Cathepsin B is part of a shear stress mechanotransduction control mechanism, which restricts leukocyte adhesion in the physiological microcirculation.
Keywords: mechanotransduction, mechanobiology, integrins, leukocytes, cell signaling
Abstract
There is compelling evidence that circulatory hemodynamics prevent neutrophil activation, including adhesion to microvessels, in the microcirculation. However, the underlying mechanism or mechanisms by which that mechanoregulation occurs remain unresolved. Here, we report evidence that exposure to fluid shear stress (FSS) promotes neutrophils to release cathepsin B (ctsB) and that this autocrine regulatory event is antiadhesive for neutrophils on endothelial surfaces through Mac1-selective regulation. We used a combined cell-engineering and immunocytochemistry approach to find that ctsB was capable of cleaving Mac1 integrins on neutrophils and demonstrated that this proteolysis alters their adhesive functions. Under no-flow conditions, ctsB enhanced neutrophil migration though a putative effect on pseudopod retraction rates. We also established a flow-based cell detachment assay to verify the role of ctsB in the control of neutrophil adhesion by fluid flow stimulation. Fluid flow promoted neutrophil detachment from platelet and endothelial layers that required ctsB, consistent with its fluid shear stress–induced release. Notably, compared with leukocytes from wild-type mice, those from ctsB-deficient (ctsB−/−) mice exhibited an impaired CD18 cleavage response to FSS, significantly elevated baseline levels of CD18 surface expression, and an enhanced adhesive capacity to mildly inflamed postcapillary venules. Taken together, the results of the present study support a role for ctsB in a hemodynamic control mechanism that is antiadhesive for leukocytes on endothelium. These results have implications in the pathogenesis of chronic inflammation, microvascular dysfunction, and cardiovascular diseases involving sustained neutrophil activation in the blood and microcirculation.
Introduction
Blood flow–derived FSS serves as an important mechanism that, in the absence of cell agonists, restricts the activity of neutrophils and ensures their passive circulation without adhesion and migration in the microvasculature [1–3]. The FSS effect occurs at low magnitudes (1–10 dyn/cm2), which, although producing relatively small, passive, viscoelastic deformation of cells, influences various cell activities. For example, nonadherent and migrating neutrophils activate and form pseudopods once removed from a flow environment but retract them on return to flow conditions [1, 2]. Stimulation with fMLP or PAF greater than critical-threshold concentrations blocks FSS-induced pseudopod retraction [1], which is consistent with the ability of blood biochemical mediators to override FSS-induced deactivation of neutrophils, such as during acute inflammation, when their activation is needed.
FSS-induced pseudopod retraction by migrating neutrophils requires interaction with substrates by means of CD18 integrins [4]. CD18 is the common β2 subunit for a family of heterodimeric integrins that mediate neutrophil recruitment to venules during acute inflammation [5]. Among the four known heterodimeric CD18 subtypes, two predominate on neutrophils [6–8]. LFA1 (CD11a/CD18) supports loose capture interactions that promote cell arrest in venular flow, whereas Mac1 (CD11b/CD18) enables neutrophils to firmly adhere and migrate when exposed to the shear stresses acting to dislodge them [9].
Under in vitro conditions that mimic a noninflamed or mildly inflamed scenario, venular-level FSS causes nonadherent or migrating neutrophils to cleave/shed the ligand-binding ectodomains of CD18 on their surface membranes [4, 6]. FSS exposure for as short as 5 min cleaves 20–30% of the CD18 integrins on neutrophils by promoting the release of the lysosomal protease ctsB [6]. That may explain how FSS exposure inhibits CD18-dependent processes, including phagocytosis and actin polymerization [10]. Recently, we demonstrated that FSS exposure was selective for Mac1 cleavage and occurred parallel to reduced binding of neutrophils to platelets and fibrinogen in suspension [8]. Fibrinogen is a Mac1 ligand that stabilizes neutrophil–platelet interactions [11]. Thus, FSS-induced ctsB release and Mac1 cleavage may serve to reduce or prevent neutrophil aggregate formation [8, 12]. It is not known, however, whether it prevents adhesion to the microvascular endothelium. The present study focused on whether an intact FSS response is responsible for restricting neutrophil-endothelial binding under physiologic conditions.
Specifically, we hypothesized that FSS mechanotransduction has a role in a hemodynamic control mechanism responsible for restricting Mac1-dependent neutrophil adhesion in the physiologic (i.e., nonpathogenic) microvasculature. For that purpose, we characterized the involvement of ctsB in Mac1 cleavage and its role in regulating neutrophil adhesive processes under flow and no-flow conditions using both in vitro and ex vivo analyses of leukocytes from human and ctsB−/− mice. From our studies, we present findings that support the involvement of flow-induced ctsB release as an antiadhesive regulatory mechanism for neutrophils on endothelial surfaces. These results implicate ctsB as part of a control mechanism responsible for minimizing neutrophil adhesion in the microcirculation. The significance of such a physiologic mechanism is underscored by literature evidence, which suggest that impaired FSS responses may promote sustained leukocyte activation in the microcirculation [13] and microvascular dysfunction [14].
MATERIALS AND METHODS
Neutrophil and platelet isolation from human blood
Human subject recruitment, informed consent, and phlebotomy were approved by the University of Kentucky Institutional Review Board. Human neutrophils were purified from whole blood harvested from asymptomatic volunteers using 2-step Histopaque-Percoll (Sigma-Aldrich, St. Louis, MO, USA) gradient centrifugation and suspending the cells in HBSS containing calcium and magnesium, as previously described [4, 15]. Platelets were extracted from plasma by removing erythrocytes and leukocytes through low-speed (100 g) centrifugation, washing the supernatant solution in HEPES buffer, and suspending the platelets in Tyrode’s buffer (with calcium and magnesium), following reported procedures [16].
Human cell cultures
HUVECs (Lifeline Cell Technology, Frederick, MD, USA) were cultured in VascuLife VEGF medium (Lifeline Cell Technology) containing calcium and magnesium, supplemented with 10% FBS and 1% penicillin-streptomycin according to the manufacturer’s instructions. HL60 cells (CCL-240; ATCC, Manassas, VA, USA) were differentiated into neutrophil-like (dHL60) cells by culturing in neutrophilic-induction medium consisting of RPMI 1640 (GE Healthcare Life Sciences, Little Chalfont, United Kingdom) containing calcium and magnesium, supplemented with 10% FBS, 1% penicillin-streptomycin, 1 mM l-glutamine, and 1.25% DMSO for 5 d, as previously reported [17]. On d 4 of differentiation, HL60 cells were transiently transfected with a ctsB-GFP expression vector using a Lonza nucleofector electroporator kit (preselected program T-019; Basel, Switzerland), followed by culture in neutrophilic induction medium on d 5.
Mice
Male ctsB−/− mice were a gift from Dr. Gregory J. Gore, whereas C57BL/6 WT (control) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Handling procedures were approved by the University of California, San Diego, Institutional Animal Care and Use Committees. For ex vivo studies, whole blood (0.4 ml) was collected from anesthetized mice (sodium-pentobarbital, 50 mg/kg) by cardiac puncture followed by exsanguination in ammonium heparin (30 U/ml). The blood was then diluted (1:20 v/v) with Plasma-Lyte (Baxter International, Deerfield, IL, USA) supplemented with 2.5 mM CaCl2 [6].
Cone–plate FSS exposure
Purified human neutrophils in HBSS or whole mice blood diluted in HBSS were exposed to a constant (5 dyn/cm2) laminar FSS for 2.5, 5, or 10 min with a custom cone–plate device (Sherline, Vista, CA, USA), as previously described [15]. Experiments were conducted with cell populations treated with and without 30 µM E64 (cysteine protease inhibitor; Sigma-Aldrich), 50 µM CA074-me (ctsB inhibitor; EMD Millipore, Billerica, MA), or 5 µM AP (negative control inhibitor; Sigma-Aldrich) for 5 min before shear exposure, as reported [17]. Immediately after shear and control experiments, neutrophils were fixed (1% p-formaldehyde in phosphate buffer) for 15 min and washed 3 times in PBS. Controls were parallel cell populations maintained under static (no-FSS), but otherwise similar, experimental conditions.
Flow cytometry analyses
Fixed human neutrophils were resuspended in a solution consisting of 1% BSA in PBS and labeled with fluorescent Abs for 1 h using standard immunochemistry. For analysis of surface integrin expression, FITC-conjugated mouse mAbs to CD18 (clones 6.7 or MEM48), a PE-conjugated mouse mAb to CD11a (clone 38), or the Alexa Fluor 488-conjugated mouse mAb to CD11b (clone ICRF44) were used to label neutrophils. For analysis of surface and total ctsB expression, rabbit polyclonal (pAb) against ctsB (clone Ab-3) and Alexa Fluor 488-conjugated goat pAb (Thermo Fisher Scientific, Waltham, MA, USA) were used to label unpermeabilized cells and permeabilized cells, respectively [8]. Nonspecific binding was assessed using an Alexa Fluor 488-conjugated mouse IgG1 isotype control (Thermo Fisher Scientific). Cells were permeabilized by adding 0.1% saponin to 1% BSA before fluorescent Ab labeling. After fluorescent labeling, neutrophil suspensions were washed 3 times in PBS to remove unbound Abs and subsequently resuspended in PBS for flow cytometric analyses using an LSR II flow cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA) interfaced with Becton-Dickinson FACsDiva software, following the manufacturer’s instructions. After data acquisition, FCS Express 4 flow cytometry software (De Novo Software, Glendale, CA, USA) was used to generate histograms of Ag-specific fluorescence intensities of the cells. The mean fluorescence intensity, representing the average amount of bound Abs for a given cell population, was used as the measure of Ag-specific expression level. Representative flow cytometry histograms were randomly selected for presentation.
For murine leukocyte analyses, blood samples were fixed, and erythrocytes were removed using FACS lysing solution (Becton Dickinson), followed by FACS buffer washes. Immunofluorescence was carried out similarly to human neutrophil labeling using FITC-conjugated rat mAbs to CD18 (clone C71/16) [6]. Flow cytometric analyses to assess Ag-specific expression levels focused on the granulocyte (i.e., neutrophil) populations identified based on forward scatter and side scatter of light as described [6].
Pseudopod activity analysis
Borosilicate glass slides were cleaned in acetone and 70% ethanol with an ultrasonic cleaner (Branson Ultrasonics, Danbury, CT, USA) for 15 min each, etched in 1N NaOH for 2 h, and coated with FBS for 10 min. For experiments, neutrophils and dHL60s were pretreated for 5 min with and without 1 μM fMLP, 0.5 U/ml ctsB, or 50 µM CA074 in HBSS and then deposited (at a density of 1 × 106/ml) on the FBS-coated glass slides for 20 min. Nonadherent cells were rinsed with PBS, and the remaining adherent cells were maintained under no-flow conditions in fresh HBSS. Bright-field micrographs of neutrophils, as well as bright-field and fluorescence images of ctsB-GFP transfected cells, were acquired every 15 s for 10 min using an Olympus IX-71 fluorescence microscope (Olympus America, Center Valley, PA, USA). Cell centroid displacement rates were estimated using ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA) by tracing individual cells and tracking the movement of their centroid between consecutive frames [18].
Neutrophil detachment assay
Detachment assays were conducted using autologous human platelets that had been deposited on cleaned glass slides and allowed to spread into a uniform coating for 30 min, following previously reported procedures [19]. Platelets were selected to ensure neutrophil adhesion would be mediated through Mac1, rather than LFA1, integrins [20]. A flow channel was assembled by interfacing the platelet-coated slides to a custom polycarbonate base having inlet and outlet ports. A precut gasket separating those 2 components formed a 200-µm-high parallel plate-flow channel. We also conducted detachment assays more representative of conditions in vivo using HUVECs seeded (5 × 105/ml) and grown on fibronectin-coated culture surfaces of 17-mm-long × 3.8-mm-wide Ibidi microslides (VI0.4; 400-µm high channel; Ibidi USA, Madison, WI, USA) for 3 d, as reported [21].
For flow studies, neutrophils (5 × 106/ml) were pretreated with or without 1 µM fMLP, 30 µM E64, 50 µM CA074-me (cell-permeable, methylated CA074), or 5 µM AP for 5 min and deposited on the platelet coatings or HUVEC monolayers for 10 min; after which, the nonadherent cells were rinsed away. A syringe pump was used to perfuse fresh HBSS through the chamber to impose an FSS of 5 dyn/cm2 on the wall of the channel. Bright-field micrographs (×100 magnification) of the neutrophils were acquired every 5 s for 10 min, and neutrophil detachment was estimated by normalizing the number of neutrophils visible in each image against that present 30 s after flow initiation. We defined this initial time point to be t = 0 to ensure nonadherent (or loosely bound) cells were rinsed away before analyses.
Micropipette shear studies with murine blood
Aliquots of diluted murine blood, preincubated with 1 µM PAF (Sigma-Aldrich) were deposited on thin (no. 1) glass coverslips to promote leukocyte adhesion without affecting their responsiveness to FSS. The selected PAF concentration was less than threshold levels known to impair the FSS response [1]. After 15 min, nonadherent cells were gently rinsed off with Plasma-Lyte, and the adherent cells were incubated with FITC-labeled mAbs to mouse CD18 (1/10 dilution) in the presence of PAF. FITC distribution and bright-field images of each adherent cell were digitally recorded during micropipette shear with a laser confocal microscope [1, 2, 6]. Mean fluorescence intensity was used as a measure of CD18 expression levels on the neutrophils.
For some experiments, diluted blood from mice was incubated on glass substrates for at least 15 min in the absence of agonists; after which, the nonadherent leukocytes and erythrocytes were rinsed with Plasma-Lyte buffer. The migrating neutrophils, identified by their granular appearance and nuclear morphology, were then exposed to a flow regime consisting of an initial 2-min no-flow period, a subsequent 2-min exposure to ∼2 dyn/cm2 FSS, and then another 2-min no-flow period using a micropipette, as previously described [1, 2, 15]. The instantaneous major axis length of each cell normalized to its average major axis length over the initial 2-min no-flow period was used as a measure of pseudopod activity.
In vivo microcirculation analyses
The femoral veins of mice were cannulated after general anesthesia (sodium-pentobarbital, 50 mg/kg). The microcirculation of the mouse cremaster muscle was visualized during superfusion with 10 nM PAF (to promote adhesion in vivo without affecting the leukocyte pseudopod retraction-response to FSS [1]) by digital, intravital fluorescence microscopy, following reported procedures [1, 14, 22]. Circulating leukocytes were labeled with carboxyfluorescein succinimidyl ester (Thermo Fisher Scientific) to facilitate visualization of leukocytes in the microvasculature. PKH-26 (Sigma-Aldrich) was used to stain erythrocytes to permit estimation of blood flow velocity [14, 22, 23]. In each animal, the number of adherent, migrating, and rolling leukocytes on blood vessel walls was measured in 20 fields every 30 min for 120 min [14, 22, 23]. Adherent leukocytes were defined as cells that adhered to endothelium along vessel lumens for at least 30 min. Migrating leukocytes were cells that migrated across the endothelium during the 30-min period. Rolling leukocytes were defined as the number of cells that rolled across a predefined vessel cross section during a 30-s period.
Statistics
Quantitative data derived using human blood and dHL60 cells were expressed as means ± sd for n ≥ 3 replicates. Statistical analyses were performed using JMP software (SAS Institute, Cary, NC, USA). One-sample t tests with P < 0.05 were used to identify significant fold changes or percentage of reductions in experimental treatments compared with a reference value. Comparisons among the means of experimental treatments were performed with ANOVA and post hoc Tukey’s test with P < 0.05 indicating significant differences. For mice studies, measurements were expressed as means ± sd. Comparisons among groups were made with ANOVA and Fischer’s protected least significant difference test, with P < 0.05 indicating significant differences.
RESULTS
FSS-induced ctsB release selectively cleaves Mac1 on human neutrophils
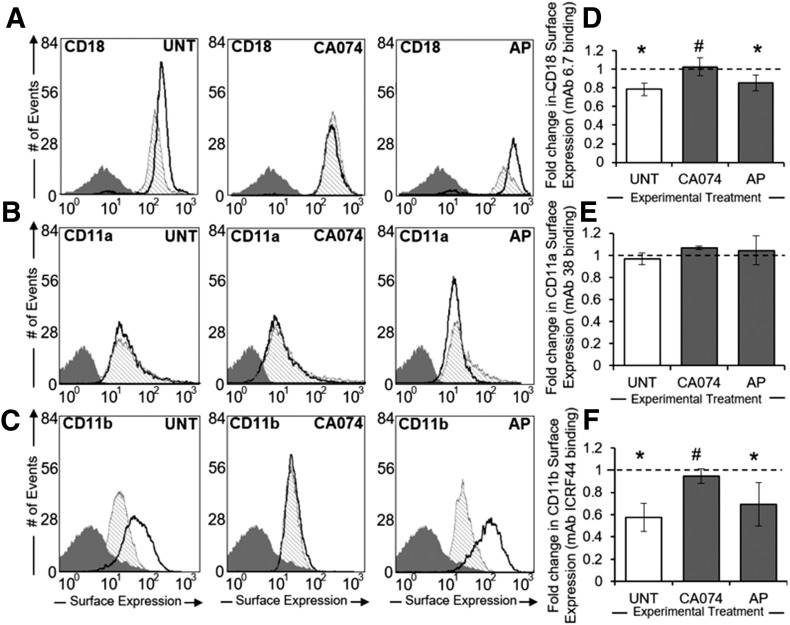
Human neutrophils exposed to FSS (5 dyn/cm2 for 10 min) with a cone–plate device exhibited reduced binding of Abs to CD18 and CD11b, but not CD11a, integrins relative to time-matched, unsheared controls (Fig. 1A–C). This reduction in CD18 Ab binding was blocked for cells pretreated with CA074, but not AP, before shearing (Fig. 1D). Notably, although exposure to FSS did not reduce binding of neutrophils to CD11a Abs (Fig. 1E), the apparent FSS-induced reductions in CD11b Ab binding to neutrophils were mitigated by CA074, but not by AP (Fig. 1F).
Figure 1. Selective cleavage of Mac1 by FSS-induced ctsB release.
(A–C) Representative flow cytometry histograms of intact CD18 (A), CD11a (B), and CD11b (C) integrin expression on the surface of neutrophil suspensions maintained under no-flow conditions (thick black line) or subjected to 5 dyn/cm2 shear stress (hatched shading) using a cone–plate viscometer. Negative (IgG) staining control is displayed in each plot (dark gray shading). Neutrophil suspensions were left UNT or treated with ctsB-specific inhibitor CA074 (50 µM) or serine inhibitor AP (5 μM). (D–F) Mean fluorescent intensities of sheared neutrophils were normalized to their respective no-flow controls and plotted as mean fold-change ± sd for CD18 (D), CD11a (E), and CD11b (F) integrins, based on fluorescent Ab binding to the neutrophil surface. n = 3 replicates in each group. *P < 0.05 compared with a hypothetical value of 1 (normalized control). #P < 0.05 compared with control.
FSS application stimulates ctsB release by human neutrophils
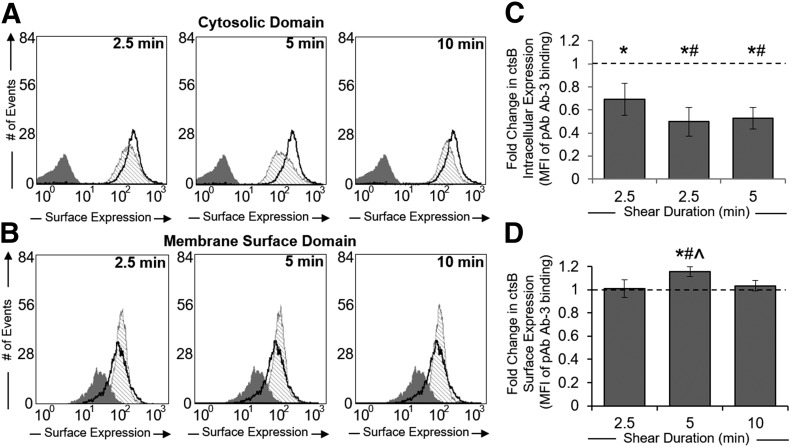
Permeabilized human neutrophils exhibited significantly reduced binding to ctsB Abs (Fig. 2A and B) after 2.5 min of FSS exposure when compared with unsheared cells (Fig. 2C). This apparent reduction in total ctsB levels was observed for the duration of the 10-min exposure. In contrast, ctsB Ab binding to unpermeabilized human neutrophils remained unchanged after 2.5 and 10 min of FSS exposure (Fig. 2D). Although surface-associated ctsB appeared to be significantly elevated after 5 min of FSS, the differences in expression levels were minor.
Figure 2. Time-dependent ctsB release in response to FSS exposure.
(A and B) Representative flow cytometry histograms of ctsB expression within the cytosolic domain (A) or membrane-surface domain (B) for neutrophil suspensions maintained under no-flow conditions (thick black line) or exposed to 5 dyn/cm2 shear stress (hatched shading) using a cone–plate viscometer. Negative (IgG) staining control is displayed in each plot (dark gray shading). Cells were permeabilized (A) or left UNT (B) to measure ctsB expression within the cytosolic or membrane surface domains. Shear duration was gradually increased (2.5, 5, and 10 min) to observe the time-dependent effects of shear. (C and D) Mean fluorescent intensities of sheared neutrophils were normalized to their respective no-flow controls and plotted as fold-change means ± sd for ctsB cytosolic (C) or membrane surface (D) expression, based on fluorescent Ab binding to ctsB. *P < 0.05 compared with a hypothetical value of 1 (normalized control). n = 3 replicates in each group. #P < 0.05 compared with 2.5 min shear exposure. ^P < 0.05 compared with 10 min shear exposure.
CtsB enhances human neutrophil pseudopod activity
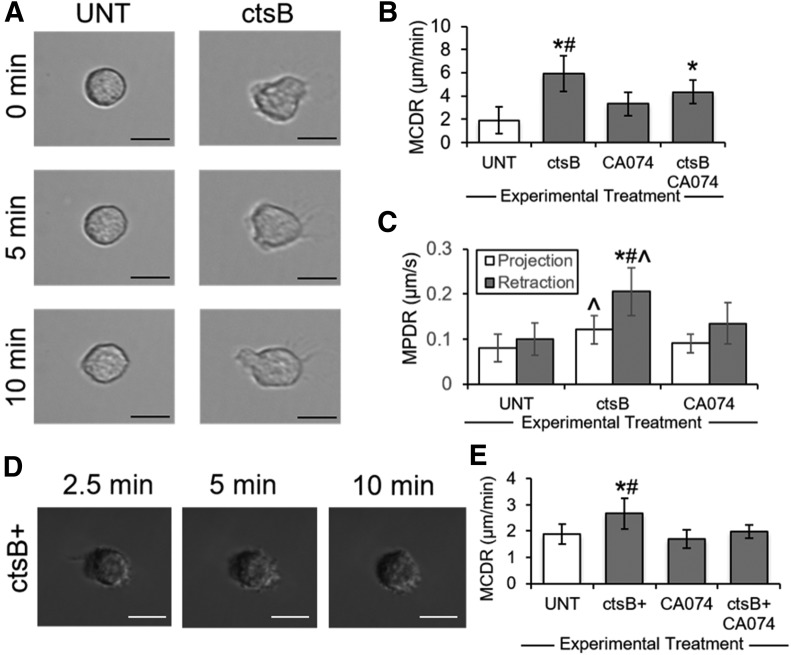
Compared with UNT human neutrophils, those treated with exogenous ctsB (Fig. 3A) displayed significantly elevated cell centroid displacement rates, consistent with increased pseudopod activity (Fig. 3B). The observed ctsB-mediated increases in pseudopod activity were attenuated by CA074. Notably, ctsB stimulated a greater increase in pseudopod retraction rates (by 2- to 2.5-fold) than in pseudopod extension rates (by approximately 1.5-fold) (Fig. 3C). CA074 attenuated the effects of ctsB in pseudopod extension and retraction.
Figure 3. Influence of ctsB on neutrophil pseudopod activity.
(A) Representative images of migrating neutrophils adhered to glass slides and maintained under no-flow conditions. Neutrophils were left UNT or treated with exogenous ctsB (0.5 μ/ml). (B) Neutrophil migration was quantified as the mean centroid displacement rate (MCDR) during a 15-s interval and plotted as means ± sd, based on cell tracings manually performed using ImageJ software. Neutrophils were treated with or without exogenous ctsB (0.5 μ/ml) and ctsB-specific inhibitor CA074 (5 μM). n = 3 replicates in each group. *P < 0.05 compared with control. #P < 0.05 compared with CA074. (C) Neutrophil pseudopod activity during migration was quantified as the mean pseudopod displacement rate (MPDR), based on manual tracings of projecting (white bars) or retracting (black bars) pseudopods using ImageJ software. Neutrophils were treated with or without exogenous ctsB (0.5 μ/ml) and ctsB-specific inhibitor CA074 (5 μM). n = 3 replicates in each group. *P < 0.05 compared with control (projection). ^P < 0.05 compared with control (retraction). #P < 0.05 compared with CA074 (projection and retraction). (D) Representative images of migrating dHL60s that were transiently transfected through electroporation with a ctsB-GFP expression vector (ctsB+) and maintained under no-flow conditions. (E) DHL60 migration was quantified as the MCDR during a 15-s interval and plotted as means ± sd, based on cell tracings manually performed using ImageJ software. Control populations were dHL60 cells transfected with an EV. DHL60 transfectants (ctsB+ or EV) were treated with or without ctsB-specific inhibitor CA074 (5 μM). n = 3 replicates in each group. *P < 0.05 compared with control. #P < 0.05 compared with CA074. Scale bars are 10 µm.
To confirm the potential regulatory influence of ctsB on neutrophil pseudopod activity, dHL60 neutrophil-like cells were transiently transfected with ctsB-GFP expression plasmids (Fig. 3D). Cells transfected with EVs exhibited baseline pseudopod activity levels, whereas ctsB-GFP transfectants (ctsB+) exhibited pseudopod activity levels that were significantly greater (Fig. 3E). CA074 mitigated the difference in pseudopod activity between these two populations.
FSS-induced human neutrophil detachment from biologic substrates involves ctsB release
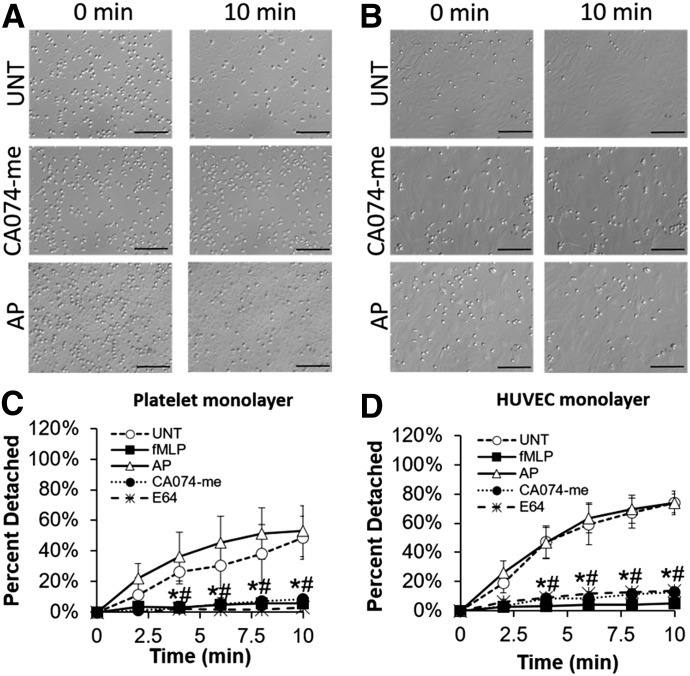
UNT human neutrophils preadhered to platelet and HUVEC monolayers exhibited time-dependent cell detachment during a 10-min period of FSS exposure (Fig. 4A and B). When human neutrophils were exposed to FSS in the presence of fMLP (above threshold levels known to block neutrophil shear responses [1, 17]), no significant detachment from platelet monolayers was observed (Fig. 4C). Blocking ctsB activity with either E64 or CA074-me also caused human neutrophils to remain largely adherent to platelet monolayers under FSS. In contrast, neutrophils pretreated with the irreversible serine protease inhibitor AP detached from platelet layers under FSS exposure in a fashion similar to that of UNT cells. Similarly, although UNT human neutrophils adhered to HUVEC monolayers exhibited time-dependent cell detachment upon acute exposure to FSS for 10 min, both fMLP (above threshold levels) and ctsB inhibitors, E64 or CA074-me, blocked that detachment response to FSS (Fig. 4D). In contrast, pretreatment of cells with AP had no effect on FSS-induced neutrophil detachment.
Figure 4. Involvement of ctsB in FSS-induced neutrophil detachment.
(A and B) Representative images depicting shear-induced neutrophil detachment from platelet (A) or HUVEC (B) monolayers in response to 5 dyn/cm2 shear stress exposure during a 10-min period. Neutrophils were left UNT or treated with ctsB-specific inhibitor CA074-me (50 µM) or serine inhibitor AP (5 μM). (C and D) The number of neutrophils remaining adherent to platelet (C) or HUVEC (D) monolayers after 10 min of shear exposure was normalized to the initial number of adherent neutrophils before shear exposure and plotted as means ± sd, based on counts manually performed using ImageJ software. Neutrophils were left UNT or pretreated for 5 min with inflammatory agonist fMLP (1 μM), serine inhibitor AP (5 μM), ctsB-specific inhibitor CA074-me (50 μM), or cysteine inhibitor E64 (30 μM). n = 4 replicates in each group. *P < 0.05 compared with control; #P < 0.05 compared with AP. Scale bars are 100 µm.
CtsB−/− neutrophils lack an FSS-mediated CD18 cleavage response
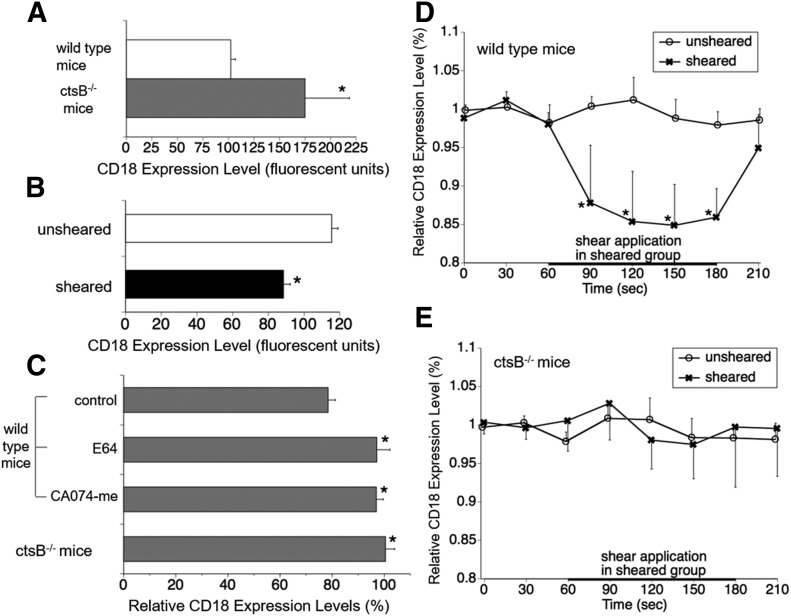
CD18 Ab binding on circulating (nonadherent) neutrophils, which were fixed immediately after blood collection from ctsB−/− mice, was significantly greater compared with that seen in neutrophils from WT mice (Fig. 5A). Notably, sheared neutrophils from WT mice exhibited significantly less binding affinities for fluorescent Abs targeting intact CD18 integrins compared with unsheared cells (Fig. 5B). In contrast, cysteine protease inhibitor E64 and ctsB-selective inhibitor CA074-me suppressed CD18 cleavage by WT neutrophils under FSS (Fig. 5C). Furthermore, FSS application had no effect on CD18 surface levels for ctsB−/− neutrophils.
Figure 5. Link between ctsB deficiency in mice and CD18 expression on leukocytes.
(A) CD18-associated FITC intensity on circulating mouse neutrophils in vivo measured with flow cytometry. The cells were fixed with 0.4% paraformaldehyde immediately after blood collection from the animals with cardiac puncture. n = 4 animals in each group. *P < 0.05 compared with WT mice. (B) Effect of fluid shear application (5 dyn/cm2) in the cone–plate device on CD18 expression of circulating neutrophils of WT mice measured with flow cytometry. n = 4 animals in each group. *P < 0.05 compared with unsheared group (ANOVA, Fisher’s protected least-significant difference). (C) CD18 label density ratio between sheared (5 dyn/cm2) and unsheared mouse neutrophils measured with flow cytometry. Neutrophils were treated with or without cysteine inhibitor E64 (100 μM) and ctsB-specific inhibitor CA074-me (10 µM). n = 4 animals in each group. *P < 0.05 compared with control in WT mice. (D and E) Time course of total membrane CD18 expression on neutrophils of WT mice (D) and ctsB−/− mice (E) adhering to a glass surface as measured by laser confocal microscopy. The intensity values are normalized by the average values at 0 s and 30 s in each group. In the sheared-cell group, fluid shear (∼1.5 dyn/cm2) was applied from 60–180 s. n = 8 cells in each group. *P < 0.05 compared with values in unsheared cells at each time point.
For migrating cells, the CD18 surface density along the perimeter of individual WT neutrophils, although almost constant under no-flow conditions, was significantly reduced under FSS (Fig. 5D). CD18-associated FITC intensity on ctsB−/− neutrophils, however, was not influenced by FSS exposure (Fig. 5E).
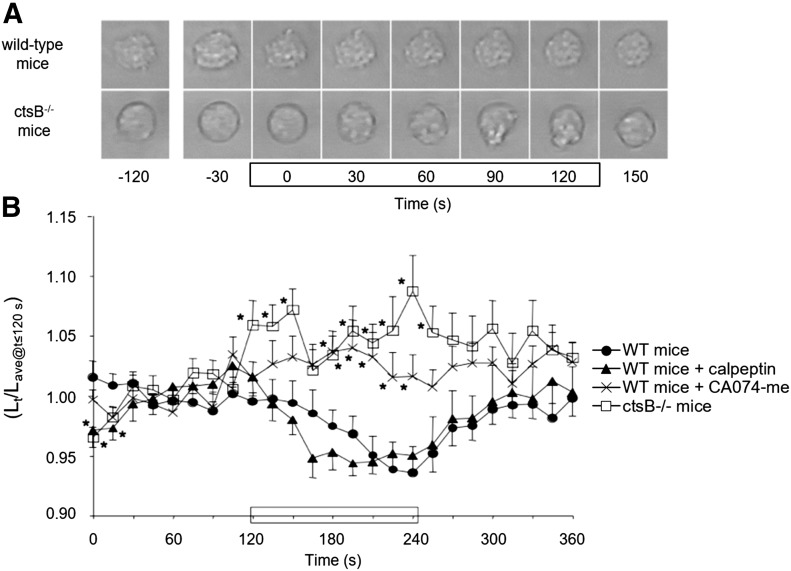
CtsB−/− neutrophils lack pseudopod retraction in response to FSS
Leukocytes from WT mice retracted pseudopods when exposed to shear (Fig. 6A and B). That shear-induced pseudopod retraction was also observed for WT leukocytes treated with calpeptin (inhibitor of cathepsin L, cathepsin K, and calpain-1 but not ctsB). However, WT leukocytes exposed to shear in the presence of CA074-me did not retract pseudopods. In addition, neutrophils from ctsB−/− mice also lacked pseudopod retraction in response to shear, confirming the requirement for ctsB in FSS regulation of pseudopod activity.
Figure 6. Link between ctsB deficiency and an impaired pseudopod retraction response to shear.
(A) Representative images of cells from either WT or ctsB−/− mice. Cells were exposed to FSS during the indicated (rectangle) 2-min period. (B) Time course of cell retraction by WT mice neutrophils migrating in the absence (solid circles) and presence of either calpeptin (solid triangles; a cathepsin G-specific inhibitor) or CA074-me (Xs; a ctsB-specific inhibitor), as well as by cells from ctsB−/− mice (open squares). Pseudopod retraction is represented by the instantaneous major length of the cell (Lt) normalized with the average major axis length (Lave) recorded for 0–120 s in each group. In each group, fluid shear (∼1.5 dyn/cm2) was applied for 120–240 s (black rectangle on x axis). n = ≥12 cells from either 4 WT mice or 7 ctsB−/− mice. *P < 0.05 compared with UNT cells at each time point using Student’s t test.
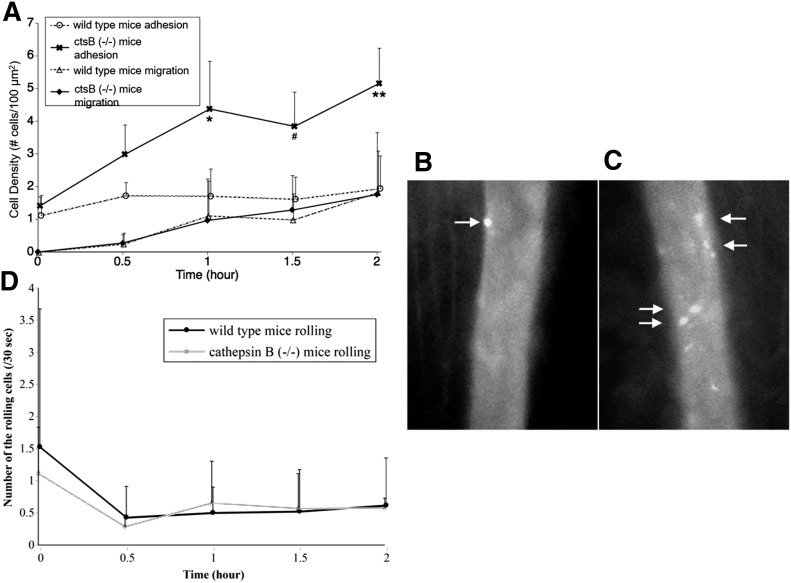
Neutrophil adhesion on inflamed, postcapillary venules is enhanced in ctsB−/− mice
Under physiologic flows, the number of adhesive leukocytes on mildly inflamed, postcapillary venules in skeletal muscle (i.e., stimulated with 1 µM PAF) was significantly greater in ctsB−/− mice relative to WT controls (Fig. 7A–C). The number of neutrophils observed to be rolling on microvasculature per 30 s in either the WT or ctsB−/− mice was similar (Fig. 7D). Furthermore, the number of leukocytes migrating across the endothelium into the adjacent interstitial space was not observed to be enhanced by deletion of ctsB in the ctsB−/− mice. Thus, the proportion of adhesive leukocytes actually migrating across the endothelium into the tissue was less in ctsB−/− mice.
Figure 7. Leukocyte behavior in the in vivo microvasculature of ctsB-deficient mice.
(A) The number of carboxyfluorescein succinimidyl ester-labeled leukocytes (number of cells per millimeter of vessel length) adhered to the endothelium of postcapillary venules and migrating into the tissues in the cremaster-muscle microcirculation of WT mice and ctsB−/− mice after superfusion, including 10 nM PAF. There is no significant difference in flow velocity between the 2 groups. *P, #P, and **P < 0.05 vs. unsheared group at each time point. n = 4 animals in each group. (B and C) Micrographs of labeled leukocytes in inflamed postcapillary venules of cremaster muscle in a WT mouse (B) and a ctsB−/− mouse (C) under normal physiologic levels of flow rates. The muscle is superfused, including 10 nM PAF for 1 h. In the WT mouse, a few adherent leukocytes are found (arrows). In the ctsB−/− mouse, more fluorescent-labeled leukocytes adhering to vascular wall are consistently present (arrows). (D) Rolling leukocyte behavior in vivo in the inflamed skeletal muscle microcirculation of WT mice and ctsB−/− mice. The number of leukocytes rolling across a predetermined vessel cross-section was counted during a 30-s period.
DISCUSSION
The results of the present study provide new insight regarding the potential role of hemodynamic FSS in the physiologic regulation of the innate immunity. Specifically, we report evidence in support of ctsB being a part of a shear stress–sensitive control mechanism, which is antiadhesive for neutrophils on endothelial surfaces. The time course of FSS-induced ctsB release agrees with that reported for FSS-induced CD11b and CD18 cleavage [8]. Furthermore, our observations, past [4] and present, support the release of ctsB in a soluble form into the neutral pH of the extracellular milieu. Although many lysosomal proteases require an acidic environment for optimal function [24], various cells, including tumor cells [25–27] and lymphocytes [28], secrete ctsB capable of remaining stable in the extracellular matrix for extended periods.
Notably, we observed substantial release of ctsB during the in vitro experiments, a result which indicates a large turnover of ctsB by neutrophils exposed to constant shear in vivo. However, additional studies are needed to support that possibility because there may be differences in the environmental factors between in vitro and in vivo neutrophils, which contributed to the altered ctsB release. The shear stress of a cone–plate viscometer, for example, exposes cells to a constant laminar shear stress, whereas in vivo cells are exposed to nonuniform shear stress [29]. Additionally, there may be in vivo regulatory mechanisms that influence FSS-induced ctsB release. For instance, neutrophil cell-surface components, normally present in vivo, may regulate the amount of ctsB released during flow exposure. The endothelial cell glycocalyx, for example, is present in vivo but missing from endothelial cells in vitro [30].
Alternatively, the synthesis of ctsB may be different within an in vivo and in vitro environment. During our studies, neutrophils were maintained in a no-flow environment before in vitro experimentation. Once exposed to FSS, the rapid release of ctsB may trigger neutrophils to increase their own ctsB production. The lifetime of neutrophils in the circulation (<24 h [31]) may somehow be linked to the point at which those cells exhaust their supply of intracellular ctsB, which causes them to be more adhesive and filtered out of the circulation.
In the present study, we not only confirmed that ctsB cleaves the extracellular domains of CD18 but also provided evidence that it targets CD11b subunits as well. Although FSS exposure reduces CD18 surface levels by only 20–30%, it decreases intact CD11b on neutrophils by up to 50% [8]. Such a proportional relationship is consistent with Mac1 cleavage 1) if CD18 cleavage occurs after CD11b proteolysis, and 2) if neutrophils express relatively similar levels of LFA1 and Mac1. Both of those appear to be the case, based on the results of this and prior studies [32]. Furthermore, FSS has no effect on CD11a subunits that make up LFA1 [8]. Thus, CD11a, which is not cleaved by ctsB, may protect its partner CD18 subunit from ctsB proteolysis, ensuring LFA1 remains intact on sheared neutrophils. Finally, our data also verify that CD18 cleavage is a sufficient measure of FSS-induced Mac1 cleavage.
The mechanism or mechanisms for Mac1 selectivity in the FSS control of CD18 expression by neutrophils remain to be examined but likely are related to structural differences between LFA1 and Mac1. Moreover, a possible implication of that selectivity may involve the specific roles of LFA1 and Mac1 in neutrophil recruitment to microvascular walls during acute inflammation. LFA1 may remain intact, even under FSS, to enable neutrophils to interact loosely with the microvascular wall as part of their immune-surveillance function. In contrast, Mac1-related binding may represent a key checkpoint against commitment into the acute inflammatory cascade that is subject to FSS control to prevent neutrophils from adhering to vessels under nonpathogenic conditions, a situation that may adversely alter tissue perfusion by either a rheological effect on peripheral resistance [33, 34] or a pathobiological action on endothelial vasomotor control [35].
Mac1 deficiency causes neutrophils to be less adhesive and to migrate at greatly diminished velocities [36]. Because of that, we expected exogenous ctsB to reduce neutrophil adhesion strength and impair cell motility, but that was not the case. In fact, ctsB increased neutrophil motility under static (i.e., no-flow) conditions, in line with our previous observations [4]. Although that may seem counterintuitive, we attribute those observations to the ability of ctsB to cleave the extracellular domains of Mac1 integrins during the uropod retraction stage in cell migration. In doing so, ctsB release may have a cooperative or additive effect on cell migration rates that are also controlled by other cellular processes involved in locomotion, such as calpain-mediated cleavage of intracellular CD18 integrins [37] and Mac1 unbinding kinetics to substrate-bound ligands [6, 13]. That possibility is consistent with the observed increases in pseudopod retraction rates of neutrophils in the presence of ctsB under static conditions. Moreover, ctsB inhibition had no effect on baseline pseudopod retraction and extension rates, further supporting an additive effect of Mac1 cleavage on cell migration rates.
It is conceivable that the small increases in pseudopod extension rates for ctsB-stimulated neutrophils were due to a secondary effect of the increased retraction rates. Enhanced pseudopod retraction because of ctsB likely involves accelerated actin depolymerization, which would increase cytosolic pools of G-actin monomers available for new pseudopod formation and thus drive an increase in projection rates; however, that possibility remains to be confirmed. Furthermore, it appears that although ctsB may be involved in FSS-induced pseudopod retraction, there is likely another component of that response charged with preventing new projections, such as by preventing F-actin polymerization [38].
FSS-related ctsB release also regulates neutrophil adhesion. Under physiologic conditions, many factors restrict neutrophil adhesion. Some are endothelial cell–derived. For example, FSS in the physiologic range is anti-inflammatory for endothelial cells by stimulating nitric oxide release or limiting expression of adhesion molecules that bind Mac1 [39, 40]; however, the endothelial cells are not the only vascular cells that regulate neutrophil adhesion. The neutrophils themselves regulate their own adhesion to endothelial cells. Mac1 cleavage promotes neutrophil detachment during chemotaxis in interstitial tissues [41]. Notably, our prior results implicated FSS-related ctsB release and subsequent CD18 cleavage to not only prevent neutrophil binding to other cells (e.g., platelets) when suspended in a flow field [8] but also reverse it during pseudopod retraction when membrane-substrate attachments must be disengaged [6].
The principal evidence for neutrophils requiring a flow environment to remain nonadherent is that fluid stasis promotes projection of pseudopods and formation of attachments with various substrates involving CD18 integrins [2, 42, 43]. Once exposed to FSS, however, neutrophils retract pseudopods in a CD18-dependent fashion [44], even in the presence of low doses of inflammatory agonists, such as <1 µM fMLP or <10 µM PAF [1]. Although neutrophils are continuously exposed to FSS in vivo, the FSS on freely suspended leukocytes is low in comparison with leukocytes attached to the microvascular wall [45]. The magnitude of FSS increases sharply as neutrophils make contact with, and initially arrest on, the endothelium [45]. That increase likely serves to enhance release of ctsB to prevent pseudopod retraction and promote neutrophil detachment back into the circulation. Based on our results, ctsB release from cytosolic granules represents a key aspect of the ability of FSS to restrict neutrophil adhesion.
Interestingly, exposure to FSS enhances neutrophil granular motion [2], which may increase the likelihood of degranulation and lysosomal protease release. Enhanced granular motion is consistent with the FSS-induced release of ctsB observed at the shear magnitudes used in our studies. Moreover, the observed degranulation is not likely due to trauma, as reported for neutrophils exposed to substantially high-shear magnitudes [46]. Additional studies are needed to link the release of ctsB with signaling pathways promoting FSS-induced degranulation at the low-shear magnitudes tested in the present study. Rac1 and Rac2, for example, may have a role in the ctsB release pathway. Inhibition of Rac1 and Rac2 because of FSS exposure has been linked with actin depolymerization, a feature that may enhance the release of cytosolic granules containing ctsB [47]. Cyclic GMP has also been implicated in pseudopod retraction under shear, further suggesting a link to actin depolymerization and potential cytosolic granule release [1]. Although these signaling molecules may be involved in the ctsB-release pathway, concentrations of Ca2+, also known to have a role in actin dynamics, reportedly remain unchanged under FSS exposure, indicating Ca2+ signaling is likely not involved [48].
Moazzam et al. [2] used intravital microscopy to show that leukocytes migrating on microvascular endothelium in the rat microcirculation during a transient period of stasis detach from venular walls and are carried away in the blood stream when the flow is reconstituted. Most of the responsive leukocytes were found to be neutrophils, with occasional monocytes [2]. The results from this study [2] formed the basis for the ex vivo, flow-based detachment assay used to quantify and demonstrate the ability of FSS to reverse neutrophil adhesion to endothelial cells. The outcomes of this analysis confirmed that, in the absence of agonists, FSS is antiadhesive for neutrophils on endothelial surfaces by promoting ctsB release, which likely reduces the Mac1-dependent adhesion of neutrophils. The results from our flow assays also suggest FSS serves to restrict/prevent neutrophil adhesion under physiologic flow conditions by minimizing their adhesive capacity. However, experimental evidence to support the prior statement is not available because of logistical limitations in the ability to distinguish adherent from nonadherent neutrophils located on endothelial monolayers immediately after flow cessation. Such a limitation may only be mitigated by imposing a finite FSS on the cells to determine the degree of leukocyte adhesion.
The role of ctsB in the antiadhesive effects of FSS on leukocytes determined from our human blood studies was substantiated by ex vivo/in vivo analyses using ctsB−/− mice. Intact ctsB and CD18 cleavage responses to FSS appear to be responsible for the low, baseline surface expression of CD18 on circulating leukocytes. Upon cessation of FSS exposure, new CD18 integrins appeared to be recruited, likely from intracellular compartments, leading to an apparent rapid up-regulation in CD18 surface expression. The rapidity of that up-regulation is on the same order as that for cytokine stimulation, such as fMLP, which reportedly up-regulates the surface expression of Mac1 within minutes [49]. We also confirmed here that FSS-induced ctsB release is important in reversing CD18-related neutrophil–substrate interactions not only during migration involving cyclical extension and retraction of pseudopods but also in preventing neutrophil adhesion in mildly inflamed microvessels.
Compared with WT mice, ctsB−/− mice had neutrophils (both adherent and passively circulating) with an elevated surface expression of CD18. That feature conceivably facilitated the firm adhesion of neutrophils to endothelium but in contrast would not affect their rolling before adhesion. Indeed, our ctsB−/− mice did not exhibit more rolling or migrating cells in the microvessels, despite the increase in adhesive cells. That observation indicates that enhanced cell adhesion is linked to an impaired, ctsB-related FSS response. It also suggests that, under flow conditions, neutrophil adhesion would require an additional trigger (i.e., stimulated endothelium) because the elevated expression of CD18 did not significantly affect neutrophil rolling in WT and ctsB−/− mice.
Moreover, although the current mice studies used Abs targeted against the CD18 subunits, it is likely, based on our human data, that the functional CD18 heterodimers cleaved off the neutrophils from the WT, but not ctsB−/−, mice were from Mac1 integrins. In support of that hypothesis, we have unpublished data confirming that neutrophils from mice (including low-density lipoprotein receptor–deficient animals) selectively cleave Mac1, and not LFA1, integrins in response to FSS exposure.
In summary, we provide new evidence that FSS-induced release of ctsB is antiadhesive for neutrophils on endothelial surfaces by selectively regulating Mac1 expression. FSS exposure appears to serve as a control function for lysosomal protease release. The evidence suggests that ctsB is released by neutrophils exposed to a flow field to restrict Mac1 surface expression to prevent new pseudopod formation, minimize neutrophil adhesion to endothelium, and ensure their ability to circulate as rounded, nonadhesive, and inactivated cells during noninflamed conditions. Failure of this control mechanism may promote neutrophil adhesion in vivo, contributing to the development of microvascular dysfunction. This possibility is supported by studies demonstrating that impaired neutrophil responses to FSS are associated with elevated leukocyte activation in the microcirculation, pathologic elevations in hemodynamic resistance, and tissue blood flow dysregulation [13, 14, 50]. In that regard, future work exploring factors that impair FSS mechanotransduction by neutrophils may potentially uncover new pathogenic mechanisms associated with microvascular dysfunction and cardiovascular disease.
AUTHORSHIP
All authors were involved in various aspects of the conception and design of the research study. M.L.A. collected and analyzed data related to Mac1 cleavage, pseudopod activity, and flow-induced detachment assays. S.F. collected and analyzed data related to ctsB−/− mice studies. M.L.A., S.F., G.W.S.S., and H.Y.S. were involved in preparing the manuscript including various aspects of study design, data analysis, data interpretation, manuscript drafting/revising and final approval of the document.
ACKNOWLEDGMENTS
This work was supported in part by the National Heart, Lung, and Blood Institute (Grant HL-10881 to G.W.S.S.), the U.S. National Institutes of Health (Grants HL-083740-01 and HL-43024 to H.Y.S. and G.W.S.S.), a National Science Foundation Integrative Graduate Education and Research Traineeship program (Grant 0653710 to M.L.A.), a National Science Foundation Kentucky Experimental Program to Stimulate Competitive Research Bioengineering Initiative (Grant 0814194 to H.Y.S.), a pilot award of an Institutional Development Award program from the National Institute of General Medical Sciences of the U.S. National Institutes of Health (Grant P20GM103527 to H.Y.S.), and a University of Kentucky Max Steckler Fellowship (to M.L.A.). We thank Dr. Christoph Peters, University of Freiburg (Freiburg, Germany), and Dr. Gregory J. Gores, Mayo Medical School, Clinic, and Foundation (Rochester, MN, USA) for kindly providing the ctsB−/− mice. We also thank Clint Branham and Jacob Schlarman, University of Kentucky, for help with flow studies.
Glossary
- AP
aprotinin
- ctsB
cathepsin B
- ctsB−/−
cathepsin B deficient
- dHL60
differentiated neutrophil-like HL60
- dyn
dyne
- EV
empty vector
- fMLP
formyl-methionyl-leucyl-phenylalanine
- FSS
fluid shear stress
- LFA1
lymphocyte function-associated Ag-1
- Mac1
macrophage-1 Ag
- PAF
platelet-activating factor
- UNT
untreated
- WT
wild type
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Fukuda S., Yasu T., Predescu D. N., Schmid-Schönbein G. W. (2000) Mechanisms for regulation of fluid shear stress response in circulating leukocytes. Circ. Res. 86, E13–E18. [DOI] [PubMed] [Google Scholar]
- 2.Moazzam F., DeLano F. A., Zweifach B. W., Schmid-Schönbein G. W. (1997) The leukocyte response to fluid stress. Proc. Natl. Acad. Sci. USA 94, 5338–5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin H. Y., Zhang X., Makino A., Schmid-Schönbein G. W.. 2011. Mechanobiological evidence for the control of neutrophil activity by fluid shear stress. Mechanobiology Handbook (Shin H. Y., Zhang X., Schmid-Schönbein A. M. G. W., ). CRC Press, Boca Raton, FL, 139–175. [Google Scholar]
- 4.Shin H. Y., Simon S. I., Schmid-Schönbein G. W. (2008) Fluid shear-induced activation and cleavage of CD18 during pseudopod retraction by human neutrophils. J. Cell. Physiol. 214, 528–536. [DOI] [PubMed] [Google Scholar]
- 5.Mazzone A., Ricevuti G. (1995) Leukocyte CD11/CD18 integrins: biological and clinical relevance. Haematologica 80, 161–175. [PubMed] [Google Scholar]
- 6.Fukuda S., Schmid-Schönbein G. W. (2003) Regulation of CD18 expression on neutrophils in response to fluid shear stress. Proc. Natl. Acad. Sci. USA 100, 13152–13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Root R. K. (1990) Leukocyte adhesion proteins: their role in neutrophil function. Trans. Am. Clin. Climatol. Assoc. 101, 207–224; discussion 224–206. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X., Zhan D., Shin H. Y. (2013) Integrin subtype-dependent CD18 cleavage under shear and its influence on leukocyte-platelet binding. J. Leukoc. Biol. 93, 251–258. [DOI] [PubMed] [Google Scholar]
- 9.Hentzen E. R., Neelamegham S., Kansas G. S., Benanti J. A., McIntire L. V., Smith C. W., Simon S. I. (2000) Sequential binding of CD11a/CD18 and CD11b/CD18 defines neutrophil capture and stable adhesion to intercellular adhesion molecule-1. Blood 95, 911–920. [PubMed] [Google Scholar]
- 10.Shive M. S., Salloum M. L., Anderson J. M. (2000) Shear stress-induced apoptosis of adherent neutrophils: a mechanism for persistence of cardiovascular device infections. Proc. Natl. Acad. Sci. USA 97, 6710–6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mine S., Fujisaki T., Suematsu M., Tanaka Y. (2001) Activated platelets and endothelial cell interaction with neutrophils under flow conditions. Intern. Med. 40, 1085–1092. [DOI] [PubMed] [Google Scholar]
- 12.Konstantopoulos K., Neelamegham S., Burns A. R., Hentzen E., Kansas G. S., Snapp K. R., Berg E. L., Hellums J. D., Smith C. W., McIntire L. V., Simon S. I. (1998) Venous levels of shear support neutrophil-platelet adhesion and neutrophil aggregation in blood via P-selectin and beta2-integrin. Circulation 98, 873–882. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X., Cheng R., Rowe D., Sethu P., Daugherty A., Yu G., Shin H. Y. (2014) Shear-sensitive regulation of neutrophil flow behavior and its potential impact on microvascular blood flow dysregulation in hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 34, 587–593. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda S., Yasu T., Kobayashi N., Ikeda N., Schmid-Schönbein G. W. (2004) Contribution of fluid shear response in leukocytes to hemodynamic resistance in the spontaneously hypertensive rat. Circ. Res. 95, 100–108. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X., Hurng J., Rateri D. L., Daugherty A., Schmid-Schönbein G. W., Shin H. Y. (2011) Membrane cholesterol modulates the fluid shear stress response of polymorphonuclear leukocytes via its effects on membrane fluidity. Am. J. Physiol. Cell Physiol. 301, C451–C460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhurat R., Sukesh M. (2014) Principles and methods of preparation of platelet-rich plasma: a review and author’s perspective. J. Cutan. Aesthet. Surg. 7, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akenhead M. L., Zhang X., Shin H. Y. (2014) Characterization of the shear stress regulation of CD18 surface expression by HL60-derived neutrophil-like cells. Biomech. Model. Mechanobiol. 13, 861–870. [DOI] [PubMed] [Google Scholar]
- 18.Meijering E., Dzyubachyk O., Smal I. (2012) Methods for cell and particle tracking. Methods Enzymol. 504, 183–200. [DOI] [PubMed] [Google Scholar]
- 19.Sheikh S., Nash G. B. (1996) Continuous activation and deactivation of integrin CD11b/CD18 during de novo expression enables rolling neutrophils to immobilize on platelets. Blood 87, 5040–5050. [PubMed] [Google Scholar]
- 20.Diacovo T. G., Roth S. J., Buccola J. M., Bainton D. F., Springer T. A. (1996) Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood 88, 146–157. [PubMed] [Google Scholar]
- 21.Ganguly A., Zhang H., Sharma R., Parsons S., Patel K. D. (2012) Isolation of human umbilical vein endothelial cells and their use in the study of neutrophil transmigration under flow conditions. J. Vis. Exp. 66, e4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuda S., Schmid-Schönbein G. W. (2002) Centrifugation attenuates the fluid shear response of circulating leukocytes. J. Leukoc. Biol. 72, 133–139. [PubMed] [Google Scholar]
- 23.Yasu T., Schmid-Schönbein G. W., Cotter B., DeMaria A. N. (1999) Flow dynamics of QW7437, a new dodecafluoropentane ultrasound contrast agent, in the microcirculation: microvascular mechanisms for persistent tissue echo enhancement. J. Am. Coll. Cardiol. 34, 578–586. [DOI] [PubMed] [Google Scholar]
- 24.Bohley P., Seglen P. O. (1992) Proteases and proteolysis in the lysosome. Experientia 48, 151–157. [DOI] [PubMed] [Google Scholar]
- 25.Frosch B. A., Berquin I., Emmert-Buck M. R., Moin K., Sloane B. F. (1999) Molecular regulation, membrane association and secretion of tumor cathepsin B. APMIS 107, 28–37. [DOI] [PubMed] [Google Scholar]
- 26.Podgorski I., Sloane B. F. (2003) Cathepsin B and its role(s) in cancer progression. Biochem. Soc. Symp. (70):263–276. [DOI] [PubMed] [Google Scholar]
- 27.Roshy S., Sloane B. F., Moin K. (2003) Pericellular cathepsin B and malignant progression. Cancer Metastasis Rev. 22, 271–286. [DOI] [PubMed] [Google Scholar]
- 28.Balaji K. N., Schaschke N., Machleidt W., Catalfamo M., Henkart P. A. (2002) Surface cathepsin B protects cytotoxic lymphocytes from self-destruction after degranulation. J. Exp. Med. 196, 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rezvan A., Ni C. W., Alberts-Grill N., Jo H. (2011) Animal, in vitro, and ex vivo models of flow-dependent atherosclerosis: role of oxidative stress. Antioxid. Redox Signal. 15, 1433–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potter D. R., Damiano E. R. (2008) The hydrodynamically relevant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ. Res. 102, 770–776. [DOI] [PubMed] [Google Scholar]
- 31.McCracken J. M., Allen L. A. (2014) Regulation of human neutrophil apoptosis and lifespan in health and disease. J. Cell Death 7, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diez-Fraile A., Duchateau L., Meyer E., Burvenich C. (2003) Expression of β2-integrin on monocytes and blood polymorphonuclear leukocytes in the periparturient period in dairy cows. Can. J. Vet. Res. 67, 235–238. [PMC free article] [PubMed] [Google Scholar]
- 33.Harris A. G., Skalak T. C. (1993) Effects of leukocyte activation on capillary hemodynamics in skeletal muscle. Am. J. Physiol. 264, H909–H916. [DOI] [PubMed] [Google Scholar]
- 34.Helmke B. P., Sugihara-Seki M., Skalak R., Schmid-Schönbein G. W. (1998) A mechanism for erythrocyte-mediated elevation of apparent viscosity by leukocytes in vivo without adhesion to the endothelium. Biorheology 35, 437–448. [DOI] [PubMed] [Google Scholar]
- 35.Singer G., Granger D. N. (2007) Inflammatory responses underlying the microvascular dysfunction associated with obesity and insulin resistance. Microcirculation 14, 375–387. [DOI] [PubMed] [Google Scholar]
- 36.Sreeramkumar V., Adrover J. M., Ballesteros I., Cuartero M. I., Rossaint J., Bilbao I., Nácher M., Pitaval C., Radovanovic I., Fukui Y., McEver R. P., Filippi M. D., Lizasoain I., Ruiz-Cabello J., Zarbock A., Moro M. A., Hidalgo A. (2014) Neutrophils scan for activated platelets to initiate inflammation. Science 346, 1234–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaff M., Du X., Ginsberg M. H. (1999) Calpain cleavage of integrin β cytoplasmic domains. FEBS Lett. 460, 17–22. [DOI] [PubMed] [Google Scholar]
- 38.Makino A., Prossnitz E. R., Bünemann M., Wang J. M., Yao W., Schmid-Schönbein G. W. (2006) G protein-coupled receptors serve as mechanosensors for fluid shear stress in neutrophils. Am. J. Physiol. Cell Physiol. 290, C1633–C1639. [DOI] [PubMed] [Google Scholar]
- 39.Berk B. C., Min W., Yan C., Surapisitchat J., Liu Y., Hoefen R. (2002) Atheroprotective mechanisms activated by fluid shear stress in endothelial cells. Drug News Perspect. 15, 133–139. [DOI] [PubMed] [Google Scholar]
- 40.Traub O., Berk B. C. (1998) Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler. Thromb. Vasc. Biol. 18, 677–685. [DOI] [PubMed] [Google Scholar]
- 41.Zen K., Guo Y. L., Li L. M., Bian Z., Zhang C. Y., Liu Y. (2011) Cleavage of the CD11b extracellular domain by the leukocyte serprocidins is critical for neutrophil detachment during chemotaxis. Blood 117, 4885–4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coughlin M. F., Schmid-Schönbein G. W. (2004) Pseudopod projection and cell spreading of passive leukocytes in response to fluid shear stress. Biophys. J. 87, 2035–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhelev D. V., Alteraifi A. M., Chodniewicz D. (2004) Controlled pseudopod extension of human neutrophils stimulated with different chemoattractants. Biophys. J. 87, 688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marschel P., Schmid-Schönbein G. W. (2002) Control of fluid shear response in circulating leukocytes by integrins. Ann. Biomed. Eng. 30, 333–343. [DOI] [PubMed] [Google Scholar]
- 45.Sugihara-Seki M., Schmid-Schönbein G. W. (2003) The fluid shear stress distribution on the membrane of leukocytes in the microcirculation. J. Biomech. Eng. 125, 628–638. [DOI] [PubMed] [Google Scholar]
- 46.Dewitz T. S., Hung T. C., Martin R. R., McIntire L. V. (1977) Mechanical trauma in leukocytes. J. Lab. Clin. Med. 90, 728–736. [PubMed] [Google Scholar]
- 47.Makino A., Glogauer M., Bokoch G. M., Chien S., Schmid-Schönbein G. W. (2005) Control of neutrophil pseudopods by fluid shear: role of Rho family GTPases. Am. J. Physiol. Cell Physiol. 288, C863–C871. [DOI] [PubMed] [Google Scholar]
- 48.Fukuda S., Mitsuoka H., Schmid-Schönbein G. W. (2004) Leukocyte fluid shear response in the presence of glucocorticoid. J. Leukoc. Biol. 75, 664–670. [DOI] [PubMed] [Google Scholar]
- 49.Anderson D. C., Miller L. J., Schmalstieg F. C., Rothlein R., Springer T. A. (1986) Contributions of the Mac-1 glycoprotein family to adherence-dependent granulocyte functions: structure-function assessments employing subunit-specific monoclonal antibodies. J. Immunol. 137, 15–27. [PubMed] [Google Scholar]
- 50.Chen A. Y., DeLano F. A., Valdez S. R., Ha J. N., Shin H. Y., Schmid-Schönbein G. W. (2010) Receptor cleavage reduces the fluid shear response in neutrophils of the spontaneously hypertensive rat. Am. J. Physiol. Cell Physiol. 299, C1441–C1449. [DOI] [PMC free article] [PubMed] [Google Scholar]