Abstract
Background
The aim of this study was to explore the accuracy of in vivo magnetic resonance imaging (MRI) in the quantitative evaluation of lipid-rich necrotic core (LRNC) in carotid atherosclerotic plaques compared with histopathology, and to assess the association of LRNC size with cerebral ischemia symptoms.
Material/Methods
Thirty patients were enrolled and 19 patients (16 men and 3 women) were analyzed. All the patients were submitted to MRI on a Siemens Avanto (1.5-Tesla) device before carotid endarterectomy (CEA). The scanning protocol included three-dimensional time of flight (3D TOF), T1-weighted image (T1WI), T2-weighted image (T2WI), turbo spin-echo T2-weighted (T2-TSE), and contrast-enhanced T1-weighted image. MRI images were reviewed for quantitative measurements of LRNC areas. LRNC specimens were collected for histology. Percentages of LRNC area to total vessel area were assessed to determine the association of MRI with histological findings.
Results
There were 151 pairs of matched MRI and pathological sections. LRNC area percentages (LRNC area/vessel area) measured by MRI and histology were 20.6±9.0% and 18.7±9.5%, respectively (r=0.69, p<0.001). Twelve out of 19 patients had symptoms (S-group; 3 had recent stroke, 3 had a recent stroke and a history of transient ischemic attack (TIA), and 6 had TIA); the remaining 7 subjects showed no symptoms (NS-group). LRNC area percentages in the S- and NS-groups were 22.2±5.8% and 12.6±10.7%, respectively (p<0.05).
Conclusions
MRI can quantitatively measure LRNC in carotid atherosclerotic plaques, and may be useful in predicting the rupture risk of plaques. These findings provide a basis for imaging use in individualized treatment plan.
MeSH Keywords: Carotid Arteries; Magnetic Resonance Imaging; Plaque, Atherosclerotic
Background
Cerebrovascular diseases result from alterations of brain vasculature, and principally encompass ischemic and hemorrhagic stroke [1]. These ailments cause high morbidity and mortality, and ischemic stroke is one of the leading causes of long-term disability throughout the world [2]. In China, stroke patients include 70% individuals with cerebral ischemic stroke [3].
Carotid artery atherosclerosis induced cerebral ischemic stroke is associated with coronary lumen stenosis as well as atherosclerotic plaque stability [4]. Lipids are the basic components of atherosclerotic plaques [5,6]. Indeed, size of the lipid-rich necrotic core (LRNC) in carotid plaques is believed to be a predictive factor for plaque rupture, and allows distinction between symptomatic and asymptomatic patients [7]. However, studies assessing the association of LRNC size and ischemic cerebrovascular diseases in Chinese patients are scarce.
Accurate determination of LRNC size is helpful in disease prognosis and optimal selection of individualized treatment plans. Magnetic resonance imaging (MRI) sequences, including three-dimensional time of flight (3D TOF), T1-weighted image (T1WI), T2-weighted image (T2WI), and contrast-enhanced T1-weighted image, have been proposed for LRNC measurements [8,9]. Currently, MRI measurement accuracy for LRNC in Chinese patients remains unclear.
Therefore, this study aimed to explore MRI accuracy in quantitatively measuring LRNC in carotid atherosclerotic plaques, compared with histopathology, and to assess the association of LRNC size with cerebral ischemia symptoms. We found that MRI can quantitatively measure LRNC in carotid atherosclerotic plaques, and may help predict the rupture risk of plaques.
Material and Methods
Patients
In this prospective study, 30 patients scheduled for carotid endarterectomy (CEA) at the Second People’s Hospital of Shenzhen from October 2014 to February 2016 were included. The included patients showed the following characteristics: (1) symptomatic patients with transient ischemic attack or stroke in last 6 months with >50% carotid stenosis; (2) asymptomatic patients with >70% carotid stenosis. Exclusion criteria were MRI contraindications. The study protocol was approved by the ethics committee of the above hospital. Signed informed consent was obtained from each patient.
MRI
For MRI, a Siemens Avanto 1.5 T MRI with an 8-channel phased-array surface coil pair specific for carotid artery assessment (Siemens, Germany) was used. Scan sequences and parameters are summarized in Table 1.
Table 1.
Scan sequences and MRI parameters.
| Sequence | TR (ms) | TE (ms) | Field of vision FOV (cm) | Depth (mm) | Matrix |
|---|---|---|---|---|---|
| T1WI | 800 | 11 | 150×150 | 2 | 256×256 |
| T2WI | 3500 | 62 | 150×150 | 2 | 256×256 |
| 3DTOF | 26 | 7.0 | 150×150 | 2 | 256×256 |
| T2-TSE | 2000 | 123 | 200×200 | 0.8 | 256×256 |
Patients’ jaws and necks were stabilized by the 8-channel phased-array surface coil pair. Patients were instructed to remain calm and to avoid swallowing during scanning. First, bilateral carotid arteries were assessed through coronal thin slice T2WI scan, in order to rebuild images and obtain carotid artery shape and stenosis position. Centering on the stenosis position, within a range of 20–24 mm (10–12 slices) in the longitudinal direction, axial 3DTOF, fast spin-echo (FSE)-based T1WI, and T2WI scans were carried out, and supplemented with fat suppression (FS). T1WI, T2WI, and 3DTOF sequence locations were kept consistent among patients. The contrast-enhanced T1 protocol had to be excluded from the analyses because some patients were allergic to contrast medium or had poor renal function.
MRI data processing and LRNC measurement
MRI data were reviewed by 2 experienced radiologists in a blinded manner for carotid plaque evaluation. Signal intensity was compared to the ipsilateral cleidomastoid muscle. Standards for reconstitution analyses were in accordance with previous reports [10,11]. The MRI images were classified in 5 grades in terms of quality, with grade 1 representing the worst quality (carotid lumen and the external contour of blood vessels were indistinguishable, low signal-to-noise ratio, and overt motion artifact), while grade 5 reflected excellent quality (carotid lumen and blood vessels were both clear, high signal-to-noise ratio, and less or no motion artifact) [12]. Images with quality scores ≤2 were excluded. T1WI, T2WI, and 3DTOF images were assessed with the plaque analysis software (MRI-PlaqueView-™, MR-P; VPDiagnostics, USA), which determined plaque areas, volumes, thicknesses, and compositions, as well as percentages of LRNC area to vessel area within the plaques [13–16].
Screening and standards for histological analyses
Samples completely resected by CEA should be complete, with annular plaque lumens. All specimens were fixed in 10% neutral formalin, decalcified with 10% formic acid, and paraffin embedded. Arteria carotis communis and internal were sectioned every 1.0 mm. The resulting slices were stained by hematoxylin-eosin (H&E). Histological sections were reviewed in a blinded manner by an MRI expert and a pathologist. Slices containing complete LRNC were selected and compared with MRI images.
Matching MRI data and histological images, and LRNC area measurements
T1WI, T2WI, and 3DTOF images were compared with histological sections. Landmarks included the bifurcation distance between the plaque and arteria carotis, as well as general morphological characteristics (such as size and shape of lumen and tube wall, and calcification level). MRI and histological sections were compared according to the above markers that could be recognized simultaneously. Since samples shrank during fixation and processing, their average lengths and widths were reduced by 30% and 15%, respectively [17]. The degree of reduction varied from sample to sample due to differences in plaque composition. Therefore, it was necessary to refer to multiple internal markers for comparison. Due to the complex lesions in each sample, different compositions resulted in distinct degrees of reduction, which might obviously change slice size, making it difficult to match accurately. As a result, we referred to LRNC area/vessel area for analysis to reduce the matching error.
Statistical analysis
Data were analyzed by the SPSS20.0 software (SPSS, USA). Pearson’s correlation analysis was used to assess the associations of quantitative LRNC parameters obtained by MRI and histological measurements. P<0.05 and P<0.01 indicated significant and highly significant correlations between the 2 data types, respectively. LRNC percentages of S and NS-groups were compared by t-test, and p<0.05 was considered statistically significant.
Results
Patient characteristics and image quality
Of the 30 patients included in this study, 7 had images quality ≤2, and were excluded. Specifically, 5 of the 7 excluded patients moved during the MRI examination, which resulted in poor MRI images or unmatched T1WI, T2WI, and 3DTOF image slices; the remaining 2 had internal carotids showing partial volume effects and blurred edges. In addition, 4 CEA samples were damaged and could not be analyzed histologically. Therefore, 19 cases were included in the final analysis. They included 16 males (84%) and 3 females (16%), aged 67.3±7.7 (range, 53–79) years. A total of 14 patients had combined hypertension (14/19, 74%); 5 had combined diabetes (5/19, 26.3%) and 8 also presented cerebral infarction (8/19, 42.1%). There were 12 symptomatic patients (S-group; 3 had recent stroke, 3 had a recent stroke and a history of transient ischemic attack (TIA), and 6 had TIA) and 7 asymptomatic patients (NS-group) (Table 2).
Table 2.
Patient characteristicsin S- and NS-groups.
| S-group (12) M±SD or % |
NS-group (7) M±SD or % |
p Value | |
|---|---|---|---|
| Age | 65.5±8.2 | 70.4±7.2 | 0.208 |
| Female | 10 (83%) | 6 (86%) | 0.913 |
| Diabetes | 3 (25%) | 2 (29%) | 0.972 |
| Hypertension | 8 (67%) | 6 (86%) | 0.415 |
MRI measurements of LRNC correlate with histological findings
MRI images of 19 patients were imported to the plaque analysis software (MR-P) (Figure 1). In total, 151 layered MRI images were obtained. Percentages of LRNC area to vessel area from MRI images and histological measurements were 20.6±9.0% and 18.7±9.5%, respectively, and indicated a strong correlation between the 2 data types (r=0.69, p<0.001) (Figure 2). Histological LRNC area to vessel area percentages in the S- and NS-groups were 22.2±5.8% and 12.6±10.7%, respectively (p<0.05) (Table 3).
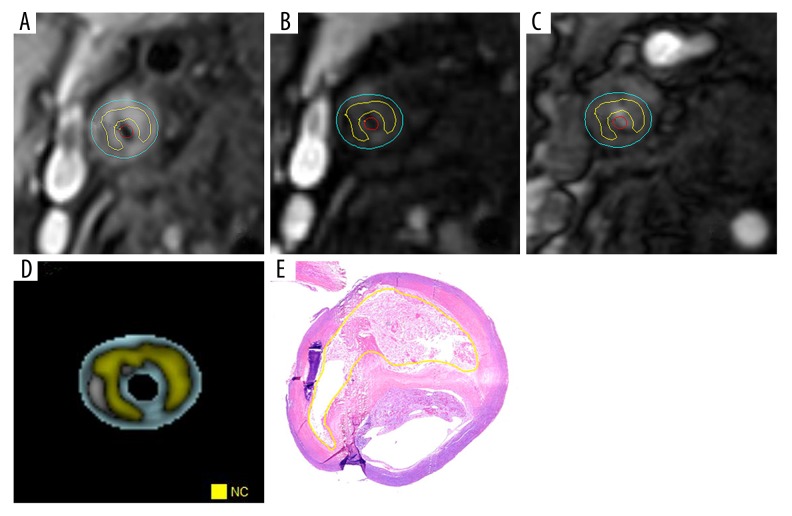
Figure 1.
Quantitative measurements of LRNC within carotid artery plaques. (A) T1WI image of lipid-rich necrotic core (LRNC; yellow line delineated area). (B) T2WI image of LRNC (yellow line delineated area). (C) 3DTOF image of LRNC (yellow line delineated area). (D) Composition analysis software MR-P merged image of carotid artery plaque, with yellow zone representing the LRNC. (E) Histological section of LRNC corresponding to MRI image layer, delineated by yellow line (H&E, ×20).
Figure 2.
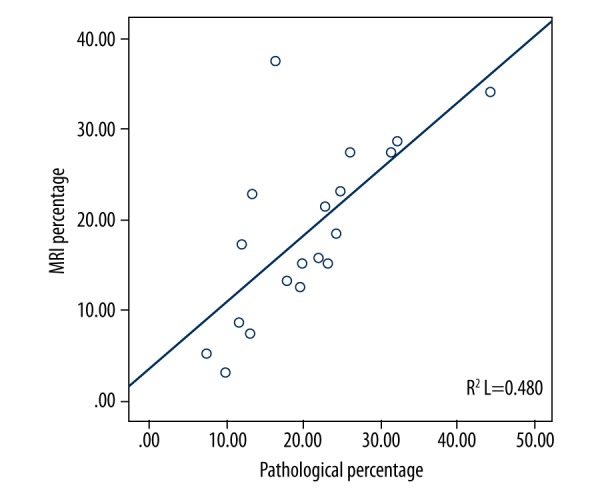
Scatter plot of carotid artery plaque MRI and histological LRNC area percentage.
Table 3.
LRNC area percentages in the S-and NS-groups.
| Measurement method | LRNC area % | p Value | |
|---|---|---|---|
| S-group | NS-group | ||
| MRI | 24.7±8.2 | 13.7±4.1 | 0.006 |
| Histology | 22.2±5.8 | 12.6±10.7 | 0.029 |
Discussion
Embolus of carotid artery plaque surface shedding and thrombosis from plaque rupture are the most common causes of cerebral ischemic strokes [18]. Multiple studies have demonstrated that vulnerable plaques normally show thin fibrous cap, large lipid necrosis nucleus, neovascularization, inflammatory cell infiltration, intra-plaque hemorrhage, and surface calcification [19,20].
This study demonstrated that MRI can quantitatively measure LRNC in carotid atherosclerotic plaques, with efficiency comparable to histological findings. As shown above, percentages of LRNC area to vessel area assessed on MRI images and histological sections were similar. Compared with histopathological findings, LRNC areas measured by MRI were larger. We hypothesized that there may be an underestimation in the histological quantification of LRNC in the plaques, likely due to the following factors: 1) tissue specimens shrunk during processing; and 2) the LRNC may fall off during operation, tissue extraction, slicing, or staining. Meanwhile, several representative points of individual cases showed a slight deviation from most points in the scatter plot (Figure 2), which may also be related to the above reasons.
This study indicated that percentages of LRNC area/vessel area measured by MRI images and histological sections were highly correlated, corroborating findings by Saam et al. [21]. These authors compared plain MRI scanning (T1WI images) and histological data, assessing 31 patients for percentages of LRNC, calcification, and hemorrhage areas over total vessel areas within the carotid artery plaques, with the image processing software (QVAS). In addition, Cai et al. [10] compared MRI enhanced T1WI images from 21 patients with respective histological results; quantitative measurements of plaque fiber cap length and LRNC size were analyzed using the QVAS software. Interestingly, average LRNC percentages measured by enhanced MRI and histology were 30.1% and 32.7%, respectively, and the values were highly correlated [10], supporting the present study. Indeed, our results suggest that quantitative evaluation of LRNCs within the plaques by MRI is highly accurate, also yielding similar results that are highly correlated between the 2 methods. Differences in percentages could be due to observed bias or different populations having different levels of LRNC.
Histology analysis demonstrated that lipid necrosis in the plaques leads to increased water percentage and increased plaque tension; meanwhile, free cholesterol in the plaques, through physical and/or humoral mechanisms, may form cholesterol crystals, whose sharp edges could destroy the fibrous tissues [22,23]. When cholesterol changes from the liquid to solid crystal state, its volume expands within the plaque. This leads to fiber cap extension and thinning or even rupture. Therefore, the greater the proportion of lipid core occupying the lumen area, the higher the risk for the plaque fiber cap to rupture. Takaya et al. [24] followed up 111 patients with lipid necrosis nucleus within the plaque for 3 years to assess the relationship between the lipid core and hazard ratio of carotid plaque. They found that each 10% increase of LRNC percentage within the plaque would lead to a hazard ratio increase of 1.6. Among the 19 patients analyzed in the current study, symptomatic patients had higher percentages than non-symptomatic ones, suggesting a relationship between carotid plaque stability and LRNC. Therefore, it is important to quantitatively evaluate LRNCs within the plaques, which may provide reliable evidence for clinical prediction of plaque risks.
Currently, multiple studies have confirmed that systematic treatment with statins is effective for plaques with LRNCs, and may even completely reverse the disease in some patients [25]. Therefore, quantitative analysis by MRI of LRNCs within the carotid artery plaques has great clinical significance for the development of treatment plans. Zhao et al. [9] performed a randomized trial assessing bevacizumab in 55 patients with carotid atherosclerotic plaques. Within the following 3 years, quantitative evaluation of LRNC areas by MRI was carried out; LRNCs had shrunk by 12 mm2 (p=0.007), 13 mm2 (p=0.004), and 1 mm2 (p>0.05) in average within the 1st, 2nd, and 3rd years, respectively. Underhill et al. also demonstrated that MRI could be used to monitor LRNC size within the carotid atherosclerotic plaques after statin treatment [25].
The measurement of LRNC by MRI has a number of merits. First, LRNC is associated with the incidence of stroke, and MRI for LRNC determination is non-invasive, allowing for monitoring plaque size and estimating stroke risk in an asymptomatic population. Vessel imaging by high resolution MRI has high reproducibility, and could sensitively recognize the composition within the carotid atherosclerotic plaques [21]. Because the risk of rupture is associated with plaque composition, it could help refining the prediction of the risk of stroke [22]. In addition, it could constitute a reliable imaging method to monitor drug therapy for carotid atherosclerotic plaques [9,25].
This study was limited by its small sample size. In addition, most included patients had complex plaques, which made LRNC delineation inaccurate, leading to bias. Furthermore, vessel imaging with high resolution and multiple sequences has only limited imaging range, and requires long scanning time (at least 30 minutes). Besides, bifurcation structures and shapes of the carotid plaques were highly variable, and even tortuous vessels were found in some carotid artery segments. Under these circumstances, volume effects could be found only in case of axial imaging with multi-sequence, affecting image interpretation. Lastly, the analysis software could show some differences when contouring the LRNC among different protocols. Therefore, more accurate plaque analysis software and faster imaging technology with wider coverage by MRI scanning are urgently needed.
Conclusions
In summary, multi-sequence high-resolution MRI quantitatively measured LRNC size in carotid artery atherosclerotic plaques. This could be helpful, not only for predicting the risk of plaque formation, but also in monitoring drug treatment for atherosclerotic plaques. Therefore, MRI may provide imaging evidence for the clinical development of individualized treatment plans.
Footnotes
Source of support: This work was supported by the Science and Technology Project of Shenzhen (No. JSGG20141020103440414), the Shenzhen Science and Technology Project (No. JCYJ20140414170821323, JCYJ20150330102720122), the Guangdong Province Science and Technology Program (No. 2014A020212038), the Natural Science Foundation of Guangdong Province (No. 2016A030313029), and the Shenzhen Science and Technology Cooperation Innovation International Cooperation Project (No. GJHZ20140415090907551, GJHZ20160301163138685)
References
- 1.Chu H, Huang C, Ding H, et al. Aquaporin-4 and cerebrovascular diseases. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17081249. pii: E1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu D, Chen J, Wang B, et al. Endovascular ischemic stroke models of adult rhesus monkeys: A comparison of two endovascular methods. Sci Rep. 2016;6:31608. doi: 10.1038/srep31608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Cui L, Ji X, et al. The China National Stroke Registry for patients with acute cerebrovascular events: Design, rationale, and baseline patient characteristics. Int J Stroke. 2011;6:355–61. doi: 10.1111/j.1747-4949.2011.00584.x. [DOI] [PubMed] [Google Scholar]
- 4.Virmani R, Ladich ER, Burke AP, Kolodgie FD. Histopathology of carotid atherosclerotic disease. Neurosurgery. 2006;59:S219–27. doi: 10.1227/01.NEU.0000239895.00373.E4. discussion S3–13. [DOI] [PubMed] [Google Scholar]
- 5.Meletta R, Borel N, Stolzmann P, et al. Ex vivo differential phase contrast and magnetic resonance imaging for characterization of human carotid atherosclerotic plaques. Int J Cardiovasc Imaging. 2015;31:1425–34. doi: 10.1007/s10554-015-0706-y. [DOI] [PubMed] [Google Scholar]
- 6.Qiao Y, Ronen I, Viereck J, et al. Identification of atherosclerotic lipid deposits by diffusion-weighted imaging. Arterioscler Thromb Vasc Biol. 2007;27:1440–46. doi: 10.1161/ATVBAHA.107.141028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cappendijk VC, Kessels AG, Heeneman S, et al. Comparison of lipid-rich necrotic core size in symptomatic and asymptomatic carotid atherosclerotic plaque: Initial results. J Magn Reson Imaging. 2008;27:1356–61. doi: 10.1002/jmri.21359. [DOI] [PubMed] [Google Scholar]
- 8.Xie Y, Yu W, Fan Z, et al. High resolution 3D diffusion cardiovascular magnetic resonance of carotid vessel wall to detect lipid core without contrast media. J Cardiovasc Magn Reson. 2014;16:1. doi: 10.1186/s12968-014-0067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao XQ, Dong L, Hatsukami T, et al. MR imaging of carotid plaque composition during lipid-lowering therapy a prospective assessment of effect and time course. JACC Cardiovasc Imaging. 2011;4:977–86. doi: 10.1016/j.jcmg.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai J, Hatsukami TS, Ferguson MS, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: Comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112:3437–44. doi: 10.1161/CIRCULATIONAHA.104.528174. [DOI] [PubMed] [Google Scholar]
- 11.Yuan C, Mitsumori LM, Ferguson MS, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001;104:2051–56. doi: 10.1161/hc4201.097839. [DOI] [PubMed] [Google Scholar]
- 12.Kerwin WS. Carotid artery disease and stroke: Assessing risk with vessel wall MRI. ISRN Cardiol. 2012;2012:180710. doi: 10.5402/2012/180710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan Z, Yu W, Xie Y, et al. Multi-contrast atherosclerosis characterization (MATCH) of carotid plaque with a single 5-min scan: Technical development and clinical feasibility. J Cardiovasc Magn Reson. 2014;16:53. doi: 10.1186/s12968-014-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu W, Fan Z, Yang L, et al. Characterization of atherosclerotic carotid plaque using MATCH: Initial clinical experience. J Cardiovasc Magn Reson. 2015;17:1. [Google Scholar]
- 15.Kerwin WS, Liu F, Yarnykh V, et al. Signal features of the atherosclerotic plaque at 3.0 Tesla versus 1.5 Tesla: impact on automatic classification. J Magn Reson Imaging. 2008;28:987–95. doi: 10.1002/jmri.21529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Engelen A, van Dijk AC, Truijman MT, et al. Multi-center MRI carotid plaque component segmentation using feature normalization and transfer learning. IEEE Trans Med Imaging. 2015;34:1294–305. doi: 10.1109/TMI.2014.2384733. [DOI] [PubMed] [Google Scholar]
- 17.Eubank WB, Yuan C, Fisher ER, et al. Endarterectomy plaque shrinkage: Comparison of T2-weighted MR imaging of ex vivo specimens to histologically processed specimens. J Vasc Invest. 1998;4:161–70. [Google Scholar]
- 18.Redgrave JN, Lovett JK, Gallagher PJ, Rothwell PM. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: The Oxford plaque study. Circulation. 2006;113:2320–28. doi: 10.1161/CIRCULATIONAHA.105.589044. [DOI] [PubMed] [Google Scholar]
- 19.Willinek WA. Looking beyond the lumen to predict cerebrovascular events: “The road less travelled by”. Stroke. 2006;37:759–60. doi: 10.1161/01.STR.0000208558.95300.9a. [DOI] [PubMed] [Google Scholar]
- 20.Saam T, Hatsukami TS, Takaya N, et al. The vulnerable, or high-risk, atherosclerotic plaque: Noninvasive MR imaging for characterization and assessment. Radiology. 2007;244:64–77. doi: 10.1148/radiol.2441051769. [DOI] [PubMed] [Google Scholar]
- 21.Saam T, Ferguson MS, Yarnykh VL, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 2005;25:234–39. doi: 10.1161/01.ATV.0000149867.61851.31. [DOI] [PubMed] [Google Scholar]
- 22.Arroyo LH, Lee RT. Mechanisms of plaque rupture: mechanical and biologic interactions. Cardiovasc Res. 1999;41:369–75. doi: 10.1016/s0008-6363(98)00308-3. [DOI] [PubMed] [Google Scholar]
- 23.Quinones A, Saric M. The cholesterol emboli syndrome in atherosclerosis. Curr Atheroscler Rep. 2013;15:315. doi: 10.1007/s11883-013-0315-y. [DOI] [PubMed] [Google Scholar]
- 24.Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: A prospective assessment with MRI – initial results. Stroke. 2006;37:818–23. doi: 10.1161/01.STR.0000204638.91099.91. [DOI] [PubMed] [Google Scholar]
- 25.Underhill HR, Yuan C, Zhao XQ, et al. Effect of rosuvastatin therapy on carotid plaque morphology and composition in moderately hypercholesterolemic patients: A high-resolution magnetic resonance imaging trial. Am Heart J. 2008;155:584e1–8. doi: 10.1016/j.ahj.2007.11.018. [DOI] [PubMed] [Google Scholar]