Abstract
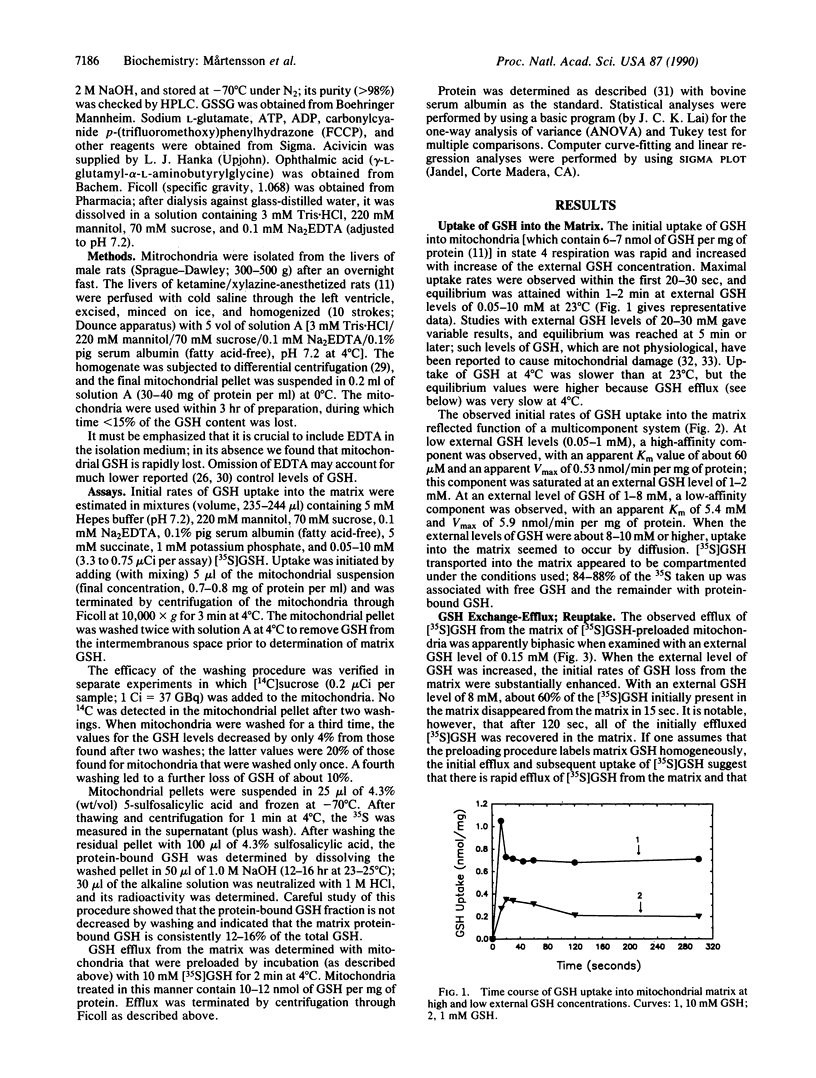
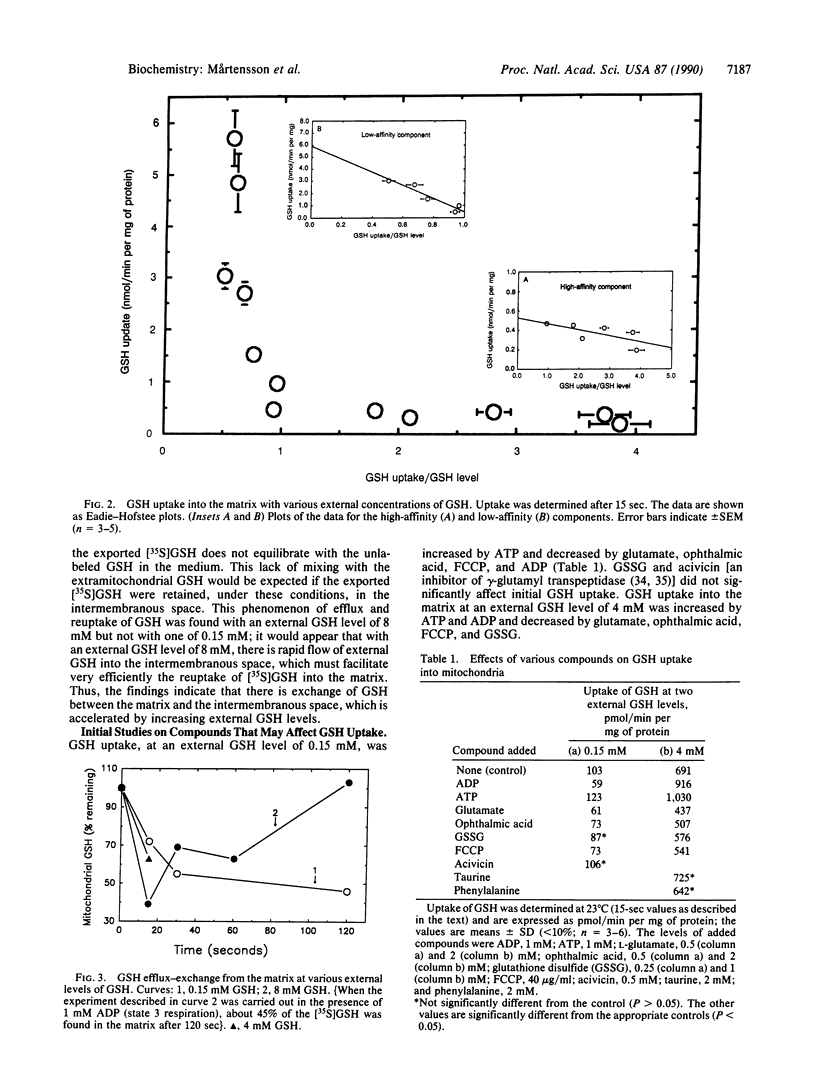
Glutathione, an essential cellular antioxidant required for mitochondrial function, is not synthesized by mitochondria but is imported from the cytosol. Rat liver mitochondria have a multicomponent system that underlies the remarkable ability of mitochondria to take up and retain glutathione. At external glutathione levels of less than 1 mM, glutathione is transported into the mitochondrial matrix by a high-affinity component (Km, approximately 60 microM; V max, approximately 0.5 nmol/min per mg of protein), which is saturated at levels of 1-2 mM and stimulated by ATP. Another component has lower affinity (Km, approximately 5.4 mM; Vmax, approximately 5.9 nmol/min per mg of protein) and is stimulated by ATP and ADP. Both components are inhibited by carbonylcyanide p-(trifluoromethoxy)phenylhydrazone (FCCP), glutamate, and ophthalmic acid. Increase of extramitochondrial glutathione promotes uptake and exchange; the intermembranous space seems to function as a recovery zone that promotes efficient recycling of matrix glutathione. The findings are in accord with in vivo data showing that (i) rapid exchange occurs between mitochondrial and cytosolic glutathione, (ii) lowering of cytosolic glutathione levels (produced by administration of buthionine sulfoximine) decreases export of glutathione from mitochondria to cytosol, and (iii) administration of glutathione esters increases glutathione levels in mitochondria more than those in the cytosol.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L., Meck R., Yunis A. The inhibition of gamma-glutamyl transpeptidase from human pancreatic carcinoma cells by (alpha S,5S)-alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid (AT-125; NSC-163501). Res Commun Chem Pathol Pharmacol. 1980 Jan;27(1):175–182. [PubMed] [Google Scholar]
- Anderson M. E., Meister A. Glutathione monoesters. Anal Biochem. 1989 Nov 15;183(1):16–20. doi: 10.1016/0003-2697(89)90164-4. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Powrie F., Puri R. N., Meister A. Glutathione monoethyl ester: preparation, uptake by tissues, and conversion to glutathione. Arch Biochem Biophys. 1985 Jun;239(2):538–548. doi: 10.1016/0003-9861(85)90723-4. [DOI] [PubMed] [Google Scholar]
- Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973 Jul;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bulter J., Spielberg S. P., Schulman J. D. Reduction of disulfide-containing amines amino acids, and small peptides. Anal Biochem. 1976 Oct;75(2):674–675. doi: 10.1016/0003-2697(76)90129-9. [DOI] [PubMed] [Google Scholar]
- Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Dennis S. C., Lai J. C., Clark J. B. Comparative studies on glutamate metabolism in synpatic and non-synaptic rat brain mitochondria. Biochem J. 1977 Jun 15;164(3):727–736. doi: 10.1042/bj1640727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlid K. D., Beavis A. D. Evidence for the existence of an inner membrane anion channel in mitochondria. Biochim Biophys Acta. 1986;853(3-4):187–204. doi: 10.1016/0304-4173(87)90001-2. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Anderson M. E., Meister A. Inhibition of glutathione biosynthesis by prothionine sulfoximine (S-n-propyl homocysteine sulfoximine), a selective inhibitor of gamma-glutamylcysteine synthetase. J Biol Chem. 1979 Feb 25;254(4):1205–1210. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Excretion of cysteine and gamma-glutamylcysteine moieties in human and experimental animal gamma-glutamyl transpeptidase deficiency. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3384–3387. doi: 10.1073/pnas.77.6.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Higashi T., Tateishi N., Naruse A., Sakamoto Y. A novel physiological role of liver glutathione as a reservoir of L-cysteine. J Biochem. 1977 Jul;82(1):117–124. doi: 10.1093/oxfordjournals.jbchem.a131659. [DOI] [PubMed] [Google Scholar]
- Jocelyn P. C., Kamminga A. The non-protein thiol of rat liver mitochondria. Biochim Biophys Acta. 1974 Apr 22;343(2):356–362. doi: 10.1016/0304-4165(74)90099-3. [DOI] [PubMed] [Google Scholar]
- Jocelyn P. C. The function of subcellular fractions in the oxidation of glutathione in rat liver homogenate. Biochem J. 1970 May;117(5):951–956. doi: 10.1042/bj1170951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa K., Hayashi N., Sato N., Kamada T., Tagawa K. Transport of glutathione across the mitochondrial membranes. Biochem Biophys Res Commun. 1990 Feb 28;167(1):367–372. doi: 10.1016/0006-291x(90)91774-m. [DOI] [PubMed] [Google Scholar]
- LEHNINGER A. L., SCHNEIDER M. Mitochondrial swelling induced by glutathione. J Biophys Biochem Cytol. 1959 Jan 25;5(1):109–116. doi: 10.1083/jcb.5.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meredith M. J., Reed D. J. Status of the mitochondrial pool of glutathione in the isolated hepatocyte. J Biol Chem. 1982 Apr 10;257(7):3747–3753. [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Frayer W., Meister A. Glutathione metabolism in the lung: inhibition of its synthesis leads to lamellar body and mitochondrial defects. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5296–5300. doi: 10.1073/pnas.86.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Meister A. Glutathione is required for intestinal function. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1715–1719. doi: 10.1073/pnas.87.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Meister A. Mitochondrial damage in muscle occurs after marked depletion of glutathione and is prevented by giving glutathione monoester. Proc Natl Acad Sci U S A. 1989 Jan;86(2):471–475. doi: 10.1073/pnas.86.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Steinherz R., Jain A., Meister A. Glutathione ester prevents buthionine sulfoximine-induced cataracts and lens epithelial cell damage. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8727–8731. doi: 10.1073/pnas.86.22.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J., Cannon B. Overview--preparation and properties of mitochondria from different sources. Methods Enzymol. 1979;55:3–28. doi: 10.1016/0076-6879(79)55003-4. [DOI] [PubMed] [Google Scholar]
- Olafsdottir K., Reed D. J. Retention of oxidized glutathione by isolated rat liver mitochondria during hydroperoxide treatment. Biochim Biophys Acta. 1988 Mar 17;964(3):377–382. doi: 10.1016/0304-4165(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Pryor W. A. Oxy-radicals and related species: their formation, lifetimes, and reactions. Annu Rev Physiol. 1986;48:657–667. doi: 10.1146/annurev.ph.48.030186.003301. [DOI] [PubMed] [Google Scholar]
- Puri R. N., Meister A. Transport of glutathione, as gamma-glutamylcysteinylglycyl ester, into liver and kidney. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5258–5260. doi: 10.1073/pnas.80.17.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapuano B. E., Maddaiah V. T. Effects of hypophysectomy and administration of growth and thyroid hormones on the hydroperoxide-induced calcium release process and glutathione levels in rat liver mitochondria. Arch Biochem Biophys. 1988 Jan;260(1):359–376. doi: 10.1016/0003-9861(88)90460-2. [DOI] [PubMed] [Google Scholar]
- Romero F. J., Sies H. Subcellular glutathione contents in isolated hepatocytes treated with L-buthionine sulfoximine. Biochem Biophys Res Commun. 1984 Sep 28;123(3):1116–1121. doi: 10.1016/s0006-291x(84)80248-x. [DOI] [PubMed] [Google Scholar]
- Tischler M. E., Pachence J., Williamson J. R., La Noue K. F. Mechanism of glutamate-aspartate translocation across the mitochondrial inner membrane. Arch Biochem Biophys. 1976 Apr;173(2):448–461. doi: 10.1016/0003-9861(76)90282-4. [DOI] [PubMed] [Google Scholar]
- Vignais P. M., Vignais P. V. Fuscin, an inhibitor of mitochondrial SH-dependent transport-linked functions. Biochim Biophys Acta. 1973 Dec 14;325(3):357–374. doi: 10.1016/0005-2728(73)90197-7. [DOI] [PubMed] [Google Scholar]
- Wahlländer A., Soboll S., Sies H., Linke I., Müller M. Hepatic mitochondrial and cytosolic glutathione content and the subcellular distribution of GSH-S-transferases. FEBS Lett. 1979 Jan 1;97(1):138–140. doi: 10.1016/0014-5793(79)80069-1. [DOI] [PubMed] [Google Scholar]
- Wellner V. P., Anderson M. E., Puri R. N., Jensen G. L., Meister A. Radioprotection by glutathione ester: transport of glutathione ester into human lymphoid cells and fibroblasts. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4732–4735. doi: 10.1073/pnas.81.15.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]


