Abstract
Objectives:
To evaluate the cost utility of cochlear implantation (CI) for severe to profound sensorineural hearing loss (SNHL) among children from rural settings in P.R. China (China).
Research Design:
A cost-utility analysis (CUA) was undertaken using data generated from a single-center substudy of the CochlearTM Pediatric Implanted Recipient Observational Study (Cochlear P-IROS). The data were projected over a 20-year time horizon using a decision tree model.
Setting:
The Chinese healthcare payer and patient perspectives were adopted.
Intervention:
Unilateral CI of children with a severe-to-profound SNHL compared with their preimplantation state of no treatment or amplification with hearing aids (“no CI” status).
Main Outcome Measure/s:
Incremental costs per quality adjusted life year (QALY) gained.
Results:
The mean total discounted cost of unilateral CI was CNY 252,506 (37,876 USD), compared with CNY 29,005 (4,351 USD) for the no CI status from the healthcare payer plus patient perspective. A total discounted benefit of 8.9 QALYs was estimated for CI recipients compared with 6.7 QALYs for the no CI status. From the healthcare payer plus patient perspective, incremental cost-effectiveness ratio (ICER) for unilateral CI compared with no CI was CNY 100,561 (15,084 USD) per QALY. The healthcare payer perspective yielded an ICER of CNY 40,929 (6,139 USD) per QALY. Both ICERs fell within one to three times China's gross domestic product per capita (GDP, 2011–2015), considered “cost-effective” by World Health Organization (WHO) standards.
Conclusions:
Treatment with unilateral CI is a cost-effective hearing solution for children with severe to profound SNHL in rural China. Increased access to mainstream education and greater opportunities for employment, are potential downstream benefits of CI that may yield further societal and economic benefits. CI may be considered favorably for broader inclusion in medical insurance schemes across China.
Keywords: Cochlear implantation, Cost-effectiveness analysis, Cost-utility analysis, Pediatric
Hearing loss affects about 5.3% (approximately 360 million) of the world's population (1), of which 9% are children. Severe to profound hearing loss can be defined as hearing loss of 61 dBHL or more in the better hearing ear (2). The prevalence and severity of hearing loss vary with some factors including geographical location, socioeconomic status, exposure to infections, consanguinity, and older age (3).
In many jurisdictions, cochlear implantation (CI) is the standard of care for permanent bilateral severe to profound sensorineural hearing loss (SNHL) (4). The intervention is safe with a major complication rate of 1.6% reported for children implanted bilaterally under the age of 18 years (5).
With increasing pressures on healthcare budgets, it is no longer sufficient to demonstrate that a device is safe and effective. It is now important to demonstrate that the intervention is also cost-effective in routine care. To improve access to CI across P. R. China (China), an economic evaluation was undertaken to assess the cost utility of unilateral CI compared with no CI in children presenting with severe to profound SNHL.
MATERIALS AND METHODS
Patient Population
A cost-utility analysis (CUA) was conducted as a substudy of the Cochlear Pediatric Implanted Recipient Observational Study (Cochlear P-IROS) in China. The Cochlear P-IROS is a prospective international patient-outcomes registry for children who are implanted in routine clinical practice with one or more hearing devices from a range of manufacturers (6,7). It aims to collect data on patient comorbidities, device use, auditory performance, quality of life, and health-related utilities, across different types of implantable hearing devices from a range of manufacturers (6,7). The CUA substudy enrolled new recipients aged less than or equal to 10 years, who underwent surgery for unilateral CI at The First Affiliated Hospital of the Anhui Medical University, Hefei, Anhui, P. R. China. All study participants were implanted with CI devices manufactured by Cochlear Limited. All children were from rural families and had bilateral severe to profound SNHL. Study participants were enrolled at the time of first fitting of the externally worn sound processor (baseline), and were followed up at 6-month and 12-month intervals. Recruitment commenced in April 2014 and ended in November 2014.
Ethics and Patient Consent
The study was conducted according to the Declaration of Helsinki (Fortaleza, Brazil, 2013) (8), and the International Conference on Harmonisation—Good Clinical Practice (ICH-GCP) Guidelines (9). The protocol was approved by the Hospital's Scientific Research Committee and the Clinical Research Ethics Committee. Patient informed consent was obtained from parent/ caregiver before enrolment.
Data Sources
Measures of Effectiveness: Utilities
At present, there is no established multi-attribute utility instrument (MAUI) appropriate for children who are deaf or hard of hearing and have undergone CI. The perceived health status of children was, therefore, observed using the modified EQ-5D version of the visual analogue scale (VAS) (10), further detailed in Supplementary Section 1.1. The VAS was administered to parents/caregivers of children at baseline (pre-CI use), and two post-CI follow-up intervals at 6 and 12 months. The VAS scores were transformed to health-related utilities using a conversion formula for time-trade off (TTO) scores, as reported previously (11).
Costs and Resource Utilization
Cost data related to the CI intervention and comparator were collected through the CUA substudy. The primary sources for costs and resource utilization were hospital billing data, and the Cochlear Implant Cost Questionnaire (CICQ) (12), which was administered to parents/caregivers of study participants at 6 and 12 months follow-up. Further details of itemised cost data collected from the primary sources are presented in Supplementary Section 1.2.
Economic Evaluation
Treatment
The treatment under investigation was unilateral CI in children age less than 10 years with bilateral severe to profound SNHL.
Comparator
An intra-subject comparator was used. The comparator under investigation was referred to as “no CI,” defined as using hearing aids (HAs) or no intervention for the treatment of bilateral severe-to-profound SNHL. The pre-CI status of recipients informed the costs and quality of life associated with “no CI” in the economic model. In the economic model, costs and quality of life of pre-CI HA-users and HA non-users were considered proportionately. This approach reflected routine clinical practices in the treatment of children eligible for unilateral CI as part of the standard continuum of the care in Anhui Province, China.
Economic Measure
Incremental cost-effectiveness ratios (ICER) were calculated for the intervention with CI compared with the no CI status. The ICER was defined as the incremental cost per quality of life year (QALY) gained. According to the World Health Organization (WHO), an ICER that falls within one to three times the national GDP per capita is considered to be “cost-effective” (13). The 2011 to 2015 GDP per capita in China was CNY 49,034 (USD 7,355) (14), which was similar to the 2015 GDP per capita of Anhui province (CNY 35,997, USD 5,400).
Perspective
Costs from the healthcare payer formed the base case and the “healthcare payer plus patient” perspective was also considered.
Time Horizon
A time horizon of 20 years was applied in the base case model, as this gives a long-term view without introducing an unreliable degree of uncertainty into the model. The impact of different time horizons was considered as part of structural sensitivity analysis.
Discounting
Direct medical costs and QALYs were discounted at 3% using the net present value formula (10).
Model Inputs
Costs of CI
Total direct medical costs related to CI were considered in the economic model. Indirect costs related to transportation, carer costs, and loss of productivity were not included in the model. The base year for costs was 2014. All direct medical costs were measured in Chinese Yuan Renminbi (CNY; 1 CNY = 0.15 USD, 2014).
Initial costs:
Initial costs of CI included the fixed cost of the initial CI system (implant and sound processor), preoperative assessments, including imaging and vestibular tests, and the hospital episode. For rural residents of Anhui Province, China, 70% of the combined costs of CI system and hospital episode were reimbursed through the New Rural Cooperative Medical System (NRCMS) of China (15). Initial costs considered in the economic model included direct medical costs related to hospital stay, salaries of ward medical and ward nursing staff, allied health, imaging, pathology, pharmacy, critical care, operating theatre, anaesthesia and consumables, nursing and ward supplies, and other minor bed costs. All costs were obtained from hospital billing data. The costs of implant and sound processor were fixed for all patients. As HA trials were not mandatory for recipients of NRCMS funding, costs related to HA trials were not considered under treatment costs for CI.
Postoperative costs:
Annual visits to the audiologist, device maintenance (batteries, microphone protectors, etc.), and costs related to hearing habilitation through speech therapy were considered under postoperative costs. A range of hearing habilitation subsidisations from the China Disabled Person's Federation (CDPF) were reported by some families for the first year of CI use. For other families these costs were borne entirely out-of-pocket. The base-case economic analysis, thus, considered cost of hearing habilitation for the first year post-CI, and the impact of prolonged hearing habilitation was tested through sensitivity analysis. The base-case analysis also considered additional expenses related to out of warranty repair and maintenance of the CI system including spare parts and accessories, sound processor replacements every 6 years, and other out-of-pocket expense for families of CI recipients. Due to uncertainty in sound processor replacement costs, a range of prices were considered in sensitivity analyses reflecting the regular retail price of a range of sound processors manufactured by the Cochlear Limited (Sydney, Australia) and sold in China. Using the price of the least expensive sound processor as a starting point, the impact of increments of CNY 5,000 (USD 750) to the retail price, up to a maximum (most expensive) device price of CNY 75,000 (USD 11,250) was investigated.
Cost of No CI
For the no CI comparator status, costs were considered proportionately to the number of children using/not using HAs at baseline (pre-CI use). The HA retail price was not available through the Cochlear P-IROS registry, or the CICQ. Cost estimates were obtained from study investigators for this model input. Due to the uncertainty of the estimate, the impact of HA retail price was further tested through sensitivity analyses. All expenses represented out-of-pocket costs for families of HA users, and included the HA device costs, costs of fitting, annual hearing checks, and costs of HA replacement every 5 years.
Utilities
The VAS data collected at 12-month follow-up informed the average utility for CI use. The VAS data collected at baseline (pre-CI use) from parents/caregivers of children using/not using HAs informed the average utility of the “no CI” status.
CI and HA Non-Use
As there were no published evidence on rates of CI or HA non-use from China, published rates from international literature as well as clinical opinion were sought. There were limited published literature on rates of pediatric CI non-use (16–18), and rates of HA non-use among children (16). An older study from the United Kingdom reported a CI non-use rate of 7.1% (18); however, more recent studies from Turkey (17) and Australia (16) reported rates of 0.96% after 11 years and 0.86% after 3 years, respectively. The one study from Australia reporting on pediatric HA non-use recorded a rate at 3.4% after 3 years (16). Based on these findings, and clinical opinion, our base case model conservatively assumed rates of CI and HA non-use in the Chinese context at 1.1 and 5.1%, respectively. Due to uncertainty in the estimate, potential rates of device non-use were considered from 0 to 10% in sensitivity analyses.
CI and HA Failure Rates
An annual rate of 1% for device failure was assumed in the base case model as per published evidence (19). Thus, a cumulative rate of revision surgery of 20% was assumed over a time horizon of 20 years. Due to limited published evidence, the rates of HA device failure were conservatively estimated at 0.5% per annum, based on clinical experience, which translated to a failure rate of 10% over 20 years. Given the uncertainties around failure rates, one-way sensitivity analyses were performed to understand the impact of higher failure rates on the cost-utility of CI. The prostheses costs related to device replacement were assumed to be covered by the manufacturer within the 10-year warranty period, and by individual families outside of this period. The costs of additional hospital episodes or clinical visits related to revision surgery were also assumed to be borne by individual families.
Model Type
A simple decision tree model informed the economic evaluation.
Sensitivity Analysis
Model sensitivity to uncertain input parameters was tested through one-way and two-way deterministic sensitivity analyses, and probabilistic sensitivity analysis. Uncertainties related to model structure were tested through: calculating ICERs from the perspective of healthcare payer plus patient, and healthcare payer only; considering time horizons from 20 to 76 years; using discount rates of 0 to 10%; and conducting scenario analyses related to medical insurance coverage, as detailed in Supplementary Section 1.3.
Statistical Analyses
Due to the small sample of 29 patients who participated in the CUA substudy, all cost estimates yielded from the study were bootstrapped over 1,000 iterations. Resulting bootstrap means and standard errors were subsequently used in the economic model. The significance of health-related utilities was tested through Mann–Whitney U tests, due to the non-normal distribution of utility parameters. For statistical analyses and decision tree modelling, Microsoft Excel (Microsoft Corp, Redmond, WA), and Ersatz software (EpiGear International, Sunrise Beach, Australia) were used.
RESULTS
Patient Characteristics
Characteristics of study participants are presented in Supplementary Section 2.1. The mean (standard deviation) and median age (interquartile range) at time of CI was 3.9 (2.8) years and 2.8 (1.9, 6.2) years, respectively. The majority (72.4%) of children were implanted at age more than or equal to 2 years. The most common type of hearing loss was congenital hearing loss (82.7%).
The pre-CI status of participants informed the costs and quality of life associated with “no CI” intervention. Less than half of the cohort (44.8%, 13/29) used HAs before CI, with 31.0% (9/29) and 13.8% (4/29) participants using HAs in bilateral and unilateral configurations, respectively. The remaining participants (55%, 16/29) had not used any HAs before CI. Before surgery, 17.2% of children (5/29) had an auditory-oral mode of communication.
All patients received CI systems manufactured by Cochlear Limited (Sydney, Australia). The most common devices were the Cochlear™ Nucleus® Freedom™ cochlear implant with Contour Advance™ electrode, CI24RE (CA) implant (51.7%), and the Nucleus Freedom sound processor (41.4%).
More than half (58.6%) of the mothers of CI recipients had attained a secondary school education, while 13.8% did not received any formal education. Similar trends were also observed among the fathers of CI recipients. Consistent with the rural locations of these families, the majority of mothers (65.5%), were not employed, while the majority of fathers (86.2%) held jobs, most commonly as laborers or helpers (34.4%). The majority of the CI recipients (82.7%) came from low income families, defined as an income of less than CNY 75,000 (USD 11,250) per annum; while the remaining came from middle income families, classified as earning CNY 75,000 to 300,000 (USD 11,250–45,000) per annum. Results from the CICQ at 12 months follow-up suggested that 17.2% of CI recipients did not attend any hearing habilitation, and only 16.7% of the remaining recipients were fully reimbursed for their visits to the speech therapist through the CDPF.
Costs
Cost of CI and No CI
The expected cost of CI from the perspective of the healthcare payer only, and the healthcare payer plus patient, are presented Tables 1 and 2, respectively. The total cost of CI over a time horizon of 20 years averaged CNY 169,452 (USD 25,418), and CNY 447,864 (USD 67,180), respectively. The total cost of no CI status over a time horizon of 20 years was CNY 4,707 (USD 706), and CNY 53,865 (USD 8,080), from each perspective, respectively.
TABLE 1.
Costs (CNY) associated with treatment with CI over a 20-year time horizon
| Average Cost (CNY) | Units | HC Payer Perspective | HC payer + Patient Perspective | Source | |
| Initial costs | |||||
| Hospital episode—reimbursed costa | 14,090 | 1 | 14,090 | 14,090 | Hospital billing data (n = 29) |
| Unilateral CI + SP system | |||||
| Reimbursed costa | 119,000 | 1 | 119,000 | 119,000 | Hospital billing data (n = 29) |
| Patient out-of-pocket costa | 39,056 | 1 | — | 39,056 | Hospital billing data (n = 29) |
| Additional clinical visits for hearing checksb | 250 | 4 | 1000 | 1000 | Hospital billing data/ clinical experts |
| Sub-total | 134,090 | 173,146 | |||
| Postoperative costsc | |||||
| Speech therapy—semi reimburseda | 12,000 | 1 | 12,000 | 12,000 | CICQ (n = 15) |
| Speech therapy—semi patient out-of-pocket costa | 6,771 | 1 | — | 6,771 | CICQ (n = 15) |
| Speech therapy—fully reimburseda | 13,861 | 1 | 13,861 | 13,861 | CICQ (n = 5) |
| Speech therapy—fully patient out-of-pocket costa | 21,755 | 1 | — | 21,755 | CICQ (n = 4) |
| No therapya | — | — | — | — | CICQ (n = 5) |
| Sub-total | 25,861 | 54,388 | |||
| CI maintenance | |||||
| Spare parts, batteries, and maintenance—patient out-of-pocket cost per year (out of warranty)d | 5,083 | 10 | — | 50,830 | In-house company database |
| Bi-annual hearing checkse | 250 | 38 | 9,500 | 9,500 | Hospital billing data/ clinical experts |
| Sub-total | 9,500 | 60,330 | |||
| Total cost of CI—20 year horizon excl. SP replacements | 169,452 | 287,864 | |||
| SP replacements | |||||
| SP end-user cost (assuming replacement every 6 yr) | 48,000 | 3.3 | — | 160,000 | Clinical experts/in-house company database |
| Total cost of CI—20 years horizon incl. SP replacements | 169,452 | 447,864 | |||
aBootstrapped costs.
bAssumes four visits for hearing checks in the year of implantation.
cPostoperative costs considers hearing habilitation-related costs for the first year of CI use.
dWarranty period = 3 years and accessories include rechargeable batteries, controller, microphone protector, drying briks/capsule, etc.
eAssumes two visits per year for hearing checks (e.g., mapping) after the first year.
CNY 1 = USD 0.15 (2014).
CI indicates cochlear implant; CICQ, cochlear implant cost questionnaire; CNY, Chinese Yuan; excl., excluding; HC, healthcare; incl., including; SP, sound processor; yrs, years.
TABLE 2.
Costs (CNY) associated with “no CI” (HA or no treatment) over a 20-year time horizon
| Average Cost (CNY) | Units | Proportion/Weight | HC Payer Perspective | HC Payer + Patient Perspective | Source | |
| Device costs | ||||||
| Bilateral HA user out-of-pocket cost | 10,000 | 2 | 0.31 | — | 6,207 | Clinical experts (cost)/P-IROS registry (weight) |
| Unilateral HA user out-of-pocket cost | 10,000 | 1 | 0.14 | — | 1,379 | Clinical experts (cost)/P-IROS registry (weight) |
| Not using HA | — | 0.55 | — | — | P-IROS registry (weight) | |
| Additional clinical visits for hearing testsa | 250 | 4 | 0.45 | 448 | 448 | Hospital billing data/clinical experts |
| Sub-total | 448 | 8,034 | ||||
| HA maintenance | ||||||
| Spare parts and maintenance—bilateral HA user out-of-pocket cost per year (out of warranty)b | 1,233 | 24 | 0.31 | — | 9,186 | Clinical experts |
| Spare parts and maintenance—unilateral HA user out-of-pocket cost per year (out of warranty)b | 1,233 | 12 | 0.14 | — | 2,041 | Clinical experts |
| Bi-annual hearing checksc | 250 | 38 | 0.45 | 4,259 | 4,259 | Hospital billing data/clinical experts |
| Sub-total | 4,259 | 15,486 | ||||
| Total cost of HA—20 years horizon | 4,707 | 23,521 | ||||
| HA Replacements | Unit Cost | Units | ||||
| Bilateral HA-user out-of-pocket cost (assuming replacement every 5 yr) | 10,000 | 8 | 0.31 | — | 24,828 | Clinical experts/P-IROS registry |
| Unilateral HA-user out-of-pocket cost (assuming replacement every 5 yr) | 10,000 | 4 | 0.14 | — | 5,517 | Clinical experts/P-IROS registry |
| Sub-total | — | 30,345 | ||||
| Total cost of CI—20 years horizon incl. HA replacements | 4,707 | 53,865 |
aAssumes four visits for hearing tests in the year of HA fitting.
bWarranty period = 2 years.
cAssumes two visits per year for hearing tests after the first year.
1 CNY = 0.15 USD (2014).
CNY indicates Chinese Yuan; HA, hearing aids; HC, healthcare; incl., including; P-IROS, pediatric implanted recipient observational study.
Utilities
The VAS scores, and corresponding utilities observed at pre-CI and post-CI intervals, are shown in Table 3. For children using/not using HA at baseline, mean VAS scores of 45.4 and 36.3 were estimated, corresponding to mean utilities of 0.61 (95% CI: 0.52–0.70) and 0.50 (95% CI: 0.43–0.58), respectively. An overall mean utility of 0.55 (95% CI: 0.49–0.61) was observed for the no CI status. At 12 months follow-up, a mean utility of 0.81 (95% CI: 0.77–0.85) was observed among children using CI, representing an incremental improvement of 0.26 (95% CI: 0.19–0.32) from the no CI status at pre-CI baseline.
TABLE 3.
Mean VAS scores and utilities of patients at baseline, 6 months, and 12 months intervals
| N | Mean VAS Score (95% CI) | Difference in Mean VAS Score from Baseline (95%CI) | Mean Utility Score (95%CI)a | Difference in Utility Score from Baseline (95% CI) | |
| Baseline (presurgery) | 29 | 40.5 (35.3–45.7) | — | 0.55 (0.49–0.61) | — |
| HA usersb | 13 | 45.8 (37.6–53.9) | — | 0.61 (0.52–0.70) | — |
| HA non-users | 16 | 36.3 (30.1–42.4) | — | 0.50 (0.43–0.58) | — |
| 6 months | 29 | 60.6 (56.8–64.4) | 20.1 (15.9–24.3) | 0.77 (0.73–0.80) | 0.21 (0.17–0.26) |
| 12 months | 29 | 65.7 (61.2–70.2) | 25.2 (18.8–31.6) | 0.81 (0.77–0.85) | 0.26 (0.19–0.32) |
aUtility scores were obtained through conversion of VAS scores to TTO scores using the formula, UVAS = 1 − (1 − V/100)1.6 published previously (11).
bIncludes nine bilateral HA users and four unilateral HA users.
95%CI indicates 95% confidence interval; HA, hearing aid; VAS, visual analogue scale.
Decision Tree Model
The decision tree from the healthcare payer plus patient perspective is shown in Supplementary Section 2.2.
ICERs
The discounted expected costs and QALYs and the corresponding ICERs for CI from each perspective are shown in Table 4. From the perspective of the healthcare payer plus patient, the ICER was CNY 100,561 (USD 15,084) per QALY. When considering the healthcare payer perspective only, the ICER was CNY 40,929 (USD 6,139) per QALY. Both ICERs fell either below or within the thresholds of CNY 49,034 (USD 7,355) and CNY 147,102 (USD 22,065), one to three times China's GDP per capita (2011–2015) (16,17).
TABLE 4.
The incremental costs per QALY gained for CI over a 20-year time horizon
| Healthcare Payer Plus Patient Perspective | |||||||
| Expected Cost (CNY) | Discounted Cost (CNY) | Expected Outcome (QALY) | Discounted Expected | Incremental Cost (CNY) (disc) | Incremental Effect (disc) | ICER (CNY) | |
| No CI | 52,386 | 29,005 | 12.1 | 6.7 | — | — | — |
| CI | 456,054 | 252,506 | 16.1 | 8.9 | 223,502 | 2.2 | 100,561a |
| Healthcare payer perspective only | |||||||
| No CI | 5,155 | 2,854 | 12.1 | 6.71 | — | — | — |
| CI | 169,451 | 93,821 | 16.1 | 8.93 | 90,967 | 2.2 | 40,929b |
aEquivalent to 15,084 USD (1 CNY = 0.15 USD, 2014).
bEquivalent to 6,139 USD (1 CNY = 0.15 USD, 2014).
CI indicates cochlear implantation; HA, hearing aid; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life years.
Sensitivity Analysis
Parameter Sensitivity Analysis
One-Way Sensitivity Analysis
Results from one-way sensitivity analyses for each perspective are presented in Supplementary Section 2.3.
From the perspective of the healthcare payer plus patient, parameters including the regular retail price of HA, cost of replacement sound processors, and duration of participation in hearing habilitation had an impact on the ICERs. Increasing HA price improved the cost-effectiveness of CI (i.e., reduced the ICER); while increasing the costs of CI replacement sound processors resulted in unfavorable ICERs. Increasing the number of years of hearing habilitation from 1 to 2, and 5 years increased the ICER from CNY 100,561 (USD 15,084) per QALY (base case), to CNY 112,341 (USD 16,851) per QALY, and CNY 152,988 (USD 22,948) per QALY, respectively. Increasing cost of maintenance of CI and HA including costs of spare parts and accessories had a moderate effect on the ICER. Parameters including rates of device failures, non-use, and frequency of visits for hearing checks, had minimal impact on the ICER.
From the perspective of the healthcare payer only, all cost parameters that represented patient out-of-pocket costs had no impact on the base case ICER of CNY 40,929 (USD 6,139) per QALY. However, the frequency of years attending hearing habilitation, and the frequency of visits for hearing checks beyond first year had substantial impact on the ICER.
Two-Way Sensitivity Analysis
Results from two-way sensitivity analyses for each perspective are presented, and discussed in Supplementary Section 2.4.
Probabilistic Sensitivity Analysis
Monte Carlo simulation results from the healthcare payer plus patient perspective are presented in Figure 1A and B. Approximately, 90% of ICERs fell within the limits of one to three times China's GDP per capita (2011–2015) (12,13) (Fig. 1A). At a threshold of one time GDP per capita (CNY 49,034, USD 7,355) CI was not cost-effective (Fig. 1B). However, at thresholds of two times GDP per capita (CNY 98,068, USD 14,710), and three times GDP per capita (CNY 147,102, USD 22,065), the probability of cost-effectiveness improved to 50 and 100%, respectively (Fig. 1B).
FIG. 1.
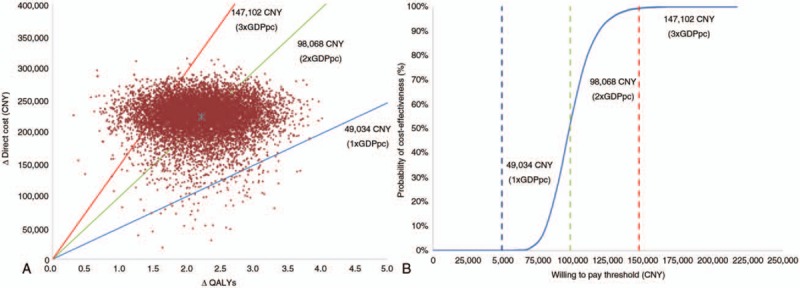
A, Scatter plot presenting results from the Monte Carlo simulations (10,000 iterations), from the healthcare payer plus patient perspective. Incremental QALYs are plotted against direct costs (CNY) observed at 20 years. B, Cost-effectiveness acceptability curve presenting results from the Monte Carlo simulations (10,000 iterations). The probabilities of cost-effectiveness (%) corresponding to various thresholds are plotted, as observed at 20 years. GDPpc indicates gross domestic product per capita; QALY, quality-adjusted life years. Note: ∗ symbol in the center represents the base case result.
The results from the perspective of the healthcare payer only, are presented in Figure 2A and B. Results from Monte Carlo simulations indicated that 76% of the ICERs fell below the threshold of one time GDP per capita, and 24% of ICERs fell between one and two times GDP per capita, indicating that CI is highly cost-effective from the perspective of the healthcare payer (Fig. 2B). At a threshold of one time GDP per capita, the probability of cost-effectiveness of CI was 76%. The probability of cost-effectiveness improved to 100% at thresholds of two times GDP per capita (Fig. 2B).
FIG. 2.
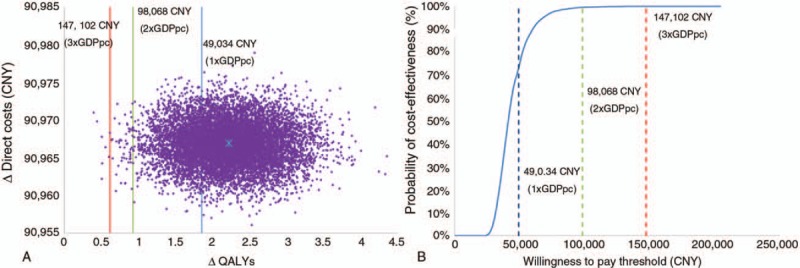
A, Scatter plot presenting results from the Monte Carlo simulations (10,000 iterations), from the healthcare payer perspective only. Incremental QALYs are plotted against direct costs (CNY) observed at 20 years. B, Cost-effectiveness acceptability curve presenting results from the Monte Carlo simulations (10,000 iterations). The probabilities of cost-effectiveness (%) corresponding to various thresholds are plotted, as observed at 20 years. GDPpc indicates gross domestic product per capita; QALY, quality-adjusted life years. Note: ∗ symbol in the center represents the base-case result.
Structural Sensitivity Analysis
Increasing the time horizon substantially improved the cost-effectiveness of CI, as per results presented in Supplementary Section 2.5. When a time horizon of 76 years (the current average life expectancy in China) (20) was considered, ICERs of CNY 58,556 (USD 8,783) per QALY, and CNY 11,784 (USD 1,768) per QALY were yielded from the healthcare payer, and the healthcare payer plus patient perspectives, respectively. Doubling of discount rate to 6% resulted in ICERs of CNY 52,836 (USD 7,925) per QALY, and CNY 139,986 (USD 20,998) per QALY, from each of the perspectives, respectively. If 100% of costs of hearing habitation were reimbursed through medical insurance, 1 to 8 years of hearing habilitation resulted in ICERs of CNY 48,035 (USD 7,205) per QALY to CNY 142,879 (USD 21,432) per QALY, from the perspective of healthcare payer. While these ICERs were higher than a threshold of one time GDP per capita (CNY 49,034, USD 7,355), they were below the threshold three times GDP per capita (CNY 147,102, USD 22,065).
DISCUSSION
This is the first economic evaluation to assess the cost-effectiveness of unilateral CI in children with severe to profound SNHL in China. CI has been well documented as a safe, clinically effective, and cost-effective intervention (5,21,22), establishing it as the gold standard treatment for children with significant sensorineural hearing disability, who cannot derive sufficient benefit from HAs. Similar health outcomes can be expected in China.
In this study, all children came from low-income rural families in the province of Anhui, China, and were supported by the NRCMS, one of the world's largest basic medical insurance schemes, mandated by China's Ministry of Health, and implemented by local and provincial governments (15). All families participating in our cost-utility substudy were reimbursed 70% of the CI system costs (CNY 119,000, USD 17,850), through the NRCMS. The remaining 30% of CI system costs represented out-of-pocket expenses for families of CI recipients. Policies such as the NRCMS, improve patient access, with approximately 95% of the population covered by an urban or rural medical scheme (23). Coverage for CI procedures varies among these schemes and across provincial and municipal jurisdictions.
The Economic Model
The costs in the model were evaluated from the healthcare payer and the “healthcare payer plus patient” perspectives to reflect the financial burden of out-of-pocket costs borne by families covered by the NRCMS. The healthcare payer perspective considered various items of standard healthcare subsidised by medical insurance, and disability provisions, of the NRCMS, and the CDPF, respectively; and financed collectively by the China central, provincial, and local governments.
A conservative approach was undertaken to estimate uncertain parameters. A time-horizon of 20 years was considered in the base case analysis, as this gives a long-term view without introducing an unreliable degree of uncertainty into the model, particularly in the era of advancing technological development. The costs for the “no CI” group were assumed entirely out-of-pocket, and were weighed according to the proportions of bilateral HA users, unilateral HA users, and HA non-users, observed at the baseline (pre-CI use) of the CUA substudy. The model also assumed 100% of CI revision surgeries were CI reimplantations, whereas recent evidence suggest this rate to be 85.5% (19). CI reimplantation surgeries are often more costly than other types of CI revision surgeries (19).
Summary of Findings
The ICERs generated by the economic model of CNY 40,929 (USD 6,139) per QALY and CNY 100,561 (USD 15,084) per QALY from each perspective, fell within one to three times China's GDP per capita (2011–2015) (16,17), suggesting treatment with unilateral CI compared with no CI is cost-effective in the Chinese province of Anhui. The base case ICER estimates may be conservative, given CI is known to enhance the opportunity for children to participate in mainstream education instead of Schools for the Deaf. Education in mainstream schools leads to greater downstream benefits such as higher employment opportunities and increased societal productivity. These downstream benefits have been shown to lead to societal cost-savings in previous economic evaluations (22,24–26).
Our sensitivity analyses indicated that the results were robust against the majority of model parameters. Sensitive parameters included the regular retail price of HA, the cost of CI sound processor replacements, and the frequency of replacements of CI sound processors and HAs. As the price of initial CI system was fixed for all patients in this setting, sensitivity analyses on initial CI device cost were not necessary. Structural sensitivity analyses revealed that our results were similarly robust against most parameters, and sensitive to assumptions around coverage of hearing habilitation, discounts rates, and time horizon. It is anticipated that the benefits of CI in children will last a lifetime. If a lifetime horizon of 76 years rather than 20 years was considered, the ICERs improved to CNY 11,784 (USD 1,768) per QALY, and CNY 58,556 (USD 8,783) per QALY, from both perspectives, respectively. These reductions are substantial, suggesting unilateral CI compared with no CI is cost-effective, over a child's lifetime.
Cost-Effectiveness of Unilateral CI from Current Literature
The cost-effectiveness of unilateral CI compared with no intervention or HA is well-established in the international literature. In a National Institute of Health and Care Excellence (NICE) evaluation in the UK, CI was found to be cost-effective when compared with HAs and/or other non-implantable treatments for hearing loss in children (ICER: USD, 2014: 27,289 per QALY) (27). In the US, unilateral CI was deemed more cost-effective from a societal economic perspective among children implanted at age less than 18 months (15,594 per QALY) compared with age 18 to 36 months (USD 18,561 per QALY), and age more than 36 months (USD 19,938 per QALY) (24). In another evaluation performed from the UK healthcare perspective, pediatric unilateral CI was found cost-effective (USD 26,034 per QALY) compared with non-surgical intervention, utilizing various methods for assessments of health-related utility, including the time-trade off (TTO) method, and the VAS (11). A similar VAS approach was also reported in Barton et al. (28), and was adopted in the current evaluation. By contrast, other economic evaluations, which additionally considered costs and benefits from mainstream education for CI recipients, showed societal cost savings from CI compared with no treatment or HAs (22,24–26).
The current evaluation revealed that unilateral CI leads to health-related utility gains over time (at 6 and 12 mo post-CI), resulting in additional QALYs for children using CI compared with no CI. An earlier study undertaken by Barton et al. (28) also reported consistent utility gains over time for pediatrics using CI. These observations are meaningful as it shows consistent utility gains were observed by patients treated with CI, cross-temporally, and across geographies. Thus, suggesting that utility gains resulting from CI are consistently universal and transferable.
Similar health-related utility gains may also observed among adult CI recipients leading to similar cost-effectiveness results. Evaluations conducted from the UK and Canadian healthcare perspectives showed that unilateral CI was cost-effective in adults when compared with HAs or no intervention (USD 33,144 per QALY; USD 9,624 per QALY; and USD 36,398 per QALY, respectively) (27,29,30). An earlier study (31) showed that unilateral CI in older adults aged more than 50 years is cost-effective from the US healthcare perspective (USD 12,954 per QALY). Lee et al. (32) demonstrated that unilateral CI in adults is cost-effective in the Korean healthcare context (USD 20,825 per QALY). These studies used the Health Utility Index (HUI), a MAUI, for measuring health-related QALYs. To-date, there has been no economic evaluation of CI performed for adults with severe to profound SNHL in China.
Impact of Early Implantation and Hearing Habilitation
The cost utility of unilateral CI may improve with earlier age at implantation, made possible through early identification of potential candidates through the continued roll out of new born hearing screening programs, and timely access to affordable care, including CI and hearing habilitation. Earlier implantation minimizes language delays, allowing age-equivalent language development (33) and possibly shorter durations of hearing habilitation (34). This translates into downstream benefits such as higher educational placement, enhanced employment opportunities, and productivity on the job (33).
The mean age at implantation is relatively high across China compared with that of countries with universal new born hearing screening, and a substantial proportion of rural recipients of CI may not attend hearing habilitation. In the current study sample, the mean and median age of implantation was age 3.9 years and age 2.8 years, respectively. Participation in speech therapy was limited to 83% of children, where only 17% received reimbursement in full by the CDPF in their first postoperative year. Factors mitigating higher participation rates in hearing habilitation include access to rehabilitation clinics, which are primarily located in urban areas, and limited subsidisation of hearing habilitation services for CI recipients under the age of 3 years. Due to inadequate evidence on the rates of prolonged participation in hearing habilitation of children in China, the present economic evaluation did not assume participation in hearing habilitation beyond the first year of CI use. For children implanted later, functional hearing performance and speech intelligibility outcomes could be further enhanced through improving access to extended hearing habilitation (speech therapy) (34). Our sensitivity analyses showed that if hearing habilitation services were fully reimbursed for up to 8 years, CI is still cost-effective, not taking into account the long-term quality of life benefits associated with speech training.
Strengths and Limitations
While our evaluation was strengthened by use of prospective data, and pragmatic sensitivity analyses, it was limited by the use of secondary sources of data for regular retail price of HAs, number of years spent in hearing habilitation, rates of device non-use, and HA device failure rates. Furthermore, due to insufficient evidence in the Chinese context, this economic evaluation did not consider the potential costs and benefits (e.g., savings) from educational outcomes of children using CI, and changes to income level and socioeconomic status in the long-term. Further research is warranted to quantify these downstream benefits of CI in the long-term in the Chinese context.
CONCLUSION
This is the first economic evaluation of pediatric unilateral CI in China. Unilateral CI is a cost-effective hearing solution for children with bilateral severe to profound SNHL. Potential downstream benefits of CI include participation in mainstream education, and improved integration in the Chinese society through greater education and employment opportunities. CI may be considered favorably for broader inclusion in medical insurance schemes across China.
Acknowledgments
The authors sincerely acknowledge the contributions of Ms. Xueling Zhu.
Supplementary Material
Supplementary Material
Footnotes
J.Q. and C.Y. have contributed equally to this work.
Competing interests: T.V.A., C.F., Z.K., and G.S. are employees of Cochlear Limited.
Authors’ Contributions: J.Q., C.Y., and T.V.A. wrote the paper. T.V.A. designed the economic model and analyzed health economic data. Z.K., Y.S., C.Y., L.Z., and F.Q., collected the data. G.S. and C.F. provided critical input to the design and review of the model, and the research design. All authors provided critical revision of the manuscript and approved the final manuscript.
Financial Disclosures/Conflicts of Interest: This research was sponsored by Cochlear Limited. All authors maintained independent control of manuscript content and were not subject to any censorship or editorial control by Cochlear Limited.
REFERENCES
- 1.World Health Organization. Global Estimates on Prevalence of Hearing Loss; 2012. Available at: http://www.who.int/pbd/deafness/WHO_GE_HL.pdf. Accessed August 8, 2015. [Google Scholar]
- 2.Mathers C, Smith A, Concha M. Global burden of hearing loss in the year 2000. Global Burden Dis 2000; 18:1–30. [Google Scholar]
- 3.Stevens G, Flaxman S, Brunskill E, et al. Global and regional hearing impairment prevalence: an analysis of 42 studies in 29 countries. Eur J Public Health 2013; 23:146–152. [DOI] [PubMed] [Google Scholar]
- 4.Balkany TJ, Hodges AV, Eshraghi AA, et al. Cochlear implants in children—a review. Acta Otolaryngol 2002; 122:356–362. [DOI] [PubMed] [Google Scholar]
- 5.Broomfield SJ, Murphy J, Wild DC, et al. Results of a prospective surgical audit of bilateral paediatric cochlear implantation in the UK. Cochlear Implants Int 2014; 15:246–253. [DOI] [PubMed] [Google Scholar]
- 6.Sanderson G, Ariyaratne TV, Wyss J, et al. A global patient outcomes registry: Cochlear paediatric implanted recipient observational study (Cochlear™ P-IROS). BMC Ear Nose Throat Disord 2014; 14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh S, Vashist S, Ariyaratne TV. One-year experience with the Cochlear™ paediatric implanted recipient observational study (Cochlear P-IROS) in New Delhi, India. J Otol 2015; 10:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Association WM. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310:2191. [DOI] [PubMed] [Google Scholar]
- 9.Dixon JR. The international conference on harmonization good clinical practice guideline. Qual Assur 1999; 6:65–74. [DOI] [PubMed] [Google Scholar]
- 10.Gray AM, Clarke PM, Wolstenholme JL, et al. Applied Methods of Cost-Effectiveness Analysis in Healthcare. Oxford: OUP; 2010. [Google Scholar]
- 11.Summerfield AQ, Lovett RE, Bellenger H, et al. Estimates of the cost-effectiveness of pediatric bilateral cochlear implantation. Ear Hear 2010; 31:611–624. [DOI] [PubMed] [Google Scholar]
- 12.Kerr GR. Long-Term Cost Implications for Cochlear Implant Recipients. Stellenbosch: Stellenbosch University; 2011. [Google Scholar]
- 13.World Health Organization. Cost effectiveness and strategic planning (WHO-CHOICE); 2014. Available at: http://www.who.int/choice/costs/CER_levels/en/. Accessed April 12, 2016. [Google Scholar]
- 14.The World Bank. GDP per capita (current US$) table; 2015. Available at: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD. Accessed April 12, 2016. [Google Scholar]
- 15.Li C, Hou Y, Sun M, et al. An evaluation of China's new rural cooperative medical system: achievements and inadequacies from policy goals. BMC Public Health 2015; 15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marnane V, Ching TYC. Hearing aid and cochlear implant use in children with hearing loss at three years of age: Predictors of use and predictors of changes in use. Int J Audiol 2015; 54:544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Özdemir S, Tuncer Ü, Tarkan Ö, et al. Factors contributing to limited or non-use in the cochlear implant systems in children: 11 years experience. Int J Pediatr Otorhinolaryngol 2013; 77:407–409. [DOI] [PubMed] [Google Scholar]
- 18.Raine CH, Summerfield Q, Strachan DR, et al. The cost and analysis of nonuse of cochlear implants. Otol Neurotol 2008; 29:221–224. [DOI] [PubMed] [Google Scholar]
- 19.Wang JT, Wang AY, Psarros C, et al. Rates of revision and device failure in cochlear implant surgery: A 30-year experience. Laryngoscope 2014; 124:2393–2399. [DOI] [PubMed] [Google Scholar]
- 20.The World Bank. Life expectancy at birth, total (years) table; 2015. Available at: http://data.worldbank.org/indicator/SP.DYN.LE00.IN. Accessed April 12, 2016. [Google Scholar]
- 21.Farinetti A, Gharbia DB, Mancini J, et al. Cochlear implant complications in 403 patients: comparative study of adults and children and review of the literature. Eur Ann Otorhinolaryngol Head Neck Dis 2014; 131:177–182. [DOI] [PubMed] [Google Scholar]
- 22.Cheng AK, Rubin HR, Powe NR, et al. Cost-utility analysis of the cochlear implant in children. JAMA 2000; 284:850–856. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Fact sheets: Health sector reform in China; 2016. Available at: http://www.wpro.who.int/china/mediacentre/factsheets/health_sector_reform/en/. Accessed November 22, 2016. [Google Scholar]
- 24.Semenov YR, Yeh ST, Seshamani M, et al. Age-dependent cost-utility of pediatric cochlear implantation. Ear Hear 2013; 34:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulze-Gattermann H, Illg A, Schoenermark M, et al. Cost-benefit analysis of pediatric cochlear implantation: German experience. Otol Neurotol 2002; 23:674–681. [DOI] [PubMed] [Google Scholar]
- 26.Foteff C, Kennedy S, Milton AH, et al. Economic evaluation of treatments for pediatric bilateral severe to profound sensorineural hearing loss: An Australian perspective. Otol Neurotol 2016; 37:462–469. [DOI] [PubMed] [Google Scholar]
- 27.Bond M, Mealing S, Anderson R, et al. The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: a systematic review and economic model. Health Technol Assess 2009; 13:1–330. [DOI] [PubMed] [Google Scholar]
- 28.Barton GR, Stacey PC, Fortnum HM, et al. Hearing-impaired children in the United Kingdom, IV: cost-effectiveness of pediatric cochlear implantation. Ear Hear 2006; 27:575–588. [DOI] [PubMed] [Google Scholar]
- 29.Chen JM, Amoodi H, Mittmann N. Cost-utility analysis of bilateral cochlear implantation in adults: A health economic assessment from the perspective of a publicly funded program. Laryngoscope 2014; 124:1452–1458. [DOI] [PubMed] [Google Scholar]
- 30.Summerfield A, Marshall DH, Barton GR, et al. A cost-utility scenario analysis of bilateral cochlear implantation. Arch Otolaryngol Head Neck Surg 2002; 128:1255–1262. [DOI] [PubMed] [Google Scholar]
- 31.Francis HW, Chee N, Yeagle J, et al. Impact of cochlear implants on the functional health status of older adults. Laryngoscope 2002; 112:1482–1488. [DOI] [PubMed] [Google Scholar]
- 32.Lee H-Y, Park E-C, Joong Kim H, et al. Cost-utility analysis of cochlear implants in Korea using different measures of utility. Acta Otolaryngol 2006; 126:817–823. [DOI] [PubMed] [Google Scholar]
- 33.Summerfield AQ, Marshall DH. Paediatric cochlear implantation and health-technology assessment. Int J Pediatr Otorhinolaryngol 1999; 47:141–151. [DOI] [PubMed] [Google Scholar]
- 34.Zhou H, Chen Z, Shi H, et al. Categories of auditory performance and speech intelligibility ratings of early-implanted children without speech training. PLoS ONE 2013; 8:e53852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


