Abstract
Optical coherence tomography (OCT) delivers 3-dimensional images of tissue microstructures. Although OCT imaging offers a promising high-resolution method, OCT images experience some artifacts that lead to misapprehension of tissue structures. Speckle, intensity decay, and blurring are 3 major artifacts in OCT images. Speckle is due to the low coherent light source used in the configuration of OCT. Intensity decay is a deterioration of light with respect to depth, and blurring is the consequence of deficiencies of optical components. In this short review, we summarize some of the image enhancement algorithms for OCT images which address the abovementioned artifacts.
Keywords: Optical coherence tomography (OCT), Image enhancement, Speckle reduction, attenuation compensation, Deblurring
Optical coherence tomography (OCT) is a noninvasive, non-ionizing optical imaging modality based on low coherence interferometry.1 Michelson, Mach-Zehnder, and common path interferometers have been used in the configuration of the OCT system.1–4 To form an OCT image, the magnitude and time delay of backscattered infrared light returned from a biological sample are measured transversally.5,6 Optical coherence tomography is similar to the ultrasound imaging, except that it uses light instead of sound.7 Providing high-resolution images and a moderate penetration depth, ie, 1 to 3 mm, OCT is currently used in several medical and biomedical applications including dermatology,8–12 dentistry,13 oncology,3 gastrointestinal endoscopy,14 intravascular imaging,15,16 cardiology,17 and neurology in addition to its initial successes in ophthalmology.18
An OCT system is characterized by several parameters such as imaging speed, lateral and axial resolutions, and penetration depth.19 Although imaging depths are not as deep as ultrasound, the resolution of OCT is more than 10 to 100 times finer than standard clinical ultrasound.7 There are 2 main types of OCT: time domain and spectral domain.5 Spectral domain OCT is a newer technology in which the scan rate is much faster than that in time domain, in addition to have a better penetration depth and signal-to-noise ratio. These characteristics are further improved in swept-source OCT, the most favorite OCT device in the market. The high scan rate diminishes the likelihood of motion artifacts and consequently enhances the image contrast and reduces the chance of missing pathology.20,21
Optical coherence tomography images visualize the morphological details of tissue microstructures, ie, stratum corneum, epidermis, dermis, hair follicles, eccrine sweat ducts, and sebaceous gland.8,22 Figure 1 illustrates some of the skin structures visible in OCT images.
Figure 1.
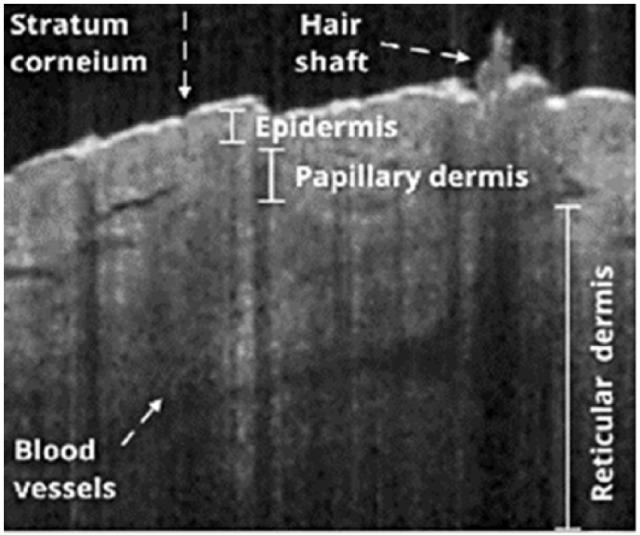
Optical coherence tomography image of skin of sole.
Since the invention of OCT, several hardware and signal processing advancements have been implemented; for instance, ultrahigh-speed OCT with the ability of generating several 3-dimensional images per second and sub-micron resolution OCT; both of which have benefitted from novel laser light sources and graphical processing units.23–25 Polarization-sensitive OCT uses the depth-resolved polarization states’ information (birefringence property of tissues) of recorded interference to provide high-resolution images.26 Endoscopic OCT is a newer modality with a miniaturized probe to image internal organs such as gastrointestinal, pulmonary, and urinary tracts as well as arteries and veins.3 Functional/molecular OCT has also been implemented and used in clinical applications such as brain tumor surgery.27 More recently, OCT has been used as an optical biopsy method for differentiating between healthy and tumorous tissues.28–30
Quantitative analysis of OCT images through optical properties’ extraction using extended Huygens-Fresnel principle31–34 has made OCT an even more powerful modality.35–38 Some of the optical properties that can be extracted from OCT images include scattering coefficient, absorption coefficient, refractive index, and anisotropy factor.39
Optical coherence tomography is a powerful high-resolution imaging method for medical and biomedical applications. Many modifications have already been applied to OCT hardware and software; however, OCT images still have artifacts.19 Five major artifacts found in OCT images are speckle noise, intensity decay, sample or device motion, refractive index change, and blurring and dispersion. In this review article, we briefly present the nature of the artifacts as well as a summary of the solutions to overcome them.
Similar to other low coherent imaging modalities, OCT images are contaminated with a grainy pattern of speckle which degrades the quality of images and conceals diagnostically relevant features.40 In fact, speckles make the images vague and tissue microstructures may become indistinct. There are 2 main speckle reduction methods: software based and hardware based.41–49 The most common hardware-based speckle reduction method is compounding. In compounding methods, the sample is imaged several times by the OCT system. There are 4 types of compounding: angular compounding, sample imaged from different angles; frequency compounding, sample imaged with different wavelengths; polarization compounding, sample imaged with different polarizations; and finally, spatial compounding, sample imaged from different positions.1,44,49–51 Then, the acquired images are averaged and a speckle-reduced image is obtained. Software-based speckle reduction methods rely on digital filtering based on a mathematical model of speckle and do not need several images to work. There are 3 main classes of digital filters: sliding window, adaptive statistical-based, and edge-preserved patch or pixel correlation–based. Sliding window filters, a class of filter including mean, median, and symmetric nearest neighbor,52 are highly efficient and can be used in real-time speckle reduction applications such as video-rate OCT imaging.52 Although they effectively reduce speckle noise in the OCT image, they smooth edges in the image and create blurriness.53,54 Adaptive statistical-based filters, a class of despeckling filter, include Kuwahara filter55 and homomorphic Wiener filter and use statistical features, eg, mean and variance, extracted from the image or a part of the image.56,57 Patch or pixel correlation–based filters, a class of despeckling filters, including nonlocal mean filter,58 total variation,59 and block matching and 3D filtering,60 are based on high inter- or intracorrelations among nearby pixels or patch of pixels.59,61–64 Wavelet-based algorithms are also considered as effective speckle reduction methods in which speckles are separated in higher level of decomposition.47,60,65,66 In addition to these 3 main categories, there are artificial neural network–based denoising methods that are considered as effective speckle reduction approaches.67–72
Intensity decay is due to decline in the incident and backscattered light amplitudes when it passes through a biological sample.1 The decay follows the Beer-Lambert law in the simplest model of skin—a single scattering model. When the light is attenuated, far less energy is deposited in deeper structures. By finding attenuation coefficients of skin layers and using the inverse Beer-Lambert law and some numerical methods, attenuation can be compensated.73,74 Optical coherence tomography images are considered logarithmic and because of that their exponential nature is changed to linear. Hence, by finding the slope of A-scan profiles in a homogeneous area, the attenuation coefficient is found and the intensity decay can be compensated.
Motion artifact in OCT is a result of sample or device motion.75,76 It is clear that longer acquisition times lead to greater motion artifacts. Optical coherence tomography systems with higher speed conserve experience with far fewer motion artifacts. To overcome the motion artifact, both hardware (using markers) and software (registration) solutions should be used.76
Being a noncontact method, the OCT image is distorted as a result of diffraction phenomenon that occur at the air/tissue interface. Therefore, algorithms are introduced to correct this distortion in OCT.77
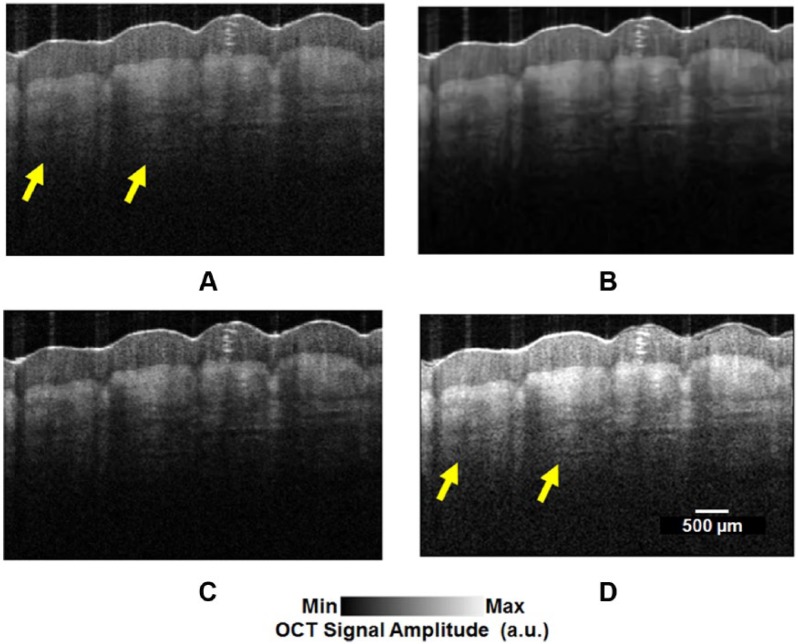
Blurring is as a result of aberration which is due to imperfections in optical devices used in the configuration of the OCT.78 Blurring deteriorates the lateral resolution of OCT images. Adaptive optics (AO) and deconvolution are 2 methods to reduce aberration and blurring.79,80 The main components in an AO system are tilt mirrors, digital mirror devices, spatial light modulators, and deformable mirrors.78,81 As the OCT signal is a convolution of the sample response with the coherence function of the light source, deconvolution methods are used to solve the depth resolution degradation caused by the depth point spread function envelope.1,80,82–86 Lucy-Richardson and Weiner deconvolution algorithms are 2 popular methods in this area.80,82,86 An OCT image on which some of the abovementioned processing, ie, speckle reduction, deblurring, and attenuation compensation, have been applied is shown in Figure 2.
Figure 2.
Optical coherence tomography images of a 24-year-old male’s thumb pad. (A) Original image, (B) despeckled image,51,57,64,66,67,72,87 (C) deconvolved image,80,86 and (D) attenuation-compensated image.88 Yellow arrows refer to the clearer structures at deeper depths before and after attenuation compensation.
Dispersion also degrades depth resolution. Therefore, equalizing dispersion between the reference and sample arms is essential to obtain a higher depth resolution. There are Fourier transform–based numerical dispersion compensation methods as well as pre-imaging optical techniques to compensate for OCT dispersion.89 The common pre-imaging method to solve distortion is to use dispersive materials (such as prisms) in the reference arm of the interferometer.90–92
Due to intermediate resolution and penetration depth, OCT has been a favorable device in many medical and biomedical applications. To make the diagnostically relevant features in OCT images more salient, and assist the specialists in making better diagnostic decisions using OCT images, quality improvement algorithms have been used. Required image quality and resolution is dependent on the application of OCT. Most of the causes of OCT image artifacts discussed in this short review could be resolved by some changes in the hardware of the OCT before imaging.93 Further image quality improvement could be accomplished by software during postprocessing of OCT images. Many novel hardware modifications and technology advancements undergo test and evaluation to make the OCT system a more useful and versatile device. There have been other high-resolution imaging modalities added to OCT to improve some of its limitations, eg, penetration depth.94,95
Footnotes
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 597 words, excluding any confidential comments to the academic editor.
FUNDING: The author(s) received no financial support for the research, authorship, and/or publication of this article.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions
MA designed the research. SA, ZT, EF and MA wrote the manuscript and all authors participated in paper revisions.
REFERENCES
- 1.Schmitt JM. Optical coherence tomography (OCT): a review. IEEE J Sel Top Quant. 1999;5:1205–1215. [Google Scholar]
- 2.Rollins AM, Izatt JA. Optimal interferometer designs for optical coherence tomography. Opt Lett. 1999;24:1484–1486. doi: 10.1364/ol.24.001484. [DOI] [PubMed] [Google Scholar]
- 3.Tearney GJ, Brezinski ME, Bouma BE, et al. In vivo endoscopic optical biopsy with optical coherence tomography. Science. 1997;276:2037–2039. doi: 10.1126/science.276.5321.2037. [DOI] [PubMed] [Google Scholar]
- 4.Vakhtin AB, Kane DJ, Wood WR, Peterson KA. Common-path interferometer for frequency-domain optical coherence tomography. Appl Opt. 2003;42:6953–6958. doi: 10.1364/ao.42.006953. [DOI] [PubMed] [Google Scholar]
- 5.Leitgeb R, Hitzenberger C, Fercher A. Performance of Fourier domain vs. time domain optical coherence tomography. Opt Exp. 2003;11:889–894. doi: 10.1364/oe.11.000889. [DOI] [PubMed] [Google Scholar]
- 6.Podoleanu AG. Optical coherence tomography. Br J Radiol. 2014;78:976–988. doi: 10.1259/bjr/55735832. [DOI] [PubMed] [Google Scholar]
- 7.Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat Biotechnol. 2003;21:1361–1367. doi: 10.1038/nbt892. [DOI] [PubMed] [Google Scholar]
- 8.Welzel J. Optical coherence tomography in dermatology: a review. Skin Res Technol. 2001;7:1–9. doi: 10.1034/j.1600-0846.2001.007001001.x. [DOI] [PubMed] [Google Scholar]
- 9.Avanaki MR, Hojjatoleslami A, Sira M, Schofield JB, Jones C, Podoleanu AG. Investigation of basal cell carcinoma using dynamic focus optical coherence tomography. Appl Opt. 2013;52:2116–2124. doi: 10.1364/AO.52.002116. [DOI] [PubMed] [Google Scholar]
- 10.Avanaki MR, Hojjat A, Podoleanu AG. Investigation of computer-based skin cancer detection using optical coherence tomography. J Mod Optic. 2009;56:1536–1544. [Google Scholar]
- 11.Taghavi A, Abad K, Adabi S, et al. A novel dermo-epidermal localization algorithm for swept source OCT images of human skin; Paper presented at: SPIE BiOS2017; San Francisco, CA. January 28, 2017. [Google Scholar]
- 12.Avanaki MR, Hojjatoleslami A. Skin layer detection of optical coherence tomography images. Optik. 2013;124:5665–5668. [Google Scholar]
- 13.Otis LL, Everett MJ, Sathyam US, Colston BW. Optical coherence tomography: a new imaging technology for dentistry. J Am Dent Assoc. 2000;131:511–514. doi: 10.14219/jada.archive.2000.0210. [DOI] [PubMed] [Google Scholar]
- 14.Sivak MV, Kobayashi K, Izatt JA, et al. High-resolution endoscopic imaging of the GI tract using optical coherence tomography. Gastrointest Endosc. 2000;51:474–479. doi: 10.1016/s0016-5107(00)70450-0. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto JG, Brezinski ME, Tearney GJ, et al. Optical biopsy and imaging using optical coherence tomography. Nat Med. 1995;1:970–972. doi: 10.1038/nm0995-970. [DOI] [PubMed] [Google Scholar]
- 16.Brezinski ME, Tearney GJ, Bouma BE, et al. Optical coherence tomography for optical biopsy properties and demonstration of vascular pathology. Circulation. 1996;93:1206–1213. doi: 10.1161/01.cir.93.6.1206. [DOI] [PubMed] [Google Scholar]
- 17.Jang I-K, Bouma BE, Kang D-H, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39:604–609. doi: 10.1016/s0735-1097(01)01799-5. [DOI] [PubMed] [Google Scholar]
- 18.Drexler W, Fujimoto JG. Optical coherence tomography in ophthalmology. J Biomed Opt. 2007;12:041201. doi: 10.1117/1.2773736. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt JM, Xiang S, Yung KM. Speckle in optical coherence tomography. J Biomed Opt. 1999;4:95–105. doi: 10.1117/1.429925. [DOI] [PubMed] [Google Scholar]
- 20.De Boer JF, Cense B, Park BH, Pierce MC, Tearney GJ, Bouma BE. Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Opt Lett. 2003;28:2067–2069. doi: 10.1364/ol.28.002067. [DOI] [PubMed] [Google Scholar]
- 21.Avanaki MR, Bradu A, Trifanov I, Ribeiro ABL, Hojjatoleslami A, Podoleanu AG. Algorithm for excitation optimization of Fabry-Pérot filters used in swept sources. IEEE Photonic Tech L. 2013;25:472–475. [Google Scholar]
- 22.Gladkova ND, Petrova G, Nikulin N, et al. In vivo optical coherence tomography imaging of human skin: norm and pathology. Skin Res Technol. 2000;6:6–16. doi: 10.1034/j.1600-0846.2000.006001006.x. [DOI] [PubMed] [Google Scholar]
- 23.Jian Y, Wong K, Sarunic MV. Graphics processing unit accelerated optical coherence tomography processing at megahertz axial scan rate and high resolution video rate volumetric rendering. J Biomed Opt. 2013;18:26002. doi: 10.1117/1.JBO.18.2.026002. [DOI] [PubMed] [Google Scholar]
- 24.Drexler W. Ultrahigh-resolution optical coherence tomography. J Biomed Opt. 2004;9:47–74. doi: 10.1117/1.1629679. [DOI] [PubMed] [Google Scholar]
- 25.Izatt J, Choma M. Theory of optical coherence tomography. In: Drexler W, Fujimoto JG, editors. Optical Coherence Tomography: Technology and Applications. Berlin: Germany: Springer; 2008. pp. 47–72. [Google Scholar]
- 26.De Boer JF, Milner TE. Review of polarization sensitive optical coherence tomography and Stokes vector determination. J Biomed Opt. 2002;7:359–371. doi: 10.1117/1.1483879. [DOI] [PubMed] [Google Scholar]
- 27.Kut C, Chaichana KL, Xi J, et al. Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci Transl Med. 2015;7:292ra100. doi: 10.1126/scitranslmed.3010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujimoto JG, Pitris C, Boppart SA, Brezinski ME. Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia. 2000;2:9–25. doi: 10.1038/sj.neo.7900071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zysk AM, Nguyen FT, Oldenburg AL, Marks DL, Boppart SA. Optical coherence tomography: a review of clinical development from bench to bedside. J Biomed Opt. 2007;12:051403. doi: 10.1117/1.2793736. [DOI] [PubMed] [Google Scholar]
- 30.Adabi S, Conforto S, Hosseinzadeh M, et al. Textural Analysis of Optical Coherence Tomography Skin Images: quantitative differentiation between healthy and cancerous tissues; Paper presented at: SPIE 2017; San Francisco, CA. January 28, 2017. [Google Scholar]
- 31.Goodman JW. Introduction to Fourier Optics. New York, NY: MacGraw-Hill; 1960. [Google Scholar]
- 32.Yura HT, Hanson SG. Optical beam wave propagation through complex optical systems. JOSA A. 1987;4:1931–1948. [Google Scholar]
- 33.Yura HT, Thrane L, Andersen PE. Closed-form solution for the Wigner phase-space distribution function for diffuse reflection and small-angle scattering in a random medium. JOSA A. 2000;17:2464–2474. doi: 10.1364/josaa.17.002464. [DOI] [PubMed] [Google Scholar]
- 34.Born M, Wolf E. Principles of Optics. 6th ed. Oxford, UK: Pergamon Press; 1980. pp. 188–189. [Google Scholar]
- 35.Drexler W, Fujimoto JG. Optical Coherence Tomography: Technology and Applications. Berlin, Germany: Springer; 2008. [Google Scholar]
- 36.Schmitt JM, Knuttel A, Yadlowsky M, Eckhaus M. Optical-coherence tomography of a dense tissue: statistics of attenuation and backscattering. Phys Med Biol. 1994;39:1705. doi: 10.1088/0031-9155/39/10/013. [DOI] [PubMed] [Google Scholar]
- 37.Thrane L, Frosz MH, Jørgensen TM, Tycho A, Yura HT, Andersen PE. Extraction of optical scattering parameters and attenuation compensation in optical coherence tomography images of multilayered tissue structures. Opt Lett. 2004;29:1641–1643. doi: 10.1364/ol.29.001641. [DOI] [PubMed] [Google Scholar]
- 38.Avanaki MR, Podoleanu AG, Schofield JB, et al. Quantitative evaluation of scattering in optical coherence tomography skin images using the extended Huygens-Fresnel theorem. Appl Opt. 2013;52:1574–1580. doi: 10.1364/AO.52.001574. [DOI] [PubMed] [Google Scholar]
- 39.Turani Z, Emad F, Avanaki MRN. Refractive index correction in optical coherence tomography images; The 23rd Iranian Conference on Optics and Photonics (ICOP 2017) and the 9th Iranian Conference on Photonics Engineering and Technology (ICPET 2017); Tehran, Iran. February 2, 2017. [Google Scholar]
- 40.Bashkansky M, Reintjes J. Statistics and reduction of speckle in optical coherence tomography. Opt Lett. 2000;25:545–547. doi: 10.1364/ol.25.000545. [DOI] [PubMed] [Google Scholar]
- 41.Hee MR, Izatt JA, Swanson EA, et al. Optical coherence tomography of the human retina. Arch Ophthalmol. 1995;113:325–332. doi: 10.1001/archopht.1995.01100030081025. [DOI] [PubMed] [Google Scholar]
- 42.Magnin PA, von Ramm OT, Thurstone FL. Frequency compounding for speckle contrast reduction in phased array images. Ultrason Imaging. 1982;4:267–281. doi: 10.1177/016173468200400303. [DOI] [PubMed] [Google Scholar]
- 43.Jørgensen TM, Thrane L, Mogensen M, Pedersen F, Andersen PE. Speckle reduction in optical coherence tomography images of human skin by a spatial diversity method; Paper presented at: European Conference on Biomedical Optics; Munich, Germany. June 17, 2007. [Google Scholar]
- 44.Iftimia N, Bouma BE, Tearney GJ. Speckle reduction in optical coherence tomography by “path length encoded” angular compounding. J Biomed Opt. 2003;8:260–263. doi: 10.1117/1.1559060. [DOI] [PubMed] [Google Scholar]
- 45.Wang RK. Reduction of speckle noise for optical coherence tomography by the use of nonlinear anisotropic diffusion; Paper presented at: Biomedical Optics 2005; San Jose, CA. January 22, 2005. [Google Scholar]
- 46.Smithies DJ, Lindmo T, Chen Z, Nelson JS, Milner TE. Signal attenuation and localization in optical coherence tomography studied by Monte Carlo simulation. Phys Med Biol. 1998;43:3025. doi: 10.1088/0031-9155/43/10/024. [DOI] [PubMed] [Google Scholar]
- 47.Adler DC, Ko TH, Fujimoto JG. Speckle reduction in optical coherence tomography images by use of a spatially adaptive wavelet filter. Opt Lett. 2004;29:2878–2880. doi: 10.1364/ol.29.002878. [DOI] [PubMed] [Google Scholar]
- 48.Goodman JW. Speckle Phenomena in Optics: Theory and Applications. Greenwood Village, CO: Roberts & Company Publishers; 2007. [Google Scholar]
- 49.Pircher M, Go E, Leitgeb R, Fercher AF, Hitzenberger CK. Speckle reduction in optical coherence tomography by frequency compounding. J Biomed Opt. 2003;8:565–569. doi: 10.1117/1.1578087. [DOI] [PubMed] [Google Scholar]
- 50.Goodman JW. Some fundamental properties of speckle*. J Opt Soc Am. 1976;66:1145–1150. [Google Scholar]
- 51.Avanaki MR, Cernat R, Tadrous PJ, Tatla T, Podoleanu AG, Hojjatoleslami SA. Spatial compounding algorithm for speckle reduction of dynamic focus OCT images. IEEE Photonic Tech L. 2013;25:1439–1442. [Google Scholar]
- 52.Ozcan A, Bilenca A, Desjardins AE, Bouma BE, Tearney GJ. Speckle reduction in optical coherence tomography images using digital filtering. JOSA A. 2007;24:1901–1910. doi: 10.1364/josaa.24.001901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harwood D, Subbarao M, Hakalahti H, Davis LS. A new class of edge-preserving smoothing filters. Pattern Recogn Lett. 1987;6:155–162. [Google Scholar]
- 54.Nieminen A, Heinonen P, Neuvo Y. A new class of detail-preserving filters for image processing. IEEE T Pattern Anal. 1987;1:74–90. doi: 10.1109/tpami.1987.4767873. [DOI] [PubMed] [Google Scholar]
- 55.Kyprianidis JE, Kang H, Döllner J. Image and video abstraction by anisotropic Kuwahara filtering. Comput Graph Forum. 2009;28:1955–1963. [Google Scholar]
- 56.Bakker P, van Vliet LJ, Verbeek PW. Edge preserving orientation adaptive filtering; Paper presented at: IEEE Computer Society Conference on Computer Vision and Pattern Recognition; Fort Collins, CO. June 23–25, 1999. [Google Scholar]
- 57.Turani Z, Fatemizadeh E, Adabi S, Mehregan D, Daveluy S, Nasiriavanaki M. Noise reduction in OCT skin images; Paper presented at: SPIE Medical Imaging; Orlando, FL. February 11, 2017. [Google Scholar]
- 58.Aum J, Kim J-h, Jeong J. Effective speckle noise suppression in optical coherence tomography images using nonlocal means denoising filter with double Gaussian anisotropic kernels. Appl Opt. 2015;54:D43–D50. [Google Scholar]
- 59.Rudin LI, Osher S, Fatemi E. Nonlinear total variation based noise removal algorithms. Physica D. 1992;60:259–268. [Google Scholar]
- 60.Chong B, Zhu YK. Speckle reduction in optical coherence tomography images of human finger skin by wavelet modified BM3D filter. Opt Commun. 2013;291:461–469. [Google Scholar]
- 61.Dabov K, Foi A, Katkovnik V, Egiazarian K. Image denoising by sparse 3-D transform-domain collaborative filtering. IEEE T Image Process. 2007;16:2080–2095. doi: 10.1109/tip.2007.901238. [DOI] [PubMed] [Google Scholar]
- 62.Dabov K, Foi A, Katkovnik V, Egiazarian K. Image restoration by sparse 3D transform-domain collaborative filtering; Electronic Imaging 2008 International Society for Optics and Photonics; Feb, 2008. pp. 681207–681207. [Google Scholar]
- 63.Buades A, Coll B, Morel JM. A non-local algorithm for image denoising; Paper presented at: IEEE Computer Society Conference on Computer Vision and Pattern Recognition (CVPR 2005); Washington, DC. June 20–26, 2005. [Google Scholar]
- 64.Adabi S, Rashedi E, Conforto S, Mehregan D, Xu Q, Nasiriavanaki M. Speckle reduction of OCT images using an adaptive cluster-based filtering; Paper presented at: Proceedings of SPIE; San Francisco, CA. January 28, 2017. [Google Scholar]
- 65.Kafieh R, Rabbani H. Optical Coherence Tomography noise reduction over learned dictionaries with introduction of complex wavelet for start dictionary; Paper presented at: SPIE Optical Engineering + Applications; San Diego, CA. August 25, 2013. [Google Scholar]
- 66.Avanaki M, Laissue P, Podoleanu AG, Aber A, Hojjatoleslami S. Evaluation of wavelet mother functions for speckle noise suppression in OCT images. Int J Graphics Bioinf Med Eng. 2011;11:1–5. [Google Scholar]
- 67.Avanaki M, Laissue PP, Eom TJ, Podoleanu AG, Hojjatoleslami A. Speckle reduction using an artificial neural network algorithm. Appl Opt. 2013;52:5050–5057. doi: 10.1364/AO.52.005050. [DOI] [PubMed] [Google Scholar]
- 68.Avanaki MR, Laissue PP, Eom TJ, Podoleanu AG, Hojjatoleslami A. Speckle reduction using an artificial neural network algorithm. Appl Opt. 2013;52:5050–5057. doi: 10.1364/AO.52.005050. [DOI] [PubMed] [Google Scholar]
- 69.Adabi S, Conforto S, Clayton A, Podoleanu AG, Hojjat A, Avanaki M. An intelligent speckle reduction algorithm for optical coherence tomography images; Paper presented at: Proceedings of the 4th International Conference on Photonics, Optics and Laser Technology; Rome, Italy. February 2016. [Google Scholar]
- 70.Avanaki MR, Laissue PP, Podoleanu AG, Hojjat A. Denoising based on noise parameter estimation in speckled OCT images using neural network; Paper presented at: 1st Canterbury Workshop and School in Optical Coherence Tomography and Adaptive Optics; Canterbury, UK. September 6, 2008. [Google Scholar]
- 71.Nasiriavanaki M, Marques MJ, Bradu A, Hojjatoleslami A, Podoleanu AG. A new algorithm for speckle reduction of optical coherence tomography images; Paper presented at: SPIE BiOS2014; San fransisco, CA. March 4, 2014. [Google Scholar]
- 72.Avanaki M, Laissue PP, Hojjatoleslami A. De-noising speckled optical coherence tomography images using an algorithm based on artificial neural network. J Neurosci Neuroeng. 2013;2:347–352. [Google Scholar]
- 73.Hojjatoleslami A, Avanaki MR. OCT skin image enhancement through attenuation compensation. Appl Opt. 2012;51:4927–4935. doi: 10.1364/AO.51.004927. [DOI] [PubMed] [Google Scholar]
- 74.Avanaki MR, Hojjatoleslami A. Speckle reduction with attenuation compensation for skin OCT images enhancement; Proceeding of Medical Image Understanding and Analysis (MIUA); London, UK. July 14–15, 2009; Kingston University; pp. 179–183. [Google Scholar]
- 75.Yun S, Tearney G, De Boer J, Bouma B. Motion artifacts in optical coherence tomography with frequency-domain ranging. Opt Exp. 2004;12:2977–2998. doi: 10.1364/opex.12.002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liew YM, McLaughlin RA, Wood FM, Sampson DD. Motion correction of in vivo three-dimensional optical coherence tomography of human skin using a fiducial marker. Biomed Opt Express. 2012;3:1774–1786. doi: 10.1364/BOE.3.001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Westphal V, Rollins A, Radhakrishnan S, Izatt J. Correction of geometric and refractive image distortions in optical coherence tomography applying Fermat’s principle. Opt Exp. 2002;10:397–404. doi: 10.1364/oe.10.000397. [DOI] [PubMed] [Google Scholar]
- 78.Nasiri-Avanaki M-R, Hojjatoleslami S, Paun H, et al. Optical coherence tomography system optimization using simulated annealing algorithm; Proceedings of Mathematical Methods and Applied Computing (WSEAS, 2009); Athens, Greece. September 28–30, 2009; pp. 669–674. [Google Scholar]
- 79.Avanaki MR, Podoleanu AG, Price MC, Corr SA, Hojjatoleslami S. Two applications of solid phantoms in performance assessment of optical coherence tomography systems. Appl Opt. 2013;52:7054–7061. doi: 10.1364/AO.52.007054. [DOI] [PubMed] [Google Scholar]
- 80.Hojjatoleslami S, Avanaki M, Podoleanu AG. Image quality improvement in optical coherence tomography using Lucy–Richardson deconvolution algorithm. Appl Opt. 2013;52:5663–5670. doi: 10.1364/AO.52.005663. [DOI] [PubMed] [Google Scholar]
- 81.Vargas-Martın F, Prieto PM, Artal P. Correction of the aberrations in the human eye with a liquid-crystal spatial light modulator: limits to performance. JOSA A. 1998;15:2552–2562. doi: 10.1364/josaa.15.002552. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y, Liang Y, Mu G, Zhu X. Deconvolution methods for image deblurring in optical coherence tomography. JOSA A. 2009;26:72–77. doi: 10.1364/josaa.26.000072. [DOI] [PubMed] [Google Scholar]
- 83.Ralston TS, Marks DL, Kamalabadi F, Boppart SA. Deconvolution methods for mitigation of transverse blurring in optical coherence tomography. IEEE Trans Image Process. 2005;14:1254–1264. doi: 10.1109/tip.2005.852469. [DOI] [PubMed] [Google Scholar]
- 84.Kulkarni MD, Izatt JA, Sivak MV. Image enhancement in optical coherence tomography using deconvolution. US5994690. Google Patents. 1999 Nov 30;
- 85.Woolliams PD, Ferguson RA, Hart C, Grimwood A, Tomlins PH. Spatially deconvolved optical coherence tomography. Appl Opt. 2010;49:2014–2021. doi: 10.1364/AO.49.002014. [DOI] [PubMed] [Google Scholar]
- 86.Almasganj M, Adabi S, Fatemizadeh E, et al. A spatially variant deconvolution method based on total variation for optical coherence tomography images; Paper presented at: SPIE Medical Imaging; Orlando, FL. February 11, 2017. [Google Scholar]
- 87.Avanaki MR, Marques MJ, Bradu A, Hojjatoleslami A, Podoleanu AG. A new algorithm for speckle reduction of optical coherence tomography images; Paper presented at: SPIE BiOS2014; San Fransisco, CA. March 4, 2014. [Google Scholar]
- 88.Hojjatoleslami A, Avanaki M. OCT skin image enhancement through attenuation compensation. Appl Opt. 2012;51:4927–4935. doi: 10.1364/AO.51.004927. [DOI] [PubMed] [Google Scholar]
- 89.Akcay AC, Lee K, Rolland JP. Dispersion manipulation in optical coherence tomography with Fourier-domain optical delay line; Paper presented at: Biomedical Optics 2005; San Jose, CA. January 22, 2005. [Google Scholar]
- 90.Wojtkowski M, Srinivasan VJ, Ko TH, Fujimoto JG, Kowalczyk A, Duker JS. Ultrahigh-resolution, high-speed, Fourier domain optical coherence tomography and methods for dispersion compensation. Opt Exp. 2004;12:2404–2422. doi: 10.1364/opex.12.002404. [DOI] [PubMed] [Google Scholar]
- 91.Fercher A, Hitzenberger C, Sticker M, Zawadzki R, Karamata B, Lasser T. Dispersion compensation for optical coherence tomography depth-scan signals by a numerical technique. Opt Commun. 2002;204:67–74. doi: 10.1364/oe.9.000610. [DOI] [PubMed] [Google Scholar]
- 92.Hakki B. Polarization mode dispersion compensation by phase diversity detection. IEEE Photonic Tech L. 1997;9:121–123. [Google Scholar]
- 93.Avanaki MR, Cernat R, Tadrous PJ, Tatla T, Podoleanu AG, Hojjatoleslami SA. Spatial compounding algorithm for speckle reduction of dynamic focus OCT images. IEEE Photonic Tech L. 2013;25:1439–1442. [Google Scholar]
- 94.Hariri A, Bely N, Chen C, Nasiriavanaki M. Towards ultrahigh resting-state functional connectivity in the mouse brain using photoacoustic microscopy; Paper presented at: SPIE BiOS2016; San Francisco, CA. February 13, 2016. [Google Scholar]
- 95.Avanaki MR, Xia J, Wang LV. High resolution functional photoacoustic computed tomography of the mouse brain during electrical stimulation; Paper presented at: SPIE BiOS2013; San Francisco, CA. February 2, 2013. [Google Scholar]