Abstract
Two studies were performed that compared a “Paired” condition in which participants studied paired associates with a “Generated” condition in which participants completed word fragments to produce paired associates. In both tasks, participants were responsible for memory of the material either studied or generated. The experiments revealed significant differences between the responses of a predefined prefrontal region and a predefined parietal region. The parietal region responded more in the Generated condition than the Paired condition, whereas there was no difference in the prefrontal region. On the other hand, the prefrontal region responded to the delay between study and test in both the Paired and Generated conditions, whereas the parietal region only responded to delay in the Generated condition. This pattern of results is consistent with the hypothesis that the parietal region is responsive to changes in problem representation and the prefrontal region to retrieval operations. An information-processing model embodying these assumptions was fit to the blood oxygen level–dependent responses in these regions.
Keywords: associative learning, encoding, memory, parietal cortex, prefrontal cortex
Introduction
The prefrontal region has been repeatedly implicated in imaging research as important for memory (e.g., Buckner et al. 1999; Lepage et al. 2000; Fletcher and Henson 2001; Wagner et al. 2001; Cabeza et al. 2002; Sohn et al. 2003, 2005; Thompson-Schill 2003; Wheeler and Buckner 2003; Kohler et al. 2004; Badre et al. 2005; Dobbins and Wagner 2005). These imaging results are not particularly surprising given evidence about the memory deficits associated with prefrontal lesions (Stuss and Benson 1984; Shimamura 1995), although such lesions tend to be not as devastating to memory as temporal lesions (Squire 1992; Cohen and Eichenbaum 1993). As noted in the recent review of Wagner et al. (2005), there is also evidence that the posterior parietal region is implicated in studies of memory (e.g., Habib and Lepage 1999; Buckner and Wheeler 2001; Rugg et al. 2002) even though lesions to this parietal region do not tend to be associated with deficits in memory. A classic finding in these studies is that there is greater parietal activation in recognition memory experiments when old items are correctly recognized than when foils are correctly rejected. Although most data implicating the parietal region come from recognition and source memory experiments, this paper will show that parietal regions are also active in associative recall tasks.
Cabeza et al. (2003) noted that the parietal and prefrontal regions are part of a general circuit that also includes the anterior cingulate and thalamic regions and that these areas tend to be involved in a number of attention tasks (see also Dosenbach et al. 2006). They argue that activity in all these regions may reflect more general processes than just memory. Based on the ACT-R theory (Anderson, Bothell, et al. 2004), we have developed an interpretation of the activity in this circuit (e.g., Anderson 2005; Anderson et al. 2007; Danker and Anderson 2007) and have argued that different regions serve different functions. In particular, the prefrontal region serves a more pure memory function, being engaged by storage and retrieval operations, whereas the posterior parietal region is engaged by changes in problem representation. There is considerable evidence to support the assumption that the parietal region plays an important role in visual–spatial and verbal representations. It is engaged in verbal encoding (Davachi et al. 2001; Clark and Wagner 2003), mental rotation (Alivisatos and Petrides 1997; Richter et al. 1997; Carpenter et al. 1999; Heil 2002; Zacks et al. 2002), and visual–spatial strategies in a variety of contexts (Dehaene et al. 1999; Reichle et al. 2000; Sohn et al. 2004). A number of other researchers have also proposed a representational role for the parietal region (e. g., Bunge et al. 2002; Shannon and Buckner 2004).
Many tasks, including memory tasks, will engage both the representational and retrieval activities. For instance, in a recall trial for a paired-associate task like the one described here, the participant must first represent the stimulus, then engage in retrieval, and then represent the retrieved response. If representational activities engage the parietal and retrieval activities engage the prefrontal, it is not surprising that the activities of these 2 regions are frequently correlated. (Perhaps the reason why the posterior parietal region is more active when old items are recognized than when foils are rejected is that participants are representing their memory of the retrieved item). To disentangle these regions, one needs a manipulation that will affect only the difficulty of the retrieval or only the difficulty of the representation. Sohn et al. (2003, 2005) report a series of studies that manipulated the difficulty of the retrieval process but not the difficulty of the representational process. According to the ACT-R theory, fan (a manipulation of associative interference) should slow the retrieval of the item but should have no effect on the representational processes that are invoked to represent the probe and the eventual result of the retrieval. Corresponding to this theoretical analysis, Sohn et al. found robust activation in both prefrontal and parietal regions, but fan only affected the level of activation in the prefrontal region. The purpose of the current experiments is to go beyond the demonstration of the previous Sohn et al. experiments by using a double dissociation, with one factor influencing the difficulty of the representation and another factor affecting the difficulty of the retrieval operation.
Although the paper will report exploratory analyses for the effects of the representational factor and the retrieval factors, the major interest will be on 2 predefined regions, which we have examined in previous studies. By using the same regions across a series of studies, it is possible to test the replicability of results. Also because we are just looking at 2 predefined regions where there are strong prior hypotheses, we avoid the need for having to correct for false positives that occur in exploratory studies that look at large numbers of regions. In particular, our past studies have focused on the following 2 left regions, each 5 voxels wide, 5 voxels long, and 4 voxels high (a voxel is 3.125 mm long and wide and 3.2 mm high):
Prefrontal. Centered at Talairach coordinates x = −40, y = 21, z = 21. This includes parts of Brodmann Areas 45 and 46 around the inferior frontal sulcus.
Parietal. Centered at x = −23, y = −64, z = 34. This includes parts of Brodmann Areas 7, 39, and 40 at the border of the intraparietal sulcus.
Within a paired-associate task, the current experiments used both a manipulation of retrieval difficulty and a manipulation of representational difficulty. The experiments used delay to manipulate retrieval difficulty, contrasting cases where the retrieval demands were minimal with cases where they were substantial. The minimal retrieval condition was created by asking participants to retrieve an item immediately after studying. The substantial retrieval condition was created by inserting a number of intervening items. This variable of delay of recall was manipulated within participants. Pilot research indicated a large effect of delay on retrieval time. The prediction was that this delay manipulation would have a greater effect on the prefrontal region.
Both experiments used a representational manipulation in which one condition involved minimal representational requirements and another condition involved more substantial requirements. The minimal condition simply involved presenting the paired associate to study and the stimulus to retrieve. The other condition required the participant to engage in extra representational activities to construct the paired associate at study and to extract the response from the paired associate at test. This was manipulated between participants. In the Paired condition, participants were presented with a paired associate such as band-2 at study. At test band was presented and participants were asked to recall 2. In the Generated condition, participants were given word phrases, such as “b-nd _ -id = adhesive strip,” and instructed to solve the phrase by finding a single letter that would complete the phrase (in this case that letter is “a”). Thus, they generated the paired associate band-aid. Pilot research had indicated that these problems were sufficiently constrained that participants could almost always solve them successfully. At test, the Generated participants were presented with the word (e.g., band) and had to respond with the position of the letter they had provided (i.e., 2 in this case). Thus, at test, participants responded with the same number to the same word in either the Paired or the Generated condition, but they had to extract the response from the pair in the Generated condition. Pilot research indicated that there was similar performance in the 2 conditions, but we expected that the Generated condition would have greater activation in the parietal region because of the need to construct the paired associate at study and to extract the response at test.
Experiment 1
The first study was a slow event-related design. Participants went through a repeating cycle of study and test with enough time between trials to let the hemodynamic response from one trial return to baseline before the next trial. Figure 1 shows the procedures for the Paired and the Generated trials. Both conditions involved fourteen 2-s scans. Both conditions consisted of a fixation period (1 scan, 2 s), a study period (3 scans, 6 s), a warning for the retrieval (1 scan, 2 s), a response interval (3 scans, 6 s), feedback (1 scan, 2 s), and then a minimal distractor task to prevent rehearsal and enable the blood oxygen level–dependent (BOLD) response to return to a baseline (5 scans, 10 s).
Figure 1.
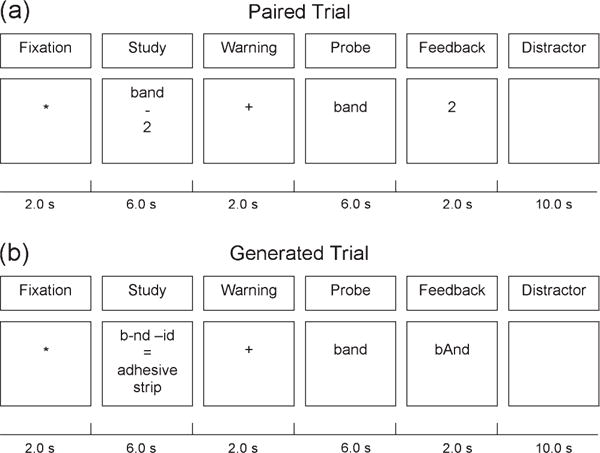
A comparison of the procedures in the Paired and Generated conditions.
The following were our basic predictions before the experiments:
In the parietal region, during the period of study and test, there will be a stronger response for the Generated condition than the Paired condition. This is because the parietal region will have to perform more representational operations in the Generated condition.
In the prefrontal region, during the period of study and test, there will be a stronger or equal response for the Paired condition. The response may be stronger than the Generated condition because the participants may devote more effort to memorizing in the Paired condition.
In the prefrontal region, only during the period of test, there will be a stronger response in the delay condition than the immediate condition. This is because of the greater retrieval times in the delay condition.
In the parietal region, there should be no difference between the immediate and delay conditions.
After describing both experiments, we will describe an ACT-R model for the experiment that enabled us to elaborate these predictions and to slightly modify them.
Method
Participants
Participants were recruited locally, and they provided written informed consent in accordance with the guidelines at the University of Pittsburgh and Carnegie Mellon University. Twenty right-handed, native English speakers (9 female/11 male, 18–27 years of age with an average age of 21.5) completed the study, which consisted of one training session and one scanning session. Eleven participants were in the Paired condition and 9 in the Generated condition.
Materials and Design
The E-Prime (Schneider et al. 2002) software package was used to present stimuli and to collect behavioral performance.
There were 8 different test versions used in the experiment, 4 for the Generated group and 4 for the Paired group. As shown in Figure 1, the test format for both Generated and Paired conditions were identical. Each version consisted of 50 practice trials and 100 test trials. Each trial consisted of a study interval, a probe or test interval, a feedback interval, and a distractor task interval.
The length between the study interval and probe interval was manipulated, ranging from no delay, where the probe was a word shown in the immediately previous study interval, to a 7-trial delay, where the probe corresponded to a study item presented 7 trials earlier or approximately 3 min earlier. There were 3 levels of delay: no delay, short delay (1–2 trials), and long delay (6–7 trials) with 25 trials of each type of delay. The remaining probe items were foil items, in which the participant was never shown the items during a previous study interval. To match the foils tests, the study items included fillers that were never tested. For purposes of generality, we used 4 orders of presentation for the items, with the same 4 orders used in both the Generated and Paired conditions.
The 4-letter word used in the Paired group was always the 4-letter word that was used in the Generated group. Also, the number in the Paired group was the position of the missing letter in the first word of the phrase used in the Generated group. The probe items were identical for both groups. However, the feedback was slightly different: The Generated group had the 4-letter word re-presented with the missing letter capitalized to indicate that it had been the correct response or with all the letters remaining lower case to indicate that this probe item had no missing letters and was indeed a foil. In the Paired group, the feedback consisted of either the correct number or an X to indicate that the item was a foil.
An n-back task (Owen et al. 2005) was used as a distractor task to prevent rehearsal between trials. Early pilot data revealed that when the participants were given 12 s to stare at a blank screen, they created numerous rehearsal strategies. The presentation of 3-letter words was chosen rather than pictures to prevent the possibility of watching the pictures while vocally rehearsing the study items. An n-back of 1 was used which simply requires participants to detect repetitions of words. Although this is a very easy task, it still served to prevent rehearsal.
Procedure
During the information session, 2–3 days prior to the scan, the experimenter gave instructions and showed the participant several sample trials. Then the participants completed a short series of practice trials. The participants were given the opportunity to ask clarifying questions or to repeat the practice trials if they were showing low accuracy.
During the scan session, participants completed 5 blocks of 30 trials each. The first 10 trials were for warm-up, and the remaining 20 trials included 5 each of no delay, short delay, long delay, and filler. Thus, there were 25 observations per participant in each condition of interest.
A trial consisted of several components as illustrated in Figure 1. A star was presented for 2 s as a fixation point. Then, during the study interval of 6 s, a phrase fragment was presented for the Generated group and a word-number pair for the Paired group. During this interval, the participants in the Generated group were required to determine which letter was needed to complete the phrase in order to successfully complete the word phrase fragment. Participants in the Paired group were to study the word-number pair and were encouraged to think of an elaborative link between the 2 (such as interpreting the pair band-2 as referring to the White Stripes—a musical band with just 2 members) to improve their performance when later presented with the probe.
Next, a brief blank screen was shown, followed by a plus sign which served as a warning signal that the probe was about to appear. The probe was always a 4-letter word and appeared for a maximum of 6 s. If the participant responded, the trial moved immediately forward. If the participant did not respond, the trial moved forward at the end of the 6 s. During this interval, the word should act as a prompt to the participant. The participants in the Generated group were to recall the position of the letter they had generated. The participants in the Paired group were to recall the number they had studied with that word. All participants used a data glove to respond. For the participants in the Generated group, each of the 4 fingers mapped to the 4-letter positions in the probe word. For the participants in the Paired group, each of the 4 fingers mapped to the numbers between 1 and 4. A thumb press indicated a foil.
Following the feedback, the 1-back task was given. A subset of 16 three-letter words was presented randomly, and the participant responded with a thumb press whenever a word was repeated. Following the end of the 1-back component, the next item was presented for study, thus beginning the next trial.
Functional Magnetic Resonance Imaging Procedures and Preprocessing
Imaging data were collected with a Siemens 3T Allegra Scanner using a standard radio frequency head coil. Each functional volume contained 34 oblique axial slices (3.20 mm thickness, 64 × 64 matrix, 3.125 mm2 in-plane resolution) parallel to the anterior commissure—posterior commissure (AC–PC) plane, with the 24th slice from the superior centered at the AC–PC line. Functional images were acquired using a gradient echo-planar image acquisition sequence (2000 ms time repetition, 30 ms time echo, 70° flip angle, 200 mm field of view (FOV), 0 slice gap). Functional acquisition was event related, with image acquisition synchronized to stimulus onset, such that 14 volumes, each with 34 slices, were acquired during each 28-s trial. Anatomical images were acquired using a standard T1-weighted spin-echo pulse sequence at the identical slice location as the functional images using a finer inplane resolution (3.2 mm thickness, 200 mm FOV, 256 × 256 matrix, 0.78125 mm2 in-plane resolution).
Preprocessing of the functional imaging data included 6-parameter rigid-body motion correction using automated image registration (Woods et al. 1992). Data were then spatially transformed into a common space using the transformation obtained by co-registering anatomical images to a common reference structural magnetic resonance imaging image by means of a 12-parameter automatic algorithm AIR (Woods et al. 1998) and then smoothed with a 6-mm full-width-half-max 3-D Gaussian filter to accommodate individual differences in anatomy.
Results
Behavioral Results
Figure 2 shows the accuracy and latency results. Analyses of variance (ANOVA) were performed on the effects of delay and study condition. With respect to accuracy, there was a significant decrease in accuracy as the length of the delay increased, from 95% at the no delay to 70% at the long delay (F2,36 = 26.57, P < 0.0001, mean standard error [MSE] = 0.011). There was no significant difference between the Paired and Generated groups for targets (F1,18 = 0.04, MSE = .034). There was also no significant difference between the 2 groups for foil items (F1,18 = 0.183, MSE = 0.003).
Figure 2.
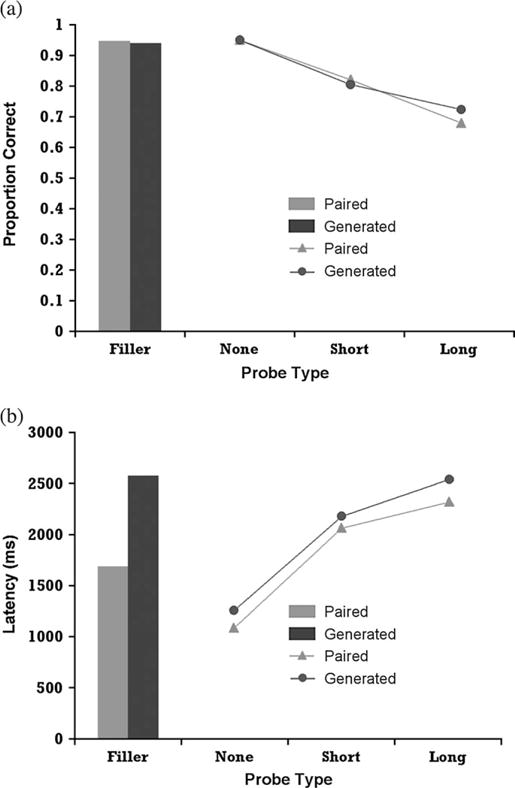
Behavioral results from Experiment 1: (a) Probability of correct recall; (b) Latency of correct recall.
Increase in delay also led to a significant increase in response time. The response times for the items answered correctly increased from 1183 ms at the no delay to 2411 ms at the long delay (F2,36 = 118.113, P < 0.0001, MSE = 70221). The increase in response time for the targets occurred for both the Generated and the Paired groups, and there was no significant difference between the 2 groups (F1,18 = 1.51, MSE = 323543). However, there was a significant difference between the 2 groups for foil items (F1,18 = 14.795, P < 0.001, MSE = 3966948) as the response time for correct responses on foil items was 2580 ms for the Generated group but 1689 ms for correct responses on foil items for the Paired group.
Whereas there is a latency difference between short and long delays (279 ms, t19 = 3 71, P < 0.005), the much larger difference is between short and no delay (949 ms, t19 = 10.76, P < 0.0001). Because the major effect of latency was between no delay versus longer delays, this contrast was used in the imaging analyses (averaging together short and long delays).
Confirmatory Imaging Analysis
BOLD responses reported throughout this paper were computed using the response of the first scan of a trial as the baseline from which percent change was calculated over the remaining time course of the trial. Figure 3 shows these percent change measures for the predefined parietal and prefrontal regions. For purposes of initial analysis scans 3–6 were defined as “early” scans reflecting the encoding (study was during scans 2–4) and scans 8–11 as “late” scans reflecting the retrieval (probe was during scans 6–8). The early and late scans were offset from the study and probe periods to reflect the lagged character of the hemodynamic response. A2 × 2 × 2 × 2 ANOVA was performed on the average values in these areas in which the factors were region (parietal vs. prefrontal), study (Paired vs. Generated), retention (immediate vs. delayed), and scan (early vs. late). There was a significant region-by-study interaction (F1,18 = 12.91, P < 0.005, MSE = 0.034). As predicted, the Generated condition showed significantly greater activation than the Paired condition in the parietal region (t18 = 2.85, P < 0.05 (all significance levels for t statistics are based on 2-tailed tests) and the difference in the prefrontal region is not significant (t18 = −0.79). The only other significant effect was a retention-by-scan interaction (F1,18 = 31.91; P < 0.0001) such that the effect of retention condition was only significant in the late scans. As predicted, there is a large and significant retention effect during the late scans in the prefrontal region (0.16%, t19 = 3.91, P < 0.001) but there is also an unpredicted and equally large retention effect in the parietal region (0.18%, t19 = 3.81, P < .005). Breaking down the retention effect by study conditions and regions, there are significant effects for Paired-prefrontal (15%, t10 = 2.45, P < 0.05), Generated-prefrontal (18%, t8 = 2.97, P < 0.05), and Generated-parietal (25%, t8 = 3.88, P < 0.001), but the effect is only marginal for the Paired-parietal (13%, t10 = 1.84, P < 0.10).
Figure 3.
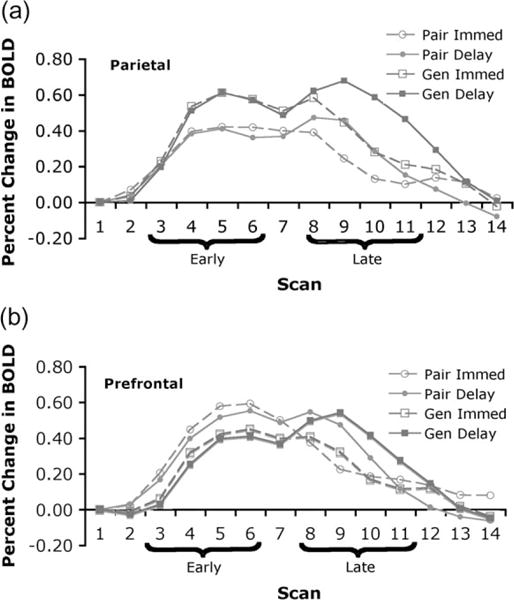
fMRI results from Experiment 1: (a) BOLD response in the predefined posterior parietal region; (b) BOLD response in the predefined prefrontal region.
While not displayed in Figure 3, if we calculate the response in the late scans for short and long delay, there is a greater response for long delay than short delay (8% difference in the prefrontal and 4% in the parietal), but neither difference is statistically significant (t19 = 1.65 for prefrontal and t19 = 1.32 for parietal). The rather weak imaging effect is not surprising given the rather weak behavioral effect (see Fig. 2).
Exploratory Imaging Analysis
This section will report exploratory analyses to determine what other regions might be reliably responding to the manipulations of the experiment. Functional imaging data were analyzed using mixed-effects ANOVA models (Braver et al. 1997; Snitz et al. 2005) within the Neuro Imaging Software system (http://kraepelin.wpic.pitt.edu/nis/index.html). Two exploratory analyses were performed. In the first analysis, participant was treated as a random factor and within-participant factors included Delay (2 values: none and delay) and Scan (14). This analysis was used to identify brain areas that showed differential BOLD profiles in response to the Delay factor, indicated by a significant Delay-by-Scan interaction. The second exploratory analysis again treated participant as a random factor, with Group (2 values: Paired and Generated) as a fixed effects between-participant factor and Scan as a within-participant factor. This analysis was used to identify brain areas that showed differential BOLD profiles in response to the varied demands in the Paired and Generated conditions, indicated by a significant Group-by-Scan interaction. In both analyses, the lower bound degrees of freedom (df) correction was applied to correct for nonsphericity due to nonindependence of scans. An alpha level of P < 0.05 was used in both analyses. To correct for multiple comparisons issues, only those regions having a contiguous cluster size of 30 or more significant voxels are reported (Forman et al. 1995).
Table 1 reports the regions found in the 2 analyses. The significant interactions that identified these regions can usually be characterized by the total magnitude of the average BOLD response (computed as percent change from scan 1) over the within-trial time course activity during a particular condition. This measure can be thought of as a proxy for total work performed over the period of a trial (Anderson et al. 2004). Table 1 reports, for each region, for each condition, the sum of the percentages over the 14 scans comprising a trial.
Table 1.
Results of Exploratory Analyses of Experiment 1 (Except were noted P < 0.05)
| Region of Interest | Brodmann Areas | Voxel Count | Stereotaxic Coordinates (mm)
|
Condition 1 | Condition 2 | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Delay-by-scan | Immed. | Delay | |||||
| Supplementary Motor, Anterior Cingulate Cortex | 6, 8, 24, 32 | 591 | 1 | 20 | 44 | 1.66% | 2.22% |
| L. Motor, Prefrontal, Posterior Parietal | 2, 4, 7, 39, 40,45,46 | 3406 | −33 | −8 | 31 | 2.59% | 3.13% |
| L. Post Parietal (P < 0.01) | 7, 40 | 220 | −25 | −57 | 41 | 3.66% | 5.10% |
| L. Prefrontal (P < 0.01) | 9, 46 | 455 | −45 | 12 | 31 | 3.24% | 4.22% |
| R. Supramarginal Gyrus | 40 | 619 | 56 | −43 | 27 | 0.49% | −0.75% |
| Precuneus | 7 | 32 | 5 | −52 | 48 | 2.07% | 1.37% |
| R. Prefrontal | 9, 45, 46 | 188 | 45 | 27 | 28 | 2.01% | 2.39% |
| R. Posterior Parietal | 7, 39, 40 | 135 | 33 | −57 | 40 | 2.82% | 3.34% |
| Polar Frontal | 9,10 | 89 | 2 | 54 | 14 | −1.13% | −2.17% |
| Posterior Cingulate | 23, 31 | 118 | −2 | −31 | 29 | 1.70% | 2.15% |
| R. Anterior Prefrontal | 10 | 94 | 32 | 47 | 3 | 0.32% | 1.34% |
| R. Precentral Gyrus | 6 | 50 | 55 | 2 | 9 | 2.41% | 0.65% |
| R. Anterior Insula | 13 | 225 | 34 | 22 | 4 | 1.57% | 2.14% |
| R. Temporal Gyrus | 21, 22 | 68 | 59 | −23 | −2 | 0.45% | 0.00% |
| L. Temporal Gyrus | 21, 22 | 45 | −61 | −26 | −2 | 0.64% | 1.06% |
| R. Occipital Gyrus | 18,19 | 60 | 26 | −85 | 4 | −2.58% | −2.33% |
| L. Fusiform Gyrus | 39 | 61 | −43 | −54 | −7 | 1.82% | 1.86% |
| L. Occipital Gyrus | 18 | 85 | −28 | −87 | −2 | −2.25% | −1.88% |
| Group-by-Scan | Paired | Generated | |||||
| Supplementary Motor, Anterior Cingulate Cortex | 6, 8, 24, 32 | 348 | 9 | 12 | 53 | 2.74% | 1.23% |
| L. Prefrontal | 6 | 61 | −32 | 2 | 45 | 2.13% | 1.55% |
| L. Posterior Parietal | 40 | 21 | −42 | −55 | 47 | 3.61% | 5.40% |
| R. Posterior Parietal | 39 | 117 | 37 | −64 | 39 | 2.25% | 2.73% |
| L. Posterior Parietal | 39 | 78 | −26 | −59 | 38 | 3.05% | 5.86% |
| L. Anterior Prefrontal | 10 | 78 | −30 | 48 | 15 | 3.49% | 1.37% |
| L. Occipital Gyrus | 17, 18 | 354 | −21 | −84 | 6 | 1.09% | 3.89% |
| R. Occipital Gyrus | 17, 18 | 177 | 17 | −88 | 12 | 1.37% | 3.57% |
Note: L, left; R, right.
The delay-by-scan interaction revealed one large left-sided region, which included the predefined prefrontal and parietal regions. To identify the foci of activity in this large region, a more conservative alpha level of P < 0.01 was used and showed 2 foci of activation, 1 in left parietal cortex overlapping our predefined parietal region and 1 in left prefrontal cortex, overlapping our predefined prefrontal region. The delay-by-scan interaction also revealed right-sided regions homologous to the predefined prefrontal and parietal regions. The group-by-scan interaction revealed a left-sided parietal region close to the predefined region. The group-by-scan analysis also found 2 left-sided prefrontal regions (b2 and b6) behaving similarly to the predefined prefrontal, but neither of these is particularly close to the predefined region. It will be important to determine which of these exploratory regions can be replicated in a second study.
Experiment 2
Experiment 2 was an attempt to replicate the first experiment with one difference. To better separate the encoding phase from the retrieval phase, we inserted 2 additional scans (4 s) between them and then took 2 scans away from the final 1-back phase. Participants were recruited locally, and they provided written informed consent in accordance with the guidelines at the University of Pittsburgh and Carnegie Mellon University. Twenty right-handed, native English speakers (10 female/10 male, 18–26 years of age with an average of age of 21.1 years) completed the study, which consisted of one information session and one scanning session. Eleven participants were in the Paired condition and 9 in the Generated condition (11 participants were recruited in the Generated condition but 2 were lost because of errors in the scanning parameters).
Results
Behavioral Results
Figure 4 shows the accuracy and latency results. The analyses contrasted no delay probes, short delay probes (1–2 trials), and long delay probes (6–7 trials). There was a significant decrease in accuracy as the length of the delay increased, from 93% at the no delay to 61% at the long delay (F2,36 = 52.52, P < 0.0001, MSE = 0.007). There was no significant difference between the 2 groups on targets (F1,18 = 0.29, MSE = 0.045) or foil items (F1,18 = 0.39, MSE = 0.023).
Figure 4.
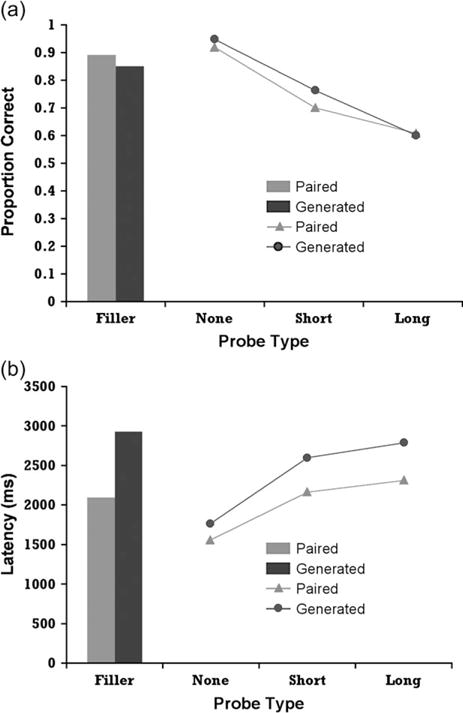
Behavioral results from Experiment 2: (a) Probability of correct recall; (b) Latency of correct recall.
Delay also had a significant impact on response time as the increase in delay led to an overall increase in response time. The response times for the items answered correctly increased from 1666 ms at the no delay to 2550 ms at the long delay (F2,36 = 67.68, P < 0.0001, MSE = 71706). The Generated group is marginally slower than the Paired group on targets (F1,18 = 3.52, P < 0.10, MSE = 645249). There was also a significant difference between the 2 groups for foil items (F1,18 = 11.294, P < 0.005, MSE = 338321) with the Generated group again longer.
The behavioral results are quite consistent between the 2 experiments. Similar to experiment 1, the short and long delays are averaged together in the functional magnetic resonance imaging (fMRI) analyses, although again the difference between the 2 delays is statistically significant (t19 = 2.18, P < 0.05).
Confirmatory Imaging Analysis
Figure 5 shows the BOLD responses as percent change from the baseline at scan 1. For purposes of initial analysis scans 3–8 were defined as early scans reflecting the encoding (study was during scans 2–4) and scans 9–14 as late scans reflecting the retrieval (probe was during scans 8–10). This analysis replicated the 2 significant interactions of the previous study. There was a significant region-by-study interaction (f1,18 = 8.49, P < 0.01, MSE = 0.023). As predicted, the Generated condition was significantly greater than the Paired condition in the parietal (t18 = 2.40, P < 0.05) and the difference in the prefrontal is not significant (t18 = −0.38). The retention-by-scan interaction was also significant (F1,18 = 25.30; P < 0.0001) such that the effect of retention condition was only significant in the late scans. This time, however, focusing just on the late scans, there was a significant retention-by-region interaction (F1,18 = 10.02, P < 0.01, MSE = 0.004). As predicted, the effect of delay was greater in prefrontal. The difference between delay and immediate was significant in 3 of the 4 comparisons: Generated-prefrontal (0.22%, t8 = 5.49, P < 0.001), Paired-prefrontal (0.12% – t10 = 4.82, P < .001), and Generated-parietal (0.14%, t8 = 2.90, P < 0.05). Again, the effect is only marginal in the Paired-parietal (0.05% – t10 = 2.14, P < 0.1).
Figure 5.
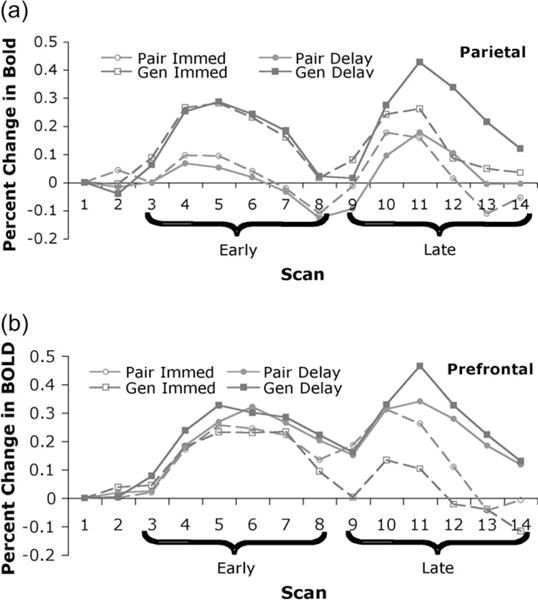
fMRI results from Experiment 2: (a) BOLD response in the predefined posterior parietal region; (b) BOLD response in the predefined prefrontal region.
The outcome of these 4 comparisons of retention effects is quite consistent between the 2 experiments. Given that both experiments show a marginal retention effect in the Paired-parietal condition, we should probably consider that there is an effect in this condition as well. Indeed, a t-test over the 2 experiments finds a significant retention effect in the Paired-parietal condition (0.09% – t10 = 2.46, P < 0.05). However, t-tests also confirm that the size of the effect in the Paired-parietal is smaller than the size of the effect in any other condition (14% Paired-prefrontal, 20% Generated-Parietal, and 20% Generated-Prefrontal).
With respect to the parietal region, both Experiments 1 and 2 show a much greater late response in the Generated delay condition than in any of other 3 conditions. Perhaps, participants in the Generated condition had to extract the answer in the delayed condition but could use their still active memory instead in the immediate condition.
If we break the delay condition down into short and long, there is a 4% greater late response for long delay than short delay in the Prefrontal but a 3% weaker response in the parietal. Neither effect is statistically significant (t19 = 1.30 for prefrontal and t19 = −1.03 for parietal). However, if we combine these effects with the first experiment, the average effect for the prefrontal is 6%, which is marginally significant (t39 = 1.76, P < 0.10), whereas the effect for the parietal is just 1% and it is not significant (t39 = 0.33). Furthermore, over the 2 experiments, the difference between the prefrontal and parietal effects is significant (t39 = 2.19, P < 0.05).
Exploratory Imaging Analysis
Exploratory analyses were performed as in Experiment 1, using mixed-effects ANOVA models and focusing on the delay-by-scan and group-by-scan interactions in order to reveal the brain areas showing BOLD response profiles differentially affected by the experimental manipulations. Table 2 summarizes the regions shown to have significant effects. The delay-by-scan analysis found two large regions containing the predefined parietal and predefined prefrontal. Raising the significance level to P < 0.01 identified 2 subregions almost identical in location to the subregions identified by the same analysis in Experiment 1. Besides the predefined regions, this delay-by-scan analysis found a number of common areas in the two experiments that display similar responses. These are as follows
A large region involving the supplementary motor area and anterior cingulate cortex that responds more strongly in the delay condition than the immediate condition.
A right supramarginal region that displays a negative response.
A posterior cingulate region. However, the direction of the response is different in the two experiments – positive in Experiment 1 and negative in Experiment 2.
A right anterior prefrontal region that only responds in the delay condition.
Right and left occipital regions that display negative responses.
Table 2.
Results of Exploratory Analyses of Experiment 2 (Except were noted P < 0.05)
| Region of Interest | Brodman Areas | Voxel Count | Stereotaxic Coordinates (mm)
|
||||
|---|---|---|---|---|---|---|---|
| x | y | z | Condition 1 | Condition 2 | |||
| Delay-by-scan | Immed. | Delay | |||||
| Supplementary Motor, Anterior Cingulate Cortex | 6, 8, 24, 32 | 519 | 1 | 19 | 43 | 0.20% | 1.30% |
| L. Posterior Parietal | 7, 40 | 664 | −40 | −47 | 46 | 0.67% | 1.49% |
| L. Post Parietal (P < 0.01) | 7, 40 | 150 | −26 | −59 | 45 | 0.95% | 1.94% |
| L. Motor and Prefrontal, Thalamus | 9, 10, 45, 46 | 1834 | −27 | 9 | 15 | 0.80% | 1.70% |
| L Prefrontal (P < 0.01) | 9, 46 | 229 | −45 | 12 | 31 | 1.72% | 3.05% |
| R. Prefrontal | 9 | 65 | 47 | 7 | 34 | −1.88% | −0.97% |
| R. Posterior Parietal | 7, 39 | 43 | 34 | −63 | 44 | −1.01% | −0.45% |
| R. Prefrontal and Insula | 13, 45 | 262 | 37 | 21 | 7 | −0.31% | 0.65% |
| R. Supramarginal Gyrus | 40 | 137 | 50 | −50 | 28 | −1.05% | −1.21% |
| R. Anterior Prefrontal | 10 | 78 | 38 | 51 | 9 | −0.79% | 0.63% |
| Posterior Cingulate | 23, 31 | 62 | 1 | −28 | 27 | −0.92% | −0.07% |
| L. Occipital Gyrus | 18, 19 | 90 | −30 | −88 | 3 | −3.73% | −3.29% |
| R. Occipital Gyrus | 18, 19 | 49 | 29 | −91 | 4 | −5.53% | −4.42% |
| Red Nucleus | 45 | 1 | −19 | −8 | 0.48% | 0.71% | |
| L. Fusiform Gyrus | 39 | 60 | −44 | −54 | −4 | −0.40% | −0.01% |
| Group-by-Scan | Paired | Generated | |||||
| Polar Frontal | 9, 10 | 42 | −5 | 54 | 18 | 1.70% | −0.39% |
| L. M. Temporal Gyrus | 39 | 41 | −45 | −65 | 28 | 1.25% | −0.57% |
| L and R Occipital | 17, 18, 19 | 2112 | 1 | −82 | 9 | 1.16% | 2.31% |
| R.S. Temporal Gyrus | 13 | 103 | 51 | −47 | 18 | −0.41% | −3.04% |
| Anterior Cingulate | 32 | 49 | −3 | 45 | −6 | 2.11% | 0.58% |
| L. Thalamus | – | 25 | −17 | −28 | 2 | 0.39% | 0.99% |
Note: L, left; R, right; M, medial; S, superior
The group-by-scan interaction failed to find any regions overlapping the predefined. In general, all the regions found with the group-by-scan interaction were lower in the brain than the predefined regions. The only region that overlaps between these experiments is the large visual region in the occipital lobe (b7 and b8 in that Experiment 1 and b3 in Experiment 2), where a much stronger response is obtained in the Generated condition. This happens during the encoding period and presumably reflects visual examination of the stimulus trying to solve the puzzle. There is no difference in activity during the recall phase in this occipital region. The occipital area that displays this pattern is much larger than the occipital areas that are revealed in the delay-by-scan analysis.
Information-Processing Model
Anderson (2005) describes a general methodology, based on the ACT-R theory (Anderson et al. 2004), for taking the processing time estimates from an information-processing model and mapping these onto predictions for the BOLD response in various brain regions. An ACT-R implementation of the information-processing model described in Sohn et al. (2005) can provide the processing times to predict the BOLD responses in the parietal and prefrontal regions for the current experiments. Figure 6 illustrates the major steps in the model (an ACT-R model implementing these steps is available from the model’s link at the ACT-R website (http://act-r.psy.cmu.edu) under the title of this paper) for each of the 4 major conditions of these experiments and can be put into correspondence with the experimental events illustrated in Figure 1. The sequence of events begins with the encoding of fixation and continues through to the generation of a response. Although we have illustrated activity in the visual (blue) and manual (yellow) modules, the predictions depend on the imaginal (green) module which is responsible for creating problem representations and declarative (red) module which responds to retrieval operations.
Figure 6.
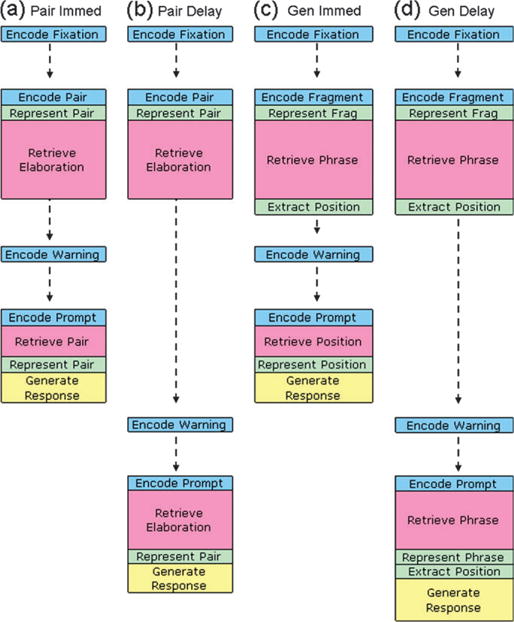
A representation of the sequence of module activity in different conditions of the experiment. Blue denotes activity in the visual module, green in the imaginal (parietal) module, red in the declarative retrieval (prefrontal) module, and yellow in the manual module. The retrieval boxes and delays between activity are not drawn to scale.
Upon the presentation of the study material, the model spends 200 ms either encoding the pair (Paired condition) or the fragment (Generated condition). In the Paired condition, the participant is instructed to retrieve an elaboration to help remember the pair, whereas in the Generated condition, they have to retrieve a phrase that completes the fragment. As a simplifying assumption, we assumed that these 2 retrieval operations took the same time. Thus, the only difference during study between the 2 conditions is that there is an extra imaginal operation required in the Generated condition to extract the position of the letter from the retrieved information.
In the immediate conditions, recall performance is very similar for the Paired and Generated conditions. In both cases the prompt is encoded, then a retrieval operation returns either the pair that has just been studied (Paired condition) or the position (Generated condition), then the result of the retrieval is represented, and finally the response is generated. The time to retrieve the pair or position is relatively brief because this material has just been encoded and is still active. However, at delay, the elaborations and phrases used in the study phase are more active (as we will detail further below) and so it is more rapid and successful to retrieve these rather than the pair or position. In the Generated condition, after the retrieval, an extra imaginal operation is required to extract the letter position from the phrase.
Table 3 presents a summary of the times for all the stages in Figure 6. Figure 7 compares the predicted times from the model for the test phase with the actual observed times.
Table 3.
Time Parameters for Model in Figure 6
| Paired No Delay | Paired Short Delay | Paired Long Delay | Generated No Delay | Generated Short Delay | Generated Long Delay | |
|---|---|---|---|---|---|---|
| Study | ||||||
| Visual Encoding | 200 ms | 200 ms | 200 ms | 200 ms | 200 ms | 200 ms |
| Represent Encoding | 250 ms | 250 ms | 250 ms | 250 ms | 250 ms | 250 ms |
| Retrieval | 2700 ms | 2700 ms | 2700 ms | 2700 ms | 2700 ms | 2700 ms |
| Position Extraction | 250 ms | 250 ms | 250 ms | |||
| Test | ||||||
| Visual Encoding | 200 ms | 200 ms | 200 ms | 200 ms | 200 ms | 200 ms |
| Retrieval | 470 ms | 1200 ms | 1540 ms | 470 ms | 1200 ms | 1540 ms |
| Represent Retrieval | 250 ms | 250 ms | 250 ms | 250 ms | 250 ms | 250 ms |
| Extract Response | 250 ms | 250 ms | ||||
| Motor | 450 ms | 450 ms | 450 ms | 450 ms | 450 ms | 450 ms |
Note: Immediate retrieval times are averaged over the 2-s delay in Experiment 1 and 6-s delay in Experiment 2. The Delay retrieval times are also averaged over short and long delays. Visual, Representational, and Manual actions include a 50-ms production.
Figure 7.
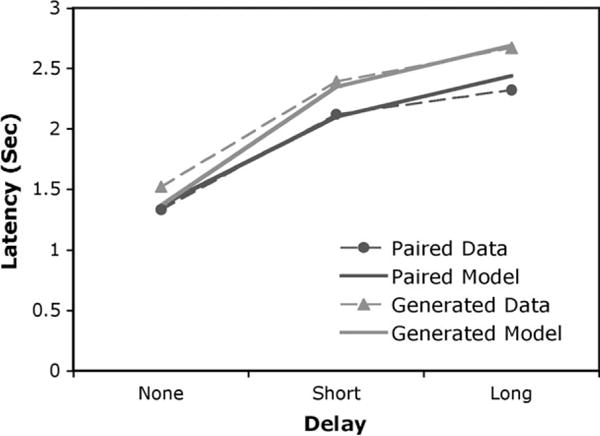
Predictions of the model for latencies and observed latencies, averaging over the 2 experiments.
The timing of all the operations, except the retrieval operations come from the default values in the ACT-R architecture. The retrieval times, T, are determined by the activation of the memories, A, according to the ACT-R retrieval equation:
where F is the latency scale parameter and was estimated at 270 ms for these experiments. The activations themselves are determined by the time, t because they were rehearsed according to the formula:
where B is a prior activation boost, which is 0 in the case of new memories and was estimated to be 0.1 (a second estimated parameter) in the case of the prior elaboration (Paired condition) or phrase (Generated condition). The 0.5 in this equation is the default decay rate assumed in the ACT-R architecture. Thus, the activation of the elaboration or phrase during study is just ln(0.1) and its latency for retrieval will be 2.7 s. At the time of test, the activation of these elements will be approximately ln(0.1 + (3 + d)−0.5), where d is the delay between study and test. The activation of the specific memory will be approximately ln(d−0.5). Note in Figure 6 that the retrieval of the elaboration or phrase completes approximately 3 s before the end of the study. In ACT-R, this results in the delay of the specific memory being approximately 3 s less than the delay for prior memory. This accounts for the d in the expression for the specific and the 3 + d in the expression for the prior memories. In an immediate test, the specific memory will be more active reflecting the shorter interval between the end of the study and beginning of recall. However, in a delayed test the long-term elaboration or phrase will be more active than the specific memory reflecting the additional 0.1 to reflect prior experience.
The 2 parameters estimated to fit the latency data were the latency scale of 270 ms and the prior strength of 0.1 for elaborations and phrases. With these parameters, we could determine the timing and duration of all the retrieval and imaginal operations. With the timing parameters set to fit the behavioral latencies, a BOLD response that reflects encoding times can be calculated for the parietal region and a BOLD response that reflects the retrieval times can be calculated for the prefrontal region. For a particular region, let D(x) be a 0–1 demand function reflecting when a region is active according to Figure 6. Then one can then get a predicted BOLD response, B(t), for a region by convolving the demand functions with a standard hemodynamic function H(t):
In past research, we have used a standard gamma function for the hemodynamic function, as is the custom (e.g., Boyton et al. 1996; Cohen 1997; Dale and Buckner 1997; Friston et al. 1998; Glover 1999):
where s is the time scale and b is exponent. However, the characterization of the BOLD response is conceptually cleaner if the simple gamma function is replaced with the gamma function from statistics, which has the convenient property that it always integrates to 1:
where Γ is the gamma function (the factorial is a special case: for integer a, Γ(a + 1) = a!).
The mode of the statistical gamma function is (a − 1) × s, the mean is a × s, and its variance is a × s2. One can convert from the first formulation to the second by setting b = a − 1 and changing the magnitude scale. The statistics formulation makes magnitude values, M, comparable across regions that might vary in their exponent and time scale.
To focus on the interaction between the study conditions of Paired and Generated and the manipulation of immediate versus delay, we averaged over the 2 experiments and fit the model to the average data. Figure 8 offers a comparison of model and average data.
Figure 8.
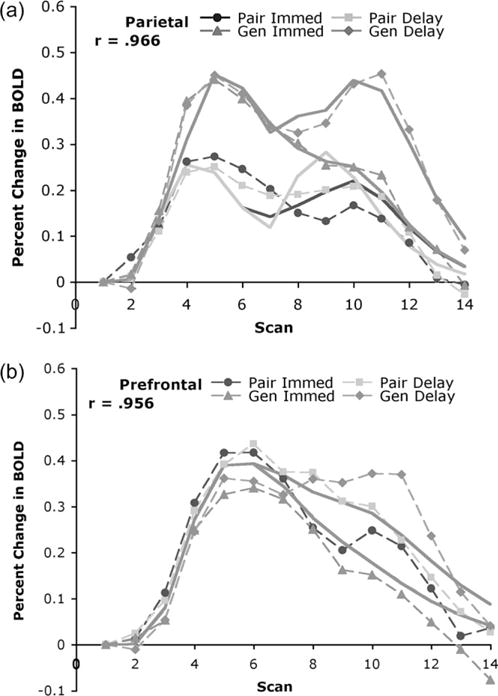
Predictions of the information-processing model for parietal and prefrontal regions of interest for the 2 experiments. Dotted lines connect the data points and solid lines are the predictions of the model.
To fit the data, we needed to estimate the parameters governing the hemodynamic function for parietal and prefrontal regions. These parameters are
M, the magnitude of activity in a region,
a, the shape of the hemodynamic function,
s, the time scale.
Constraining the shape parameter to be an integer, the best-fitting parameters for the parietal region were M = 9.89%, a = 4, s = 1.79 s and for the prefrontal region the values were M = 1.61%, a = 3, s = 2.95 s.
Each condition for each region has 13 free data points (the first is constrained to equal 0 as baseline). Thus, there are 13 free scans × 2 regions × 4 conditions for 104 df. Subtracting the 6 parameters estimated for the hemodynamic function means that the fit to the data has 98 df per experiment.
The significance of the deviations between data and prediction can be measured by a chi-square statistic
where the numerator is the sum of the squared deviations between the predictions and the mean values observed and the denominator gives the variance of the mean estimated from the interaction between condition and participants. The chi-square value was 77.43, which is not a significant deviation between prediction and observation. Therefore, the fits to these experiments are acceptable.
To test how much evidence the model fits provided for the proposed interpretations of the prefrontal and parietal regions, we tried crossing the associations and using the imaginal activity to predict the prefrontal region and the declarative activity to predict the parietal region. Again 6 parameters were estimated for each experiment. The chi-square value was 344.44, which indicates highly significant deviations in the predictions.
While we have fit the model averaging together the short and long delay, we noted that there was a difference in the prefrontal response to these 2 delays. Figure 9 shows the predictions of the model to the prefrontal data with short and long delay separated, using the same parameters as Figure 8.
Figure 9.
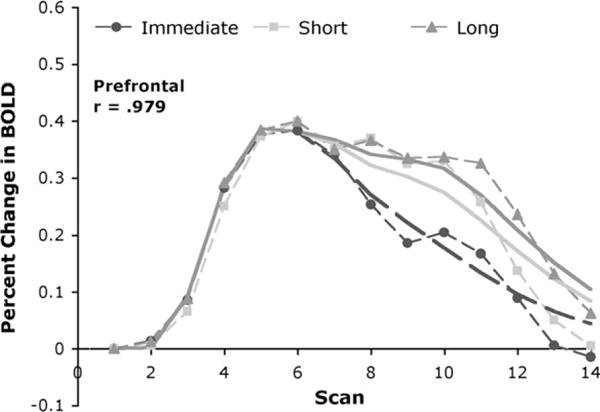
Response in the prefrontal region for each delay condition. Dotted lines connect the data points and solid lines are the predictions of the model.
Conclusions
The predictions given at the beginning of the paper were made in advance of the detailed model in Figure 6. It is worth revisiting these predictions in light of the average results and model predictions in Figure 8.
In the parietal region, during the period of study and test, there will be a stronger response for the Generated condition than the Paired condition. This was clearly confirmed and was produced in the model by the extra encoding times in the Generated condition to extract the position during study and test when there was a delay (see Fig. 6).
In the prefrontal region, during the period of study and test, there will be a stronger or equal response for the Paired condition. The model in Figure 6 predicts no difference and Figure 8 confirms that there was virtually no difference.
In the prefrontal region, only during the period of test, there will be a stronger response in the delay condition than the immediate condition. This was confirmed and produced in the model because of the longer retrieval times.
In the parietal region, there should be no difference between the immediate and delay condition. This prediction was not confirmed but the model actually produced a delay effect in the parietal region for the Generated condition. This is because of the extra encoding operation to extract the position in the delay condition.
One value of an explicit model is that it can reveal errors in predictions based on an informal understanding of a theory. This is the case for prediction 4 above. We failed to recognize that the time to extract the position in the delay condition would produce a greater parietal response in the Generated delay condition. The model predicts no difference between the late parietal activation in 3 conditions—Generated immediate, Paired immediate, and Paired delay and that these should all show less activation than the late parietal activation in the Generated delay condition. Figure 8(a) appears to support this prediction. This implies a significant t-value comparing late parietal activity in the Generated delay condition with late parietal activity in any other condition. Over the 2 experiments, there were 6 such t-tests and all were significant. It also predicts no significant t-tests comparing late parietal activity in the other 3 conditions. Over the 2 experiments, there were 6 such t-tests and none were significant, although as noted earlier if one combines the 2 experiments there is greater parietal activity in the delay Paired condition than the immediate Paired condition.
More generally, note that this study once again replicates the finding of both parietal and prefrontal involvement in a memory task. As is particularly apparent in Experiment 2 with a longer delay between study and test, both regions respond to the memory structure of the experiment (see Fig. 5). The goal of this research was to try to separate out the degree to which the regions respond to the representational versus the mnemonic demands of the task. These experiments and the accompanying model support the view that the prefrontal region responds to the memory demands and the parietal region responds to the representational demands.
Although the interpretation of the prefrontal region seems uniformly supported by these experiments, one result does complicate the interpretation of the parietal region. By combining the 2 experiments, we were able to confirm a weak but significant retention effect in the parietal region in the Paired condition. This is an effect not predicted by the model and does suggest that the parietal region does respond to a manipulation that we have interpreted as only affecting memory demands. Perhaps, our predefined parietal region does not uniquely reflect a single encoding function that we ascribe to it. However, an alternative interpretation was suggested by Bunge et al. (2002) in another effort to separate parietal from prefrontal function in a different task. They found a parietal region with nearly the same coordinates as ours. This region appeared to respond to a representational function that, they too, had assigned to this region, but also a selection function that they had assigned to prefrontal regions. They proposed that the parietal region needed to represent the choices longer in conditions where selection took longer. Similarly, it may be the case that there is some increased demand in our experiment for re-representation of the stimulus during periods of longer retrieval. This might create the weak but significant effect of delay in the parietal region that we found in the Paired condition.
The results of these experiments are relevant to a number of other research efforts concerned with the function of the prefrontal and parietal cortices. With respect to the prefrontal, there has been a considerable amount of research suggesting that a region very close to our predefined prefrontal region is activated in conditions that require difficult selections among retrieved information (e.g., Thompson-Schill et al. 1997; Moss et al. 2005). On the other hand, it has been argued that these effects are due to greater retrieval demands in the more difficult conditions (Wagner et al. 2001; Martin and Cheng 2006). It has also been argued that a more anterior prefrontal region is sensitive to retrieval while this region is sensitive to selection demands (Badre et al. 2005; Gold et al. 2006). Recently, Thompson-Schill and Botvinick (2006) suggest that the distinction between retrieval and selection may be a false dichotomy, and certainly, it is not a distinction that has any meaning in the ACT-R architecture. What drives the magnitude of response is the amount of time that this region has to hold the cues for retrieval and this time will increase when selection is hard. That being said, the effect of delay on activation in this region does seem difficult to accommodate in views that ascribe a distinct role to selection different from retrieval. The selection demands should remain constant with delay; the only effect of delay should be to make the information less available for retrieval.
It is clear from the literature that different prefrontal regions are involved in retrieval and that they are responsive to somewhat different factors. Our experiments did not find an anterior prefrontal region corresponding to the one found by Badre et al. and Gold et al. (the exploratory analysis did find a right anterior prefrontal region responsive to delay, but the other research has found left regions), and therefore, these experiments do not contribute to an understanding of the difference between this region and the one found in this experiment. Going beyond the results of this experiment or any other specific experiment, however, one can ask how the ACT-R theory would deal with evidence for functional heterogeneity along the inferior frontal gyrus during retrieval. No one has yet successfully articulated the functional differences among prefrontal regions in terms of the ACT-R architecture, and so this remains a challenge for further development of the theory. The ACT-R theory has evolved in response to brain imaging data (Anderson 2005), separating out control functions (ascribed to the anterior cingulate cortex) from representational functions (ascribed to the parietal cortex). Originally, both functions were ascribed to a single module in the theory. We might imagine a similar development in declarative memory functions.
In their review of parietal contributions to memory, Wagner et al. (2005) note that most of the fMRI data come from recognition and source recognition paradigms. Wagner et al. identify 2 regions that consistently show an old–new effect, a precuneus regions and a region in the lateral parietal cortex. Curiously, our parietal region lies intermediate between these 2 regions. Wagner et al. identify as a question for future research whether paradigms like cued recall will provide additional evidence for a parietal role in memory. Our research has indicated that our parietal region is at most weakly affected by delay in a standard paired associate condition. Sohn et al. (2005) also found a weak effect of fan that was not significant. It would seem that the prefrontal cortex is much more sensitive to memory manipulations in associative tasks.
Acknowledgments
Funding
National Institute of Mental Health (MH068243 to J.R.A.).
We would like to thank Jared Danker, Jennifer Ferris, Myeong-Ho, Sohn, and Anthony Wagner for their comments on the paper.
Footnotes
Conflicts of Interest: None declared.
References
- Alivisatos B, Petrides M. Functional activation of the human brain during mental rotation. Neuropsychologia. 1997;35:111–118. doi: 10.1016/s0028-3932(96)00083-8. [DOI] [PubMed] [Google Scholar]
- Anderson JR. Human symbol manipulation within an integrated cognitive architecture. Cogn Sci. 2005;29:313–342. doi: 10.1207/s15516709cog0000_22. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Bothell D, Byrne MD, Douglass S, Lebiere C, Qin Y. An integrated theory of mind. Psychol Rev. 2004;111:1036–1060. doi: 10.1037/0033-295X.111.4.1036. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Qin Y, Stenger VA, Carter CS. The relationship of three cortical regions to an information-processing model. J Cogn Neurosci. 2004;16:637–653. doi: 10.1162/089892904323057353. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Qin Y, Yung K-J, Carter CS. Information-processing modules and their relative modality specificity. Cogn Psychol. 2007;54:185–217. doi: 10.1016/j.cogpsych.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Paré-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith E, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Kelley WM, Petersen SE. Frontal cortex contributes to human memory formation. Nat Neurosci. 1999;2:311–314. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME. The cognitive neuroscience of remembering. Nat Rev Neurosci. 2001;2:624–634. doi: 10.1038/35090048. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JDE. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage. 2002;17:1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16:317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Prince SE, Rice HJ, Weissman DH, Nyberg L. Attention-related activity during episodic memory retrieval: a crossfunction fMRI study. Neuropsychologia. 2003;41:390–399. doi: 10.1016/s0028-3932(02)00170-7. [DOI] [PubMed] [Google Scholar]
- Carpenter PA, Just MA, Keller TA, Eddy W, Thulborn K. Graded function activation in the visuospatial system with the amount of task demand. J Cogn Neurosci. 1999;11:9–24. doi: 10.1162/089892999563210. [DOI] [PubMed] [Google Scholar]
- Clark D, Wagner AD. Assembling and encoding word representations: fMRI subsequent memory effects implicate a role for phonological control. Neuropsychologia. 2003;41:304–317. doi: 10.1016/s0028-3932(02)00163-x. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum HE. Memory, amnesia, and the hippocampal system. Cambridge (MA): MIT Press; 1993. [Google Scholar]
- Danker JF, Anderson JR. The roles of prefrontal and posterior parietal cortex in algebra problem solving: a case of using cognitive-modeling to inform neuroimaging data. Neuroimage. 2007;35:1365–1377. doi: 10.1016/j.neuroimage.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Davachi L, Maril A, Wagner AD. When keeping in mind supports later bringing to mind: neural markers of phonological rehearsal predict subsequent remembering. J Cogn Neurosci. 2001;13:1059–1070. doi: 10.1162/089892901753294356. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science. 1999;284:970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cereb Cortex. 2005;15:1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Henson RNA. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging fMRI: use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Jones SJ, Powell DK, Smith CD, Andersen AH. Dissociation of automatic and strategic lexical semantics: fMRI evidence for differing roles of multiple frontotemporal regions. J Neurosci. 2006;26:6523–6532. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib R, Lepage M. Novelty assessment in the brain. In: Tulving E, editor. Memory, consciousness, and the brain. Philadelphia (PA): Psychology Press; 1999. pp. 265–277. [Google Scholar]
- Heil M. Early career award: the functional significance of ERP effects during mental rotation. Psychophysiology. 2002;39:535–545. doi: 10.1017.S0048577202020449. [DOI] [PubMed] [Google Scholar]
- Kohler S, Paus T, Buckner RL, Milner B. Effects of left inferior prefrontal stimulation on episodic memory formation: Two-stage fMRI-rTMS study. J Cog Neurosci. 2004;16:178–188. doi: 10.1162/089892904322984490. [DOI] [PubMed] [Google Scholar]
- Lepage M, Ghaffar O, Nyberg L, Tulving E. Prefrontal cortex and episodic memory retrieval mode. Proc Natl Acad Sci USA. 2000;97:506–511. doi: 10.1073/pnas.97.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC, Cheng Y. Selection demands versus association strength in the verb generation task. Psychon Bull Rev. 2006;13:396–401. doi: 10.3758/bf03193859. [DOI] [PubMed] [Google Scholar]
- Moss HE, Fletcher PC, Abdallah S, Pilgrim LK, Acres K, Bright P, Tyler LK. Selecting among competing alternatives: selection and controlled retrieval in the left prefrontal cortex. Cereb Cortex. 2005;15:1723–1735. doi: 10.1093/cercor/bhi049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A, Laird A, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichle ED, Carpenter PA, Just MA. The neural basis of strategy and skill in sentence-picture verification. Cogn Psychol. 2000;40:261–295. doi: 10.1006/cogp.2000.0733. [DOI] [PubMed] [Google Scholar]
- Richter W, Ugurbil K, Georgopoulos A, Kim SG. Time-resolved fMRI of mental rotation. Neuroreport. 1997;8:3697–3702. doi: 10.1097/00001756-199712010-00008. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Olten IJ, Henson RN. The neural basis of episodic memory: evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci. 2002;357:1097–1110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-prime user’s guide. Pittsburgh (PA): Psychology Software Tools Inc; 2002. [Google Scholar]
- Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP. Memory and frontal lobe function. In: Gazzaniga MS, editor. The cognitive neurosciences. Cambridge (MA): MIT Press; 1995. pp. 803–813. [Google Scholar]
- Snitz BE, MacDonald A, 3rd, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naïve state and effects of short-term atypical antipsychotic treatment. Am J Psychiatry. 2005;162:2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- Sohn M-H, Goode A, Koedinger KR, Stenger VA, Carter CS, Anderson JR. Behavioral equivalence does not necessarily imply neural equivalence: evidence in mathematical problem solving. Nat Neurosci. 2004;7:1193–1194. doi: 10.1038/nn1337. [DOI] [PubMed] [Google Scholar]
- Sohn M-H, Goode A, Stenger VA, Carter CS, Anderson JR. Competition and representation during memory retrieval: roles of the prefrontal cortex and the posterior parietal cortex. Proc Natl Acad Sci USA. 2003;100:7412–7417. doi: 10.1073/pnas.0832374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn M-H, Goode A, Stenger VA, Jung K-J, Carter CS, Anderson JR. An information-processing model of three cortical regions: evidence in episodic memory retrieval. Neuroimage. 2005;25:21–33. doi: 10.1016/j.neuroimage.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Benson DF. Neuropsychological studies of the frontal lobes. Psychol Bull. 1984;95:3–28. [PubMed] [Google Scholar]
- Thompson-Schill SL. Neuroimaging studies of semantic memory: inferring “how” from “where”. Neuropsychologia. 2003;41:280–292. doi: 10.1016/s0028-3932(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Botvinick MM. Resolving conflict: a response to Martin and Cheng 2006. Psychon Bull Rev. 2006;13:402–408. doi: 10.3758/bf03193860. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left prefrontal cortex in retrieval of semantic knowledge: a re-evaluation. Proc Natl Acad Sci USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. Neuroimage. 2001;14:1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional dissociation among components of remembering: control, perceived oldness, and content. J Neurosci. 2003;23:3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intraparticipant, intramodality validation. J Comput Assisted Tomogr. 1998;22:139. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Ollinger JM, Sheridan MA, Tversky B. A parametric study of mental spatial transformation of bodies. Neuroimage. 2002;16:857–872. doi: 10.1006/nimg.2002.1129. [DOI] [PubMed] [Google Scholar]


