Abstract
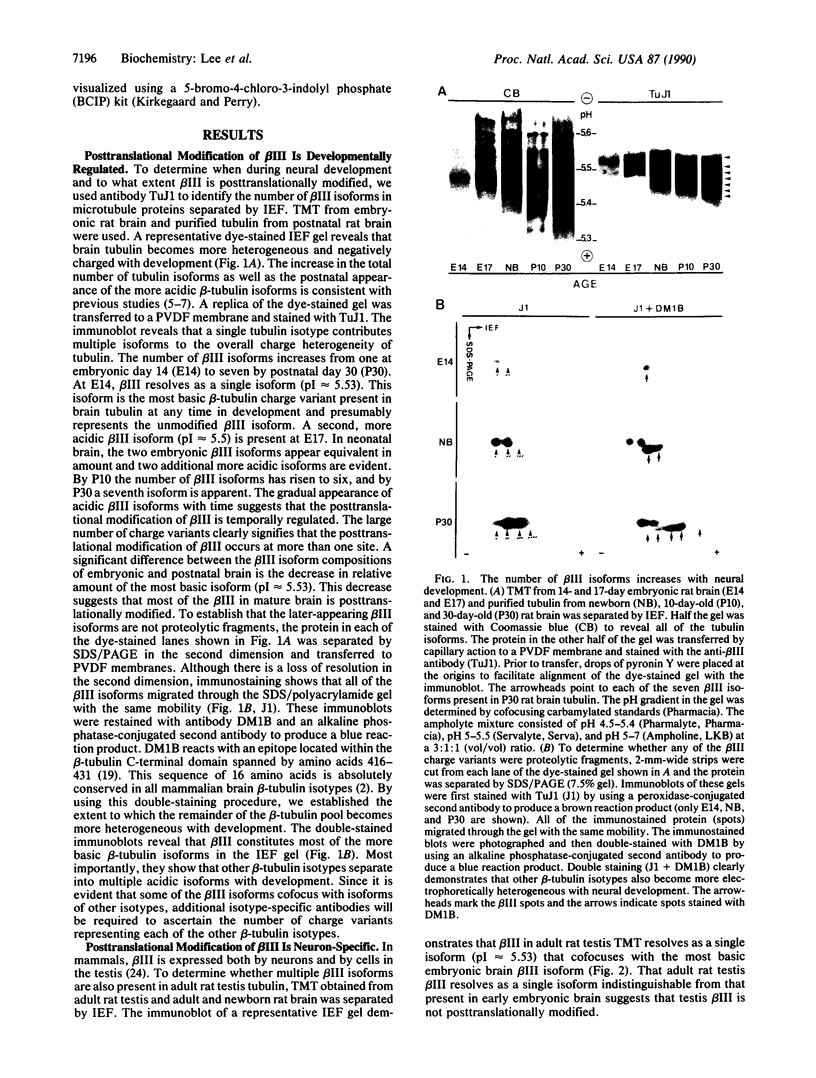
The charge heterogeneity of class III beta-tubulin (beta III) during neural development was analyzed by high-resolution isoelectric focusing/two-dimensional polyacrylamide gel electrophoresis in combination with site-specific proteolytic digestion and immunological detection. The number of beta III isoforms (charge variants) gradually increases from one in embryonic brain to seven in adult brain. All of the charge heterogeneity is due to posttranslationally modified sites located within the extreme C-terminal region of the beta III polypeptide. One beta III isoform is present in testis, the only other tissue in which this isotype is expressed. The testis beta III isoform cofocuses with the earliest-appearing embryonic brain beta III charge variant. Our results indicate that the posttranslational modifications of beta III are developmentally regulated, occur at more than one site, and are neuron-specific. The location of these modifications within the extreme C-terminal domain suggests that their function is to modulate the interaction of tubulin with microtubule-associated proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barra H. S., Arce C. A., Rodríguez J. A., Caputto R. Some common properties of the protein that incorporates tyrosine as a single unit and the microtubule proteins. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1384–1390. doi: 10.1016/0006-291x(74)90351-9. [DOI] [PubMed] [Google Scholar]
- Eddé B., Denoulet P., de Néchaud B., Koulakoff A., Berwald-Netter Y., Gros F. Posttranslational modifications of tubulin in cultured mouse brain neurons and astroglia. Biol Cell. 1989;65(2):109–117. doi: 10.1111/j.1768-322x.1989.tb00779.x. [DOI] [PubMed] [Google Scholar]
- Eddé B., Rossier J., Le Caer J. P., Desbruyères E., Gros F., Denoulet P. Posttranslational glutamylation of alpha-tubulin. Science. 1990 Jan 5;247(4938):83–85. doi: 10.1126/science.1967194. [DOI] [PubMed] [Google Scholar]
- Eipper B. A. Properties of rat brain tubulin. J Biol Chem. 1974 Mar 10;249(5):1407–1416. [PubMed] [Google Scholar]
- Elkon K. B. Isoelectric focusing of human IgA and secretory proteins using thin layer agarose gels and nitrocellulose capillary blotting. J Immunol Methods. 1984 Feb 10;66(2):313–321. doi: 10.1016/0022-1759(84)90343-0. [DOI] [PubMed] [Google Scholar]
- Field D. J., Collins R. A., Lee J. C. Heterogeneity of vertebrate brain tubulins. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4041–4045. doi: 10.1073/pnas.81.13.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgue S. T., Dahl J. L. Rat brain tubulin: subunit heterogeneity and phosphorylation. J Neurochem. 1979 Mar;32(3):1015–1025. doi: 10.1111/j.1471-4159.1979.tb04588.x. [DOI] [PubMed] [Google Scholar]
- Gard D. L., Kirschner M. W. A polymer-dependent increase in phosphorylation of beta-tubulin accompanies differentiation of a mouse neuroblastoma cell line. J Cell Biol. 1985 Mar;100(3):764–774. doi: 10.1083/jcb.100.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havercroft J. C., Cleveland D. W. Programmed expression of beta-tubulin genes during development and differentiation of the chicken. J Cell Biol. 1984 Dec;99(6):1927–1935. doi: 10.1083/jcb.99.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly J. C., Flynn G., Purich D. L. The microtubule-binding fragment of microtubule-associated protein-2: location of the protease-accessible site and identification of an assembly-promoting peptide. J Cell Biol. 1989 Nov;109(5):2289–2294. doi: 10.1083/jcb.109.5.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Hernault S. W., Rosenbaum J. L. Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry. 1985 Jan 15;24(2):473–478. doi: 10.1021/bi00323a034. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Field D. J., George H. J., Head J. Biochemical and chemical properties of tubulin subspecies. Ann N Y Acad Sci. 1986;466:111–128. doi: 10.1111/j.1749-6632.1986.tb38388.x. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Cowan N. J. Complex regulation and functional versatility of mammalian alpha- and beta-tubulin isotypes during the differentiation of testis and muscle cells. J Cell Biol. 1988 Jun;106(6):2023–2033. doi: 10.1083/jcb.106.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Lee M. G., Cowan N. J. Five mouse tubulin isotypes and their regulated expression during development. J Cell Biol. 1985 Sep;101(3):852–861. doi: 10.1083/jcb.101.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludueña R. F., Zimmermann H. P., Little M. Identification of the phosphorylated beta-tubulin isotype in differentiated neuroblastoma cells. FEBS Lett. 1988 Mar 28;230(1-2):142–146. doi: 10.1016/0014-5793(88)80658-6. [DOI] [PubMed] [Google Scholar]
- Piperno G., Fuller M. T. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol. 1985 Dec;101(6):2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybin D., Flavin M. An enzyme tyrosylating alpha-tubulin and its role in microtubule assembly. Biochem Biophys Res Commun. 1975 Aug 4;65(3):1088–1095. doi: 10.1016/s0006-291x(75)80497-9. [DOI] [PubMed] [Google Scholar]
- Sackett D. L., Bhattacharyya B., Wolff J. Tubulin subunit carboxyl termini determine polymerization efficiency. J Biol Chem. 1985 Jan 10;260(1):43–45. [PubMed] [Google Scholar]
- Sackett D. L., Wolff J. Proteolysis of tubulin and the substructure of the tubulin dimer. J Biol Chem. 1986 Jul 5;261(19):9070–9076. [PubMed] [Google Scholar]
- Serrano L., Avila J., Maccioni R. B. Controlled proteolysis of tubulin by subtilisin: localization of the site for MAP2 interaction. Biochemistry. 1984 Sep 25;23(20):4675–4681. doi: 10.1021/bi00315a024. [DOI] [PubMed] [Google Scholar]
- Serrano L., de la Torre J., Maccioni R. B., Avila J. Involvement of the carboxyl-terminal domain of tubulin in the regulation of its assembly. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5989–5993. doi: 10.1073/pnas.81.19.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K. F. Structure and utilization of tubulin isotypes. Annu Rev Cell Biol. 1988;4:687–716. doi: 10.1146/annurev.cb.04.110188.003351. [DOI] [PubMed] [Google Scholar]
- Vallee R. B. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs). J Cell Biol. 1982 Feb;92(2):435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera J. C., Rivas C. I., Maccioni R. B. Biochemical dissection of the role of the one-kilodalton carboxyl-terminal moiety of tubulin in its assembly into microtubules. Biochemistry. 1989 Jan 10;28(1):333–339. doi: 10.1021/bi00427a045. [DOI] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff A., Denoulet P., Jeantet C. High level of tubulin microheterogeneity in the mouse brain. Neurosci Lett. 1982 Aug 31;31(3):323–328. doi: 10.1016/0304-3940(82)90041-6. [DOI] [PubMed] [Google Scholar]
- de la Viña S., Andreu D., Medrano F. J., Nieto J. M., Andreu J. M. Tubulin structure probed with antibodies to synthetic peptides. Mapping of three major types of limited proteolysis fragments. Biochemistry. 1988 Jul 12;27(14):5352–5365. doi: 10.1021/bi00414a060. [DOI] [PubMed] [Google Scholar]












