Abstract
Objective
To estimate the incidence and mortality rates of physician-diagnosed primary Sjögren’s syndrome (pSS) among residents of Olmsted County, Minnesota, and their evolution over time.
Patients and Methods
All medical records of patients with a diagnosis or suspicion of SS in Olmsted County, MN, from January 1, 2006 to December 31, 2015 were reviewed to identify incident cases of pSS (defined according to physician diagnosis). These cases were combined with a previous 1976–2005 incident cohort from the same population. Incidence rates were age and sex adjusted to the US white 2010 population. Survival rates were compared with the expected rates in the population of Minnesota.
Results
With 61 incident cases of pSS diagnosed in Olmsted County in 2006–2015, the total cohort included 172 patients with incident pSS in 1976–2015. Of the 172 patients, 151 (88%) were women and 161 (94%) were white, with a mean (SD) age at diagnosis of 58.3 (16.7) years. The average age- and sex- adjusted annual incidence for 2006–2015 was 5.9 per 100,000 population (95%CI 4.4–7.4), and overall incidence for the entire period was 5.8 (95% CI: 4.9–6.6) per 100,000. The incidence increased with calendar time over the 40-year period (P=.005). There was no difference in mortality in the pSS cohort compared to expected (standardized mortality ratio 1.15, 95%CI 0.86–1.50).
Conclusions
The average annual incidence of pSS in this population based-cohort was 5.8/100,000, with a progressive increase over the 40 years covered by the study. Overall survival of pSS patients was not different from the general population.
Introduction
Primary Sjögren’s syndrome (pSS) is a systemic autoimmune disorder characterized by an inflammatory infiltrate and progressive dysfunction of exocrine glands, particularly the lachrymal and salivary glands 1. Cardinal symptoms include mouth and eye dryness, marked fatigue and wide-spread pain, which have a profound effect on the quality of life of patients with pSS 2. These symptoms are non-specific and can occur with many other conditions 3, 4. Up to one third of patients also experience extraglandular inflammatory involvement including polysynovitis, neuropathy and inflammatory lung disease 5–7.
The etiology of pSS is not well understood 8, and probably includes environmental triggers in genetically predisposed subjects, as illustrated by its familial aggregation 9 and predisposing genetic variants 10. It mainly affects middle-aged females, with a strong sex bias potentially explained by an X chromosome dose effect 11. pSS occurs as a primary disorder, and secondary SS may occur with other systemic inflammatory diseases such as rheumatoid arthritis and systemic lupus erythematosus 12, 13. pSS may be associated with malignancies, especially non-Hodgkin lymphoma 14.
There are no specific diagnostic tests for pSS. To facilitate study of the disease and improve uniformity of the patient populations in clinical studies, researchers have developed classification criteria based upon the signs and symptoms and diagnostic tests used in evaluating patients with suspected SS. The coexistence of two different sets of classification criteria for pSS (the 2002 American European Consensus Group (AECG) 15 and the 2012 American College of Rheumatology (ACR) classification criteria 16) justified new consensual criteria17–19, which have been developed through an ACR-European League Against Rheumatism (EULAR) collaboration and were recently published 20, 21.
The epidemiology of pSS is poorly defined 22, 23. We recently reported that the 2015 prevalence of pSS in Olmsted County was 10.3/10,000 inhabitants according to the physician diagnosis, but only 2.2/10,000 according to classification criteria 24. Estimates of pSS incidence also vary considerably depending on population studied and methods used for case detection and ascertainment, with published incidence rates ranging from 3.1 to 10.7 cases per 100,000 25,26. The only incidence data available in the US were published by our group, which reported an average incidence of 3.9 per 100,000 for the 1976–1992 period 27 and 5.1 per 100,000 for the 1976–2005 period 28, suggesting a progressive increase of the incidence of the disease over time. It is unclear whether patients with pSS have an increased risk of mortality compared to the general population 29. The objectives of this study were to estimate the evolution over time of incidence of pSS among residents of Olmsted County, Minnesota and examine its effect on mortality.
Methods
Case identification and ascertainment
The study population included patients with incident pSS diagnosed in Olmsted County, Minnesota, a county with 113,306 adult (age ≥ 18 years) inhabitants as of January 1, 2015. This population is well suited for investigation of the epidemiology of pSS because of the availability of comprehensive medical record information for the entire population 30, 31. The potential cases were selected based on diagnostic codes for Sjögren’s syndrome, auto-antibody positivity (anti-SSA and/or – SSB), sicca syndrome and keratoconjunctivitis sicca (KCS) using the resources of the Rochester Epidemiology Project (REP). This is a medical records linkage system that allows ready access to the complete (inpatient and outpatient) records from all healthcare providers for the local population, including the Mayo Clinic and its affiliated hospital, the Olmsted Medical Center and its affiliated community hospital, local nursing homes and a few private practitioners 32. This system ensures virtually complete clinical information on all clinically recognized cases of pSS among Olmsted County residents. For the current update of pSS incidence, potential cases were all residents of Olmsted County, Minnesota at the time of their diagnosis, made between January 1, 2006 and December 31, 2015. These new cases were analyzed together with a previously reported incident pSS cohort including patients first diagnosed in 1976–2005 from the same population 28.
In addition to screening by diagnostic codes for Sjögren’s syndrome and relevant autoantibodies, 50 randomly selected patient records with diagnostic codes of xerostomia and 50 with KCS but without diagnosis of Sjögren’s syndrome or relevant autoantibodies were also screened to assess whether any additional cases of pSS case were missed using the other codes. As no patients with incident pSS were identified in these samples, the probability of missed cases is very low.
All individual clinical charts from patients selected during the first screening phase were reviewed. All patients with a definite physician diagnosis of pSS were included. Almost all diagnoses were made or confirmed by rheumatologists. Cases of uncertain diagnosis and those with secondary SS were excluded. Collected data included date of first pSS diagnosis, age, sex, ethnicity, smoking status, presence of dry eyes and dry mouth, serologic tests (anti-SSA, anti-SSB and antinuclear antibodies, rheumatoid factor), presence of hypergammaglobulinemia and results of diagnostic tests if performed such as Schirmer’s test, ocular surface staining, salivary scintigraphy, parotid sialography, unstimulated salivary flow measurement and minor salivary gland biopsy.
The 2002 AECG and 2012 ACR classification criteria were applied to all physician diagnosed cases. Systemic involvement was analyzed at the time of the diagnosis in patients diagnosed between 2006 and 2015 according to the EULAR Sjögren’s syndrome disease activity index (ESSDAI) 33–35, with no or low systemic activity category defined as an ESSDAI of less than 5, moderate activity as an ESSDAI comprised between 5 and 13, and high activity as an ESSDAI of 14 or more 36.
Statistical analyses
Descriptive statistics (means, percentages, etc.) were used to summarize the data. Comparisons of patient characteristics between time periods were performed using Chi-square and rank sum tests. Age- and sex-specific incidence rates for adults (age ≥ 18 years) were calculated using the number of incidence cases as the numerator and population estimates based on decennial census counts as the denominator, with linear interpolation to estimate population size for intercensal years. Annual incidence rates were illustrated using a 3 year centered moving average. Overall rates were age and sex adjusted to the 2010 United States white population. Ninety-five percent confidence intervals were computed for incidence rates assuming that the observed number of cases follows a Poisson distribution. Poisson regression models with smoothing splines to allow for non-linear effects were used to evaluate time trends in incidence rates.
Survival following the diagnosis of pSS was estimated using Kaplan-Meier methods. Observed and expected survival rates were compared using the log-rank test, where expected survival was based on the sex and age of the study population and on death rates from the Minnesota Caucasian life tables. The standardized mortality ratio (SMR), the ratio of observed number of deaths to the expected number, was estimated and a 95% CI obtained assuming that the observed number of deaths follows a Poisson distribution. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of the cohort
Between 2006 and 2015, 61 new patients were diagnosed with incident pSS in Olmsted County, and analyzed together with the incident cases diagnosed between 1976 and 2005. One patient age 17 years at diagnosis was excluded from incidence calculations but was included for survival analysis. Among these 172 patients (Table 1), 151 (88%) were women and 161 (94%) were white. The mean (SD) age at diagnosis was 58.3 (16.7) years. A majority (n=94, 58%) were never smokers. More patients reported dry eye symptoms (n=156, 91%) than dry mouth symptoms (n=144, 84%). There were no significant differences concerning these general characteristics between patients diagnosed before or after 2005. Features of systemic involvement at diagnosis were retrospectively collected for patients diagnosed between 2006 and 2015 using the ESSDAI (Table 2). The majority of patients (70%) had no or low systemic activity at incidence, 21% had moderate and 8% had high systemic disease activity. The median (range) ESSDAI score was 1 (0, 23). Among the different ESSDAI domains, most of the systemic activity was scored for the biological domain (in 26 patients), with only 12 patients scored for the glandular, 6 for the peripheral nerve, and 3 for the articular domains, respectively.
Table 1.
Characteristics of the patients with incident primary Sjögren’s syndrome diagnosed in Olmsted County, Minnesota between 1976 and 2015
| Table 1. Characteristics of patients with incident primary Sjögren’s syndrome by time period c | ||||
|---|---|---|---|---|
| 1976–2005 (N=111) | 2006–2015 (N=61) | Total (N=172) | P valueb | |
| Age, years, mean (SD) | 58.0 (16.6) | 58.9 (17.0) | 58.3 (16.7) | .73 |
| Sex (female) | 100 (90%) | 51 (84%) | 151 (88%) | .21 |
| Ethnicity, White | 105 (95%) | 56 (92%) | 161 (94%) | .06 |
| Smoking status at diagnosis of pSS (never smoked) | 58/102 (57%) | 36/60 (60%) | 94/162 (58%) | .13 |
| Ocular Symptoms | 101/111 (91%) | 55/61 (90%) | 156/172 (91%) | .86 |
| Oral symptoms | 94/111 (85%) | 50/61 (82%) | 144/172 (84%) | .64 |
| Rheumatoid factor | ||||
| Not done | 23 (21%) | 10 (16%) | 33 (19%) | .49 |
| Positive | 51/88 (58%) | 23/51 (45%) | 74/139 (53%) | .14 |
| Anti-nuclear antibody | ||||
| Not done | 14 (13%) | 2 (3%) | 16 (9%) | .04 |
| Positive | 67/97 (69%) | 43/59 (73%) | 110/156 (71%) | .61 |
| Anti-SSA (anti-Ro) | ||||
| Not done | 37 (33%) | 1 (2%) | 38 (22%) | <.001 |
| Positive | 51/74 (69%) | 48/60 (80%) | 99/134 (74%) | .15 |
| Anti-SSB (anti-La) | ||||
| Not done | 35 (32%) | 2 (3%) | 37 (22%) | <.001 |
| Positive | 42/76 (55%) | 34/59 (58%) | 76/135 (56%) | .78 |
| Hypergammaglobulinemia | ||||
| Not done | 24 (22%) | 11 (18%) | 35 (20%) | .58 |
| Present | 45/87 (52%) | 24/50 (48%) | 69/137 (50%) | .68 |
| Ocular objective tests | ||||
| Not done | 72 (65%) | 39 (64%) | 111 (65%) | .90 |
| Abnormal | 38/39 (97%) | 15/22 (68%) | 53/61 (87%) | .001 |
| Oral objective tests | ||||
| Not done | 56 (50%) | 51 (84%) | 107 (62%) | <.001 |
| Abnormal | 6/55 (11%) | 9/10 (90%) | 15/65 (23%) | <.001 |
| Salivary gland biopsy | ||||
| Not done | 100 (90%) | 51 (84%) | 151 (88%) | .21 |
| Abnormal | 3/11 (27%) | 9/10 (90%) | 12/21 (57%) | .004 |
| Met AECG criteria a | 15 (14%) | 17 (28%) | 32 (19%) | .02 |
| Met ACR criteria | 20 (18%) | 16 (26%) | 36 (21%) | .20 |
Abbreviations: AECG, 2002 American-European Consensus Group; ACR, 2012 American College of Rheumatology
P value for comparison between patients diagnosed during the 1976–2005 period vs the 2006–2015 period.
Values in table are n (%) or n present / n available (%) unless otherwise specified.
Table 2.
Systemic involvement in 61 patients with primary Sjögren’s syndrome diagnosed between 2006 and 2015 according to ESSDAI a criteria
| Domain | N (%) |
|---|---|
| Constitutional domain | |
| 0 | 59 (97%) |
| 1 | 1 (2%) |
| 2 | 1 (2%) |
| Lymphadenopathy domain | |
| 0 | 59 (97%) |
| 1 | 2 (3%) |
| Glandular domain | |
| 0 | 49 (80%) |
| 1 | 8 (13%) |
| 2 | 4 (7%) |
| Articular domain | |
| 0 | 58 (95%) |
| 1 | 1 (2%) |
| 2 | 2 (3%) |
| Cutaneous domain | |
| 0 | 60 (98%) |
| 2 | 1 (2%) |
| Respiratory domain | |
| 0 | 59 (97%) |
| 1 | 1 (2%) |
| 2 | 1 (2%) |
| Renal domain | |
| 0 | 59 (97%) |
| 3 | 2 (3%) |
| Muscular domain | |
| 0 | 59 (97%) |
| 2 | 1 (2%) |
| 3 | 1 (2%) |
| Peripheral nervous domain | |
| 0 | 55 (90%) |
| 1 | 2 (3%) |
| 2 | 3 (5%) |
| 3 | 1 (2%) |
| Central nervous domain | |
| 0 | 61 (100%) |
| Hematological domain | |
| 0 | 59 (98%) |
| 1 | 1 (2%) |
| Biological domain | |
| 0 | 35 (57%) |
| 1 | 25 (41%) |
| 2 | 1 (2%) |
| ESSDAI score, median (range) | 1.0 (0.0–23.0) |
| ESSDAI categories | |
| Low activity (<5) | 43 (70%) |
| Moderate activity (5–13) | 13 (21%) |
| High activity (≥14) | 5 (8%) |
ESSDAI, EULAR Sjögren’s syndrome disease activity index
Among the objective tests used by physicians to make a pSS diagnosis, serologic studies were the most frequently performed (Table 1). When performed, rheumatoid factor was positive in 53% of cases, antinuclear antibodies in 71%, anti-SSA in 74% and anti-SSB antibodies in 56%. Hypergammaglobulinemia was present in 50% of patients with available data. Objective tests to assess for sicca complaints were rarely performed. Ocular objective procedures (Schirmer’s test or ocular surface staining) were performed in 61 patients and were positive in 53 (87%). Salivary gland morphologic or functional tests (salivary scintigraphy, parotid sialography or unstimulated salivary flow) were performed in 65 patients and were positive in 15 (23%). Minor salivary gland lip biopsy was obtained in only 21 patients and was positive in 12 (57%). Comparing the two periods, physicians more often used serologic tests, especially anti-SSA/SSB antibodies, to diagnose the disease after 2005. Accordingly, only 32 (19%) patients met AECG criteria for classification of pSS, and 36 (21%) fulfilled ACR criteria.
Analysis of pSS incidence over time and impact of seasonality
The overall age and sex adjusted annual incidence of pSS was 5.8 per 100,000 population age ≥ 18 years over the 40-year period covered by the study (Table 3). Incidence was 2 to 7 times higher in females compared to males in the different age classes (5.9 times higher on average), and increased progressively with age, culminating at 19.6 per 100,000 in females aged 65–74 years, with a slight decline thereafter to 15.9 per 100,000 among females aged 75 years and more.
Table 3.
Incidence of primary Sjögren’s syndrome among residents of Olmsted County, Minnesota in 1976–2015 by sex and age group, per 100,000 population age ≥ 18 years.
| Male | Female | Total | ||||
|---|---|---|---|---|---|---|
| Age group | N | Rate | N | Rate | N | Rate |
| 18–44 | 6 | 0.6 | 30 | 3.1 | 36 | 1.9 |
| 45–54 | 3 | 1.0 | 36 | 12.0 | 39 | 6.6 |
| 55–64 | 4 | 2.0 | 30 | 14.1 | 34 | 8.2 |
| 65–74 | 3 | 2.5 | 29 | 19.6 | 32 | 11.9 |
| 75+ | 5 | 5.7 | 25 | 15.9 | 30 | 12.3 |
| Overallc | 21 | 1.6a (0.9, 2.3) | 150 | 9.5 a (8.0, 11.1) | 171 | 5.8b (4.9, 6.6) |
Age adjusted to US white 2010 population
Age and sex adjusted to US white 2010 population
One patient age 17 years at diagnosis was excluded from incidence calculations.
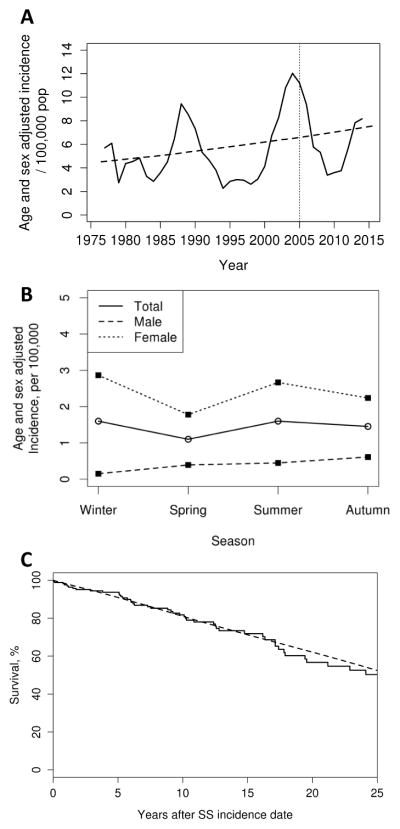
There was some fluctuation in annual incidence of pSS with higher values around 1990, 2005 and 2015. Overall, however, there was a progressive increase of pSS incidence over time (Figure 1A, P=.005). The overall age and sex adjusted incidence rate was 4.2 (95% CI: 2.3, 6.1) for the period 1976–1985, 5.2 (95% CI: 3.4, 7.1) for the period 1986–1995, 7.0 (95% CI: 5.1, 8.9) for the period 1996–2005,and 5.9 (95% CI: 4.4, 7.4) per 100,000 population for the period 2006–2015.
Figure 1.
Incidence over time (A), seasonality (B) and survival (C) in Olmsted County, Minnesota residents with incident primary Sjögren’s syndrome (SS) in 1976–2015 (n=172).
No effect of seasonality on disease incidence was observed (figure 1B), with an age and sex adjusted incidence rate (95% CI) of 1.6 (1.1, 2.1) during winter, 1.1 (0.7, 1.5) during spring, 1.6 (1.1, 2.1) during summer and 1.5 (1.0, 1.9) per 100,000 population during fall (P=.38 for the four season comparison).
Mortality of pSS patients compared to the general population
There were 52 deaths in the 172 patients. Compared to the general population, in which about 45 deaths would be expected, the standardized mortality for patients with pSS incident 1976–2015 was 1.15 (95% CI: 0.86, 1.50) (Figure 1C). No evidence of a time trend in survival according to year of diagnosis was found (P=.90, data not shown). Overall, 5, 10, and 20 year survival rates were 56.7 (95% CI: 46.6–68.9%), 93.7% (95% CI: 90.0–97.6%), 81.8 (95% CI: 75.5–88.6%), and 71.8 (95% CI: 63.8–80.8%), respectively.
Discussion
This study reports estimates of the incidence and mortality rates of pSS patients in a geographically well-defined region in the U.S., and their evolution over a period of 40 years. The estimated incidence of pSS in Olmsted County in 1976–2015 was 5.8/100,000, with a significant progressive increase of pSS incidence over time. Mortality rates of pSS patients were similar to expected rates in the general population.
Better description of the epidemiology of pSS is important for public health stakeholders, regulating agencies and drug companies. To date, no disease-modifying drug has been proven effective to improve pSS symptoms and change disease natural course, as all published large randomized controlled trials are negative 37–39. These trial failures could be explained by design issues and outcome definitions 40, 41. Treatment algorithms are currently based on expert opinion, usually by analogy to other systemic autoimmune diseases 42–44. However, recent years have witnessed an increase in interest of pharmaceutical companies for developing new drugs in the disease, and more than 10 industry-sponsored randomized controlled studies are currently ongoing 45. Hopefully, some of these innovative drugs tested in the disease will demonstrate their efficacy, and better knowledge of the number and characteristics of patients in the general population who could potentially benefit from these drugs will be of major importance at the time of their commercialization.
All previous studies reporting pSS incidence worldwide are summarized in table 4. Our incidence rate is in the range of what was described in those previous studies, despite study design differences and case definitions. Strengths of the current study include the broad screening methodology, using the REP, allowing complete full access to a performant coding system and to all individual medical records of the screened patients, which resulted in an exhaustive case detection and ascertainment in the general population living in Olmsted County. Most of these other studies used hospital-based medical record search 25, 26, 46, 47, but it is likely that some patients with mild presentation not requiring specific therapies are diagnosed and followed outside of the hospital setting, and are therefore missed by such case-detection strategies. The study by Weng et al in Taiwan used administrative database search with no case ascertainment, which could be biased by coding errors and therefore false diagnoses 48.
Table 4.
Comparison of published incidence studies of primary Sjögren’s syndrome worldwide
| Reference | Region | Study Period | Source of cases | Case definition | pSS cases | Background population | Reported incidence rate (95%CI)/100,000 |
|---|---|---|---|---|---|---|---|
| Plesivcnik Novljan et al., 200446 | Slovenia | 2000–2002 | Hospital-based case screening (Rheumatology department) | 1996 European criteria | 71 | 599,895 | 3.9 (1.1, 10.2) |
| Alamanos et al., 200647 | Greece | 1982–2003 | Medical record search (hospital + private rheumatologists) | AECG a criteria | 422 | 488,435 | 5.3 4.5, 6.1) |
| Weng et al., 201148 b | Taiwan | 2005–2007 | National Health Insurance database“ | Catastrophic Illness Certificate”, no case review | 3352 | 22,823,550 | 6.0 (5.8, 6.2) |
| Kvarnstrom et al., 201525 | Sweden | 2007–2011 | Hospital-based case screening (Rheumatology department) | AECG | 199 | ≈1,300,000 | 3.1 (2.3, 4.3) |
| Elfving et al., 201626 | Finland | 2010 | Hospital-based case screening (Rheumatology department) | Physician diagnosis | 22 | 206,441 | 10.7 (6.7–16.1) |
| Present study | USA (Minnesota) | 1976–2015 | Rochester Epidemiology Project: coding system screening for the whole population, and medical records review | Physician diagnosis | 172 | 113,306 | 5.8 (4.9, 6.6) |
Abbreviations: AECG, American-European Consensus Group classification criteria; ICD, International Classification of the Diseases; ACR, American College of Rheumatology; ND, not done; pSS, primary Sjögren’s syndrome.
The case-ascertainment method in this study was based on definite physician diagnosis of pSS. Even if not standardized, this reflects how the disease is actually diagnosed, and therefore treated, in routine practice in a community setting. Using only administrative coding systems without analyzing actual medical data from the clinical charts may lead to both inclusion of cases that do not have the diagnoses and failure to detect actual cases.
As demonstrated in our study, current classification criteria for pSS are not applicable to epidemiologic studies in a community-based setting, since physicians in Olmsted County do not use several of the tests included in the different versions of the classification criteria (mainly functional salivary and ocular tests and salivary gland biopsy) to diagnose the disease. In our study, the main explanation for not fulfilling the criteria was that the required tests were not performed, and not that they were negative. Of note, a similar observation was made in previous population-based pSS studies. In the prevalence study by Maldini et al, a substantial proportion of patients received a clinical diagnosis of pSS but did not fulfil AECG criteria because oral/ocular tests were not performed, and the authors used “enlarged” criteria to define pSS cases 49.
The low number of patients who underwent a salivary gland biopsy in our cohort probably indicates that treating physicians consider that patients without autoantibodies (anti-SSA, anti-SSB, but also ANA or RF) do not actually have pSS. The main therapies offered to patients with sicca symptoms with or without pSS are directed toward management of dryness, including tear and saliva substitutes. Therefore, physicians may not consider it clinically useful to perform invasive diagnostic tests such as salivary gland biopsy if results of these tests would not affect clinical decisions regarding diagnosis and management. However, even if “seronegative” pSS patients probably have a lower risk of lymphoma development 50, they have symptoms severe enough to require medical attention 51. Besides its diagnostic value, salivary gland biopsy also gives important prognostic information, as patients with higher focus scores or ectopic germinal centers have a higher risk of systemic complications and lymphoma development 52–54. Therefore, minor salivary gland biopsy should be considered in every patient with suspected pSS.
Several reasons could explain the progressive increase of pSS incidence over time seen in this study. Aging of the general population is not a factor, since all incidence estimates are age and sex adjusted to the general population. Interestingly, general characteristics of patients diagnosed with pSS in Olmsted County did not change over time. Physicians could be more aware of the diagnosis in the recent years compared to the early years (1970s–1980s) of the study period. The greater availability of anti-SSA and anti-SSB antibody testing over the 40 years of our study could also have contributed to the increased incidence over time, facilitating a diagnosis in those with milder disease. Patients may seek more often medical care for subjective symptoms that impair their quality of life but may still be considered benign. Changes in lifestyle and environmental exposures could also lead to a real increase of the number of individuals developing this autoimmune disease.
The mortality in patients with incident pSS in Olmsted County is not higher that the general population. Previous studies have shown that subgroups of pSS patients have a decreased survival, especially patients with high systemic disease activity 55, and notably patients who develop severe systemic involvement such as cryoglobulinemic vasculitis 56 or who develop lymphoma 57. However, those severe presentations are rare among patient with incident pSS, and do not affect the overall survival. Systemic involvement in our cohort was analyzed at the time of diagnosis based on retrospective chart review, which could lead to an under-evaluation of the ESSDAI score, especially its biological domain, if tests required for the scoring were not performed (such as cryoglobulin, IgG or complement levels).
Conclusion
To conclude, the average annual incidence of physician-diagnosed pSS in Olmsted County was 5.8/100,000, with a progressive increase over the 40 years covered by the study. Current classification criteria do not perform well to study the epidemiology of pSS in a community setting. Overall survival of pSS patients was not different from the general population.
Acknowledgments
Financial support: This work was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676 and CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Divi Cornec received a grant from the French Society of Rheumatology (SFR) and from Brest University Hospital for his current fellowship at Mayo Clinic.
List of abbreviations
- pSS
Primary Sjögren’s syndrome
- AECG
American European Consensus Group
- ACR
American College of Rheumatology
- EULAR
European League Against Rheumatism
- KCS
keratoconjunctivitis sicca
- REP
Rochester Epidemiology Project
- SMR
standardized mortality ratio
Footnotes
Conflict of interest disclosure: The authors have no other conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brito-Zeron P, Baldini C, Bootsma H, et al. Sjogren syndrome. Nat Rev Dis Primers. 2016;2:16047. doi: 10.1038/nrdp.2016.47. [DOI] [PubMed] [Google Scholar]
- 2.Cornec D, Devauchelle-Pensec V, Mariette X, et al. Severe Health-Related Quality-of-life Impairment in Active Primary Sjogren’s Syndrome Is Driven by Patient-Reported Outcomes: Data from a Large Therapeutic Trial. Arthritis Care Res (Hoboken) 2016 doi: 10.1002/acr.22974. [DOI] [PubMed] [Google Scholar]
- 3.Cornec D, Saraux A, Jousse-Joulin S, et al. The Differential Diagnosis of Dry Eyes, Dry Mouth, and Parotidomegaly: A Comprehensive Review. Clin Rev Allergy Immunol. 2014 doi: 10.1007/s12016-014-8431-1. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen A, Radfar L, Lewis D, et al. Previous diagnosis of Sjogren’s Syndrome as rheumatoid arthritis or systemic lupus erythematosus. Rheumatology (Oxford) 2016;55:1195–1201. doi: 10.1093/rheumatology/kew023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malladi AS, Sack KE, Shiboski SC, et al. Primary Sjogren’s syndrome as a systemic disease: a study of participants enrolled in an international Sjogren’s syndrome registry. Arthritis Care Res (Hoboken) 2012;64:911–918. doi: 10.1002/acr.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos-Casals M, Brito-Zeron P, Seror R, et al. Characterization of systemic disease in primary Sjogren’s syndrome: EULAR-SS Task Force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology (Oxford) 2015;54:2230–2238. doi: 10.1093/rheumatology/kev200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvajal Alegria G, Guellec D, Mariette X, et al. Epidemiology of neurological manifestations in Sjogren’s syndrome: data from the French ASSESS Cohort. RMD Open. 2016;2:e000179. doi: 10.1136/rmdopen-2015-000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nocturne G, Mariette X. Advances in understanding the pathogenesis of primary Sjogren’s syndrome. Nat Rev Rheumatol. 2013;9:544–556. doi: 10.1038/nrrheum.2013.110. [DOI] [PubMed] [Google Scholar]
- 9.Kuo CF, Grainge MJ, Valdes AM, et al. Familial Risk of Sjogren’s Syndrome and Co-aggregation of Autoimmune Diseases in Affected Families: A Nationwide Population Study. Arthritis Rheumatol. 2015;67:1904–1912. doi: 10.1002/art.39127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lessard CJ, Li H, Adrianto I, et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjogren’s syndrome. Nat Genet. 2013;45:1284–1292. doi: 10.1038/ng.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu K, Kurien BT, Zimmerman SL, et al. X Chromosome Dose and Sex Bias in Autoimmune Diseases: Increased Prevalence of 47,XXX in Systemic Lupus Erythematosus and Sjogren’s Syndrome. Arthritis Rheumatol. 2016;68:1290–1300. doi: 10.1002/art.39560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown LE, Frits ML, Iannaccone CK, Weinblatt ME, Shadick NA, Liao KP. Clinical characteristics of RA patients with secondary SS and association with joint damage. Rheumatology (Oxford) 2015;54:816–820. doi: 10.1093/rheumatology/keu400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fragoulis GE, Fragkioudaki S, Reilly JH, Kerr SC, McInnes IB, Moutsopoulos HM. Analysis of the cell populations composing the mononuclear cell infiltrates in the labial minor salivary glands from patients with rheumatoid arthritis and sicca syndrome. J Autoimmun. 2016;73:85–91. doi: 10.1016/j.jaut.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Nocturne G, Virone A, Ng WF, et al. Rheumatoid Factor and Disease Activity Are Independent Predictors of Lymphoma in Primary Sjogren’s Syndrome. Arthritis Rheumatol. 2016;68:977–985. doi: 10.1002/art.39518. [DOI] [PubMed] [Google Scholar]
- 15.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiboski SC, Shiboski CH, Criswell L, et al. American College of Rheumatology classification criteria for Sjogren’s syndrome: a data-driven, expert consensus approach in the Sjogren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 2012;64:475–487. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmussen A, Ice JA, Li H, et al. Comparison of the American-European Consensus Group Sjogren’s syndrome classification criteria to newly proposed American College of Rheumatology criteria in a large, carefully characterised sicca cohort. Ann Rheum Dis. 2014;73:31–38. doi: 10.1136/annrheumdis-2013-203845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornec D, Saraux A, Cochener B, et al. Level of agreement between 2002 American-European Consensus Group and 2012 American College of Rheumatology classification criteria for Sjogren’s syndrome and reasons for discrepancies. Arthritis Res Ther. 2014;16:R74. doi: 10.1186/ar4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitali C, Bootsma H, Bowman SJ, et al. Classification criteria for Sjogren’s syndrome: we actually need to definitively resolve the long debate on the issue. Ann Rheum Dis. 2013;72:476–478. doi: 10.1136/annrheumdis-2012-202565. [DOI] [PubMed] [Google Scholar]
- 20.Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren’s syndrome A consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-210571. [DOI] [PubMed] [Google Scholar]
- 21.Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjogren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2016 doi: 10.1002/art.39859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin B, Wang J, Yang Z, et al. Epidemiology of primary Sjogren’s syndrome: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:1983–1989. doi: 10.1136/annrheumdis-2014-205375. [DOI] [PubMed] [Google Scholar]
- 23.Cornec D, Chiche L. Is primary Sjogren’s syndrome an orphan disease? A critical appraisal of prevalence studies in Europe. Ann Rheum Dis. 2015;74:e25. doi: 10.1136/annrheumdis-2014-206860. [DOI] [PubMed] [Google Scholar]
- 24.Maciel G, Crowson CS, Matteson EL, Cornec D. Prevalence of Primary Sjogren’s Syndrome in a Population-Based Cohort in the United States. Arthritis Care Res (Hoboken) 2016 doi: 10.1002/acr.23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kvarnstrom M, Ottosson V, Nordmark B, Wahren-Herlenius M. Incident cases of primary Sjogren’s syndrome during a 5-year period in Stockholm County: a descriptive study of the patients and their characteristics. Scand J Rheumatol. 2015;44:135–142. doi: 10.3109/03009742.2014.931457. [DOI] [PubMed] [Google Scholar]
- 26.Elfving P, Marjoniemi O, Niinisalo H, et al. Estimating the incidence of connective tissue diseases and vasculitides in a defined population in Northern Savo area in 2010. Rheumatol Int. 2016;36:917–924. doi: 10.1007/s00296-016-3474-7. [DOI] [PubMed] [Google Scholar]
- 27.Pillemer SR, Matteson EL, Jacobsson LT, et al. Incidence of physician-diagnosed primary Sjogren syndrome in residents of Olmsted County, Minnesota. Mayo Clin Proc. 2001;76:593–599. doi: 10.4065/76.6.593. [DOI] [PubMed] [Google Scholar]
- 28.Nannini C, Jebakumar AJ, Crowson CS, Ryu JH, Matteson EL. Primary Sjogren’s syndrome 1976–2005 and associated interstitial lung disease: a population-based study of incidence and mortality. BMJ Open. 2013;3:e003569. doi: 10.1136/bmjopen-2013-003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh AG, Singh S, Matteson EL. Rate, risk factors and causes of mortality in patients with Sjogren’s syndrome: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 2016;55:450–460. doi: 10.1093/rheumatology/kev354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seror R, Ravaud P, Bowman SJ, et al. EULAR Sjogren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann Rheum Dis. 2010;69:1103–1109. doi: 10.1136/ard.2009.110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seror R, Theander E, Brun JG, et al. Validation of EULAR primary Sjogren’s syndrome disease activity (ESSDAI) and patient indexes (ESSPRI) Ann Rheum Dis. 2015;74:859–866. doi: 10.1136/annrheumdis-2013-204615. [DOI] [PubMed] [Google Scholar]
- 35.Seror R, Bowman SJ, Brito-Zeron P, et al. EULAR Sjogren’s syndrome disease activity index (ESSDAI): a user guide. RMD Open. 2015;1:e000022. doi: 10.1136/rmdopen-2014-000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seror R, Bootsma H, Saraux A, et al. Defining disease activity states and clinically meaningful improvement in primary Sjogren’s syndrome with EULAR primary Sjogren’s syndrome disease activity (ESSDAI) and patient-reported indexes (ESSPRI) Ann Rheum Dis. 2016;75:382–389. doi: 10.1136/annrheumdis-2014-206008. [DOI] [PubMed] [Google Scholar]
- 37.Mariette X, Ravaud P, Steinfeld S, et al. Inefficacy of infliximab in primary Sjogren’s syndrome: results of the randomized, controlled Trial of Remicade in Primary Sjogren’s Syndrome (TRIPSS) Arthritis Rheum. 2004;50:1270–1276. doi: 10.1002/art.20146. [DOI] [PubMed] [Google Scholar]
- 38.Devauchelle-Pensec V, Mariette X, Jousse-Joulin S, et al. Treatment of primary Sjogren syndrome with rituximab: a randomized trial. Ann Intern Med. 2014;160:233–242. doi: 10.7326/M13-1085. [DOI] [PubMed] [Google Scholar]
- 39.Gottenberg JE, Ravaud P, Puechal X, et al. Effects of hydroxychloroquine on symptomatic improvement in primary Sjogren syndrome: the JOQUER randomized clinical trial. JAMA. 2014;312:249–258. doi: 10.1001/jama.2014.7682. [DOI] [PubMed] [Google Scholar]
- 40.Cornec D, Devauchelle-Pensec V, Mariette X, et al. Development of the Sjogren’s Syndrome Responder Index, a data-driven composite endpoint for assessing treatment efficacy. Rheumatology (Oxford) 2015;54:1699–1708. doi: 10.1093/rheumatology/kev114. [DOI] [PubMed] [Google Scholar]
- 41.Devauchelle-Pensec V, Gottenberg JE, Jousse-Joulin S, et al. Which and How Many Patients Should Be Included in Randomised Controlled Trials to Demonstrate the Efficacy of Biologics in Primary Sjogren’s Syndrome? PLoS One. 2015;10:e0133907. doi: 10.1371/journal.pone.0133907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saraux A, Pers JO, Devauchelle-Pensec V. Treatment of primary Sjogren syndrome. Nat Rev Rheumatol. 2016;12:456–471. doi: 10.1038/nrrheum.2016.100. [DOI] [PubMed] [Google Scholar]
- 43.Carsons SE, Vivino FB, Parke A, et al. Treatment Guidelines for Rheumatologic Manifestations of Sjogren’s: Use of Biologics, Management of Fatigue and Inflammatory Musculoskeletal Pain. Arthritis Care Res (Hoboken) 2016 doi: 10.1002/acr.22968. [DOI] [PubMed] [Google Scholar]
- 44.Vivino FB, Carsons SE, Foulks G, et al. New Treatment Guidelines for Sjogren’s Disease. Rheum Dis Clin North Am. 2016;42:531–551. doi: 10.1016/j.rdc.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nocturne G, Cornec D, Seror R, Mariette X. New biological therapies in Sjogren’s syndrome. Best Pract Res Clin Rheumatol. 2015;29:783–793. doi: 10.1016/j.berh.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Plesivcnik Novljan M, Rozman B, Hocevar A, Grmek M, Kveder T, Tomsic M. Incidence of primary Sjogren’s syndrome in Slovenia. Ann Rheum Dis. 2004;63:874–876. doi: 10.1136/ard.2003.014027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alamanos Y, Tsifetaki N, Voulgari PV, Venetsanopoulou AI, Siozos C, Drosos AA. Epidemiology of primary Sjogren’s syndrome in north-west Greece, 1982–2003. Rheumatology (Oxford) 2006;45:187–191. doi: 10.1093/rheumatology/kei107. [DOI] [PubMed] [Google Scholar]
- 48.Weng MY, Huang YT, Liu MF, Lu TH. Incidence and mortality of treated primary Sjogren’s syndrome in Taiwan: a population-based study. J Rheumatol. 2011;38:706–708. doi: 10.3899/jrheum.100883. [DOI] [PubMed] [Google Scholar]
- 49.Maldini C, Seror R, Fain O, et al. Epidemiology of primary Sjogren’s syndrome in a French multiracial/multiethnic area. Arthritis Care Res (Hoboken) 2014;66:454–463. doi: 10.1002/acr.22115. [DOI] [PubMed] [Google Scholar]
- 50.Quartuccio L, Baldini C, Bartoloni E, et al. Anti-SSA/SSB-negative Sjogren’s syndrome shows a lower prevalence of lymphoproliferative manifestations, and a lower risk of lymphoma evolution. Autoimmun Rev. 2015;14:1019–1022. doi: 10.1016/j.autrev.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Segal BM, Pogatchnik B, Henn L, Rudser K, Sivils KM. Pain severity and neuropathic pain symptoms in primary Sjogren’s syndrome: a comparison study of seropositive and seronegative Sjogren’s syndrome patients. Arthritis Care Res (Hoboken) 2013;65:1291–1298. doi: 10.1002/acr.21956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Risselada AP, Kruize AA, Goldschmeding R, Lafeber FP, Bijlsma JW, van Roon JA. The prognostic value of routinely performed minor salivary gland assessments in primary Sjogren’s syndrome. Ann Rheum Dis. 2014;73:1537–1540. doi: 10.1136/annrheumdis-2013-204634. [DOI] [PubMed] [Google Scholar]
- 53.Carubbi F, Alunno A, Cipriani P, et al. Is minor salivary gland biopsy more than a diagnostic tool in primary Sjogrens syndrome? Association between clinical, histopathological, and molecular features: a retrospective study. Semin Arthritis Rheum. 2014;44:314–324. doi: 10.1016/j.semarthrit.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 54.Carubbi F, Alunno A, Cipriani P, et al. A retrospective, multicenter study evaluating the prognostic value of minor salivary gland histology in a large cohort of patients with primary Sjogren’s syndrome. Lupus. 2015;24:315–320. doi: 10.1177/0961203314554251. [DOI] [PubMed] [Google Scholar]
- 55.Brito-Zeron P, Kostov B, Solans R, et al. Systemic activity and mortality in primary Sjogren syndrome: predicting survival using the EULAR-SS Disease Activity Index (ESSDAI) in 1045 patients. Ann Rheum Dis. 2016;75:348–355. doi: 10.1136/annrheumdis-2014-206418. [DOI] [PubMed] [Google Scholar]
- 56.Retamozo S, Gheitasi H, Quartuccio L, et al. Cryoglobulinaemic vasculitis at diagnosis predicts mortality in primary Sjogren syndrome: analysis of 515 patients. Rheumatology (Oxford) 2016;55:1443–1451. doi: 10.1093/rheumatology/kew194. [DOI] [PubMed] [Google Scholar]
- 57.Theander E, Manthorpe R, Jacobsson LT. Mortality and causes of death in primary Sjogren’s syndrome: a prospective cohort study. Arthritis Rheum. 2004;50:1262–1269. doi: 10.1002/art.20176. [DOI] [PubMed] [Google Scholar]
- 58.See LC, Kuo CF, Chou IJ, Chiou MJ, Yu KH. Sex- and age-specific incidence of autoimmune rheumatic diseases in the Chinese population: a Taiwan population-based study. Semin Arthritis Rheum. 2013;43:381–386. doi: 10.1016/j.semarthrit.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Yu KH, See LC, Kuo CF, Chou IJ, Chou MJ. Prevalence and incidence in patients with autoimmune rheumatic diseases: a nationwide population-based study in Taiwan. Arthritis Care Res (Hoboken) 2013;65:244–250. doi: 10.1002/acr.21820. [DOI] [PubMed] [Google Scholar]