Supplemental Digital Content is available in the text.
Keywords: China, cost, intensive care unit, mechanical ventilation, outcome
Abstract
Objective:
To evaluate the contemporary practice, outcomes, and costs related to mechanical ventilation among ICUs in China.
Design:
A prospective observational cohort study.
Setting:
Fourteen ICUs among 13 hospitals in Beijing, China.
Patients:
Seven hundred ninety-three patients who received at least 24 hours of mechanical ventilation within the first 48 hours of ICU stay.
Intervention:
None.
Measurements and results:
The mean age was 64 years. Sixty-three percent were male. New acute respiratory failure accounted for 85.5% of mechanical ventilation cases. Only 4.7% of the patients received mechanical ventilation for acute exacerbation of chronic obstructive pulmonary disease. The most widely used ventilation mode was the combination of synchronized intermittent mandatory ventilation and pressure support (43.6%). Use of lung-protective ventilation is widespread with tidal volumes of 7.1 mL/kg (2.1 mL/kg). The ICU/hospital mortality was 27.6%/29.3%, respectively (8.5%/9.7% for surgical patients and 41.3%/43.2% for medical patients, respectively). The mean level of ICU/hospital cost per patient was $15,271 (18,940)/$22,946 (25,575), respectively. The mean daily ICU cost per patient was $1,212.
Conclusion:
For the first time, we obtained a preliminary epidemiology data of mechanical ventilation in Beijing, China, through the study. Compared with the other nations, our patients are older, predominantly male, and treated according to prevailing international guidelines yet at a relatively high cost and high mortality. The expanding elderly population predicts increase demand for mechanical ventilation that must be met by continuous improvement in quality and efficiency of critical care services.
Mechanical ventilation (MV) is a cornerstone of critical care medicine (1). Although descriptions of the practice and costs of MV in western critical care units have appeared over the past 2 decades, little has been reported from the Far East and nothing from China (2–7).
Different from western nations, MV did not become widely available in China until the 1980s (8). Three forces catalyzed rapid spread: economic growth, improvement and integration of the healthcare system, and—like the west—a rapidly aging population. The aim of this study was to obtain the information and evaluate the contemporary acute MV practice, outcomes, and costs for ICU patients in major urban Chinese hospitals.
PATIENTS AND METHODS
Prior to the study, we obtained approval from the ethical oversight committees of each participating units. To minimize the potential effects of observation on behavior (Hawthorn effect) and thereby obtain the most accurate assessment of contemporary practice, only the investigator and research coordinator in each ICU were aware that the study was being conducted.
A prospective observational cohort study was performed in 14 ICUs in Beijing, China, from March 2011 to March 2012. All 13 hospitals included in the study were tertiary hospitals. Each hospital is affiliated with a medical university; each has more than 600 beds. Among the 14 participating ICUs, 10 were medical-surgical ICU, two surgical ICU, one respiratory ICU, and one medical ICU. In order to qualify for inclusion in the cohort, each ICU was required to have at least six beds and at least 60% of the physicians were required to have had to undergone ICU training or have more than 5 years of experience in ICU.
The study population consisted of adults (> 18 yr) admitted to the participating ICUs and received invasive MV for more than 24 consecutive hours within the first 48 hours of ICU admission. Patients received ventilation support less than 24 hours, less than 18 years old, and discharged against medical advice were excluded. Patients with neuromuscular disease were excluded too owing to their often very prolonged dependence on MV, a dependence that would skew the results.
The investigator and research coordinator selected from each participating ICU were trained at the core center and were provided with a data dictionary and a manual for data collection. Investigators collected the data on eligible patients and filled out the paper forms in their own hospital; coordinators responded to all ambiguities regarding data collection. Patients in the cohort were followed-up until hospital discharge. Costs in the ICU and hospital were obtained from the central information center of each hospital (Supplemental Digital Content 1, http://links.lww.com/CCM/C481). At the end of the study period, the data collection forms were sent to Fu Xing Hospital, where data were manually entered into software forms. Each questionnaire was independently checked by two study coordinators to identify omissions and inconsistencies. Inconsistent data were reviewed and reconciled by review of the original data.
The patient’s data collection form consisted of three sections:
1) Basic clinical data: including patients demographic characteristics (age, sex, height, actual body weight, and predicted body weight), the admission Acute Physiology and Chronic Health Evaluation (APACHE) II score; diagnoses and comorbidities (identified according to the International Classification of Disease, 10th Edition). The predicted body weight for men/women (kg) = actual body height (cm) – 110/actual body height (cm) – 105.
2) Data related to MV: the time and the indication for initiation of MV, arterial blood gas analysis just before the start of ventilation, ventilator mode and setting in the first hour from the initiation of the MV; sedative, analgesic, and neuromuscular blocking agents (given for ≥ 3 hr during a 24-hr period) recorded daily.
3) Data describing the clinical outcome and the cost of each patient.
Outcomes of interest included ICU mortality and hospital mortality, duration of MV, length of stay (LOS) in the ICU and hospital, and ICU and hospital costs.
The indication for the initiation of MV was selected from the following predefined categories: 1) acute respiratory failure (ARF); 2) acute exacerbation of chronic respiratory failure, consisted of acute exacerbation of chronic obstructive pulmonary disease (AECOPD), asthma, and other chronic pulmonary diseases (other-CPDs); and 3) coma. The patients categorized as ARF were separated into the following subgroups: 1) postoperative state; 2) pneumonia; 3) acute respiratory distress syndrome (ARDS); 4) sepsis; 5) congestive heart failure; 6) cardiac arrest; 7) aspiration; 8) trauma; and 9) others. The definitions of ARDS, sepsis, and chronic obstructive pulmonary disease (COPD) were used as described previously (9–11). The definition of the other indications can be seen in the reference article by Esteban et al (4). We defined patients with other CPDs excluding COPD and asthma as other-CPDs, including those with pulmonary fibrosis, bronchiectasis, and tuberculosis-destroyed lung obsolete, mainly. Admission was defined as surgical (postoperative) if the patient was admitted to the ICU from the operation theatre or recover room. Whenever a patient had more than one indication for MV, the investigator judged according to the dominant indication.
All analyses were undertaken using SPSS 19.0 (IBM Corp, New York, NY). Data are expressed as mean (sd), median (interquartile range), and absolute and relative frequencies, as appropriate. Student t test for continuously normally distributed data, Mann-Whitney U test for non-normally distributed data, and chi-square test for categorical variables were used. Two-tailed p values of less than 0.05 were used as the threshold for statistical significance. Univariate analysis and 95% CIs were calculated and those variables with p value of less than 0.05 were included in the multiple variables stepwise logistic regression model to estimate the effects of variables on ICU mortality. The Hosmer-Lemeshow test and C-statistic were used to evaluate the goodness-of-fit for logistic regression model.
RESULTS
A total of 793 cases were included, and the database was constructed for the study. Four hundred sixty-three were medical patients, and 330 were postoperative patients.
Basic Data
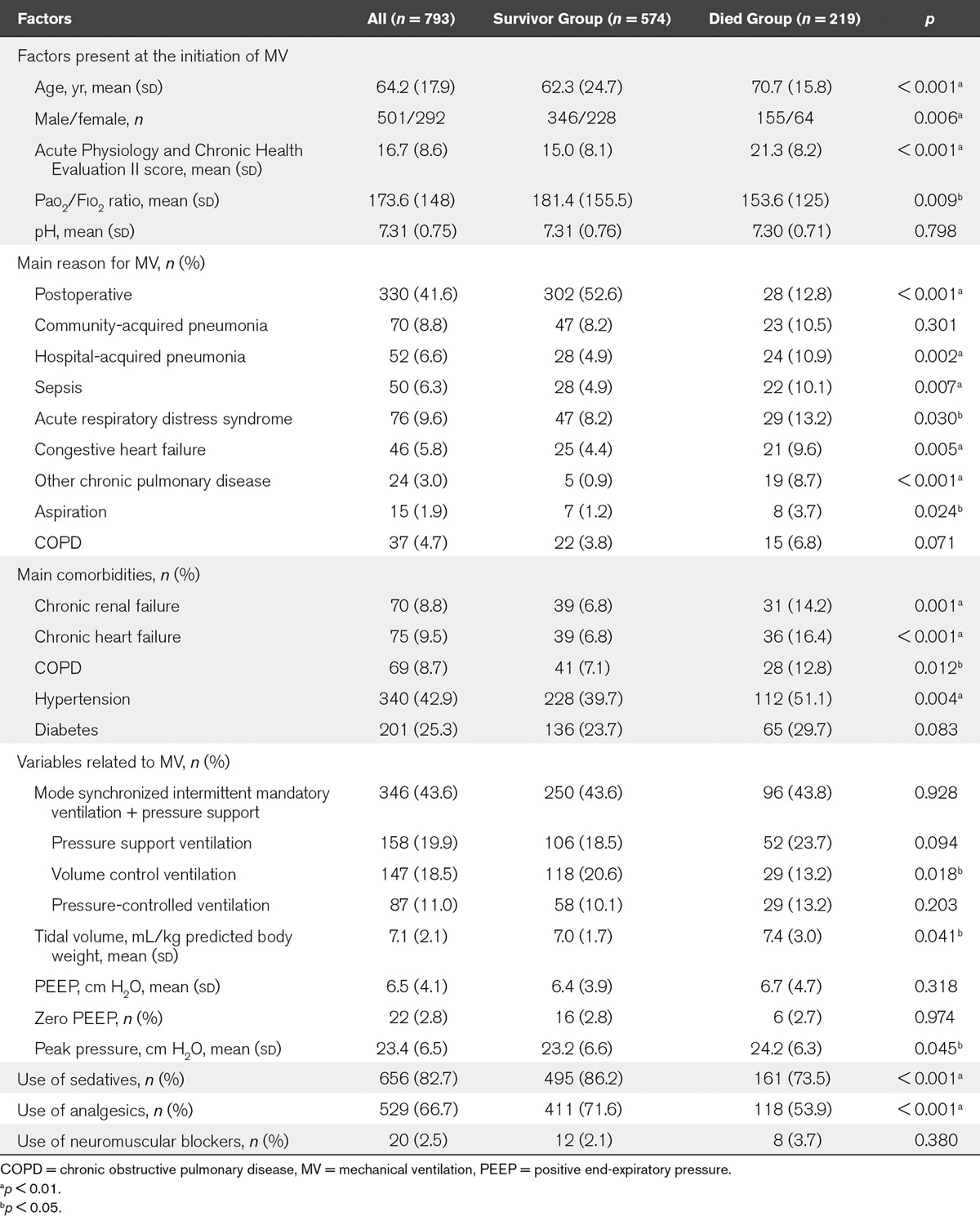
The patients’ age ranged from 18 to 97 years old, the mean age was 64 years (18 yr). Men accounted for 63.2% of the patients, with a significant difference (p < 0.001). The mean APACHE II score was 16.7 (8.6) for all patients. The APACHE II score for medical patients was much higher than that for surgical patients (19.8 [7.9] vs 12.3 [7.1]; p < 0.001).
Indication for MV
ARF was the most frequent reason for the initiation of MV, representing 85.5% of the study population. Acute exacerbation of CPD only accounted for 8.5% of the total patients. The leading causes of ARF were postoperative (41.6%), pneumonia (15.4%), ARDS (9.6%), sepsis (6.3%), and congestive heart failure (5.8%). The AECOPD and coma accounted for 4.7% and 5.7% of the total patients, respectively.
The detail of percent of indications for MV and cause of ARF for all the patients are shown in Figure 1, A and B, respectively.
Figure 1.
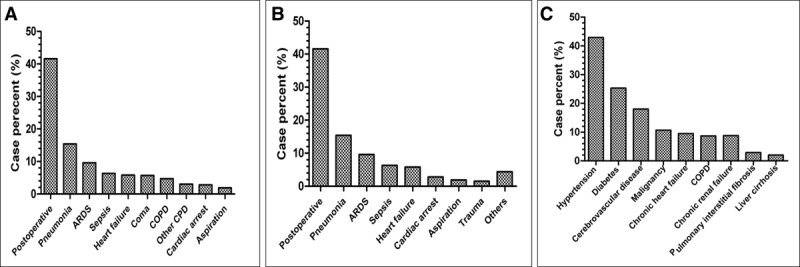
Distribution of indications for (A) mechanical ventilation; (B) cause of acute respiratory failure; and (C) the main comorbidities of all the patients (n = 793). ARDS = acute respiratory distress syndrome, COPD = chronic obstructive pulmonary disease, CPD = chronic pulmonary disease.
The major common comorbidities were hypertension (42.9%), diabetes (25.3%), cerebrovascular disease (18%), and malignancy (10.7%). The prevalence of comorbidities for the population is shown in Figure 1C.
Management of MV
The combination of synchronized intermittent mandatory ventilation and pressure support mode (SIMV+PS) was the most preferred mode and used in 43.6% of the patients, followed by PS alone (19.9%), volume assist/control ventilation (VCV, 18.5%), pressure control ventilation (PCV, 11%), SIMV (5.5%), and pressure-regulated volume control (0.8%).
The mean tidal volume setting was 7.1 mL/kg (2.1 mL/kg) in all patients. There were no significant differences on tidal volume setting between medical and surgical patients (7.1 [2.5] vs 7.1 [1.6] mL/kg; p = 0.123), and between COPD and ARDS patients (7.4 [4.9] vs 6.9 [1.6] mL/kg; p = 0.068).
The mean level of positive end-expiratory pressure (PEEP) for all patients was 6.5 (4.1) cm H2O. Patients with ARDS received higher PEEP than patients with COPD (8.0 [3.7] vs 6.0 [2.4] cm H2O; p = 0.043). Only 22 patients (2.9%) ventilated without PEEP.
In total, 82.6% of the patients were given with sedative therapy, 66.7% were given with analgesics, and only 2.5% required a neuromuscular blocker.
Outcomes
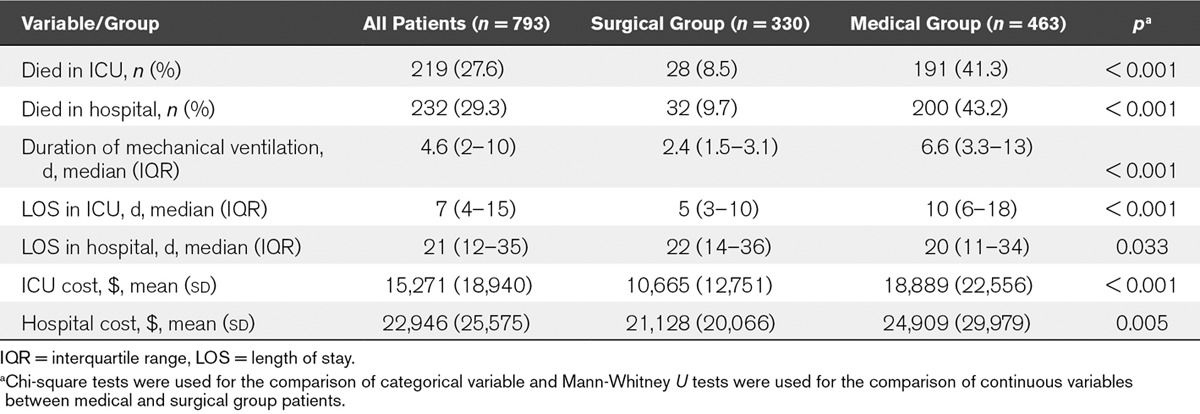
Overall durations of ventilator support were 4.6 days (2–10 d). The median LOS in the ICU was 7 days (4–15 d). LOS in the hospital was 21 days (12–35 d). Compared with surgical patients, medical patients received longer MV (6.6 [3.3–13] vs 2.4 [1.5–3.1] d; p < 0.001) and longer LOS in the ICU (10 [6–18] vs 5 [3–10] d; p < 0.001). The LOS in the hospital for surgical patients was longer than that of medical patients (22 [4–36] vs 20 [11–34]; p = 0.033).
The total ICU mortality and hospital mortality were 27.6% and 29.3%, respectively. Compared with surgical patients, medical patients have much higher ICUs mortality (41.3% vs 8.5%; p < 0.001) and hospital mortality (43.2% vs 9.7%; p < 0.001).
Cost
The mean level of ICU/hospital cost per patient was $15,271 (18,940)/$22,946 (25,575), respectively. The mean ICU cost for medical patients was higher than that for surgical patients ($18,889 [22,556] vs $10,665 [12,751]; p < 0.001), and the mean hospital cost for medical patients was higher than that for surgical patients ($24,909 [29,979] vs $21,128 [20,066]; p = 0.005) as well.
The observed outcomes and costs of all the patients are shown in Table 1.
TABLE 1.
Outcomes and Cost for Patients Who Received Mechanical Ventilation: Surgical Versus Medical Comparison
The mean daily ICU cost per patient was $1,212, and among the total ICU cost, the medicine costs accounted for 42.2%, followed by disposable medical materials (20.1%), laboratory investigation cost (15%), and labor cost (8.7%). The cost estimate methodology and the hospital costs composition in percentage are detailed in Supplemental Digital Content 1 (http://links.lww.com/CCM/C481).
Outcomes and Indications and Management of MV
According to the patient’s outcome in ICU, all patients were divided into two groups (survivor or died). Compared with the survivor group patients, died group patients appeared with higher age, higher APACHE scores, more medical patients, and comorbidities. Detailed results are shown in Table 2.
TABLE 2.
Comparisons of Clinical Characteristics, Main Indications, Comorbidities, and the Management of Mechanical Ventilation Between Survivor and Died Group Patients in ICU
The multiple logistic regression mode reveals that APACHE score at ICU admission (odds ratio [OR], 1.061; 95% CI, 1.038–1.085; p < 0.001) and other-CPDs (OR, 8.044; 95% CI, 2.842–22.769; p < 0.001) were independently significant risk factors for the ICU mortality. The Hosmer-Lemeshow test (p = 0.196) and C-statistic (0.774; 95% CI, 0.737–0.810) demonstrated that the logistic regression model is appropriate.
The detailed information referred in the Supplemental Digital Content 2 (http://links.lww.com/CCM/C482).
Outcomes and Costs
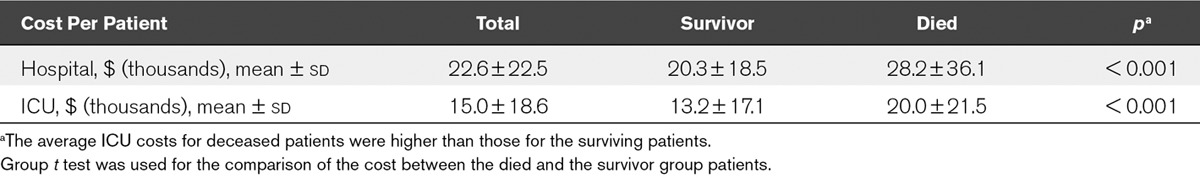
The mean ICU cost for ICU died group patients was higher than that for ICU survivor group patients ($ [thousands], 20.0 [21.5] vs 13.2 [17.1]; p < 0.001). The detailed costs and outcomes for ICU and hospital were shown in Table 3.
TABLE 3.
Cost for Patients Who Received Mechanical Ventilation: Survivor Versus Died Group
The ICU and hospital costs were analyzed according to the outcome (live or die) and different age group, the results also revealed that patients in died groups with higher costs than patients in survivor groups (Fig. 2).
Figure 2.
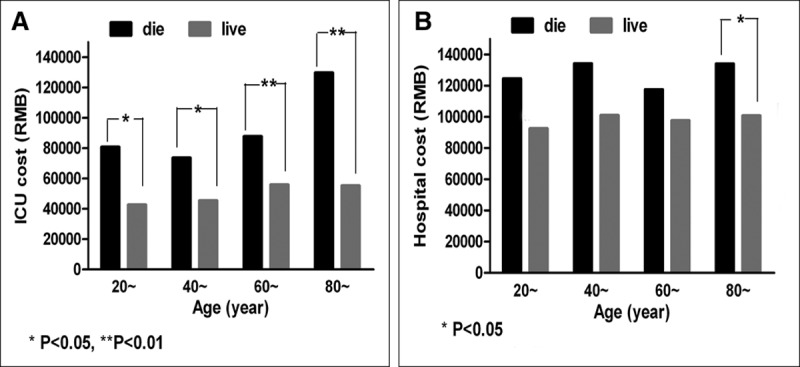
Comparison of ICU and hospital costs by outcome (survival or died) and by different age groups. RMB = Ren Min Bi (or China Yuan).
DISCUSSION
The study focused on patients admitted to tertiary hospital’s ICU and received MV for more than 24 hours in Beijing, China. We investigated characteristics, indications, and management of MV, outcomes, and costs of all the included patients. This information will be useful for us to evaluate our contemporary practice on MV and purse quality improvement and research in the future.
The mean age of our patients was 64 years (18 yr), being older than 61 years (17 yr) reported by Esteban et al (6). The majority of patients (63.2%) were male, but it is not clear whether the extraordinary predominance of male patients in this sample is representative of the national ICU population. It will therefore be important to determine whether men have more critical illness or alternatively whether they simply enjoy better access to critical care in China. Each has an important implication for management of health and resources in our nation.
Compared with the result reported by Esteban et al (5), more ARF (85.5% vs 68.8%) and less AECOPD (4.7% vs 10.1%) patients appeared in our cohort, too many postoperative patients included in our study was likely the main reason (41.6% in our study vs 21% in the study by Esteban et al [5]).
There appear to be national preferences in selection of the mode of ventilation: SIMV+PS in this cohort; PCV in the Korean cohort versus VCV in other cohorts (3–7). A recent study reported that there was no significant difference in outcomes of the patients ventilated with either VCV or PCV mode (12), but our study showed that VCV mode used in the survival group patients was more than used in nonsurvival group patients. Further studies will be needed to clarify which mode is better exactly.
Patients ventilated with a lower tidal volume are now understood to decrease mortality compared with traditional high tidal volumes (13–16). Epidemiology data from different countries (3–6) showed that 6–8 mL/kg predicted body weight has been adopted as a strategy. The mean tidal volume was 7.1 mL/kg (2.1 mL/kg) in our cohort. This suggested that Chinese physicians in the ICUs have embraced low-tidal volume ventilation as a lung-protective strategy.
PEEP settings appeared to correlate with the indication for ventilation: patients with ARDS in the cohort received a relatively higher level of PEEP. Clinical trials (17–20) have confirmed that a significant reduction in mortality was found only in patients with moderate and severe ARDS assigned to higher level PEEP. There is an ongoing research on how to set optimal PEEP in individual patients (21), and our initial data point to the need to understand how Chinese intensivists are choosing PEEP settings and whether there is a national opportunity to write, evaluate, and establish a guideline.
Sedative agents are commonly used in ICUs (22). A larger proportion of the patients were given with sedatives (83%) compared with other regional reports (71% and 56%) (6, 7).We noted that although there was extensive use of sedatives, there was little use of neuromuscular blocking agents in our cohort (3% in our study vs 11% and 26% in other nations) (6, 7). Again, what matters are clinical outcomes including preservation of strength and avoidance of awareness-related sequelae such as posttraumatic stress disorder.
Several studies have observed ICU/hospital mortality of patients receiving MV, 33.8%/35.6% reported in India in 2016 (23) and 28%/35% in the third International Survey in 2010 (6). A retrospective cohort study in United States also reported that the hospital mortality for all adult patients undergoing MV during hospitalization was 34.5% (2).
The total ICU/hospital mortality was 27.6%/29.3% in our study. It was superficially lower than that reported in the studies mentioned above. But the low-observed mortality should be interpreted with caution, for there was an excess of postoperative patients in our study (42% in this cohort vs 21% in the cohort by Esteban et al [6]) who had much lower mortality than medical patients. Namely, the ICU/hospital mortality was 8.5%/9.7% for surgical patients and 41.3%/43.2% for medical patients, respectively; when recalculated the mortality hypothesized, the ratio of surgical/medical patients was found to be same as 21%:79% in the cohort by Esteban et al (6); the standardized expected ICU/hospital mortality was 34.4%/36.2% in our cohort, and it was found to be higher than 28%/35% reported by Esteban et al (6) in 2010.
Mortality reported after MV may reflect patient selection, hospital-level variations in organizational structure (24–28), care given, or disposition (in some countries, patients are discharged “alive” but to hospice for terminal care). Thus, improvements are required in the organization and delivery of critical care in the ICU in China.
It has been reported that MV was associated with higher daily costs for patients in ICUs (29–32). The mean daily ICU costs per patient was $1,212 in our study, being lower than $3,500 in United States in 2005 (29), higher than approximately $299 in India in 2012 (30). Considering the differences in per capita gross domestic product (GDP) of the three countries, we calculated the percentages of mean daily ICU costs per capita GDP were 8.1% in America (2005), 19.8% in China (2012), and 20.2% in India (2012), respectively, in gross (Supplemental Digital Content 3, http://links.lww.com/CCM/C483). The ICU costs seem high due to relative higher ratio in China than that in the United States, and rational for the little difference between China and India being developing countries.
The main cost in our study was medicine (42% of the total), direct labor cost only accounted for 8.7% of the total, which were different from the other studies reported that the main cost of critical care was the labor cost (over 40% of the total), and the drug cost only accounted for 10–25% (33–35). For prices of medical services (such as doctor’s consultation, nursing care, and surgical operation) were solely formulated by the Chinese government and were pitifully low. The fees for 90% of the services are less than their average unit costs, although the State Price Commission allowed a drug profit margin of 15% over the wholesale price (36).
We also found that average ICU costs for deceased patients were higher than those for the surviving patients, different from that reported in United States (2). It might be that patients are admitted to the ICU in the United States for terminal care that is expected to be short and therefore not costly. It might be that Chinese physicians persist longer in hopeless cases. Understanding the origin of the differences by comparing national practices seems important if scarce critical care resources are to be effectively allocated.
This study has some limitations. First, the data from 14 Beijing’s tertiary hospital ICUs still do not represent the whole country. Second, the classified information of the cost was analyzed retrospectively and only included 87 patients, with relatively small sample size, case selection bias may exist.
In summary, we found that our patients are older, predominantly male, and treated according to prevailing international guidelines yet at a relatively high cost and high mortality. Our data suggesting that a disproportionate amount of our expense is applied to patients who do not survive the hospitalization has already prompted further analysis toward more efficient use of our ICU resources, and we suggest that tracking costs with respect to meaningful outcomes should be part of every ICU’s ongoing evaluation of the value it provided to patients, to hospitals, and to the government.
ACKNOWLEDGMENTS
We thank the following participating units: Departments of Critical Care Medicine in Peking University Third Hospital, China-Japan Friendship Hospital, Beijing Shijitan Hospital, the 309th Hospital of Chinese People’s Liberation Army, Beijing Tong Ren Hospital of Capital Medical University, Beijing Tian Tan Hospital of Capital Medical University, Beijing Haidian Hospital, the 304th Hospital of Chinese People’s Liberation Army, and Beijing Friendship Hospital of Capital Medical University; Respiratory Intensive Care Unit and Surgery Intensive Care Unit of Beijing Chao-Yang Hospital of Capital Medical University; Medical Intensive Care Unit of Peking Union Medical College Hospital; and Surgery Intensive Care Unit of Xuan Wu Hospital of Capital Medical University, Beijing, China.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by the Capital Health Research and Development of Special. (2007-1042).
Dr. Ye’s institution received funding from Beijing Municipal Commission of Health and Family Planning. Drs. Zhu, Q. Jiang, and Wang’s institution received funding from the Capital Health Research and Development of Special (2007-1042). Dr. Xi received support for article research from the Capital Health Research and Development of Special (2007-1042) and disclosed government work. The remaining authors have disclosed that they do not have any potential conflicts of interest
REFERENCES
- 1.Goligher E, Ferguson ND. Mechanical ventilation: Epidemiological insights into current practices. Curr Opin Crit Care 2009; 15:44–51. [DOI] [PubMed] [Google Scholar]
- 2.Wunsch H, Linde-Zwirble WT, Angus DC, et al. The epidemiology of mechanical ventilation use in the United States. Crit Care Med 2010; 38:1947–1953. [DOI] [PubMed] [Google Scholar]
- 3.Metnitz PG, Metnitz B, Moreno RP, et al. ; SAPS 3 Investigators: Epidemiology of mechanical ventilation: Analysis of the SAPS 3 database. Intensive Care Med 2009; 35:816–825. [DOI] [PubMed] [Google Scholar]
- 4.Esteban A, Anzueto A, Alía I, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med 2000; 161:1450–1458. [DOI] [PubMed] [Google Scholar]
- 5.Esteban A, Anzueto A, Frutos F, et al. ; Mechanical Ventilation International Study Group: Characteristics and outcomes in adult patients receiving mechanical ventilation: A 28-day international study. JAMA 2002; 287:345–355. [DOI] [PubMed] [Google Scholar]
- 6.Esteban A, Frutos-Vivar F, Muriel A, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med 2013; 188:220–230. [DOI] [PubMed] [Google Scholar]
- 7.Jeong BH, Suh GY, An JY, et al. Clinical demographics and outcomes in mechanically ventilated patients in Korean intensive care units. J Korean Med Sci 2014; 29:864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du B, Xi X, Chen D, et al. ; China Critical Care Clinical Trial Group (CCCCTG): Clinical review: Critical care medicine in mainland China. Crit Care 2010; 14:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS: Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824. [DOI] [PubMed] [Google Scholar]
- 10.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis: The ACCP/SCCM Consensus Conference Committee: American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–1655. [DOI] [PubMed] [Google Scholar]
- 11.Rabe KF, Hurd S, Anzueto A, et al. ; Global Initiative for Chronic Obstructive Lung Disease: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176:532–555. [DOI] [PubMed] [Google Scholar]
- 12.Rittayamai N, Katsios CM, Beloncle F, et al. Pressure-controlled vs volume-controlled ventilation in acute respiratory failure: A physiology-based narrative and systematic review. Chest 2015; 148:340–355. [DOI] [PubMed] [Google Scholar]
- 13.Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 14.Villar J, Kacmarek RM, Pérez-Méndez L, et al. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: A randomized, controlled trial. Crit Care Med 2006; 34:1311–1318. [DOI] [PubMed] [Google Scholar]
- 15.Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: A preventive randomized controlled trial. Crit Care 2010; 14:R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: A meta-analysis. JAMA 2012; 308:1651–1659. [DOI] [PubMed] [Google Scholar]
- 17.Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: Systematic review and meta-analysis. JAMA 2010; 303:865–873. [DOI] [PubMed] [Google Scholar]
- 18.Meade MO, Cook DJ, Guyatt GH, et al. ; Lung Open Ventilation Study Investigators: Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: A randomized controlled trial. JAMA 2008; 299:637–645. [DOI] [PubMed] [Google Scholar]
- 19.Mercat A, Richard JC, Vielle B, et al. ; Expiratory Pressure (Express) Study Group: Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: A randomized controlled trial. JAMA 2008; 299:646–655. [DOI] [PubMed] [Google Scholar]
- 20.Brower RG, Lanken PN, MacIntyre N, et al. ; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network: Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004; 351:327–336. [DOI] [PubMed] [Google Scholar]
- 21.Rittayamai N, Brochard L. Recent advances in mechanical ventilation in patients with acute respiratory distress syndrome. Eur Respir Rev 2015; 24:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett S, Hurford WE. When should sedation or neuromuscular blockade be used during mechanical ventilation? Respir Care 2011; 56:168–176. [DOI] [PubMed] [Google Scholar]
- 23.Divatia JV, Amin PR, Ramakrishnan N, et al. ; INDICAPS Study Investigators: Intensive care in India: The Indian intensive care case mix and practice patterns study. Indian J Crit Care Med 2016; 20:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuraz A, Guérin C, Payet C, et al. Patient mortality is associated with staff resources and workload in the ICU: A multicenter observational study. Crit Care Med 2015; 43:1587–1594. [DOI] [PubMed] [Google Scholar]
- 25.Sasabuchi Y, Yasunaga H, Matsui H, et al. The volume-outcome relationship in critically ill patients in relation to the ICU-to-hospital bed ratio. Crit Care Med 2015; 43:1239–1245. [DOI] [PubMed] [Google Scholar]
- 26.Needham DM, Bronskill SE, Rothwell DM, et al. Hospital volume and mortality for mechanical ventilation of medical and surgical patients: A population-based analysis using administrative data. Crit Care Med 2006; 34:2349–2354. [DOI] [PubMed] [Google Scholar]
- 27.Cavalcanti AB, Bozza FA, Machado FR. Effect of a quality improvement intervention with daily round checklists, goal setting, and clinician prompting on mortality of critically ill patients. JAMA 2016; 315:1480–1490. [DOI] [PubMed] [Google Scholar]
- 28.Checkley W, Martin GS, Brown SM, et al. ; United States Critical Illness and Injury Trials Group Critical Illness Outcomes Study Investigators: Structure, process, and annual ICU mortality across 69 centers: United States critical illness and injury trials group critical illness outcomes study. Crit Care Med 2014; 42:344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dasta JF, McLaughlin TP, Mody SH, et al. Daily cost of an intensive care unit day: The contribution of mechanical ventilation. Crit Care Med 2005; 33:1266–1271. [DOI] [PubMed] [Google Scholar]
- 30.Kumar P, Jithesh V, Gupta SK. Does a single specialty intensive care unit make better business sense than a multi-specialty intensive care unit? A costing study in a trauma center in India. Saudi J Anaesth 2015; 9:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zilberberg MD, Luippold RS, Sulsky S, et al. Prolonged acute mechanical ventilation, hospital resource utilization, and mortality in the United States. Crit Care Med 2008; 36:724–730. [DOI] [PubMed] [Google Scholar]
- 32.Cox CE, Carson SS, Govert JA, et al. An economic evaluation of prolonged mechanical ventilation. Crit Care Med 2007; 35:1918–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan SS, Bakker J, Hoogendoorn ME, et al. Direct cost analysis of intensive care unit stay in four European countries: Applying a standardized costing methodology. Value Health 2012; 15:81–86. [DOI] [PubMed] [Google Scholar]
- 34.Lefrant JY, Garrigues B, Pribil C, et al. ; CRREA Study Group; AzuRea Group: The daily cost of ICU patients: A micro-costing study in 23 French intensive care units. Anaesth Crit Care Pain Med 2015; 34:151–157. [DOI] [PubMed] [Google Scholar]
- 35.Shelat PR, Kumbar SK. A retrospective analysis of direct medical cost and cost of drug therapy in hospitalized patients at private hospital in western India. J Clin Diagn Res 2015; 9:9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Liu Y, Chen N. The Chinese experience of hospital price regulation. Health Policy Plan 2000; 15:157–163. [DOI] [PubMed] [Google Scholar]


