Abstract
Larger numbers of pneumococci were detected in the nasal tract compared to the lung, cervical lymph nodes, and spleen 1, 2, 4, 7, 14, and 21 days after nasal challenge with Streptococcus pneumoniae strain EF3030. In this mouse model of pneumococcal carriage, peripheral S. pneumoniae pneumococcal surface adhesin A (PsaA)-specific humoral responses (immunoglobulin G2a [IgG2a] ≫ IgG1 = IgG2b > IgG3) were significantly higher than pneumococcal surface protein A (PspA)-specific, genetic toxoid derivative of pneumolysin (PdB)-specific, or pneumococcal surface protein C (PspC)-specific serum antibody levels. However, PspA-specific mucosal IgA antibody levels were significantly higher than those against PsaA, PdB, and PspC. In general, both PsaA- and PspA-specific lung-, cervical lymph node-, nasal tract-, and spleen-derived CD4+ T-cell cytokine (interleukin-4, interleukin-6, granulocyte-macrophage colony-stimulating factor, gamma interferon, and tumor necrosis factor alpha) and proliferative responses were higher than those for either PspC or PdB. Taken together, these findings suggest that PsaA- and PspA-specific mucosal responses as well as systemic humoral and T helper cell cytokine responses are predominantly yet differentially induced during pneumococcal carriage.
The immunodominant antigen of Streptococcus pneumoniae is the capsular polysaccharide; however, susceptibility to pneumococcal carriage by humans does not correlate with anticapsular polysaccharide antibody responses (30). While 7-valent polysaccharide-protein conjugate vaccines elicit modest protection against pneumococcal carriage (16, 23, 24), they have not been reported to eliminate carriage in human or mouse models (15, 29). However, several pneumococcal proteins are known to elicit protective immunity. The pneumococcal surface protein C gene (pspC) of S. pneumoniae encodes PspC (also known as CbpA and SpsA), which provides protective pneumococcal immunity (13, 35). PspC is present in approximately 75% of all S. pneumoniae strains and binds human complement factor H (17) to avoid complement attack and opsonophagocytosis (20). Additionally, PspC inhibits the binding of secretory immunoglobulin A (IgA) antibodies to pneumococci (19). Mice immunized with PspC alone are significantly protected against systemic challenge with the highly virulent capsular type 2 pneumococcal strain D39 (35).
Pneumolysin (its genetic toxoid derivative of pneumolysin [PdB]) is a cytoplasmic cytolytic toxin of S. pneumoniae that can interfere with phagocyte function (36). It also slows ciliary beating and disrupts the surface integrity of the human respiratory epithelium (44). This important virulence factor is released in vivo during the autolysis of pneumococci and is believed to pave the way for invasion and disease. Nasopharyngeal IgA antibodies to PdB can be produced early in life by pneumococcal colonization and may be important for carriage in adults (49).
Pneumococcal surface protein A (PspA) is a surface protein of S. pneumoniae that inhibits complement activation (47). In mice, PspA has been shown to elicit protective systemic immunity against pneumococcal infection as well as to be necessary for full pneumococcal virulence (14). Intranasal immunization with PspA plus adjuvant in mice can protect against carriage as well as systemic infection (3). All S. pneumoniae isolates tested to date express a 37-kDa lipoprotein, pneumococcal surface adhesin A (PsaA), which is present on strains of all 90 serotypes (32). Furthermore, certain psaA mutants are significantly less virulent, as judged by intraperitoneal challenge of mice, but intranasal low-dose challenge of mice with similar psaA mutants does not result in comparable differences in virulence (7). Antibodies to this pneumococcal protein are protective against nasal colonization in mice (8), and higher concentrations of antibodies to PsaA in humans are associated with lower risks of pneumococcal carriage and disease progression (33, 40).
To provide adequate herd immunity or widespread protection against pneumococcal infection, a new or modified vaccine that takes into consideration the pathogenesis of S. pneumoniae is needed. With an experimental model of human pneumococcal carriage, it was shown that human subjects nasally colonized with S. pneumoniae (capsular type 23F or 6B) mounted IgG antibody responses against PspA and PspC but lower antibody titers against PsaA and PdB (31). However, local protection against pneumococcal carriage may require both mucosal and peripheral (systemic) immunity. If carriage could be controlled, then pneumococcal transmission and invasive disease progression could be eliminated or at least mitigated. The present study is among the first that compares the mucosal and systemic adaptive (humoral and cellular) immune responses generated against PsaA, PspA, PspC, and PdB during pneumococcal carriage.
MATERIALS AND METHODS
S. pneumoniae strain EF3030 growth and carriage.
S. pneumoniae capsular group 19 strain EF3030 (12) was obtained from Alan Parkinson at the Arctic Investigations Laboratory of the Centers for Disease Control. S. pneumoniae strain EF3030 was among the human isolates of capsular group 19 that were examined previously and found to be relatively noninvasive in mice (9); specifically, this strain was chosen because it colonizes the upper respiratory tract in the absence of bacteremia. Pneumococci were grown in Todd Hewitt broth and stored frozen in aliquots at −70°C in 20% glycerol in sterile lactated Ringer's injection solution (Abbott Labs, North Chicago, Ill.) (1, 11).
Pneumococcal antigens.
Recombinant lipidated PsaA was prepared with the Qiaexpress system (Qiagen, Chatsworth, Calif.). The expression host, Escherichia coli SG 13009, was transformed with pAB247, the recombinant plasmid that carries psaA from the serotype 2 S. pneumoniae strain D39 cloned into pQE30 (2). Recombinant PspA from S. pneumoniae strain Rx1 (for this study expressing amino acids 1 to 303 of the mature PspA protein); PdB, a derivative of pneumolysin with a Trp433-Phe mutation that reduces hemolytic activity without affecting antigenicity; and PspC, a 59- to 105-kDa paralogue of PspA, were purified and prepared as previously described (8, 13, 37).
Animals.
Female BALB/c mice, 8 to 12 weeks old, were procured from Jackson Laboratories (Bar Harbor, Mass.). All mice were housed in horizontal laminar flow cabinets. Routine antibody screening for a large panel of pathogens and routine histological analyses of organs and tissues were performed to ensure that mice were pathogen free before the start of this study. To establish nasal carriage, groups of BALB/c mice were nasally administered ≈7.5 × 106 CFU/ml of S. pneumoniae strain EF3030 in 15 μl of Ringer's solution (22). Experimental groups consisted of five mice, and studies were repeated three to five times. The guidelines proposed by the committee for the Care of Laboratory Animal Resources Commission of Life Sciences, National Research Council, were followed to minimize animal pain and distress. All procedures involving mice were approved by the Institutional Review Board of the Morehouse School of Medicine.
Sample and tissue collection.
To obtain individual nasal wash samples from each mouse, the interior of the nasal tract was flushed by placing polyethylene tubing (≈1 mm diameter; Becton Dickinson, Sparks, Md.), attached to a 1-ml syringe, through the trachea towards the nasal cavity and rinsed with 100 μl of sterile phosphate-buffered saline (PBS). Approximately 200 μl of blood was collected from each mouse by retroorbital plexus puncture, with heparinized capillary tubes, and serum was obtained following centrifugation. Serum and nasal washes that were collected on days 0 and 28, stored at −20°C, and subsequently measured for antigen-specific (PsaA, PspA, PspC, and PdB) antibodies by enzyme-linked immunosorbent assay (ELISA). Mice were sacrificed by CO2 inhalation to collect the spleen and mucosal lymphoid tissues (nasal tract, cervical lymph nodes, and lung).
Antigen-specific antibody detection by ELISA.
PsaA-, PspA-, PdB-, and PspC-specific antibodies in nasal secretion and serum samples were measured by ELISA (26). Briefly, 96-well ELISA plates were coated with 50 μl of 5-μg/ml PsaA, PspA, PspC, or PdB in coating buffer (sodium carbonate-bicarbonate buffer) overnight at 4°C and blocked with 200 μl of 10% fetal calf serum (FCS; Atlanta Biologicals, Norcross, Ga.) in phosphate-buffered saline (PBS) (FCS-PBS) for 3 h at room temperature. Individual samples (100 μl) were added and serially diluted in FCS-PBS. After overnight incubation, plates were washed three times with 250 μl of PBS containing 0.05% Tween 20 (PBS-T), and titers of IgG and IgA were determined by the addition of 100 μl of 0.33-μg/ml horseradish peroxidase-conjugated goat anti-mouse α, γ, or μ heavy-chain-specific antiserum (Southern Biotechnology Associates, Inc., Birmingham, Ala.) in FCS-PBS-T. Similarly, 100 μl of biotin-conjugated rat anti-mouse γ1 (clone G1-7.3 at 12.5 ng/ml), γ2a (clone R19-15 at 125 ng/ml), γ2b (clone R12-3 at 12.5 ng/ml), and γ3 (clone R40-82 at 50 ng/ml) (BD-PharMingen, San Diego, Calif.) heavy-chain-specific monoclonal antibodies were used to determine the antigen-specific IgG subclasses (26). After incubation and washing three times with 250 μl of PBS-T, 100 μl of 0.5-μg/ml horseradish peroxidase-antibiotin antibody (BD-PharMingen, San Diego, Calif.) in FCS-PBS-T was added to IgG subclass detection wells, followed by incubation for 3 h at room temperature. Following incubation, the plates were washed six times and the color reaction for this ELISA was developed by adding 100 μl of tetramethylbenzidine substrate (eBioscience, San Diego, Calif.). The ELISA was allowed to react for 20 min and stopped with 50 μl of 2% H2SO4. The optical density was read at 450 nm and compared against a standard curve that was generated with control standards of IgG, IgA, and IgM antibodies.
Cell isolation.
Single-cell suspensions of spleen, lung, cervical lymph node, and nasal tract cells were prepared by aseptically removing tissues and passing them through a sterile wire screen. The lower respiratory tract (lungs and mediastinal lymph nodes) were instilled with 10 ml of cold PBS to remove blood, dissected into small pieces, and subjected to collagenase digestion with 1 mg of collagenase type IV (Sigma) per ml in RPMI 1640 (collagenase solution) (26). Lymphocytes were further purified with a discontinuous Percoll (Pharmacia, Uppsala, Sweden) gradient, and the lymphocytes were collected at the 40 to 75% interface (25, 26).
Nasal tract lymphocytes were isolated by gently washing nasal cavities with 200 μl of cold PBS to remove blood. Next, the nasal tract mucosal tissue was removed by scraping, and the resulting tissue was then passed through sterile glass wool (25). T-cell fractions were obtained by passing single-cell suspensions over nylon wool (Polysciences, Inc., Warrington, Pa.) for 1 h at 37°C (>98% purity, as determined by flow cytometry, described below). Subsequently, CD4+ T cells were enriched with mouse CD4 Cellect Plus columns according to the manufacturer's protocols (Biotex Laboratories, Inc. Edmonton, Alberta, Canada). Furthermore, the purity (>99%) of CD4+ T-cell isolation was assessed by flow cytometry. Briefly, cells were stained with fluorescein isothiocyanate-conjugated hamster anti-mouse CD3ɛ IgG1 (clone 145-2C11; BD-Pharmingen) and phycoerythrin-conjugated rat anti-mouse CD4 IgG2b (clone GK1.5; BD-Pharmingen) or isotype fluorescein isothiocyanate-conjugated nonspecific hamster IgG1 (clone A19-3; BD-Pharmingen) and phycoerythrin-conjugated nonspecific rat IgG2b (cloneA95-1; BD-Pharmingen) controls for 30 min with shaking. Lymphocytes were then washed twice with flow cytometry buffer (PBS with 1% FCS) and fixed in 2% paraformaldehyde in PBS and analyzed by flow cytometry (Becton Dickinson, San Diego, Calif.).
A fluorescence intensity lower limit was set based on isotype controls (i.e., negative). CD3+ CD4+ lymphocytes with fluorescent intensity above this threshold cutoff represented positive cell surface expression. Cell suspensions were washed twice in RPMI 1640, and lymphocytes were maintained in complete medium, which consisted of RPMI 1640 supplemented with 10 ml of nonessential amino acids (Mediatech, Washington, D.C.) per liter, 1 mM sodium pyruvate (Sigma), 10 mM HEPES (Mediatech), 100 U of penicillin per ml, 100 μg of streptomycin per ml, 40 μg of gentamicin (Elkins-Sinn, Inc., Cherry Hill, N.J.) per ml, and 10% FCS.
Pneumococcal quantification.
Single-cell suspensions of spleen, lung, cervical lymph node, and nasal tract tissues were prepared by passage through a sterile wire screen as above, with the exception that lungs and nasal tracts were not instilled or rinsed, respectively, with PBS. Cells from each tissue were suspended in 2 ml of sterile Ringer's solution. Five serial 10-fold dilutions were made and plated (in quadruplicate) on blood agar plates containing 4 μg of gentamicin sulfate per ml. The numbers of CFU per organ were enumerated 24 h after plating and incubation in a candle jar at 37°C.
Cytokine detection by ELISA.
Purified CD4+ T cells and irradiated feeder cells were cultured at densities of 5 × 106 and 1 × 106 cells per ml, respectively, in complete medium containing 5 μg of antigen (PsaA, PspA, PspC, or PdB) per ml at 37°C in 5% CO2. To assess cytokine production, 1 ml of culture supernatant from 12-well flat-bottomed plates (Corning Glass Works) were harvested after 3 days of antigen stimulation. The amounts of interleukin (IL)-2, IL-4, IL-6, IL-10, tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and granulocyte-macrophage colony-stimulating factor (GM-CSF) in cell culture supernatants were determined by ELISA, following the manufacturer's instructions (e-Biosciences, San Diego, Calif.). Briefly, Falcon 3912 Microtest plates (Fisher Scientific) were coated with 100 μl of 2.5-μg/ml rat anti-mouse IFN-γ, TNF-α, IL-2, IL-4, IL-6, and IL-10 in 0.1 M bicarbonate buffer (pH 8.2) overnight at 4°C and blocked with 10% FCS at room temperature for 3 h. Next, 100 μl of serially diluted recombinant murine cytokines as standards or cultured supernatant samples were added in duplicate and incubated overnight at 4°C. The plates were washed with PBS containing 0.05% Tween 20 (PBS-T) and incubated with 0.2 μg of biotinylated secondary murine cytokine detection antibodies per ml in FBS-PBS-T for 3 h at room temperature. After washing three times with 250 μl of PBS-T followed by three washes with 250 μl of PBS alone, wells were incubated for 2 h in 100 μl of 0.5-μg/ml horseradish peroxidase-conjugated antibiotin antibody. Following incubation, the plates were washed six times and the color reaction for this ELISA was developed by adding 100 μl of tetramethylbenzidine substrate (eBioscience, San Diego, Calif.). The ELISA was allowed to react for 20 min and stopped with 50 μl of 2% H2SO4. The optical density was read at 450 nm and compared against a standard curve that was generated with control standards. The cytokine ELISAs were capable of detecting 8 pg/ml of IFN-γ and TNF-α, 2 pg/ml of IL-2, 4 pg/ml of IL-4, IL-6, and GM-CSF, or 15 pg/ml of IL-10.
Cell proliferation.
Lymphocyte proliferation was measured by 5-bromo-2′-deoxyuridine absorption and detection (Roche Diagnostics, Düsseldorf, Germany). In brief, purified CD4+ T cells were cultured at a density of 5 × 106 cells/ml with 106 irradiated feeder cells/ml in complete medium containing 5 μg of antigen (PsaA, PspA, PspC, or PdB) per ml at 37°C in 5% CO2. After 2 days of antigen stimulation, cells, at a density of 106/ml, were transferred to polystyrene 96-well plates (Corning Glass Work). After 10 μl of bromodeoxyuridine labeling solution (10 μM final concentration per well) was added, the cells were incubated for 18 h at 37°C with 5% CO2. The cells were then fixed and incubated with 100 μl of nuclease solution in each well for 30 min at 37°C. Next, cells were washed with complete medium and incubated with horseradish peroxidase-conjugated antibromodeoxyuridine antibody for 30 min at 37°C. The incorporation of bromodeoxyuridine was correspondingly developed by adding 100 μl of tetramethylbenzidine substrate. The substrate reaction was allowed to continue for 20 min and stopped with 50 μl of 2% H2SO4. The optical density was read at 450 nm and compared against a standard curve.
Statistics.
The data are expressed as the mean ± standard error of the mean and compared with a two-tailed Student's t test or an unpaired Mann-Whitney U test. The results were analyzed with the Statview II statistical program (Abacus Concepts, Inc., Berkeley, Calif.) for Macintosh computers and were considered statistically significant if P values were less than 0.05. When cytokine or antibody levels were below the detection limit, they were recorded as one-half the lower detection limit (e.g., 2 pg/ml for IL-6) for statistical analysis.
RESULTS
PsaA-, PspA-, PspC-, and PdB-specific antibody responses during pneumococcal carriage.
Intranasal infection of mice with certain strains of capsular group 19 S. pneumoniae can result in carriage or focal pneumonia in the absence of bacteremia (10, 12, 46). We used S. pneumoniae strain EF3030 (group 19) to study mucosal as well as systemic humoral and cellular immune responses against pneumococcal proteins during carriage (12). Following the establishment of carriage, high CFU of S. pneumoniae strain EF3030 were detected in the nasal tract compared to the lungs, cervical lymph nodes, and spleen (Table 1). The next highest numbers of pneumococci were detected in the lung (≈78 CFU) 2 days after challenge. As many as ≈1.4 × 105 CFU were detected in the nasal tract 5 days after challenge. Subsequently, this nasopharyngeal bacterial load varied from 316 to 3,981 CFU from 7 to 21 days, respectively, post-S. pneumoniae strain EF3030 inoculation.
TABLE 1.
S. pneumoniae strain EF3030 in tissuea
| Day post Challenge | Mean level of S. pneumoniae strain EF3030 (102 CFU) (± SEM)
|
|||
|---|---|---|---|---|
| Spleen | Cervical lymph nodes | Lung | Nasal tract | |
| 1 | <0.02 | <0.02 | <0.02 | 5.56 (± 0.83) |
| 2 | <0.02 | 0.16 (± 0.02) | 0.78 (± 0.12) | 562.34 (± 74.35) |
| 4 | <0.02 | <0.02 | 0.28 (± 0.08) | 1,318.26 (± 183.58) |
| 7 | <0.02 | <0.02 | 0.12 (± 0.02) | 3.16 (± 0.46) |
| 14 | <0.02 | <0.02 | 0.03 (± 0.02) | 5.01 (± 0.72) |
| 21 | <0.02 | 0.04 (± 0.02) | <0.02 | 39.81 (± 5.87) |
BALB/c mice were nasally challenged with ∼7.5 × 106 CFU of S. pneumoniae strain EF3030 in a 15-μl volume of Ringer's solution. Nasal tract-, lung-, cervical lymph node-, and spleen-associated pneumococci were enumerated 1, 2, 4, 7, 14, and 21 days postchallenge and are expressed as mean CFU per mouse for five mice; data are representative of three different experiments. Uninfected (negative control) mice had no detectable S. pneumoniae (i.e., < 0.02 × 102), and these values are not shown.
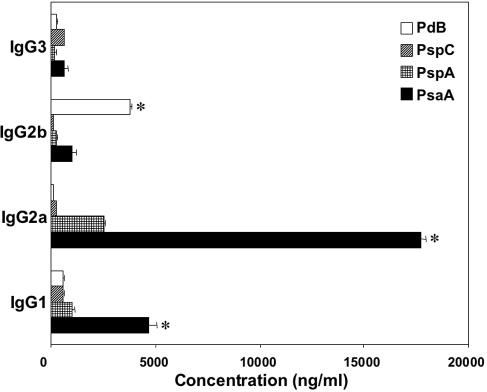
After the establishment of pneumococcal carriage, the immunogenicity of potential pneumococcal vaccine antigens was also evaluated 28 days later. All of the pneumococcal antigens tested elicited antigen-specific plasma as well as nasal antibody responses in mice challenged with S. pneumoniae strain EF3030 (Fig. 1). Pneumococcus-specific plasma and nasal antibodies were below the detectable limit in naïve mice (data not shown). While there were no differences among PsaA-, PspA-, PspC-, and PdB-specific IgG3 plasma antibody responses, PsaA-specific IgG1 and IgG2a antibodies were significantly higher than similar IgG responses to PspA, PspC, or PdB. PdB-specific IgG2b plasma antibody levels were significantly higher than PsaA-, PspA-, or PspC-specific plasma IgG2b responses. Interestingly, the highest levels of IgG subclass antibody responses were directed to PsaA, the most immunogenic (systemic) antigen following pneumococcal carriage.
FIG. 1.
PsaA-, PspA-, PspC-, and PdB-specific plasma antibody responses during pneumococcal carriage. Groups of five BALB/c mice were intranasally challenged with ≈7.5 × 106 CFU of Streptococcus pneumoniae EF3030 in 15 μl of Ringer's solution. Serum samples were collected 28 days postinfection, and S. pneumoniae PsaA-, PspA-, PspC-, and PdB-specific IgG subclass antibody levels in plasma were measured by ELISA, which was capable of detecting >20 pg/ml of IgG1, IgG2a, IgG2b, and IgG3 antibodies. The data presented are the mean antibody concentration ± standard error of the mean of three separate experiments with infected mice. Naïve mice displayed responses that were below the detection limit of the ELISA and are not shown. Asterisks indicate significant differences (P < 0.05) between the maximum and next highest antigen-specific IgG1, IgG2a, IgG2b, or IgG3 antibody level in infected mice.
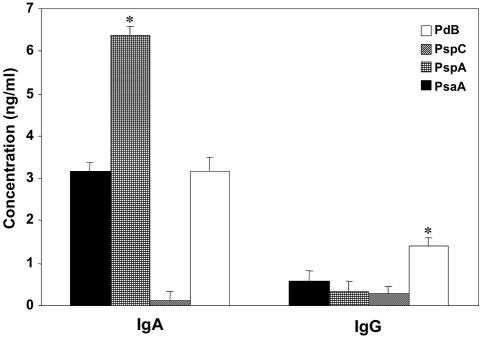
Antigen-specific IgA and IgG antibody responses in nasal washes were also characterized to determine the most immunogenic mucosal pneumococcal antigen during carriage. As with PdB-specific IgG2b systemic antibody responses, nasal PdB-specific IgG antibodies were significantly higher than those against PsaA, PspA, and PspC (Fig. 2). PspA-specific IgA antibody levels were higher than either PsaA, PspC, or PdB levels; however, PsaA- and PdB-specific IgA antibody concentrations were greater than those raised to PspC. Taken together, the host mounts disparate humoral responses to PsaA, PspA, PspC, and PdB, which not only highlights the differences in antigen immunogenicity but also suggests differential expression of these antigens in the mucosa (nasal tract) versus the systemic compartment (spleen) during carriage.
FIG. 2.
PsaA-, PspA-, PspC-, and PdB-specific nasal antibody responses during pneumococcal carriage. Groups of five BALB/c mice were intranasally challenged with ≈7.5 × 106 CFU of Streptococcus pneumoniae EF3030 in 15 μl of Ringer's solution. Nasal wash samples were collected 28 days postinfection, and S. pneumoniae PsaA-, PspA-, PspC-, and PdB-specific antibody titers were measured by ELISA that was capable of detecting >20 pg/ml of IgA or IgG antibodies. The data presented are the mean antibody concentrations ± standard error of the mean of three separate experiments with infected mice. Naïve mice displayed responses that were below the detection limit of the ELISA and are not shown. Asterisks indicate significant differences (P < 0.05) between the maximum and next highest antigen-specific IgA or IgG antibody levels in infected mice.
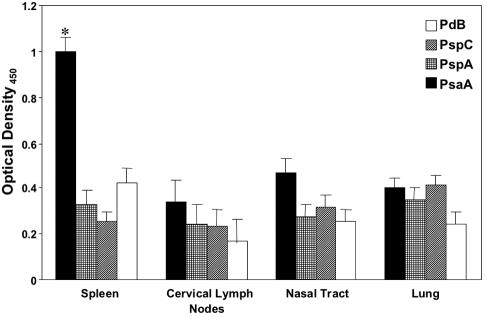
Antigen-specific CD4+ T-cell proliferation.
Mucosal (nasal tract, lung, and cervical lymph node) and spleen-derived CD4+ T cells were purified from naïve mice (data not shown) or from mice that had been nasally challenged 28 days earlier with S. pneumoniae strain EF3030. To determine antigen-specific T helper responses, these purified CD4+ T cells were restimulated ex vivo with PsaA, PspA, PspC, and PdB. In this regard, PdB was used ex vivo to mitigate pneumolysin’s potential cytotoxic effects. Proliferative responses to PsaA, PspA, PspC, and PdB by T cells from the nasal tract, cervical lymph node, and lung to S. pneumoniae strain EF3030 (Fig. 3) were comparatively higher than those in naïve mice (data not shown). In confirmation with the high PsaA-specific serum antibody levels, CD4+ T cells from the spleen had the highest response to PsaA stimulation relative to PspA, PspC, and PdB.
FIG. 3.
PsaA-, PspA-, PspC-, and PdB-specific proliferative responses by antigen-stimulated CD4+ T cells following pneumococcal carriage. BALB/c mice were intranasally challenged with ≈7.5 × 106 CFU of Streptococcus pneumoniae EF3030 in 15 μl of Ringer's solution. Nasal tract-, lung-, cervical lymph node-, and spleen-derived CD4+ T cells were purified and antigen stimulated with 5 μg of PsaA, PspA, PspC, and PdB per ml for 3 days in complete medium. Proliferation was measured by detecting the level of bromodeoxyuridine incorporation by ELISA. The data presented are the mean ± standard error of the mean optical densities of quadruplicate cultures from infected mice. Naïve mice displayed responses that were below the detection limit of the ELISA and are not shown. Asterisks indicate significant differences (P < 0.05) between the maximum and next highest antigen-specific CD4+ T-cell proliferation response from infected mice.
Pneumococcal protein-specific T helper cytokine responses.
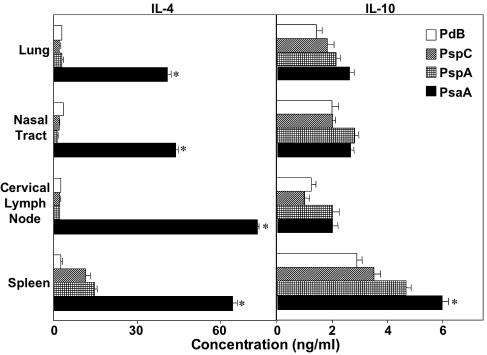
Since our studies showed that carriage-induced systemic antibody responses of S. pneumoniae strain EF3030 were in large part IgG2a mediated, we next examined the antigen-specific T helper cytokine responses generated during pneumococcal carriage compared with those from naïve mice. CD4+ T cells from the spleen, cervical lymph nodes, nasal tract, and lungs exhibited increased production of GM-CSF, IFN-γ, IL-2, IL-4, IL-6, and TNF-α compared with uninfected controls (Fig. 4, 5, and 6). Despite the Th1-biased humoral responses, IL-4 and IL-10 secreted by Th2 cells were also present after nasal carriage (Fig. 4). PsaA stimulation resulted in the highest levels of IL-4 secretion, indicative of large numbers of PsaA-specific Th2 cells in mucosal and systemic tissues.
FIG. 4.
PsaA-, PspA-, PspC-, and PdB-specific T helper 2 cytokine secretion by antigen-stimulated CD4+ T cells following pneumococcal carriage. Mice were intranasally challenged with ≈7.5 × 106 CFU of Streptococcus pneumoniae EF3030 in 15 μl of Ringer's solution; 28 days postinfection, spleen-, lung-, and cervical lymph node-derived CD4+ T cells were purified and cultured with 5 μg of PsaA, PspA, PspC, or PdB per ml for 5 days in complete medium. Cytokine levels in culture supernatants were determined by ELISA capable of detecting >20 pg/ml of IL-4 or IL-10. The data presented are the mean cytokine concentrations ± standard error of the mean. Naïve mice displayed responses that were below the detection limit of the ELISA and are not shown. Asterisks indicate significant differences (P < 0.05) between the maximum and next highest antigen-specific CD4+ T-cell cytokine response from infected mice.
FIG. 5.
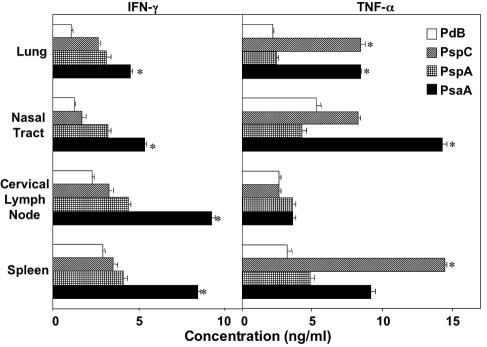
PsaA-, PspA-, PspC-, and PdB-specific IFN-γ and TNF-α secretion by antigen-stimulated CD4+ T cells following pneumococcal carriage. Mice were intranasally challenged with ≈7.5 × 106 CFU of Streptococcus pneumoniae EF3030 in 15 μl of Ringer's solution; 28 days postinfection, spleen-, lung-, and cervical lymph node-derived T cells were purified and cultured with 5 μg of PsaA, PspA, PspC, or PdB per ml for 5 days in complete medium. Cytokine levels in culture supernatants were determined by ELISA capable of detecting >10 pg/ml of IFN-γ or TNF-α. The data presented are the mean cytokine concentrations ± standard error of the mean. Naïve mice displayed responses that were below the detection limit of the ELISA and are not shown. Asterisks indicate significant differences (P < 0.05) between the maximum and next highest antigen-specific CD4+ T-cell cytokine response from infected mice.
FIG. 6.
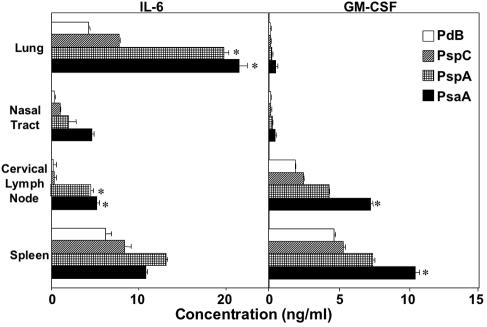
PsaA-, PspA-, PspC-, and PdB-specific IL-6 and GM-CSF secretion by antigen-stimulated CD4+ T cells following pneumococcal carriage. Mice were intranasally challenged with ≈7.5 × 106 CFU of Streptococcus pneumoniae EF3030 in 15 μl of Ringer's solution; 28 days postinfection, spleen-, lung-, and cervical lymph node-derived CD4+ T cells were purified and cultured with 5 μg of PsaA, PspA, PspC, or PdB per ml for 5 days in complete medium. Cytokine levels in culture supernatants was determined by ELISA, capable of detecting >10 pg/ml of IL-6 or GM-CSF. The data presented are the mean cytokine concentrations ± standard error of the mean. Naïve mice displayed responses that were below the detection limit of the ELISA and are not shown. Asterisks indicate significant differences (P < 0.05) between the maximum and next highest antigen-specific CD4+ T-cell cytokine response from infected mice.
The importance of IFN-γ, IL-6, and TNF-α responses in pneumococcal disease has been mentioned previously (6, 21, 27, 45, 48). These cytokines were also significantly elevated following antigen stimulation of CD4+ T cells from pneumococcus-carrying (infected) mice compared with naïve mice, with levels below detection (Fig. 5 and 6). In this regard, PsaA- and PspA-specific CD4+ T cells produced the highest levels of these cytokines compared with PspC- and PdB-specific cells. Of particular interest, lung-derived CD4+ T cells secreted the highest levels of IL-6 after stimulation with PsaA or PspA. Cells isolated from cervical lymph nodes also exhibited similar trends in IL-6 production. Conversely, GM-CSF levels were the highest from cervical lymph node- and spleen-derived PsaA- and PspA-specific CD4+ T cells compared with the levels from the lung or nasal tract (Fig. 5).
In general, PsaA stimulation resulted in significantly higher levels of IFN-γ and TNF-α secretion, compared with the other pneumococcal antigens tested, with PdB-specific mucosal and systemic CD4+ T cells producing the least (Fig. 6). While PspC stimulation did not frequently result in significant levels of cytokine secretion, TNF-α levels after PspC stimulation were as high or higher than the levels produced by PsaA-restimulated T cells from the spleen, nasal tract, lung, or cervical lymph node. Taken together, pneumococcal antigen-specific CD4+ T-cell cytokine responses differ in antigen, cytokine secretion pattern, and T helper cell source.
DISCUSSION
S. pneumoniae is carried asymptomatically by the nasopharyngeal mucosa and spread among individuals. Nasopharyngeal carriage of S. pneumoniae constitutes its major human reservoir and is thought to be the prelude to nearly all pneumococcal diseases. Current studies show that resistance to carriage is dependent on mucosal rather than systemic immunity (51). The possibility of including more than one protein along with capsular polysaccharides in future pneumococcal vaccines offers the potential of targeting multiple virulence mechanisms as well as of blocking factors important for mucosal versus systemic protection. This study mechanistically dissects the mucosal and systemic immunogenicity of PdB, PsaA, PspA, and PspC as well as addresses potential hallmarks of pneumococcal immunopathogenesis.
Serum antibodies to PsaA, PspA, and PdB measured in children <24 months old and in their mothers show that they were capable of producing antibodies to these proteins and that the presence of these antibodies was strongly associated with pneumococcal exposure (39). Antibody responses to pneumococcal surface antigens have generally been regarded as the primary mechanism of protection against pneumococcal infection. However, the different immunogenicities of pneumococcal antigens have not previously been evaluated during S. pneumoniae carriage. We show that following pneumococcal carriage of S. pneumoniae strain EF3030, IgG antibodies in serum specific for PsaA ≫ PspA > PspC = PdB are significantly elevated compared with uninfected controls. However, a different immunogenic profile was observed in nasal secretions, PspA > PsaA = PdB > PspC.
The different pneumococcal antigen-specific antibody profiles in the sera and nasal washes suggest that PsaA, PspA, PspC, and PdB are presented to the host at different levels in mucosal versus systemic compartments. Our results show that nasal wash anti-PspA IgA antibody levels are significantly higher than the levels for PsaA, PspC, and PdB. Perhaps this is due to increased expression by S. pneumoniae or to host presentation of PspA in the nasal tract (34); indeed, we show that pneumococci are present from the onset of carriage to at least 21 days postchallenge. Moreover, others have shown that the expression of pspA mRNA increases 36-fold at 24 h postinfection in mucosal samples (34). Carriage of a pspC mutant strain was reduced 100-fold (41); however, other pneumococcal strains with mutations in pspC are significantly attenuated in their ability to infect or colonize the lung (4).
The S. pneumoniae strain EF3030 mouse model of carriage, with infection primarily confined to the nasal tract, may not be the best method to determine the immunogenic and pathogenic (lung expression) contribution of PspC, which demonstrates moderate humoral immunogenicity in mucosal and systemic compartments. To this end, the lower plasma antibody responses to PdB, PspC and PspA during pneumococcal carriage could be partially attributed to the ability of these proteins to modulate complement activity (5, 20, 47).
Anti-PsaA antibody levels were among the highest relative to humoral responses against PspA and PspC, while anti-PdB humoral responses were often the lowest. PsaA is highly immunogenic in young children and in the elderly (18). Accordingly, PsaA is a hydrophobic and (palmitoylated) lipoprotein which elicits inherently higher plasma antibody responses than PspA and PdB (43). It is important to mention that PspA- and PspC-specific serum IgG responses during pneumococcal nasal colonization in humans are higher than those directed against PsaA and PdB (31). However, it is not certain whether the PsaA antigen used in these studies was palmitoylated, which would affect this protein's immunogenicity. The higher immunogenicity of PsaA could also be due to the increased expression or host presentation of this virulence factor in the blood or lungs (34). For example, the expression of pspA and psaA mRNAs was upregulated threefold and fivefold, respectively, 12 h postinfection (34).
In contrast, anti-PdB antibody levels were among the lowest compared to the other pneumococcal protein antigen antibody responses detected in nasal washes and plasma. While this could be due to PdB's having an inherently lower immunogenicity than PsaA, PspA, and PspC, these patterns most likely are a result of PdB's being predominantly intracellularly expressed by living S. pneumoniae. This intracellular expression could reduce the recognition of this antigen by antibody or major histocompatibility class II-restricted CD4+ T cells. Our results suggest that PdB is modestly expressed during pneumococcal carriage and/or poorly immunogenic. In either case, additional studies are necessary to determine the potential of PdB as a pneumococcal vaccine antigen.
The pathogenic requirements of S. pneumoniae for differential (nasal tract, lung, systemic) expression and the activity of PsaA, PspA, and PspC may also induce the host to mount similarly compensatory antibody responses to mitigate disease. The different pneumococcus-specific antibody responses and the degree of these differences are also a direct outcome of T helper cell cytokine support. In confirmation of the significant PsaA-specific IgG2a serum antibody levels, CD4+ T cells from the spleen demonstrated the highest proliferation and IL-2 responses after PsaA stimulation. T helper cells from the mucosal and systemic tissues also exhibited increased production of IL-10, IL-4, IFN-γ, IL-6, TNF-α, and GM-CSF following stimulation with PsaA, PspA, PspC, or PdB.
IL-10 reduces pulmonary vascular leakage and the appearance of red blood cells in the alveoli during murine pneumococcal pneumonia (50). IL-10 may also have adjuvant activity; it has been shown to enhance vaccine responses in aged mice that had been immunized with pneumococcal polysaccharides (28). In this study, pneumococcal antigen-specific IL-10 responses were generated during pneumococcal carriage in mice. Despite the Th1-biased humoral responses induced by our model, PsaA stimulation induced the highest levels of IL-4 secretion, which is indicative of large numbers of PsaA-specific Th2 cells in both mucosal and systemic tissues. The Th2 responses to PsaA could have been due to its palmitoylate modification, which could increase antibody responses to this protein compared with the other pneumococcal antigens tested. As a result, higher antibody responses would in turn promote the increase of both the uptake of antigens and their presentation to precursor T helper cells for subsequent differentiation of more PsaA-specific effector T helper cells.
IFN-γ, IL-6, and TNF-α responses also play an important and protective role during pneumococcal disease (6, 21, 27, 45, 48). IFN-γ is absolutely required for protective host immunity against pneumococcal disease (42). TNF-α is a predictor of S. pneumoniae bacteremia even in asymptomatic children (45). Similarly, IL-6 enhances the differentiation of in vivo pneumococcus-activated human B cells to antibody-secreting plasma cells (27) and is essential for protection against pneumococcal pneumonia (48). Since IL-6, TNF-α, and IFN-γ responses are required for protective pneumococcal immunity, future S. pneumoniae vaccine formulations that increase these responses might impart better protection against pneumococcal disease.
While the observed T helper cytokine responses may indicate differences in PsaA-, PspA-, PspC-, and PdB-specific CD4+ T-cell immunogenicity, these studies may also point out some of the hallmarks of S. pneumoniae immunopathogenesis. To explain, PsaA- and PspA-specific CD4+ T cells displayed higher IFN-γ, IL-6, and TNF-α secretion. The increased levels of GM-CSF produced in response to PspA and PsaA from cervical lymph node and splenic CD4+ T cells corresponded to relatively higher nasal tract and serum responses to these antigens as well as the diverse bacterial loads in the nasal tract and lung during carriage. This suggests that PspA and PsaA were expressed or presented at higher levels to naïve T helper cells in these immune tissues. The higher PspA antibody responses in the nasal tract corresponded with the greater cytokine levels produced by CD4+ T cells from cervical lymph nodes, which are the inductive tissues of this mucosal effector site.
Until now, the precise responses of CD4+ T cells to pneumococcal surface protein-specific T helper cytokines following pneumococcal carriage were not known. While the systemic immune system appears to mount the highest humoral and cellular immune responses to PsaA, significant mucosal immune responses are directed to PspA. The findings have important implications for pneumococcal vaccine development. In general, our studies suggest that in order for a vaccine to prevent pneumococcal carriage, it should direct an effective mucosal PspA response, while effective pulmonary pneumococcal immunity may require an immunization strategy that induces a robust PsaA systemic response. However, additional studies will be required to determine the host factors that mediate the differential PdB-, PsaA-, PspA-, and PspC-specific mucosal and systemic immunity as well as to determine the mechanisms of pneumococcal protein antigen expression and pathogenesis in vivo.
Acknowledgments
This manuscript benefited from the technical editing services of Susan Kniebes.
This work was supported in part by NIH grants AI057808, RR03034, and GM08248.
Editor: J. T. Barbieri
REFERENCES
- 1.Aaberge, I. S., J. Eng, G. Lermark, and M. Lovik. 1995. Virulence of Streptococcus pneumoniae in mice: a standardized method for preparation and frozen storage of the experimental bacterial inoculum. Microb. Pathog. 18:141-152. [DOI] [PubMed] [Google Scholar]
- 2.Aaskov, J. G., and W. J. Halliday. 1971. Requirement for lymphocyte-macrophage interaction in the response of mouse spleen cultures to pneumococcal polysaccharide. Cell. Immunol. 2:335-340. [DOI] [PubMed] [Google Scholar]
- 3.Arulanandam, B. P., J. M. Lynch, D. E. Briles, S. Hollingshead, and D. W. Metzger. 2001. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 69:6718-6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balachandran, P., A. Brooks-Walter, A. Virolainen-Julkunen, S. K. Hollingshead, and D. E. Briles. 2002. Role of pneumococcal surface protein C in nasopharyngeal carriage and pneumonia and its ability to elicit protection against carriage of Streptococcus pneumoniae. Infect. Immun. 70:2526-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benton, K. A., J. C. Paton, and D. E. Briles. 1997. The hemolytic and complement-activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb. Pathog. 23:201-209. [DOI] [PubMed] [Google Scholar]
- 6.Benton, K. A., J. L. VanCott, and D. E. Briles. 1998. Role of tumor necrosis factor alpha in the host response of mice to bacteremia caused by pneumolysin-deficient Streptococcus pneumoniae. Infect. Immun. 66:839-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry, A. M., and J. C. Paton. 1996. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 64:5255-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briles, D. E., E. Ades, J. C. Paton, J. S. Sampson, G. M. Carlone, R. C. Huebner, A. Virolainen, E. Swiatlo, and S. K. Hollingshead. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briles, D. E., M. J. Crain, B. M. Gray, C. Forman, and J. Yother. 1992. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect. Immun. 60:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briles, D. E., C. Forman, and M. Crain. 1992. Mouse antibody to phosphocholine can protect mice from infection with mouse-virulent human isolates of Streptococcus pneumoniae. Infect. Immun. 60:1957-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briles, D. E., S. K. Hollingshead, J. King, A. Swift, P. A. Braun, M. K. Park, L. M. Ferguson, M. H. Nahm, and G. S. Nabors. 2000. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 182:1694-1701. [DOI] [PubMed] [Google Scholar]
- 12.Briles, D. E., S. K. Hollingshead, J. C. Paton, E. W. Ades, L. Novak, F. W. Van Ginkel, and W. H. Benjamin Jr. 2003. Immunizations with Pneumococcal Surface Protein A and Pneumolysin Are Protective against Pneumonia in a Murine Model of Pulmonary Infection with Streptococcus pneumoniae. J. Infect. Dis. 188:339-348. [DOI] [PubMed] [Google Scholar]
- 13.Brooks-Walter, A., D. E. Briles, and S. K. Hollingshead. 1999. The pspC gene of Streptococcus pneumoniae encodes a polymorphic protein, PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect. Immun. 67:6533-6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crain, M. J., W. D. Waltman, 2nd, J. S. Turner, J. Yother, D. F. Talkington, L. S. McDaniel, B. M. Gray, and D. E. Briles. 1990. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect. Immun. 58:3293-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dagan, R., N. Givon-Lavi, O. Zamir, and D. Fraser. 2003. Effect of a nonavalent conjugate vaccine on carriage of antibiotic-resistant Streptococcus pneumoniae in day-care centers. Pediatr. Infect. Dis. J. 22:532-540. [DOI] [PubMed] [Google Scholar]
- 16.Dagan, R., R. Melamed, M. Muallem, L. Piglansky, D. Greenberg, O. Abramson, P. M. Mendelman, N. Bohidar, and P. Yagupsky. 1996. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J. Infect. Dis. 174:1271-1278. [DOI] [PubMed] [Google Scholar]
- 17.Dave, S., A. Brooks-Walter, M. K. Pangburn, and L. S. McDaniel. 2001. PspC, a pneumococcal surface protein, binds human factor H. Infect. Immun. 69:3435-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gleich, S., Y. Morad, R. Echague, J. R. Miller, J. Kornblum, J. S. Sampson, and J. C. Butler. 2000. Streptococcus pneumoniae serotype 4 outbreak in a home for the aged: report and review of recent outbreaks. Infect. Control Hosp. Epidemiol. 21:711-717. [DOI] [PubMed] [Google Scholar]
- 19.Hammerschmidt, S., S. R. Talay, P. Brandtzaeg, and G. S. Chhatwal. 1997. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol. Microbiol.. 25:1113-1124. [DOI] [PubMed] [Google Scholar]
- 20.Jarva, H., R. Janulczyk, J. Hellwage, P. F. Zipfel, L. Bjorck, and S. Meri. 2002. Streptococcus pneumoniae evades complement attack and opsonophagocytosis by expressing the pspC locus-encoded Hic protein that binds to short consensus repeats 8-11 of factor H. J. Immunol. 168:1886-1894. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, M. D., J. E. Fitzgerald, G. Leonard, J. A. Burleson, and D. L. Kreutzer. 1994. Cytokines in experimental otitis media with effusion. Laryngoscope 104:191-196. [DOI] [PubMed] [Google Scholar]
- 22.Kadioglu, A., N. A. Gingles, K. Grattan, A. Kerr, T. J. Mitchell, and P. W. Andrew. 2000. Host cellular immune response to pneumococcal lung infection in mice. Infect. Immun. 68:492-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klugman, K. P. 2001. Efficacy of pneumococcal conjugate vaccines and their effect on carriage and antimicrobial resistance. Lancet Infect. Dis. 1:85-91. [DOI] [PubMed] [Google Scholar]
- 24.Lakshman, R., C. Murdoch, G. Race, R. Burkinshaw, L. Shaw, and A. Finn. 2003. Pneumococcal nasopharyngeal carriage in children following heptavalent pneumococcal conjugate vaccination in infancy. Arch. Dis. Child. 88:211-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lillard, J. W., Jr., P. N. Boyaka, J. A. Hedrick, A. Zlotnik, and J. R. McGhee. 1999. Lymphotactin acts as an innate mucosal adjuvant. J. Immunol. 162:1959-1965. [PubMed] [Google Scholar]
- 26.Lillard, J. W. J., P. N. Boyaka, O. Chertov, J. J. Oppenheim, and J. R. McGhee. 1999. Mechanism for induction of acquired host immunity by neutrophil peptide defensins. Proc. Natl. Acad. Sci. USA 96:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lue, C., H. Kiyono, J. R. McGhee, K. Fujihashi, T. Kishimoto, T. Hirano, and J. Mestecky. 1991. Recombinant human interleukin 6 (rhIL-6) promotes the terminal differentiation of in vivo-activated human B cells into antibody-secreting cells. Cell. Immunol. 132:423-432. [DOI] [PubMed] [Google Scholar]
- 28.Luo, W., J. Fine, M. Garg, A. M. Kaplan, and S. Bondada. 1999. Interleukin-10 enhances immune responses to pneumococcal polysaccharides and sheep erythrocytes in young and aged mice. Cell. Immunol. 195:1-9. [DOI] [PubMed] [Google Scholar]
- 29.Lynch, J. M., D. E. Briles, and D. W. Metzger. 2003. Increased protection against pneumococcal disease by mucosal administration of conjugate vaccine plus interleukin-12. Infect. Immun. 71:4780-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCool, T. L., T. R. Cate, G. Moy, and J. N. Weiser. 2002. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 195:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCool, T. L., T. R. Cate, E. I. Tuomanen, P. Adrian, T. J. Mitchell, and J. N. Weiser. 2003. Serum immunoglobulin G responses to candidate vaccine antigens during experimental human pneumococcal colonization. Infect. Immun. 71:5724-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison, K. E., D. Lake, J. Crook, G. M. Carlone, E. Ades, R. Facklam, and J. S. Sampson. 2000. Confirmation of psaA in all 90 serotypes of Streptococcus pneumoniae by PCR and potential of this assay for identification and diagnosis. J. Clin. Microbiol. 38:434-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obaro, S. K., R. A. Adegbola, J. A. Tharpe, E. W. Ades, K. P. McAdam, G. Carlone, and J. S. Sampson. 2000. Pneumococcal surface adhesin A antibody concentration in serum and nasopharyngeal carriage of Streptococcus pneumoniae in young African infants. Vaccine 19:411-412. [DOI] [PubMed] [Google Scholar]
- 34.Ogunniyi, A. D., P. Giammarinaro, and J. C. Paton. 2002. The genes encoding virulence-associated proteins and the capsule of Streptococcus pneumoniae are upregulated and differentially expressed in vivo. Microbiology 148:2045-2053. [DOI] [PubMed] [Google Scholar]
- 35.Ogunniyi, A. D., M. C. Woodrow, J. T. Poolman, and J. C. Paton. 2001. Protection against Streptococcus pneumoniae elicited by immunization with pneumolysin and CbpA. Infect. Immun. 69:5997-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paton, J. C. 1996. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol.. 4:103-106. [DOI] [PubMed] [Google Scholar]
- 37.Paton, J. C., R. A. Lock, C. J. Lee, J. P. Li, A. M. Berry, T. J. Mitchell, P. W. Andrew, D. Hansman, and G. J. Boulnois. 1991. Purification and immunogenicity of genetically obtained pneumolysin toxoids and their conjugation to Streptococcus pneumoniae type 19F polysaccharide. Infect. Immun.. 59:2297-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pilling, P. A., L. M. Berry, A. M. Ogunniyi, A. D. Lock, and R. A. Paton. 1998. Expression, purification and preliminary X-ray crystallographic analysis of PsaA, a putative metal-transporter protein of streptococcus pneumoniae. Acta Crystallogr. D Biol. Crystallogr. 54:1464-1466. [DOI] [PubMed] [Google Scholar]
- 39.Rapola, S., V. Jantti, R. Haikala, R. Syrjanen, G. M. Carlone, J. S. Sampson, D. E. Briles, J. C. Paton, A. K. Takala, T. M. Kilpi, and H. Kayhty. 2000. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J. Infect. Dis. 182:1146-1152. [DOI] [PubMed] [Google Scholar]
- 40.Rapola, S., T. Kilpi, M. Lahdenkari, A. K. Takala, P. H. Makela, and H. Kayhty. 2001. Do antibodies to pneumococcal surface adhesin a prevent pneumococcal involvement in acute otitis media? J. Infect. Dis. 184:577-581. [DOI] [PubMed] [Google Scholar]
- 41.Rosenow, C., P. Ryan, J. N. Weiser, S. Johnson, P. Fontan, A. Ortqvist, and H. R. Masure. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25:819-829. [DOI] [PubMed] [Google Scholar]
- 42.Rubins, J. B., and C. Pomeroy. 1997. Role of gamma interferon in the pathogenesis of bacteremic pneumococcal pneumonia. Infect. Immun.. 65:2975-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srivastava, N., J. L. Zeiler, S. L. Smithson, G. M. Carlone, E. W. Ades, J. S. Sampson, S. E. Johnson, T. Kieber-Emmons, and M. A. Westerink. 2000. Selection of an immunogenic and protective epitope of the PsaA protein of Streptococcus pneumoniae using a phage display library. Hybridoma 19:23-31. [DOI] [PubMed] [Google Scholar]
- 44.Steinfort, C., R. Wilson, T. Mitchell, C. Feldman, A. Rutman, H. Todd, D. Sykes, J. Walker, K. Saunders, and P. W. Andrew. 1989. Effect of Streptococcus pneumoniae on human respiratory epithelium in vitro. Infect. Immun.. 57:2006-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strait, R. T., R. M. Ruddy, L. R. Friedland, K. M. Duncan, and R. W. Wilmott. 1997. A pilot study of the predictive value of plasma tumor necrosis factor alpha and interleukin 1 beta for Streptococcus pneumoniae bacteremia in febrile children. Acad. Emerg. Med. 4:44-51. [DOI] [PubMed] [Google Scholar]
- 46.Takashima, A., R. S. Taylor, and K. Takashima. 1997. Role of tumor necrosis factor alpha in pathogenesis of pneumococcal pneumonia in mice. J. Immunol.. 158:622-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tu, A. H., R. L. Fulgham, M. A. McCrory, D. E. Briles, and A. J. Szalai. 1999. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect. Immun.. 67:4720-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Poll, T., C. V. Keogh, X. Guirao, W. A. Buurman, M. Kopf, and S. F. Lowry. 1997. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J. Infect. Dis.. 176:439-444. [DOI] [PubMed] [Google Scholar]
- 49.Virolainen, A., J. Jero, H. Kayhty, P. Karma, J. Eskola, and M. Leinonen. 1995. Nasopharyngeal antibodies to pneumococcal pneumolysin in children with acute otitis media. Clin. Diagn. Lab. Immunol.. 2:704-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, E., M. Simard, N. Ouellet, Y. Bergeron, D. Beauchamp, and M. G. Bergeron. 2000. Modulation of cytokines and chemokines, limited pulmonary vascular bed permeability, and prevention of septicemia and death with ceftriaxone and interleukin-10 in pneumococcal pneumonia. J. Infect. Dis.. 182:1255-1259. [DOI] [PubMed] [Google Scholar]
- 51.Wu, H. Y., M. H. Nahm, Y. Guo, M. W. Russell, and D. E. Briles. 1997. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J. Infect. Dis.. 175:839-846. [DOI] [PubMed] [Google Scholar]
- 52.Wu, H. Y., A. Virolainen, B. Mathews, J. King, M. W. Russell, and D. E. Briles. 1997. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microb. Pathog.. 23:127-137. [DOI] [PubMed] [Google Scholar]