Abstract
Context:
Whether primary aldosteronism (PA) is the consequence of a monoclonal or multiclonal process is unclear.
Case Description:
A 48-year-old man with severe bilateral PA refractory to medical therapy underwent unilateral adrenalectomy of the dominant adrenal. Although computed tomography showed three left-sided cortical nodules, postsurgical histopathology and genetic analysis revealed five different adrenocortical adenomas. Two zona fasciculata (ZF)–like aldosterone-producing adenomas (APAs) each harbored distinct known somatic KCNJ5 mutations (L168R and T158A). A zona glomerulosa–like APA harbored a known CACNA1D G403R somatic mutation, whereas a zona reticularis–like adenoma, which was grossly black in pigmentation with histologic characteristics more associated with cortisol-producing adenomas, expressed CYP11B2, CYP17, and DHEA-ST by immunohistochemistry (IHC) and harbored no known somatic mutations. The fifth adenoma was ZF-type, negative for CYP11B2 and CYP17 IHC, and harbored no known somatic mutations.
Conclusions:
This case highlights complex intertumor heterogeneity in histology, steroidogenesis, and somatic mutations in multiple adrenocortical adenomas arising in a single patient with PA. These findings suggest that the syndrome of PA can involve heterogeneous and multiclonal functional adrenal adenomas.
This study of a patient with multiple aldosterone-producing adenomas revealed complex intertumor heterogeneity in genetics, histology, and steroidogenesis, suggesting a multiclonal process.
We report a 48-year-old white man with an 11-year history of hypertension. He was initially diagnosed at age 31 and treated with hydrochlorothiazide and amlodipine. Prior to the age of 31, he had documented normal blood pressures by his physicians (<140/90 mm Hg) and no history of childhood or adolescent hypertension, nor a family history of early-onset hypertension, stroke, primary aldosteronism (PA), multiple endocrine neoplasia, or Carney’s complex. He was noted to have hypokalemia to 2.9 mmol/L following initiation of these medications that normalized with potassium supplementation. Four years later, he was seen at our institution with a blood pressure of 139/82 mm Hg while taking amlodipine 10 mg daily, benazepril 40 mg daily, atenolol 25 mg daily, and potassium chloride 80 mmol daily. PA was diagnosed based on a markedly elevated aldosterone-to-renin ratio (Supplemental Table 1 (933.7KB, docx) ). Although dexamethasone suppression test was not performed, he did not have physical signs of Cushing syndrome, his baseline plasma adrenocorticotropic hormone and serum cortisol levels were normal, and 24-hour urinary cortisol was not elevated (Supplemental Table 1 (933.7KB, docx) ). Sex hormones were not measured. Computed tomography showed three right adrenal nodules (22 mm, 18 mm, and 4 mm in maximum diameter) and a 7- × 5-mm left adrenal nodule, all with an unenhanced attenuation of <10 Hounsfield units and exhibiting >70% washout of contrast 15 minutes after administration. Adrenal venous sampling revealed a lateralization ratio of 2, suggesting bilateral rather than unilateral aldosterone secretion, with the right adrenal gland secreting ∼twofold more aldosterone than the left when normalized for adrenal venous cortisol concentrations (Supplemental Table 2 (933.7KB, docx) ). He was treated with eplerenone for 4 years, but developed worsening hypertension and hypokalemia and had a suppressed plasma renin activity (<0.6 ng/mL/h) despite treatment with eplerenone 500 mg daily. An abdominal magnetic resonance imaging at this time demonstrated growth in the largest of the three right adrenal nodules (31 mm from 22 mm in maximum diameter) and stability in the other right- and left-sided nodules. Given his inadequate response to medical therapy, a right laparoscopic adrenalectomy was performed. Postoperatively, his potassium normalized. As expected based on the results of the adrenal venous sampling, he continued to have PA postoperatively (serum aldosterone 30 ng/dL and plasma renin activity <0.6 ng/mL/h); however, his blood pressure was <130/80 mm Hg with eplerenone 50 mg daily, amlodipine 10 mg daily, and atenolol 25 mg daily. Informed consent was obtained to review the patient’s medical records and to study surgical tissue and DNA analysis with approval from our Institutional Human Research and Ethics Committee.
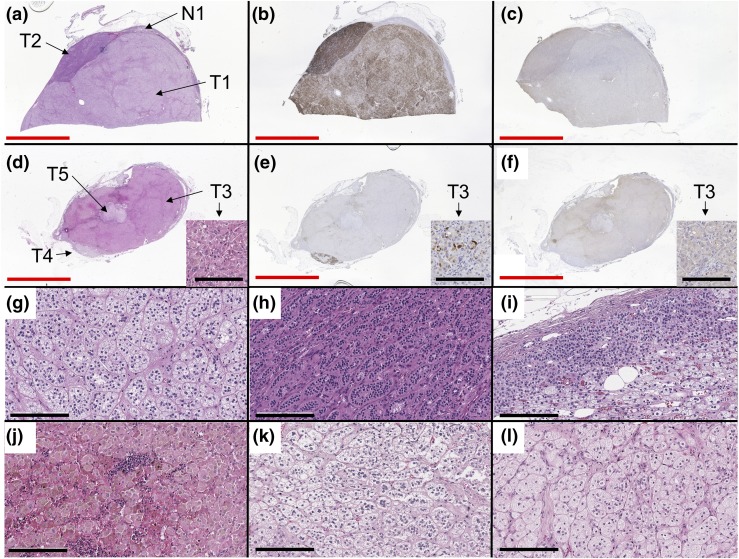
Grossly, the right adrenal weighed 40.0 g. Macroscopic findings of resected specimens revealed multiple golden-yellow nodules and a dark or black pigmented adenoma (Supplemental Fig. 1 (933.7KB, docx) ). Representative formalin-fixed paraffin-embedded tissue blocks (Supplemental Fig. 1 (933.7KB, docx) ) were serially sectioned into four 4-μm slides for hematoxylin and eosin staining; immunohistochemistry (IHC) for CYP11B2, CYP17A, and DHEA-ST; and eight 10-μm unstained slides for DNA capture. One block [Fig. 1(a–c); Table 1] showed two adjacent adrenocortical adenomas consisting of zona fasciculata (ZF)–like cells [T1, Fig. 1(g)] and zona glomerulosa (ZG)–like cells [T2, Fig. 1(h)]. The other tissue block [Fig. 1(d–f)] showed three cortical adenomas consisting of zona reticularis (ZR)–like cells [T3, Fig. 1(j)] and ZF cells [T4 and T5, Fig. 1(k) and 1(l)]. T3, the black adenoma, was comprised of interconnecting short cords of compact cells containing eosinophilic cytoplasm and abundant lipofuscin, harbored foci of lipomatous degeneration, and intratumoral lymphocyte infiltration, and resembled a cortisol-producing adenoma [Fig. 1(j)]. Adjacent normal adrenal tissue showed paradoxical hyperplasia without cortical atrophy [N1, Fig. 1(i)]. Each tumor was separated by a capsule. By Weiss criteria, all five tumors were adrenocortical adenomas. IHC of steroidogenic enzymes revealed that CYP11B2 was positive in all of the adenomas except for T5, and CYP17A was weakly positive in T2 and T3 (Fig. 1; Table 1). In the ZR-like adenoma (T3), CYP11B2, CYP17A, and DHEA-ST were all focally positive in adenoma cells [Fig. 1(d–f)].
Figure 1.
Images of histology and IHC. (a and d) Hematoxylin and eosin, (b and e) CYP11B2 IHC, (c and f) CYP17A IHC, and high magnitude images (200×) of (g) T1, (h) T2, (i) N1, (j) T3, (k) T4, and (l) T5. One block (a–c) showed two collision adenomas consisting of ZF-like cells (g) and ZG-like cells (h) and adjacent normal adrenal tissue with paradoxical hyperplasia (i). The other block (d–f) showed three collision adenomas consisting of ZR-like cells (j) and ZF-like cells (k and l). Some cells in T3 were positive for CYP11B2 and CYP17A IHC, as shown in small pictures in (d–f) (note the same structure of interstitial tissues arising from left bottom to right middle). Red and black bars indicate 10 mm and 200 µm, respectively.
Table 1.
Histology, IHC, and Genetic Analysis of Adrenal Tumors and Adjacent Normal Adrenal Tissue
| Size (mm) | ZF-Like Cell (Area %) | ZG-Like Cell (Area %) | ZR-Like Cell (Area %) | Histological Diagnosis | CYP11B2 | CYP17A | Somatic Mutation | ||
|---|---|---|---|---|---|---|---|---|---|
| T1 | 21 × 13 | 85 | 15 | 0 | Adenoma | Heterogeneously positive | (−) | KCNJ5 | L168R |
| T2 | 12 × 4 | 0 | 100 | 0 | Adenoma | Homogeneously positive | Weak positive | CACNA1D | G403R |
| T3 | 17 × 11 | 0 | 0 | 100 | Adenoma | Positive in 10% of the cells | Weak positive | N/D | N/D |
| T4 | 7 × 1 | 90 | 10 | 0 | Adenoma | 50% of ZF-like cells positive | (−) | KCNJ5 | T158A |
| T5 | 4 × 3 | 80 | 20 | 0 | Adenoma | (−) | (−) | N/D | N/D |
| N1 | Normal | N/D | N/D | ||||||
The results of histology, IHC, and targeted next-generation sequencing are shown by each component. The sample numbers match those in Fig. 1(a) and 1(d). Two different KCNJ5 somatic mutations were each identified in two APAs predominantly consisting of ZF-like cells (T1, T4), whereas CACNA1D somatic mutation was detected in the APA predominantly consisting of ZG-like cells (T2). No somatic mutations were detected in the APA with ZR-like cells (T4), the APA without CYP11B2 IHC (T5), and the adjacent normal adrenal tissue (N1). Reference transcript sequences used for determining amino acid changes are as follows: NM_000890.3 (KCNJ5), NM_001128839.2 (CACNA1D), NM_021949.3 (ATP2B3), and NM_000701.7 (ATP1A1).
Abbreviation: N/D, not detected.
DNA was isolated from each distinct tumor and the adjacent normal tissue (N1) off unstained sections and for next-generation sequencing targeting the full coding sequence of aldosterone-regulating genes (KCNJ5, CACNA1D, ATP1A1, and ATP2B3) and other genes related to adrenal diseases (CACNA1H, ARMC5, PDE11A, PDE8B, PRKACA, and PRKAR1A, and recurrent hotspots in GNAS and CTNNB1), as previously described (1). The average frequency of on-target reads, coverage uniformity, and flow-corrected depth were 96%, 92%, and 771x, respectively, consistent with high-quality sequencing results. Multiple distinct but known KCNJ5 somatic mutations (L168R or T158A) were detected in the two ZF-like tumors (T1, T4, respectively), whereas a known CACNA1D somatic mutation (G403R) was detected in the ZG-like tumor (T2) (Table 1). Variant allele frequencies were 33%, 28%, and 34%, respectively, indicating that ∼60% to 70% of the cells in each tumor harbored the single mutation, supporting independent clonal origin. Other tumors and the adjacent normal tissue did not harbor these somatic mutations (despite adequate coverage), nor other reported or novel candidate functional somatic mutations.
Finally, targeted sequencing of patient’s whole blood revealed no pathogenic germline mutations or variants of unknown significance in the following genes: CYP11B1, CYP11B2, ARMC5, GNAS, ATP1A1, ATP2B3, CACNA1D, KCNJ5, and PRKAR1A.
Discussion
In this work, we report a case of a patient with PA due to bilateral adrenal abnormalities wherein the dominant adrenal gland was comprised of at least five distinct adrenocortical adenomas based on histological, steroidogenic, and genetic profiles. To the best of our knowledge, there has only been one published report of the coexistence of the three different cortical components (ZF/ZG/ZR) in three different adrenocortical adenomas in one adrenal gland (2). In that case, the patient was clinically diagnosed with PA and subclinical Cushing syndrome, and histology and IHC of steroidogenic enzymes demonstrated that the ZF-like adenoma only produced aldosterone and the ZG-like and ZR-like adenomas produced cortisol. Of particular interest, the ZR-like adenoma in this prior case was also black in pigmentation, similar to our current case. However, this prior report lacked genetic analyses to make definitive assessment of cellular origins and the clonal relationship of the tumors.
Exome sequencing of aldosterone-producing adenoma (APA) has recently revealed several recurrent somatic mutations in genes that mediate aldosterone production. In whites, the most frequently mutated gene is KCNJ5, occurring in ∼40% of APAs, whereas CACNA1D, ATP1A1, and ATP2B3 mutations each occur in another ∼10% of APAs (3). Interestingly, the predominant cells in KCNJ5-mutated APAs are ZF-like cells (clear cells), whereas those in CACNA1D-mutated APAs are ZG-like cells (compact cells) (4). This tendency was also observed in the present case. Furthermore, the somatic mutations were detected at high variant frequencies, supporting independent clonal processes in each tumor.
More recently, we described localized small clusters of cells that express CYP11B2 beneath the adrenal capsule in normal adrenals, termed aldosterone-producing cell clusters (APCC). Using next-generation sequencing, we demonstrated that a third of APCCs harbored somatic mutations in the same genes recurrently mutated in APAs (1). In the present case, the ZG-like adenoma with CACNA1D mutation (T2) was adjacent to the main ZF-like adenoma with KCNJ5 mutation (T1). Given the shared nature of aldosterone-stimulating somatic mutations, we speculate that this patient’s multiple adrenal tumors may have originated as multifocal APCCs that then developed proliferative abnormalities resulting in a transition to an APA.
The appearance of black adrenal adenomas has been explained by the presence of abundant lipofuscin (5). Most produce both cortisol and aldosterone, and black adenomas that only produce aldosterone are rare (5). The current patient had no evidence of hypercortisolism, although IHC supported expression of the enzymatic machinery necessary for cortisol synthesis.
In conclusion, this case highlights a complex example of intertumor heterogeneity in PA, whereby distinct features of histology, steroidogenic proteins, and somatic driver mutations point to a multiclonal pathogenesis. These findings expand our understanding of intra- and intertumor heterogeneity in PA (6) and support the existence of multiple tumors from distinct clonal origins in PA. Combined IHC for steroidogenic enzymes and somatic mutation assessment may inform the pathobiology of different cortical lesions arising in the same adrenal gland. Our findings also support additional investigation into the extent of multiclonality in PA, including apparent unifocal tumors by morphology.
Acknowledgments
We thank Dr. Adi Barlev-Ehrenberg (Brigham and Women’s Hospital) for performing and interpreting germline sequencing. We thank Dr. Richard Parker (University of Alabama) for providing CYP17A monoclonal antibody (7). We also thank Drs. Fumitoshi Satoh and Sadayoshi Ito (Tohoku University) for reviewing the manuscript.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK106618 to W.E.R. and S.A.T. S.A.T. is supported as the A. Alfred Taubman Emerging Scholar by the A. Alfred Taubman Medical Research Institute. S.A.T. has received travel support and a separate sponsored research agreement with Thermo Fisher Scientific. A.V. was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK107407, Doris Duke Charitable Foundation Grant 2015085, and National Institutes of Health National Heart, Lung, and Blood Institute Grant K23HL111771.
Disclosure Summary: None of the study described in this work was supported by Thermo Fisher Scientific, and it had no role in the data collection, interpretation, or analysis, and did not participate in the study design or the decision to submit for publication. The authors have nothing to disclose.
Footnotes
- APA
- aldosterone-producing adenoma
- APCC
- aldosterone-producing cell cluster
- IHC
- immunohistochemistry
- PA
- primary aldosteronism
- ZF
- zona fasciculata
- ZG
- zona glomerulosa
- ZR
- zona reticularis.
References
- 1.Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, Liu CJ, Sanjanwala AR, Edwards MA, Gomez-Sanchez CE, Nanba K, Rainey WE. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci USA. 2015;112(33):E4591–E4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okura T, Miyoshi K, Watanabe S, Kurata M, Irita J, Manabe S, Fukuoka T, Higaki J, Sasano H. Coexistence of three distinct adrenal tumors in the same adrenal gland in a patient with primary aldosteronism and preclinical Cushing’s syndrome. Clin Exp Nephrol. 2006;10(2):127–130. [DOI] [PubMed] [Google Scholar]
- 3.Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, Strom TM, Monticone S, Amar L, Meatchi T, Mantero F, Cicala MV, Quinkler M, Fallo F, Allolio B, Bernini G, Maccario M, Giacchetti G, Jeunemaitre X, Mulatero P, Reincke M, Zennaro MC. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014;64(2):354–361. [DOI] [PubMed] [Google Scholar]
- 4.Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, Maniero C, Garg S, Bochukova EG, Zhao W, Shaikh LH, Brighton CA, Teo AE, Davenport AP, Dekkers T, Tops B, Küsters B, Ceral J, Yeo GS, Neogi SG, McFarlane I, Rosenfeld N, Marass F, Hadfield J, Margas W, Chaggar K, Solar M, Deinum J, Dolphin AC, Farooqi IS, Striessnig J, Nissen P, Brown MJ. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45(9):1055–1060. [DOI] [PubMed] [Google Scholar]
- 5.Fonseca JJ, Pirola S. Black adenoma of the adrenal gland. Int J Surg Pathol. 2012;20(3):257–259. [DOI] [PubMed] [Google Scholar]
- 6.Nanba K, Chen AX, Omata K, Vinco M, Giordano TJ, Else T, Hammer GD, Tomlins SA, Rainey WE. Molecular heterogeneity in aldosterone-producing adenomas. J Clin Endocrinol Metab. 2016;101(3):999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dharia S, Slane A, Jian M, Conner M, Conley AJ, Parker CR Jr. Colocalization of P450c17 and cytochrome b5 in androgen-synthesizing tissues of the human. Biol Reprod. 2004;71(1):83–88. [DOI] [PubMed] [Google Scholar]