Abstract
Parkinson’s disease (PD) is a progressive neurodegenerative disease with devastating clinical manifestations. In PD, neuronal death is associated with intracellular aggregates of the neuronal protein α-synuclein known as Lewy bodies. Although the cause of sporadic PD is not well understood, abundant clinical and pathological evidence show that misfolded α-synuclein is found in enteric nerves before it appears in the brain. This suggests a model in which PD pathology originates in the gut and spreads to the central nervous system via cell-to-cell prion-like propagation, such that transfer of misfolded α-synuclein initiates misfolding of native α-synuclein in recipient cells. We recently discovered that enteroendocrine cells (EECs), which are part of the gut epithelium and directly face the gut lumen, also possess many neuron-like properties and connect to enteric nerves. In this report, we demonstrate that α-synuclein is expressed in the EEC line, STC-1, and native EECs of mouse and human intestine. Furthermore, α-synuclein–containing EECs directly connect to α-synuclein–containing nerves, forming a neural circuit between the gut and the nervous system in which toxins or other environmental influences in the gut lumen could affect α-synuclein folding in the EECs, thereby beginning a process by which misfolded α-synuclein could propagate from the gut epithelium to the brain.
Keywords: Gastroenterology, Neuroscience
Enteroendocrine cells express α-synuclein, raising the possibility that these cells could be linked to pathogenesis in Parkinson’s disease.
Introduction
Parkinson’s disease (PD) is a debilitating neurodegenerative disease characterized by motor disturbances, including resting tremor, rigidity, and slow movements, as well as gastrointestinal symptoms, such as constipation and gastroparesis. The pathological hallmarks of PD are cytoplasmic inclusions known as Lewy bodies (within the cell body) and Lewy neurites (in axons) of the brain and enteric nervous system (1). These inclusions are associated with degeneration of dopaminergic neurons in the substantia nigra pars compacta (2, 3), which produces the distinctive disorders of movement and vagal nerve dysfunction. The major component of Lewy pathology is aggregated α-synuclein, a synaptic protein with the propensity to misfold and aggregate (4). Misfolded α-synuclein plays a critical role in PD pathogenesis, and recent evidence supports a model in which propagation of Lewy pathology occurs via cell-to-cell transmission of misfolded α-synuclein onto recipient cells (5–9). Misfolded α-synuclein recruits native α-synuclein in the recipient cell and acts as a template or nidus for the development of aggregates that eventually lead to formation of Lewy bodies and ultimately PD (1, 8, 10).
Although the pathogenesis of PD is incompletely understood, Braak and colleagues suggest that the pathological process begins in the enteric nervous system (11, 12). Both clinical and experimental data support such a model. Clinically, PD patients frequently experience gastrointestinal symptoms many years before motor deficits develop (13, 14), and α-synuclein aggregates appear in enteric nerves before they are found in the brain (12, 15, 16). α-Synuclein immunoreactive inclusions have been found in neurons of the submucosal plexus, whose axons project to the mucosa (15, 17, 18). Moreover, it has been reported recently that bilateral vagotomy reduces the risk of PD (19).
Experimentally, direct transmission of α-synuclein from the gut to the brain was demonstrated in a key experiment in which α-synuclein injected into the intestine was transported via the vagus nerve to the dorsal motor nucleus of the vagus in the brainstem (20, 21). This finding is consistent with the original observation that Lewy pathology appears in the projection neurons of the dorsal motor nucleus of the vagus in the early stages of PD (12). Experimentally, the vagal route of α-synuclein transport has also been documented following exposure to the environmental toxin rotenone (22) as well as following direct injection of adenoassociated viral vectors overexpressing human α-synuclein into the vagus nerve (23). A route from intrinsic enteric neurons to the vagus nerve is supported by the observation that both myenteric neurons and preganglionic vagal nerves express α-synuclein (24). These findings are consistent with the hypothesis that α-synuclein aggregation begins in the gut and spreads to the central nervous system via the vagus nerve.
Recently, it has been shown that individuals with PD have an altered gut microbial composition (25–28), raising the possibility that gut microbes affect PD pathogenesis. In a mouse model of PD, gut microbiota promoted α-synuclein aggregation and the development of motor disturbances (29). Moreover, colonization of mice with microbiota from PD-affected individuals enhanced physical deterioration (29), further emphasizing the role of the gut in PD. However, the mechanism by which gut microbes affect the progression of PD is not well understood.
Furthermore, an environmental basis for PD has been suspected for over 50 years, and a number of studies have shown an increased incidence of PD in individuals exposed to pesticides and herbicides (30–32). Enteric nerves are limited by the intestinal epithelium and do not extend into the intestinal lumen. Therefore, nerves of the gut do not have direct contact with luminal contents. Thus, even though data suggest that PD pathology begins in the gut, the mechanism by which luminal environmental toxins might induce changes in enteric neurons is unknown (12–16, 20).
Enteroendocrine cells (EECs) are chemosensory cells that are dispersed throughout the mucosal lining of the intestine and oriented with their apical surface open to the lumen of the intestine, so that they can sense luminal contents, such as ingested nutrients or gut microbes. Traditionally, EECs were viewed exclusively as hormone-producing cells of the gastrointestinal tract; however, we recently discovered that EECs also connect to neurons (33, 34). Their location places EECs at the interface between gut contents and the nervous system and provides a direct route for substances in the gut to affect neural function. In addition, EECs are electrically excitable and possess many neuronal features, including neurotrophin receptors, presynaptic and postsynaptic proteins, small clear secretory vesicles, neurofilaments, and basal processes known as neuropods (35). A functional synaptic connection between EECs and enteric nerves was established using rabies viral tracing, in which a modified rabies virus placed into the lumen of the intestine infected EECs and was transmitted into enteric nerves (33). These neuronal features suggest that EECs are sensory cells in the gut. Impressed by their neuron-like properties, we sought to determine if EECs express α-synuclein. If they do, their location at the interface of the gut lumen and the nervous system suggests that EECs could be a target for the induction of abnormal α-synuclein and initiation of the prion-like cascade leading to PD.
Results
α-Synuclein is expressed in enteroendocrine STC-1 cells.
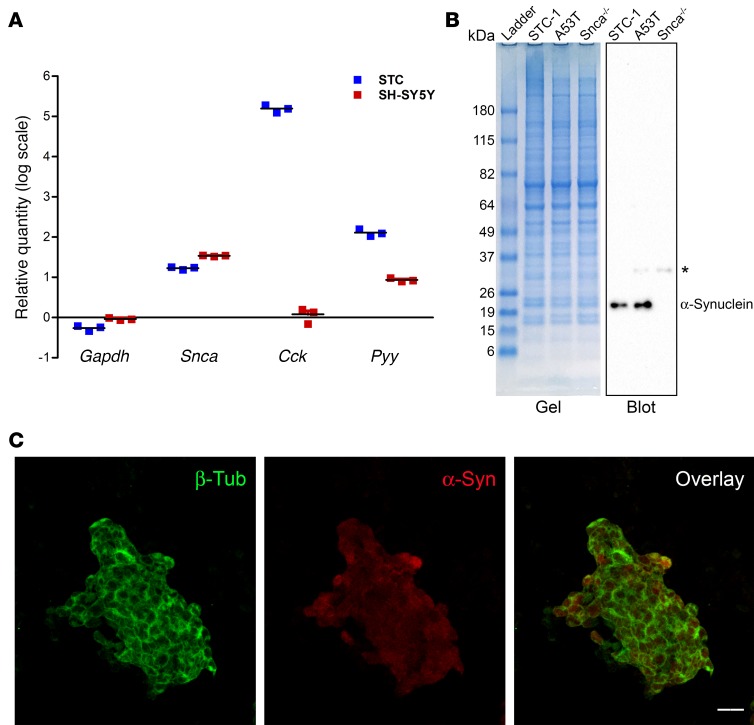
STC-1 cells were derived from the duodenum of transgenic mice that expressed SV40 large T antigen downstream from a rat insulin promoter (36); they are widely accepted as a model of native EECs (37). STC-1 cells express several gastrointestinal hormones, such as cholecystokinin (CCK) and peptide YY (PYY), whose secretion is regulated in a manner similar to native EECs (38–40). The inability to culture EECs or obtain sufficient numbers from intestinal tissue for in vitro assays makes STC-1 cells attractive for evaluating properties of EECs. Expression of α-synuclein in STC-1 cells was analyzed by real-time PCR, immunoblotting, and immunofluorescence. Figure 1A shows the relative quantitation of Gapdh, α-synuclein (Snca), Cck, and Pyy in STC-1 cells compared with the SH-SY5Y neuroblastoma cell line. HeLa cells were used as the comparator and β-actin (Actb) transcript level was used to normalize RNA abundance. The relative amount of Snca mRNA in STC-1 cells (~15-fold) was comparable in magnitude to that present in SH-SY5Y cells (~34-fold), whereas STC-1 cells expressed a much higher amount of the Cck transcript (1.5 × 105–fold versus ~1-fold). The Pyy transcript was also expressed at a higher level in STC-1 cells (~150-fold), although SH-SY5Y cells appeared to express some PYY transcript (~8-fold) relative to HeLa cells. The relative amount of Gapdh/GAPDH mRNA was similar between the 3 cell lines examined.
Figure 1. α-Synuclein protein is expressed in STC-1 cells.
(A) Relative quantitation of Gapdh, α-synuclein (Snca), cholecystokinin (Cck), and peptide YY (Pyy) mRNAs in STC-1 and SH-SY5Y cells showing that α-synuclein mRNA is present in STC-1 cells. β-Actin (Actb) was used as the normalizer, and RNA isolated from HeLa cells served as a comparator. Data represent mean ± SEM of 3 individual experiments. (B) Coomassie Blue–stained 4%–12% gradient SDS-PAGE gel and corresponding immunoblot of STC-1 cells, A53T mouse brain, and Snca-/- mouse brain extracts with α-synuclein monoclonal antibody. A nonspecific band (asterisk) was detected in A53T and Snca-/- mouse brain extracts. The molecular weights of protein bands present in the ladder are indicated in kDa. (C) 3D Z-stack image of STC-1 cells showing α-synuclein (red) in the cytoplasm. Cytoskeletal staining is shown with β-tubulin (green). Scale bar: 20 μm.
To examine α-synuclein protein levels, a cellular extract of STC-1 cells was electrophoresed, along with whole-brain lysate from A53T mice and α-synuclein–knockout (Snca-/-)mice. A53T transgenic mice contain 4 copies of the human α-synuclein gene carrying the A53T mutation on a Snca-/- background (41) (Figure 1B). Using an α-synuclein antibody that has been extensively characterized (41), α-synuclein was found to be present in both STC-1 cell and A53T mouse brain extracts but not in brain extracts of Snca-/- mice (41). A faint nonspecific band was observed in both brain samples and has previously been noted with this antibody (Y.-M. Kuo and R.L. Nussbaum, unpublished observations) (42). We also examined the cellular localization of α-synuclein in STC-1 cells by immunofluorescence. A general low level of α-synuclein immunofluorescence was present in the entire cytoplasm (Figure 1C). No immunofluorescence was detected in the absence of primary antibodies (Supplemental Figure 1; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.92295DS1).
Intestinal EECs express α-synuclein.
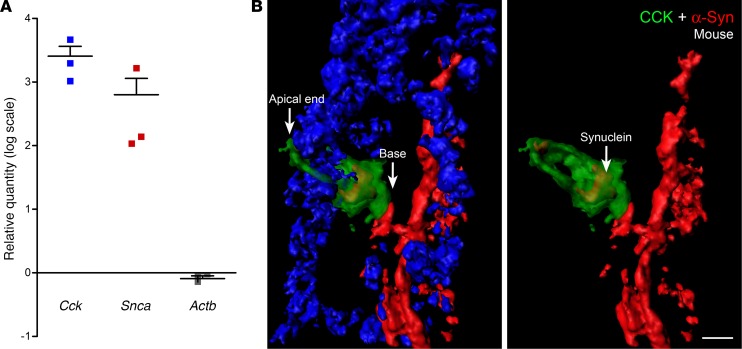
The presence of α-synuclein in STC-1 cells suggested that this protein is expressed in EECs of the intestine. To evaluate this possibility, we purified GFP-positive CCK cells from the duodenums of CCK-GFP mice using fluorescence-activated cell sorting and quantitated gene expression by real-time PCR as described previously (43, 44). Cck gene expression was almost 2,000-fold greater than Actb in GFP-positive cells and Snca was over 150-fold increased over the control gene Actb (Figure 2A), indicating that α-synuclein mRNA is greatly enriched in CCK cells. In this experiment, α-synuclein RNA expression was compared between GFP-positive CCK cells and GFP-negative mucosal cells that contained non–CCK-GFP EECs. Since α-synuclein is expressed in non–CCK EECs (see data below), it is likely that this relative quantitation of Snca gene in CCK-GFP cells is an underestimation of the actual abundance of Snca transcript in CCK cells.
Figure 2. α-Synuclein is present in mouse duodenal CCK cells.
(A) Relative quantitation of cholecystokinin (Cck), α-synuclein (Snca), and β-actin (Actb) mRNAs in FAC-sorted CCK-GFP cells from mouse duodenum. Gapdh was used as the normalizer, and RNA isolated from CCK-GFP–negative cells served as a comparator. Data represent mean ± SEM of 3 individual experiments. (B) A frozen section (10-μm thickness) of A53T mouse duodenum was fixed in a mixture of methanol and acetone and stained for α-synuclein and CCK. The image shows a small section of the villus with a CCK cell. Nuclei (blue channel) have been removed from the image on the right for an uninterrupted view. α-Synuclein (red) is present in the cytoplasm of the CCK cell (green), which has been rendered 50% transparent. α-Synuclein is also expressed in enteric nerves of the villus. This CCK cell is present in close juxtaposition to enteric nerves. Scale bar: 5 μm.
Characterizing α-synuclein in mice has been challenging, due, in part, to low endogenous levels of protein; thus, new genetic models have been used to enhance α-synuclein expression and assess its function. Therefore, as a first step, we examined α-synuclein expression in A53T mice. Our goal was to determine if α-synuclein expressed from the human promoter in A53T transgenic mice could be visualized in EECs. Figure 2B shows α-synuclein immunofluorescence in the villus of the A53T mouse duodenum. α-Synuclein–positive enteric nerves were also present in the crypt region (Supplemental Figure 2). The CCK cell also expressed α-synuclein (Figure 2B, right), and the basolateral surface of this cell rested on an α-synuclein–containing nerve. No fluorescence in EECs was observed in the absence of CCK primary antibody (Supplemental Figure 3). In wild-type (CCK-GFP) mice, α-synuclein staining was detected within some CCK cells but could not easily be visualized in the enteric nerves (Supplemental Figure 4). The striking difference in immunofluorescence intensity between A53T and wild-type mouse intestine could be attributed to higher levels of α-synuclein in A53T mice (41). It is also possible that the human protein is recognized better by the primary antibody used in these experiments. No α-synuclein staining was detected in the small intestines of Snca-/- mice (Supplemental Figure 5), suggesting that the staining observed in A53T mice was antigen specific (41).
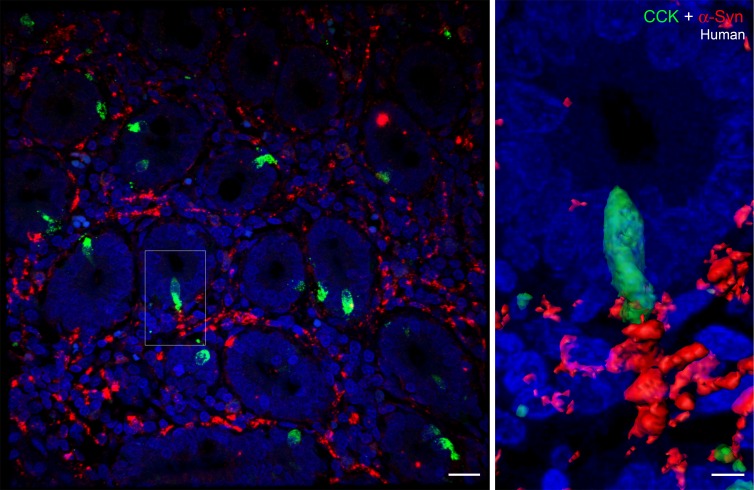
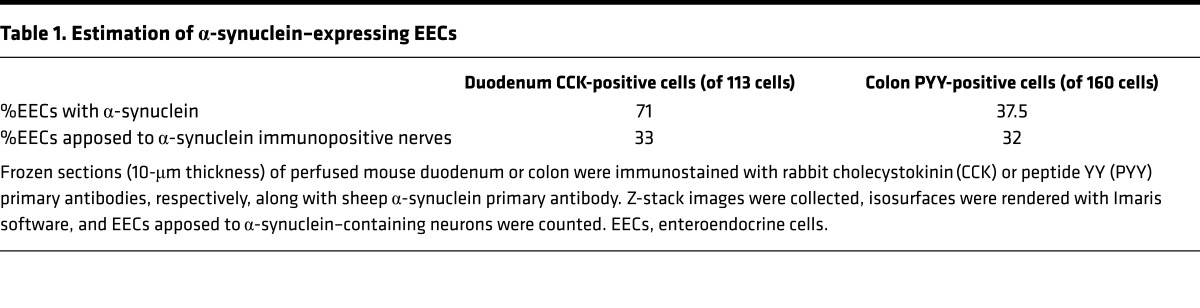
Importantly, α-synuclein staining was also present in EECs in human duodenum, as shown in Figure 3 (a CCK cell present in the center of the image on the left is shown at higher magnification on the right). A small amount of α-synuclein was visible inside the CCK cell, and this cell was adjacent to the terminus of an α-synuclein–positive enteric nerve. However, using this technique, not all CCK cells were positive for α-synuclein and only a minority of cells were found in contact with enteric nerves. Approximately 71% of CCK cells contained intracellular α-synuclein, while 33% of the cells were apposed to α-synuclein in nerves or glia of A53T;CCK-GFP mice (Table 1).
Figure 3. α-Synuclein expression in CCK cells and enteric nerves of human duodenum.
Paraffin-embedded section (5-μm thickness) of human duodenum showing a cross section of villi. α-Synuclein (red) staining is visible in enteric nerves located between the villi. One of the cholecystokinin (CCK) cells (boxed) is shown at higher magnification on the right. α-Synuclein is present inside the CCK cell, and this cell is present in close proximity to an α-synuclein–containing enteric nerve. Scale bar: 20 μm (left); 5 μm (right).
Table 1. Estimation of α-synuclein–expressing EECs.
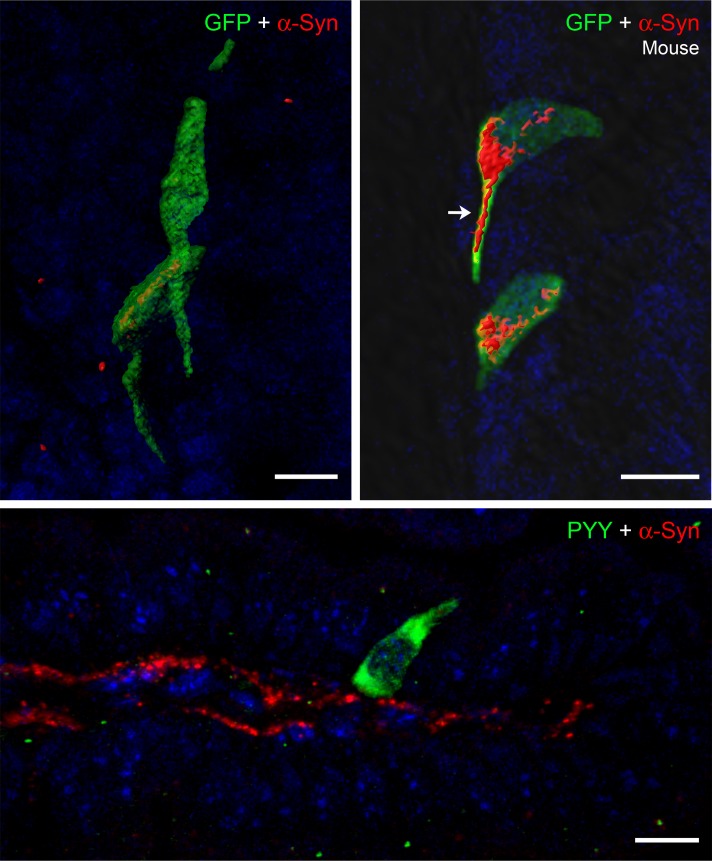
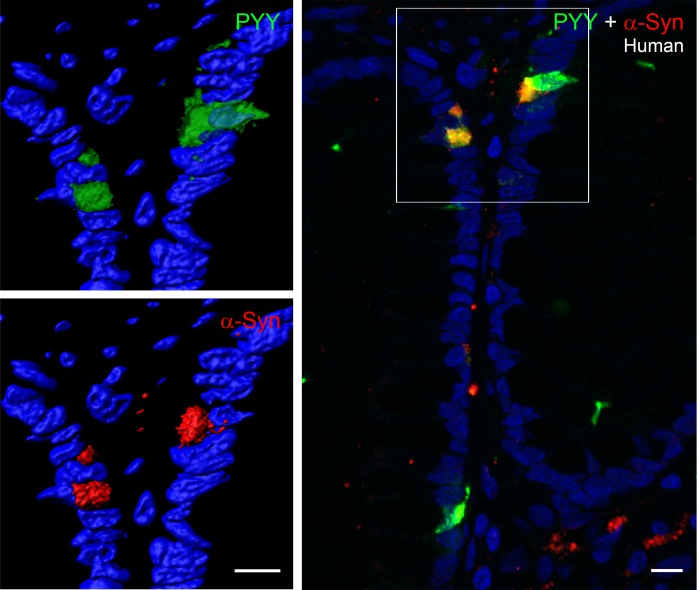
To determine if α-synuclein is expressed in other EECs, we examined PYY-containing cells of the mouse and human intestine. As shown in Figures 4 and 5, α-synuclein is prominent in mouse and human PYY cells. The top row of images of Figure 4 show 4 GFP-positive PYY cells present in the colon of a PYY-GFP mouse. These cells expressed α-synuclein, and in one of the cells, the α-synuclein protein extended into the neuropod of the PYY cell. Enteric nerves were not visible in these sections, possibly because immunofluorescence of α-synuclein was very weak in the enteric nervous system of wild-type mice (45). The image on the bottom of Figure 4 shows a PYY-positive cell in the small intestine of an A53T mouse that is located in close juxtaposition to an α-synuclein–containing enteric nerve, which runs in the lamina propria of the villus. α-Synuclein is also expressed in PYY cells of the human colon. The left side of Figure 5 shows two cells that express PYY and α-synuclein. The right side of Figure 5 shows colocalization of PYY and α-synuclein in 3 cells of the human colon. Approximately 37% of PYY cells in the colon contain intracellular synuclein, and 32% of these cells are in contact with α-synuclein in nerves or glia (Table 1).
Figure 4. α-Synuclein is expressed in PYY cells of mouse colon.
Top row: Frozen sections (10-μm thickness) of PYY-GFP mouse colon were fixed with formalin and stained for GFP (green) and α-synuclein (red). α-Synuclein is present in the cytoplasm of PYY-GFP cells and extends into the neuropod of one of the cells (arrow). Bottom row: Paraffin-embedded section (5-μm thickness) of A53T mouse intestine showing a PYY cell (green) in close proximity to a α-synuclein–stained (red) nerve in the lamina propria. Scale bar: 10 μm. PYY, peptide YY.
Figure 5. α-Synuclein is expressed in PYY cells of human colonic crypts.
Paraffin-embedded section (5-μm thickness) of human colon showing PYY cells (green) that contain α-synuclein (red). The merged image on the right shows colocalization of these two proteins in yellow. Scale bar: 10 μm. PYY, peptide YY.
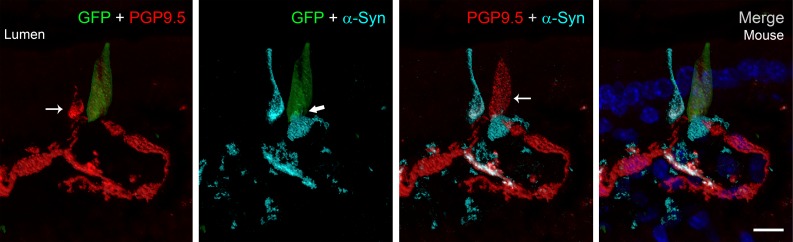
Chromogranin A is located in secretory vesicles of endocrine cells and has been used as a marker of most EECs. We identified α-synuclein in chromogranin A–positive cells (Supplemental Figure 6) in the intestine. α-Synuclein staining was also observed in rare mucosal cells that did not stain with one of the three EEC markers (CCK, PYY, and chromogranin A) used in this study. Figure 6 shows a CCK-GFP cell located near an α-synuclein–positive but GFP-negative mucosal cell. The CCK cell expresses α-synuclein in the basolateral region and is located near a nerve identified with the pan-neuronal marker, protein gene product 9.5 (PGP9.5), that also contains α-synuclein. Notably, both the CCK and non-CCK α-synuclein–positive mucosal cells contained intracellular PGP9.5. Since only one EEC marker was evaluated in any given experiment, we cannot rule out the possibility that α-synuclein is expressed in an occasional non-EEC intestinal cell; however, the CCK and non-CCK cells possess the characteristic flask shape typical of EECs, and their α-synuclein and PGP9.5 expression imply neuronal properties.
Figure 6. Identification of α-synuclein in cholecystokinin-positive and -negative cells of the intestinal mucosa.
Frozen sections (16-μm thickness) of A53T;CCK-GFP mouse duodenum showing a CCK-GFP (green) mucosal cell adjacent to an α-synuclein–positive (turquoise) cell that does not contain CCK. Protein gene product 9.5 (PGP9.5) (red) is identified in both mucosal cells (thin arrows), which lie in close proximity to a PGP9.5-positive neuron. α-Synuclein within the CCK cell (thick arrow) lies in close proximity to extracellular α-synuclein. Colocalization of α-synuclein (turquoise) and PGP9.5 (red) within the cell and in the nerve appears white. Note the similar flask shape of both the CCK-positive and -negative α-synuclein–containing mucosal cells, which is typical for enteroendocrine cells. Scale bar: 10 μm. CCK, cholecystokinin.
α-Synuclein is expressed in the enteric nervous system.
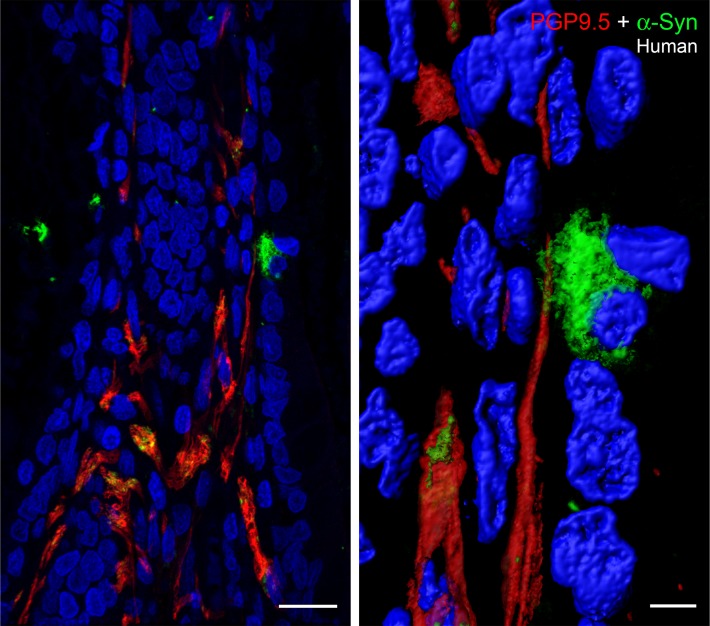
We also examined α-synuclein in submucosal enteric nerves using PGP9.5 immunostaining. Previous studies have shown that α-synuclein and PGP9.5 colocalize in cutaneous autonomic nerves (46). Figure 7 shows that α-synuclein is present in PGP9.5-positive nerves. However, the distribution of α-synuclein in the nerves is not continuous but appears to be localized to distinct zones or domains. This observation was also apparent in Figure 2B and Figure 3, where the α-synuclein staining is not continuous, but patchy, although a pattern of fluidity can be constructed. Higher magnification of the α-synuclein–positive mucosal cell revealed numerous microscopic processes that extend from the surface of the cell and appear to make contact with the PGP9.5-positive enteric nerve. The images in Figure 7 clearly demonstrate that α-synuclein is present in mucosal cells (e.g., EECs) and suggest that α-synuclein has the potential to migrate from specific mucosal cells (EECs) into enteric nerves not only via endocytosis of extracellular α-synuclein (47, 48) but also through direct physical contact between mucosal cells and enteric nerves.
Figure 7. α-Synuclein–containing mucosal cell is in contact with an enteric nerve.
Left: Paraffin-embedded section of human duodenum (5-μm thickness) showing colocalization (yellow) of α-synuclein in certain regions of protein gene product 9.5–positive (PGP9.5-positive) enteric nerves (red). Right: A high-magnification image of the α-synuclein–positive cell (green) exhibiting numerous fiber-like cellular processes, which encircle the PGP9.5-positive nerve (red). Scale bar: 20 μm (left); 5 μm (right).
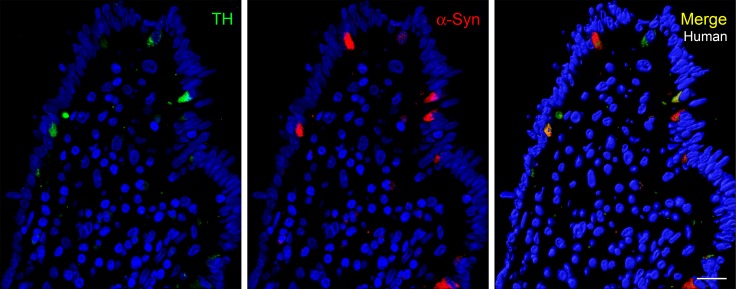
Previous studies have shown that α-synuclein and tyrosine hydroxylase (TH) are coexpressed in the brain and dopaminergic cell lines and that α-synuclein may play a role in regulating dopamine synthesis (49, 50). TH is also known to be expressed in neurons of the enteric nervous system, and TH mRNA was previously detected in intestinal PYY cells by real-time PCR (33, 51). Interestingly, EECs, like PD-affected neurons, are TH positive. Figure 8 shows that TH protein is detected by immunofluorescence in α-synuclein–positive mucosal cells of the human colon.
Figure 8. TH is expressed in α-synuclein–expressing mucosal cells.
Paraffin-embedded section (5-μm thickness) of human colon showing colocalization of TH (green) and α-synuclein (red). Not all α-synuclein–containing mucosal cells are positive for TH. Scale bar: 20 μm. TH, tyrosine hydroxylase.
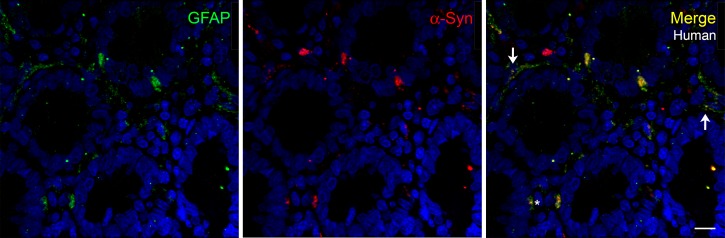
Glial fibrillary acidic protein (GFAP) is a common marker for identification of glia. To determine if glia also express α-synuclein, we examined the expression of GFAP in the human duodenum (Figure 9). Glial processes were not very abundant in the human duodenal sections used for this work, and the immunofluorescence was not bright; however, α-synuclein was present in certain regions of GFAP-positive glial processes. Supplemental Figure 7 shows that there is some colocalization of α-synuclein and GFAP in frozen sections of the perfused mouse duodenum. In other regions of the tissue, GFAP staining appeared to surround α-synuclein–positive nerves. The pattern of α-synuclein expression was similar to that described above for enteric nerves, where it was not distributed evenly in the entire glial process but visible in small areas or domains. The factors that cause the segregation and maintenance of α-synuclein in these domains remain to be established.
Figure 9. α-Synuclein colocalizes with glial marker GFAP.
Paraffin-embedded sections (5-μm thickness) of human duodenum showing colocalization of GFAP (green) and α-synuclein in some mucosal cells (asterisk) and in certain regions of fine glial processes (arrows). Scale bar: 10 μm. GFAP, glial fibrillary acidic protein.
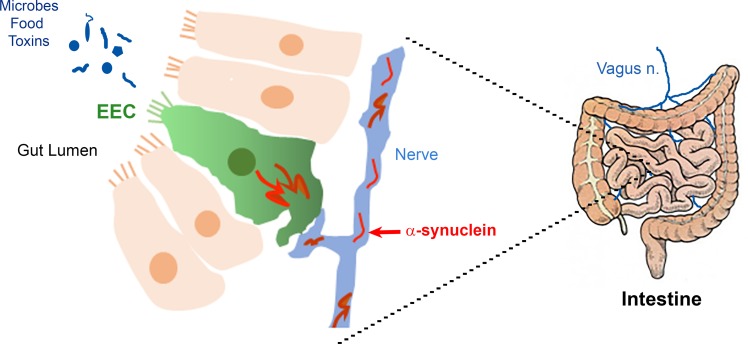
In summary, here we demonstrate that α-synuclein is expressed in the EEC line STC-1 as well as in mouse and human EECs of the duodenum and colon. α-Synuclein is also expressed in enteric nerves and to a lesser extent in enteric glia. Some EECs are in direct contact with α-synuclein–containing nerves, which could lead to the transmission of aggregated α-synuclein from EECs to the enteric nervous system (Figure 10).
Figure 10. Hypothetical pathway for pathogenic migration of α-synuclein in the gut.
The apical surface of enteroendocrine cells (EECs) is exposed to the lumen and thus is in contact with ingested toxins and metabolites produced by gut microbes. The basolateral surface of EECs is in contact with enteric nerves and glia. We propose that toxin uptake by EEC can cause aggregation of α-synuclein inside these cells and this aggregated protein can migrate to enteric nerves, thereby initiating a pathogenic cascade leading to α-synucleinopathies.
Discussion
In our earlier studies, we were impressed by the abundance of neuronal features found in EECs. Using confocal microscopy and 3D serial block face electron microscopy, we noticed that EECs possessed axon-like basal processes containing neurofilaments, presynaptic and postsynaptic proteins, prominent secretory vesicles, and neurotrophin receptors (33, 35, 52). Gene expression profiling revealed that EECs express mRNA for DOPA decarboxylase and TH, enzymes essential for dopamine synthesis (33). Our identification of PGP9.5 in EECs further expands the neuronal repertoire of EECs. The discovery that EECs connect to nerves raises a number of intriguing possibilities for how nutrients, bacteria, toxins, and potential pathogens gain access to and communicate with the nervous system. This neural circuit may have implications for the origin of neurological diseases that involve the gut or whose cause remains obscure.
PD is a neurodegenerative disease with prominent gut manifestations. Lewy pathology spreads along neural pathways and involves vagal nerves (12, 15, 53), and the recent observation that bilateral vagotomy reduces the risk of PD supports the concept that the causative agent in PD is transmitted from gut to the brain (19). Realizing that the disease origin has been elusive, we postulated that if EECs contained α-synuclein, they could be involved in the pathogenesis of PD. To this end, we made several key observations. First and central to this hypothesis is our discovery that α-synuclein is expressed in EECs. The histological appearance within cells is consistent with cytoplasmic expression of α-synuclein that is concentrated at the basal surface of the EEC, where we previously identified synaptic vesicles (35). α-Synuclein expression is another example of the neuron-like properties of EECs, and, together with the expression of other presynaptic proteins, it suggests that EECs are involved in neurotransmission.
Second, α-synuclein is expressed in EECs in both the small and large intestine. We used several complimentary approaches to characterize EECs, including peptide hormone–specific antisera in combination with antibodies to α-synuclein, neuronal proteins (e.g., PGP9.5), and biosynthetic enzymes (e.g., TH). α-Synuclein was confirmed in CCK cells, which are most prominent in the proximal intestine as well as in PYY-containing cells of the distal small intestine and colon in both mice and humans. However, even though we characterized these two types of EECs, we do not believe α-synuclein expression is limited to CCK and PYY EECs, as it was present in certain non-CCK and PYY mucosal cells. Specifically, we identified α-synuclein in mucosal cells containing chromogranin A, a more general marker of EECs. Therefore, it is likely that α-synuclein expression is common to multiple types of EECs. Within the gastrointestinal tract, EECs containing α-synuclein were more abundant in the proximal small intestine, where vagal neural innervation is more extensive. It is likely that exposure of EECs to ingested toxins or pathogens would be greater in the small intestine than the colon, making EECs in the proximal region of the gut more susceptible to changes that could affect the nervous system.
Third, EECs in which α-synuclein is expressed lie in close proximity to α-synuclein–expressing enteric neurons. The hypothesis that abnormal α-synuclein arises in EECs and spreads to enteric neurons requires cellular contact. Such an association was apparent, as α-synuclein expression was observed in juxtaposed EECs and α-synuclein–containing nerves. However, α-synuclein was not observed in neurons or glia apposing every α-synuclein–containing EEC, suggesting that α-synuclein does not move in a retrograde direction from the nervous system onto EECs. Finally, finding α-synuclein expression in EECs of both the mouse and human intestine is encouraging for the use of rodent models to study PD pathogenesis.
Glial cells are closely associated with enteric neurons, in that they provide support for neuronal function (54). Recently, it has been demonstrated that enteric glia may be adversely affected in PD (55). Our observation that α-synuclein is expressed in enteric glia has additional implications for the possible cell-to-cell transfer of α-synuclein. Cell-to-cell transfer of α-synuclein requires close cell contact but may or may not require synaptic transmission (8). We have previously demonstrated the EECs come into intimate contact with enteric glia (35), and our current demonstration that α-synuclein is coexpressed with glial markers raises the possibility that glia could participate in transfer of α-synuclein from EECs to the nervous system (56).
As cells residing in the epithelium of the gastrointestinal tract, EECs are uniquely positioned to sample gut contents and to relay those signals to the brain through a direct neural connection. However, the stability of an EEC-neural connection seemed unlikely when it was assumed that EECs behaved like enterocytes, which are born in the intestinal crypt, migrate up the villus, and are sloughed into the gut lumen within 3–5 days. This dynamic relationship in which cells are constantly moving and dying did not seem conducive to establishing long-lived contact with the nervous system. However, this concern dissipated when we discovered that some EECs reside in the mucosa for months (33). Therefore, the EEC-neuronal connection is likely to be long lived and sufficient for conveying pathological events.
Alterations in gut microbiota have been associated with PD, and recent evidence suggests that microbes from PD patients are pathogenic (29). Although it is not clear how gut microbes influence the nervous system, it is possible that this occurs through EECs. Microbial generation of short-chain fatty acids (SCFA) has been implicated in PD progression (28, 29), and it is conceivable that this occurs through EECs, including CCK and PYY cells, which have been shown to express SCFA receptors (e.g., FFAR2 and -3) (57–60).
Gastrointestinal complications, including constipation and gastroparesis, are common in PD and are believed to be secondary to impaired enteric neuronal activity. However, EECs have profound effects on gastrointestinal motility and secretion (61, 62); based on their multitude of neuronal-like characteristics, including their dopaminergic properties and α-synuclein expression, it is possible that EEC function is also adversely affected in PD. Therefore, it is interesting to speculate that EEC dysfunction occurs in PD and contributes to gastrointestinal manifestations.
The lining of the gastrointestinal tract is readily accessible to endoscopic biopsy, and attempts have been made to evaluate gastrointestinal tissue for abnormal α-synuclein (63–65). These approaches have not yet led to a diagnostic test for PD, but, with identification of α-synuclein in EECs (that are easily visible in even the most superficial of tissue samples), it may now be possible to use gastrointestinal biopsy tissues to detect abnormal α-synuclein in EECs in individuals with early or preclinical PD.
The discovery of α-synuclein in EECs, which are exposed to substances in the gut lumen, provides a previously unrecognized location in which changes to α-synuclein could occur. Should changes in α-synuclein conformation develop, leading to assembly and spread of α-synuclein in a prion-like manner, it is possible that EECs, by virtue of their neuronal connection, play a critical role in transmitting PD pathology from the gut to the brain.
Methods
Mouse and human intestinal tissue samples.
CCK-GFP transgenic mice (52) were procured from Mutant Mouse Resource and Research Centers (University of Missouri). FVB;129S6-Snca1Nbm Tg(SNCA*A53T)1Nbm Tg(SNCA*A53T)2Nbm/J mice (41) and Snca-/- (66) mice were obtained from R.L. Nussbaum. CCK-GFP mice were mated with FVB;129S6-Snca1Nbm Tg(SNCA*A53T)1Nbm Tg(SNCA*A53T)2Nbm/J mice (41) to generate the A53T;CCK-GFP line of mice that expresses GFP in CCK cells. FVB;129S6-Snca1Nbm Tg(SNCA*A53T)1Nbm Tg(SNCA*A53T)2Nbm/J (41), Snca-/- (66), A53T;CCK-GFP, or PYY-GFP (67) mice were euthanized and perfused with 3.5% freshly depolymerized paraformaldehyde. Intestinal tissue was harvested and paraffin embedded or cryopreserved in sucrose and embedded in OCT. Sections (5- to 20-μm thick) were collected on plus charged slides and used for immunostaining.
Paraffin-embedded sections of human duodenum or colon on deidentified slides were provided by A. Hiniker.
Real-time PCR.
Total RNA from STC-1, SH-SY5Y, and HeLa cells (ATCC) was isolated using an RNeasy kit (Qiagen). RNA was treated with DNase I (Ambion, ThermoFisher Scientific), and 2 μg RNA was reverse transcribed using the High Capacity cDNA reverse transcription kit (ThermoFisher Scientific). Real-time PCR was performed using inventoried TaqMan assays that spanned an exon: Actb (Mm4394036_g1, Hs01060665_g1), Cck (Mm00446170_m1, Hs00174937_m1), Gapdh (Mm99999915_g1, Hs02758991_g1), Pyy (Mm00520716_g1, Hs00373890_g1), and Snca (Mm01188700_m1, Hs00240906_m1).
For real-time PCR of murine intestinal CCK cells, GFP-positive cells and an equal number of GFP-negative cells were collected from the duodenum of CCK-GFP transgenic mice as described previously (44). Total RNA was isolated and reverse transcribed to cDNA using Superscript reverse transcriptase (Invitrogen), preamplified using TaqMan Preamp Master Mix, and used for real-time PCR quantitation (Applied Biosytems) as described previously (44). TaqMan gene expression assay IDs are as follows: Actb (Mm00607939_s1), Cck (Mm00446170_m1), Gapdh (Mm99999915_g1), and Snca (Mn00447331_m1).
Western blot.
STC-1 cells grown to 70% confluency were rinsed briefly with cold PBS (10 mM sodium phosphate, pH 7.4, 0.9% NaCl) and lysed in 30 mM Tris HCl (pH 7.5), 2.5% SDS, and 5% glycerol. The lysate was briefly homogenized with a Polytron, heated at 95°C for 10 minutes, and centrifuged at 2,800 g for 10 minutes at 4°C. Mouse brains were harvested rapidly after euthanasia and added to 4 ml prechilled RIPA buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 5 mM EDTA) and homogenized with a Polytron. The homogenate was centrifuged at 36,000 g for 30 minutes at 4°C in a Type 70 Ti fixed angle rotor (Beckman Coulter). The supernate was collected and protein concentration was measured using the Micro BCA kit (Thermo Scientific Pierce). The protein extract was mixed with Bolt 4X Sample buffer (Invitrogen Novex) and 5% β-mercaptoethanol to obtain a final concentration of 2 mg/ml total protein. STC-1 cell and brain extracts (10 μg) were loaded on a 4%–12% Bis-Tris Bolt gel and electrophoresed at 165 V for 30 minutes. The proteins were transferred to PVDF membrane and the membrane was incubated for 30 minutes in 0.4% freshly depolymerized paraformaldehyde in PBS (68), rinsed with PBS, and incubated with α-synuclein mouse monoclonal antibody (BD Transduction Laboratories; Supplemental Table 1). After incubation with an HRP-conjugated goat anti-mouse antibody (Thermo Scientific Pierce), α-synuclein protein was detected by chemiluminescence (ECL Prime Western Blotting Detection Reagent, GE HealthCare) and the signal was captured using ChemiDoc MP system (Bio-Rad Laboratories Inc.).
Immunochemistry.
All antibodies used for immunofluorescence experiments are listed in Supplemental Table 1.
STC-1 cells.
STC-1 cells were grown on poly-D lysine–coated chamber slides. When cells had reached the desired confluency, they were washed with PBS and fixed for 10 minutes in 10% formalin. β-Tubulin polyclonal antibody raised in rabbit (Novus Biologicals LLC) and mouse α-synuclein antibody (BD Transduction Laboratories) were used for immunostaining as described below. Cells were imaged in the absence of primary antibody to assess the specificity of the primary antibody, and images were captured at the same gain as images with primary antibody.
Tissue sections.
Paraffin-embedded sections were cleared in xylene and hydrated by passing through graded alcohols. Antigen retrieval was performed by one of three methods described below depending on the antigens being visualized: (a) slides were heated in a 2100 Retriever (Aptum Biologics Ltd.) for 20 minutes in 10 mM sodium citrate, pH 6.0 (Sigma-Aldrich), and allowed to cool slowly for 2 hours; (b) slides were pulse heated in boiling 0.1 mM EDTA (pH 8.0) for 10 minutes in a microwave and allowed to cool to room temperature over 30 minutes; and (c) slides were incubated in 70% formic acid (Fisher Scientific) for 30 minutes at room temperature. After antigen retrieval, sections were rinsed in water and PBS. Frozen sections were thawed, fixed for 10 minutes in either 10% formalin or a chilled 1:1 mixture of methanol and acetone, and used for immunostaining. All sections were blocked in 10% donkey serum in 10 mM Tris, pH 7.4, 0.9% NaCl, 0.1% Triton X-100 (TBST) for 30 minutes at room temperature to limit nonspecific antibody association. In experiments in which the α-synuclein signal was amplified, sections were treated with 2% H2O2 in PBS for 30 minutes and incubated in 2% blocking reagent (Tyramide signal amplification kit, Invitrogen). Incubation with primary antibodies was performed at 4°C in TBST plus 0.2% BSA. Sections were washed 3 times with TBST (5 minutes each time) and then incubated with secondary antibodies (see Supplemental Table 2) (minimal cross reactivity, Jackson ImmunoResearch Laboratories) for 1 hour at room temperature. After 3 washes with TBST, tyramide signal amplification was performed using directions provided by the manufacturer. Nuclei were stained with Hoechst dye, and sections were mounted using ProLong Gold (Invitrogen). Images were acquired using a Zeiss LSM 780 or 880 Airy Scan inverted confocal microscope, with a Zeiss ×20/0.8 NA or ×40/1.4 NA Oil Plan-Apochromat DIC, (UV) VIS-IR (420762-9900) objective. Images were collected using 405-, 488-, 561-, and 647-nm laser lines for excitation, and emission filters of BP420-480 (Hoechst dye), BP505-550 (DyLight/Alexa Fluor 488) LP575 (Cy3), and BP 640/30 (Alexa Fluor 647) were used. Pinholes were set to 1 airy unit for each channel, and line averaging of 4 or 8 was used with ×0.7–1 optical zoom. Z-stacks with an interval of 0.4 μm were acquired and 3D images were rendered using Imaris software (Bitplane Inc.). Sections were imaged in the absence of primary antibodies, and images were captured at the same gain as images with primary antibody. No endogenous tissue fluorescence was observed in the absence of primary antibodies.
Study approval.
This study was conducted in accordance with policies approved by Duke University and UCSF for use of human biospecimens (IRB-exempt Duke University Pro00067581 and UCSF IRB 11-07609). Paraffin-embedded sections of human duodenum or colon on deidentified slides were provided in accordance with IRB-approved protocols. A waiver for informed consent was granted by the UCSF IRB, as this retrospective research study presented minimal risk to subjects. All research involving animals was conducted in accordance with protocols approved by the Institutional Animal Care and Use Committees at Duke University and UCSF.
Author contributions
RC designed the research study, conducted experiments, acquired and analyzed data, and wrote the manuscript. AH contributed reagents, analyzed data, and edited the manuscript. YMK and RLN contributed reagents and edited the manuscript. RAL designed the research study, analyzed data, and wrote manuscript.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 DK098796 and R01 DK109368, Department of Veterans Affairs grant BX002230, Clinical and Translational Science Award grant UL1TR001117, and the UCSF Program for Breakthrough Biomedical Research, which is funded in part by the Sandler Foundation. The authors thank Stanley B. Prusiner for advice and encouragement. We also acknowledge Duke University’s Light Microscopy Core Facility, the Duke Cancer Institute Flow Cytometry Core Facility, and the Duke Cell Culture Facility.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:JCI Insight. 2017;2(12):e92295. https://doi.org/10.1172/jci.insight.92295.
Contributor Information
Rashmi Chandra, Email: rashmi.chandra@duke.edu.
Annie Hiniker, Email: Anne.Hiniker@ucsf.edu.
Yien-Ming Kuo, Email: Yien-ming.Kuo@ucsf.edu.
References
- 1.Angot E, Brundin P. Dissecting the potential molecular mechanisms underlying alpha-synuclein cell-to-cell transfer in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15 Suppl 3:S143–S147. doi: 10.1016/S1353-8020(09)70802-8. [DOI] [PubMed] [Google Scholar]
- 2.Bisaglia M, Mammi S, Bubacco L. Structural insights on physiological functions and pathological effects of alpha-synuclein. FASEB J. 2009;23(2):329–340. doi: 10.1096/fj.08-119784. [DOI] [PubMed] [Google Scholar]
- 3.Soto C. Transmissible proteins: expanding the prion heresy. Cell. 2012;149(5):968–977. doi: 10.1016/j.cell.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angot E, et al. Alpha-synuclein cell-to-cell transfer and seeding in grafted dopaminergic neurons in vivo. PLoS ONE. 2012;7(6):e39465. doi: 10.1371/journal.pone.0039465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desplats P, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106(31):13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen C, et al. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121(2):715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olanow CW, Prusiner SB. Is Parkinson’s disease a prion disorder? Proc Natl Acad Sci USA. 2009;106(31):12571–12572. doi: 10.1073/pnas.0906759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goedert M. NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science. 2015;349(6248):1255555. doi: 10.1126/science.1255555. [DOI] [PubMed] [Google Scholar]
- 9.Luk KC, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steiner JA, Angot E, Brundin P. A deadly spread: cellular mechanisms of α-synuclein transfer. Cell Death Differ. 2011;18(9):1425–1433. doi: 10.1038/cdd.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Tredici K, Rüb U, De Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61(5):413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna) 2003;110(5):517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 13.Natale G, Pasquali L, Ruggieri S, Paparelli A, Fornai F. Parkinson’s disease and the gut: a well known clinical association in need of an effective cure and explanation. Neurogastroenterol Motil. 2008;20(7):741–749. doi: 10.1111/j.1365-2982.2008.01162.x. [DOI] [PubMed] [Google Scholar]
- 14.Lebouvier T, et al. The second brain and Parkinson’s disease. Eur J Neurosci. 2009;30(5):735–741. doi: 10.1111/j.1460-9568.2009.06873.x. [DOI] [PubMed] [Google Scholar]
- 15.Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- 16.Hawkes CH, Del Tredici K, Braak H. A timeline for Parkinson’s disease. Parkinsonism Relat Disord. 2010;16(2):79–84. doi: 10.1016/j.parkreldis.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396(1):67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 19.Svensson E, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78(4):522–529. doi: 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- 20.Holmqvist S, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128(6):805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- 21.Visanji NP, Brooks PL, Hazrati LN, Lang AE. The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol Commun. 2013;1:2. doi: 10.1186/2051-5960-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan-Montojo F, et al. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep. 2012;2:898. doi: 10.1038/srep00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulusoy A, et al. Caudo-rostral brain spreading of α-synuclein through vagal connections. EMBO Mol Med. 2013;5(7):1119–1127. doi: 10.1002/emmm.201302475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips RJ, Walter GC, Wilder SL, Baronowsky EA, Powley TL. Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: autonomic pathway implicated in Parkinson’s disease? Neuroscience. 2008;153(3):733–750. doi: 10.1016/j.neuroscience.2008.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasegawa S, et al. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in parkinson’s disease. PLoS ONE. 2015;10(11):e0142164. doi: 10.1371/journal.pone.0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keshavarzian A, et al. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30(10):1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 27.Scheperjans F, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30(3):350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 28.Unger MM, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Sampson TR, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167(6):1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H, et al. Research on the premotor symptoms of Parkinson’s disease: clinical and etiological implications. Environ Health Perspect. 2013;121(11-12):1245–1252. doi: 10.1289/ehp.1306967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanner CM, et al. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect. 2011;119(6):866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldman SM. Environmental toxins and Parkinson’s disease. Annu Rev Pharmacol Toxicol. 2014;54:141–164. doi: 10.1146/annurev-pharmtox-011613-135937. [DOI] [PubMed] [Google Scholar]
- 33.Bohórquez DV, et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest. 2015;125(2):782–786. doi: 10.1172/JCI78361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liddle RA. Regulation of pancreatic secretion. In: Johnson LR ed. Physiology of the Gastrointestinal Tract. San Diego: Elsevier; 2012:1425–1460. [Google Scholar]
- 35.Bohórquez DV, Samsa LA, Roholt A, Medicetty S, Chandra R, Liddle RA. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PLoS ONE. 2014;9(2):e89881. doi: 10.1371/journal.pone.0089881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rindi G, et al. Development of neuroendocrine tumors in the gastrointestinal tract of transgenic mice. Heterogeneity of hormone expression. Am J Pathol. 1990;136(6):1349–1363. [PMC free article] [PubMed] [Google Scholar]
- 37. McCarthy T, Green BD, Calderwood D, Gillespie A, Cryan JF, Giblin L. STC-1 Cells. In: Verhoeckx K, et al, eds. The Impact of Food Bioactives on Health: in vitro and ex vivo models. Cham: Springer International Publishing; 2015:211–220. [Google Scholar]
- 38.Wang Y, Prpic V, Green GM, Reeve JR, Liddle RA. Luminal CCK-releasing factor stimulates CCK release from human intestinal endocrine and STC-1 cells. Am J Physiol Gastrointest Liver Physiol. 2002;282(1):G16–G22. doi: 10.1152/ajpgi.2002.282.1.G16. [DOI] [PubMed] [Google Scholar]
- 39.Sundaresan S, et al. CD36-dependent signaling mediates fatty acid-induced gut release of secretin and cholecystokinin. FASEB J. 2013;27(3):1191–1202. doi: 10.1096/fj.12-217703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hand KV, Giblin L, Green BD. Hormone profiling in a novel enteroendocrine cell line pGIP/neo: STC-1. Metab Clin Exp. 2012;61(12):1683–1686. doi: 10.1016/j.metabol.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 41.Kuo YM, et al. Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum Mol Genet. 2010;19(9):1633–1650. doi: 10.1093/hmg/ddq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corbillé AG, Neunlist M, Derkinderen P. Cross-linking for the analysis of α-synuclein in the enteric nervous system. J Neurochem. 2016;139(5):839–847. doi: 10.1111/jnc.13845. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, et al. Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am J Physiol Gastrointest Liver Physiol. 2011;300(4):G528–G537. doi: 10.1152/ajpgi.00387.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chandra R, Wang Y, Shahid RA, Vigna SR, Freedman NJ, Liddle RA. Immunoglobulin-like domain containing receptor 1 mediates fat-stimulated cholecystokinin secretion. J Clin Invest. 2013;123(8):3343–3352. doi: 10.1172/JCI68587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, et al. Mice overexpressing wild-type human alpha-synuclein display alterations in colonic myenteric ganglia and defecation. Neurogastroenterol Motil. 2012;24(9):e425–e436. doi: 10.1111/j.1365-2982.2012.01974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang N, Gibbons CH, Lafo J, Freeman R. α-Synuclein in cutaneous autonomic nerves. Neurology. 2013;81(18):1604–1610. doi: 10.1212/WNL.0b013e3182a9f449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25(25):6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sung JY, Kim J, Paik SR, Park JH, Ahn YS, Chung KC. Induction of neuronal cell death by Rab5A-dependent endocytosis of alpha-synuclein. J Biol Chem. 2001;276(29):27441–27448. doi: 10.1074/jbc.M101318200. [DOI] [PubMed] [Google Scholar]
- 49.Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22(8):3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu S, et al. Inhibition of tyrosine hydroxylase expression in alpha-synuclein-transfected dopaminergic neuronal cells. Neurosci Lett. 2004;367(1):34–39. doi: 10.1016/j.neulet.2004.05.118. [DOI] [PubMed] [Google Scholar]
- 51.Li ZS, Pham TD, Tamir H, Chen JJ, Gershon MD. Enteric dopaminergic neurons: definition, developmental lineage, and effects of extrinsic denervation. J Neurosci. 2004;24(6):1330–1339. doi: 10.1523/JNEUROSCI.3982-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chandra R, Samsa LA, Vigna SR, Liddle RA. Pseudopod-like basal cell processes in intestinal cholecystokinin cells. Cell Tissue Res. 2010;341(2):289–297. doi: 10.1007/s00441-010-0997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol. 2013;9(1):13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 54.Sharkey KA. Emerging roles for enteric glia in gastrointestinal disorders. J Clin Invest. 2015;125(3):918–925. doi: 10.1172/JCI76303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clairembault T, Leclair-Visonneau L, Neunlist M, Derkinderen P. Enteric glial cells: new players in Parkinson’s disease? Mov Disord. 2015;30(4):494–498. doi: 10.1002/mds.25979. [DOI] [PubMed] [Google Scholar]
- 56.Wong YC, Krainc D. α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med. 2017;23(2):1–13. doi: 10.1038/nm.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaji I, Karaki S, Tanaka R, Kuwahara A. Density distribution of free fatty acid receptor 2 (FFA2)-expressing and GLP-1-producing enteroendocrine L cells in human and rat lower intestine, and increased cell numbers after ingestion of fructo-oligosaccharide. J Mol Histol. 2011;42(1):27–38. doi: 10.1007/s10735-010-9304-4. [DOI] [PubMed] [Google Scholar]
- 58.Sykaras AG, Demenis C, Case RM, McLaughlin JT, Smith CP. Duodenal enteroendocrine I-cells contain mRNA transcripts encoding key endocannabinoid and fatty acid receptors. PLoS ONE. 2012;7(8):e42373. doi: 10.1371/journal.pone.0042373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nøhr MK, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154(10):3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 60.Psichas A, et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond) 2015;39(3):424–429. doi: 10.1038/ijo.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El-Salhy M, Norrgård O, Spinnell S. Abnormal colonic endocrine cells in patients with chronic idiopathic slow-transit constipation. Scand J Gastroenterol. 1999;34(10):1007–1011. doi: 10.1080/003655299750025110. [DOI] [PubMed] [Google Scholar]
- 62.Posovszky C, et al. Loss of enteroendocrine cells in autoimmune-polyendocrine-candidiasis-ectodermal-dystrophy (APECED) syndrome with gastrointestinal dysfunction. J Clin Endocrinol Metab. 2012;97(2):E292–E300. doi: 10.1210/jc.2011-2044. [DOI] [PubMed] [Google Scholar]
- 63.Ruffmann C, Parkkinen L. Gut feelings about α-synuclein in gastrointestinal biopsies: biomarker in the making? Mov Disord. 2016;31(2):193–202. doi: 10.1002/mds.26480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corbillé AG, et al. Evaluation of alpha-synuclein immunohistochemical methods for the detection of Lewy-type synucleinopathy in gastrointestinal biopsies. Acta Neuropathol Commun. 2016;4:35. doi: 10.1186/s40478-016-0305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corbillé AG, et al. What a gastrointestinal biopsy can tell us about Parkinson’s disease? Neurogastroenterol Motil. 2016;28(7):966–974. doi: 10.1111/nmo.12797. [DOI] [PubMed] [Google Scholar]
- 66.Cabin DE, et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22(20):8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bohórquez DV, Chandra R, Samsa LA, Vigna SR, Liddle RA. Characterization of basal pseudopod-like processes in ileal and colonic PYY cells. J Mol Histol. 2011;42(1):3–13. doi: 10.1007/s10735-010-9302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee BR, Kamitani T. Improved immunodetection of endogenous α-synuclein. PLoS One. 2011;6(8):e23939. doi: 10.1371/journal.pone.0023939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.