaz B. Impact of obesity on infertility in women. J Turk Ger Gynecol Assoc. 2015;16(2):111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421–439. [DOI] [PubMed] [Google Scholar]
- 4.Cardozo ER, Karmon AE, Gold J, Petrozza JC, Styer AK. Reproductive outcomes in oocyte donation cycles are associated with donor BMI. Hum Reprod. 2016;31(2):385–392. [DOI] [PubMed] [Google Scholar]
- 5.Xue Z, Huang K, Cai C, Cai L, Jiang CY, Feng Y, Liu Z, Zeng Q, Cheng L, Sun YE, Liu JY, Horvath S, Fan G. Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature. 2013;500(7464):593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purcell SA, Elliott SA, Baracos VE, Chu QS, Prado CM. Key determinants of energy expenditure in cancer and implications for clinical practice. Eur J Clin Nutr. 2016;70(11):1230–1238. [DOI] [PubMed] [Google Scholar]
- 7.Glaser C, Demmelmair H, Koletzko B. High-throughput analysis of total plasma fatty acid composition with direct in situ transesterification. PLoS One. 2010;5(8):e12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren J, Mozurkewich EL, Sen A, Vahratian AM, Ferreri TG, Morse AN, Djuric Z. Total serum fatty acid analysis by GC-MS: assay validation and serum sample stability. Curr Pharm Anal. 2013;9(4):331–339. [DOI] [PMC free article] [PubMed]
- 9.Shankar K, Zhong Y, Kang P, Lau F, Blackburn ML, Chen JR, Borengasser SJ, Ronis MJ, Badger TM. Maternal obesity promotes a proinflammatory signature in rat uterus and blastocyst. Endocrinology. 2011;152(11):4158–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie F, Anderson CL, Timme KR, Kurz SG, Fernando SC, Wood JR. Obesity-dependent increases in oocyte mRNAs are associated with increases in proinflammatory signaling and gut microbial abundance of Lachnospiraceae in female mice. Endocrinology. 2016;157(4):1630–1643. [DOI] [PMC free article] [PubMed]
- 11.Merhi Z, Buyuk E, Berger DS, Zapantis A, Israel DD, Chua S Jr, Jindal S. Leptin suppresses anti-Mullerian hormone gene expression through the JAK2/STAT3 pathway in luteinized granulosa cells of women undergoing IVF. Hum Reprod. 2013;28(6):1661–1669. [DOI] [PubMed] [Google Scholar]
- 12.Merhi Z, Polotsky AJ, Bradford AP, Buyuk E, Chosich J, Phang T, Jindal S, Santoro N. Adiposity alters genes important in inflammation and cell cycle division in human cumulus granulosa cell. Reprod Sci. 2015;22(10):1220–1228. [DOI] [PMC free article] [PubMed]
- 13.Yerushalmi GM, Salmon-Divon M, Yung Y, Maman E, Kedem A, Ophir L, Elemento O, Coticchio G, Dal Canto M, Mignini Renzinu M, Fadini R, Hourvitz A. Characterization of the human cumulus cell transcriptome during final follicular maturation and ovulation. Mol Hum Reprod. 2014;20(8):719–735. [DOI] [PubMed] [Google Scholar]
- 14.Pettersson AT, Mejhert N, Jernås M, Carlsson LM, Dahlman I, Laurencikiene J, Arner P, Rydén M. Twist1 in human white adipose tissue and obesity. J Clin Endocrinol Metab. 2011;96(1):133–141. [DOI] [PubMed] [Google Scholar]
- 15.Dobrian AD. A tale with a Twist: a developmental gene with potential relevance for metabolic dysfunction and inflammation in adipose tissue. Front Endocrinol (Lausanne). 2012;3:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu L, Liu H, Gu X, Boots C, Moley KH, Wang Q. Metabolic control of oocyte development: linking maternal nutrition and reproductive outcomes. Cell Mol Life Sci. 2015;72(2):251–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robker RL, Wu LL, Yang X. Inflammatory pathways linking obesity and ovarian dysfunction. J Reprod Immunol. 2011;88(2):142–148. [DOI] [PubMed] [Google Scholar]
- 18. Robker RL. Evidence that obesity alters the quality of oocytes and embryos. Pathophysiology. 2008;15(2):115–121. [DOI] [PubMed]
- 19.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26145566&dopt=Abstract Penfold NC, Ozanne SE. Developmental programming by maternal obesity in 2015: outcomes, mechanisms, and potential interventions. Horm Behav. 2015;76:143–152. [DOI] [PubMed]
- 20.Jabbour HN, Sales KJ, Catalano RD, Norman JE. Inflammatory pathways in female reproductive health and disease. Reproduction. 2009;138(6):903–919. [DOI] [PubMed] [Google Scholar]
- 21.Ruebel M, Shankar K, Gaddy D, Lindsey F, Badger T, Andres A. Maternal obesity is associated with ovarian inflammation and upregulation of early growth response factor 1. Am J Physiol Endocrinol Metab. 2016;311(1):E269–E277. [DOI] [PubMed] [Google Scholar]
- 22.Huang X, Hao C, Shen X, Zhang Y, Liu X. RUNX2, GPX3 and PTX3 gene expression profiling in cumulus cells are reflective oocyte/embryo competence and potentially reliable predictors of embryo developmental competence in PCOS patients. Reprod Biol Endocrinol. 2013;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rouault C, Pellegrinelli V, Schilch R, Cotillard A, Poitou C, Tordjman J, Sell H, Clément K, Lacasa D. Roles of chemokine ligand-2 (CXCL2) and neutrophils in influencing endothelial cell function and inflammation of human adipose tissue. Endocrinology. 2013;154(3):1069–1079. [DOI] [PubMed] [Google Scholar]
- 24.Farquharson AJ, Steele RJ, Carey FA, Drew JE. Novel multiplex method to assess insulin, leptin and adiponectin regulation of inflammatory cytokines associated with colon cancer. Mol Biol Rep. 2012;39(5):5727–5736. [DOI] [PubMed] [Google Scholar]
- 25.Kawano Y, Fukuda J, Nasu K, Nishida M, Narahara H, Miyakawa I. Production of macrophage inflammatory protein-3alpha in human follicular fluid and cultured granulosa cells. Fertil Steril. 2004;82(Suppl 3):1206–1211. [DOI] [PubMed] [Google Scholar]
- 26.O’Shea LC, Mehta J, Lonergan P, Hensey C, Fair T. Developmental competence in oocytes and cumulus cells: candidate genes and networks. Syst Biol Reprod Med. 2012;58(2):88–101. [DOI] [PubMed] [Google Scholar]
- 27.Gordo AC, He CL, Smith S, Fissore RA. Mitogen activated protein kinase plays a significant role in metaphase II arrest, spindle morphology, and maintenance of maturation promoting factor activity in bovine oocytes. Mol Reprod Dev. 2001;59(1):106–114. [DOI] [PubMed] [Google Scholar]
- 28. Ferguson BS, Nam H, Stephens JM, Morrison RF. Mitogen-dependent regulation of DUSP1 governs ERK and p38 signaling during early 3T3-L1 adipocyte differentiation. J Cell Physiol. 2016;231(7):1562–1574. [DOI] [PMC free article] [PubMed]
- 29.Hoffmann MS, Singh P, Wolk R, Narkiewicz K, Somers VK. Obstructive sleep apnea and intermittent hypoxia increase expression of dual specificity phosphatase 1. Atherosclerosis. 2013;231(2):378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salhab M, Dhorne-Pollet S, Auclair S, Guyader-Joly C, Brisard D, Dalbies-Tran R, Dupont J, Ponsart C, Mermillod P, Uzbekova S. In vitro maturation of oocytes alters gene expression and signaling pathways in bovine cumulus cells. Mol Reprod Dev. 2013;80(2):166–182. [DOI] [PubMed] [Google Scholar]
- 31.Lee SY, Lee HS, Kim EY, Ko JJ, Yoon TK, Lee WS, Lee KA. Thioredoxin-interacting protein regulates glucose metabolism and affects cytoplasmic streaming in mouse oocytes. PLoS One. 2013;8(8):e70708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verbraak EJ, van ’t Veld EM, Groot Koerkamp M, Roelen BA, van Haeften T, Stoorvogel W, Zijlstra C. Identification of genes targeted by FSH and oocytes in porcine granulosa cells. Theriogenology. 2011;75(2):362–376. [DOI] [PubMed] [Google Scholar]
- 33.Thélie A, Papillier P, Pennetier S, Perreau C, Traverso JM, Uzbekova S, Mermillod P, Joly C, Humblot P, Dalbiès-Tran R. Differential regulation of abundance and deadenylation of maternal transcripts during bovine oocyte maturation in vitro and in vivo. BMC Dev Biol. 2007;7:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moorthy PP, Kumar AA, Devaraj H. Expression of the Gas7 gene and Oct4 in embryonic stem cells of mice. Stem Cells Dev. 2005;14(6):664–670. [DOI] [PubMed] [Google Scholar]
- 35.Ou X, Thakali KM, Shankar K, Andres A, Badger TM. Maternal adiposity negatively influences infant brain white matter development. Obesity (Silver Spring). 2015;23(5):1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shankar K, Kang P, Harrell A, Zhong Y, Marecki JC, Ronis MJ, Badger TM. Maternal overweight programs insulin and adiponectin signaling in the offspring. Endocrinology. 2010;151(6):2577–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu X, He Y, Zhu C, Wang H, Chen S, Lin HY. Twist1 is involved in trophoblast syncytialization by regulating GCM1. Placenta. 2016;39:45–54. [DOI] [PubMed] [Google Scholar]
- 38.Robker RL, Akison LK, Bennett BD, Thrupp PN, Chura LR, Russell DL, Lane M, Norman RJ. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J Clin Endocrinol Metab. 2009;94(5):1533–1540. [DOI] [PubMed] [Google Scholar]
- 39.Valckx SD, De Pauw I, De Neubourg D, Inion I, Berth M, Fransen E, Bols PE, Leroy JL. BMI-related metabolic composition of the follicular fluid of women undergoing assisted reproductive treatment and the consequences for oocyte and embryo quality. Hum Reprod. 2012;27(12):3531–3539. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]