Abstract
Anaplasma phagocytophilum infection induces functional neutrophil changes. Using both Candida albicans and fluorescent-aggregate phagocytosis assays, we examined whether neutrophil and dimethyl sulfoxide-differentiated HL-60 cell infection impairs internalization. A. phagocytophilum infection significantly decreased phagocytosis compared to that of controls (P < 0.05). This further impairment of neutrophil function may promote opportunistic infections and exacerbate disease.
Anaplasma phagocytophilum causes tick-borne fever (TBF) in sheep, cattle, and goats (21); disease in canids (15); and human granulocytic anaplasmosis (2). Human infection is characterized by fever, headache, myalgias, thrombocytopenia, and leukopenia (2). Severe complications include opportunistic infections in humans and animals alike (2, 5, 13, 19, 21, 24). This obligate intracellular bacterium is unique because it is adapted to survive the harsh environment within neutrophils by seizing control of host cell function and regulation critical for antimicrobial activity (3, 7, 9, 22, 25). A. phagocytophilum-infected cells are also altered in other phenotypic features, such as expression of chemokines that promote recruitment of neutrophils—potential new infection hosts that may increase the proportion of impaired neutrophils (1, 16, 23).
In addition to having these defects, in vivo and ex vivo neutrophils of A. phagocytophilum-infected sheep with TBF demonstrate a reduced phagocytic capacity (4, 26, 27), a situation that may further alter neutrophil function, and predispose the sheep to opportunistic infections and dysregulation of inflammation (17). However, infection-induced phagocytosis defects are not universally observed ex vivo (20). Thus, the purpose of the present study was to determine, under carefully controlled in vitro conditions, whether A. phagocytophilum-infected neutrophils have a diminished phagocytic capacity.
(This work was presented in part at the 15th Meeting of the American Society for Rickettsiology, Captiva Island, Fla., April 2000.)
A. phagocytophilum was propagated in the HL-60 cell line (14) in RPMI 1640 medium with 2 mM l-glutamine and 1 to 5% fetal bovine serum (FBS) at 37°C in 5% CO2. HL-60 cell concentrations were maintained between 5.0 × 105 and 1 × 106 cells/ml. For some experiments, HL-60 cells were differentiated with 1.25% dimethyl sulfoxide (DMSO) for 3 to 5 days as described previously (9). Such a protocol is established to enhance neutrophil differentiation (8-10). Heavily infected HL-60 cells (>90% infected) were used as a source of cell-free A. phagocytophilum for neutrophil infections.
Neutrophils were obtained from EDTA-anticoagulated peripheral blood of healthy donors under a protocol approved by the Johns Hopkins Medicine Institutional Review Board. Neutrophils were isolated by Ficoll-Hypaque density gradient centrifugation from dextran-sedimented blood. The residual neutrophils were obtained after hypotonic-erythrocyte lysis, as described previously (9), resulting in >95% purity by morphology and >90% viability by trypan blue exclusion. Neutrophils were infected as per the method of Choi et al. (9) with the following modifications. A quantity of A. phagocytophilum-infected HL-60 cells (106 to 107 cells) equal to that of the neutrophils to be infected was centrifuged at 450 × g for 10 min, the supernatant was removed, and the cell pellet was suspended in 2 ml of medium and sonicated just until complete HL-60 cell lysis was achieved. Thereafter, this sonicate and the free bacteria in the supernatant were pooled, centrifuged at 15,000 × g, resuspended in 2 ml of medium, and centrifuged again at 200 × g for 5 min to pellet cellular debris and nuclei. The A. phagocytophilum-enriched supernatant was used to infect neutrophils for 18 h at 37°C in 5% CO2. Since neutrophil morulae were small after only 18 h, Romanowsky staining (Hema-3; Fisher, Middletown, Va.) and immunofluorescence with an A. phagocytophilum polyclonal antibody were used to confirm infection in at least 90% of the neutrophils.
Candida albicans (ATCC 90026) was grown overnight in Luria-Bertani medium with shaking and aeration at 25 to 37°C. Yeast cells were counted and washed in phosphate-buffered saline (PBS) before 5 × 105 cells were incubated with 106 infected or uninfected neutrophils, undifferentiated HL-60 cells, or differentiated HL-60 cells in RPMI 1640 medium for 30 min to 1 h. Thereafter, the cells were washed three times in PBS to remove uningested yeast cells, and the final cell suspensions were used to prepare slides for microscopic examination. Slides were stained with Hema-3, and the proportions of at least 140 neutrophils containing ingested yeast cells, as well as the numbers of ingested yeast cells per neutrophil, were counted. This experiment was repeated at least two times.
After one wash in sterile 0.1 M PBS, 106 infected and uninfected neutrophils were mixed with PKH2-PCL fluorescent aggregates (4 × 10−6 M; Sigma Chemical Co., St. Louis, Mo.) at room temperature for 5 min. Thereafter, 1 ml of sterile FBS was added for 1 min to compete against excess fluorescent-aggregate binding to cell surfaces, and cells were washed three times in medium with 5% FBS. To control for endogenous fluorescence and nonspecific cell surface binding of fluorescent aggregates, phagocytosis was inhibited in some neutrophils by 10-min fixation in 4% paraformaldehyde prior to the phagocytic assay. Samples were analyzed by flow cytometry gating on viable cells based on forward- and side-scatter characteristics. The fluorescent threshold was set to exclude >97% of endogenous fluorescence in unstained uninfected and infected neutrophils. Fluorescence that corresponded to surface-adherent aggregates in neutrophils whose ability to phagocytose was inhibited was subtracted from the final histograms to yield the plots that most accurately reflected phagocytosed fluorescent aggregates alone.
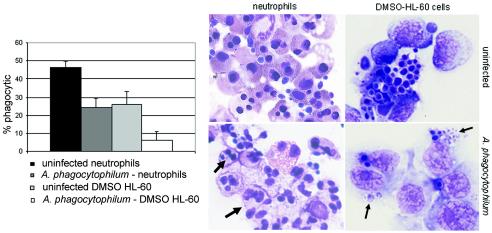
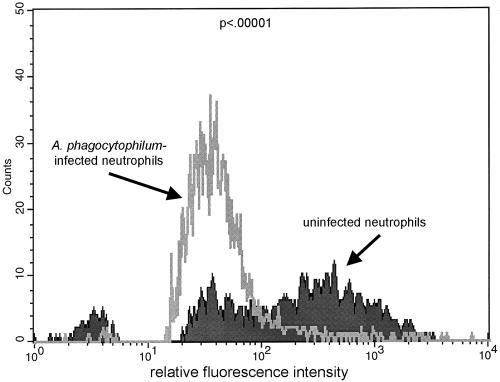
When incubated with C. albicans targets, heavily and slightly infected undifferentiated HL-60 cells ingested a low proportion of yeast cells (11.5%), similar to the proportion ingested by uninfected controls (10.5%; P = 0.23). As shown in Fig. 1, with differentiation in DMSO, fewer infected HL-60 cells ingested targets than did uninfected cells (2 to 12% versus 21 to 31%; P = 0.05) in two replicated experiments. Significantly fewer A. phagocytophilum-infected neutrophils than uninfected cells ingested C. albicans targets (24% versus 47%; P = 0.003), and fewer infected and phagocytic neutrophils than uninfected neutrophils ingested yeast cells (2.0 ± 1.0 [mean ± standard deviation] versus 3.4 ± 2.5 yeast cells/neutrophil; P < 0.001). For confirmation, infected and uninfected neutrophils were assayed for phagocytosis by a flow cytometric method that examined at least 5,000 cells per condition. As shown in Fig. 2, after adjustment for endogenous and surface-bound fluorescence, the flow cytometric method confirmed that infected neutrophils ingested significantly fewer fluorescent aggregates than did uninfected cells (P < 0.005) in three replicated experiments.
FIG. 1.
Phagocytosis of C. albicans is impaired in A. phagocytophilum-infected neutrophils and DMSO-differentiated HL-60 cells compared with that of uninfected control cells. The graph on the left represents the means of results of at least two replicated experiments; the images on the right illustrate typical patterns observed with Hema-3 staining (arrows show A. phagocytophilum morulae that are typically small after 18 h of incubation in neutrophils).
FIG. 2.
Flow cytometric analysis of uninfected and A. phagocytophilum-infected neutrophils after phagocytosis of PKH2-PCL fluorescent aggregates. As phagocytic activity increases, so does the fluorescence intensity of phagocytic cells; defective phagocytosis is indicated by reduced fluorescence intensity compared with that of uninfected control neutrophils. Note two populations among the uninfected cells, one of which demonstrated increased fluorescence corresponding to actively phagocytic cells. A. phagocytophilum-infected neutrophils had a significantly lower fluorescence mean value and distribution (P < 0.005). The histogram shown is representative of the results of three repeated experiments.
A. phagocytophilum propagates in neutrophils, altering cell functions by (i) reducing endothelial cell adhesion, transmigration, and respiratory burst; (ii) increasing degranulation and chemokine synthesis; and (iii) delaying apoptosis (3, 6, 8-10, 16, 22, 28). Overall, these neutrophil changes may enhance bacterial survival by prolonging the circulation of infected neutrophils prior to tick bites and may contribute to host disease by promoting inflammation and dysregulating microbicidal activity.
Such abnormalities may be directly related to disease in humans and animals by compromising neutrophil responses to commensal or opportunistic infectious agents. For example, TBF in sheep is associated with bacterial, fungal, and viral infections, particularly disseminated staphylococcal infections (13). Similarly, A. phagocytophilum-infected humans usually have self-limited infections but can occasionally develop severe opportunistic infections by pathogens typically controlled by neutrophils (2, 19, 24). Previous ex vivo investigations of neutrophils from infected sheep show defective phagocytosis and microbicidal activity (20, 26, 27). However, the proportion of infected cells in vivo is highly variable and usually low, and such situations are poorly amenable to experimental manipulations. Thus, in vitro cultivation of A. phagocytophilum in both human differentiated promyelocytic HL-60 cells and neutrophils allows assessment of neutrophil function, including phagocytosis, in a more carefully controlled environment.
These studies confirm previous investigations using ex vivo neutrophils from infected animals (4, 27). Moreover, the results extend to include an assessment of defects in phagocytosis after A. phagocytophilum infection of differentiated HL-60 cells and primary human neutrophils. The mechanism for phagocytosis inhibition is not known; however, with infection, Carlyon et al. (6) showed diminished RAC2 expression, an important component of some phagocytic signal transduction pathways (18). Given the broad effects of A. phagocytophilum on neutrophil function, other signaling pathways will also require investigation to understand this defect.
The potential advantages of neutrophil phagocytosis inhibition for the bacterium may relate to “infection exclusion” as noted for the related organism Anaplasma marginale, which lives in bovine erythrocytes (11, 12). The putative advantage is to permit the survival of one strain of A. marginale against competing strains. Whether any clear advantage for infection exclusion with A. phagocytophilum exists in natural-reservoir hosts remains to be determined. However, Pitzer et al. recently showed that simultaneous infection of laboratory mice by the Webster (Wisconsin) and NY10 (New York) strains results in Webster strain dominance after several weeks in vivo, suggesting a role in small-mammal reservoirs (V. Pitzer, L. Kostelnik, M. Levin, and R. Massung, Abstr. 104th Gen. Meet. Am. Soc. Microbiol., abstr. D-188, 2004). The specific mechanisms responsible for these observations are not known but may include the inhibition of phagocytosis, as observed here, by the Webster strain of A. phagocytophilum.
What is beneficial to A. phagocytophilum may be detrimental to infected hosts, especially those that develop significant clinical disease, such as humans. For example, inhibition of respiratory burst and assembly of phagocyte oxidase impairs the killing of ingested microorganisms (7), while protracted degranulation may promote inflammation and associated tissue injury (10), even in the absence of substantial numbers of A. phagocytophilum organisms. Consistent with the observations of infected animals and, rarely, of humans, a defect in phagocytosis would be expected to further modify the host's ability to contain infections.
Acknowledgments
This work was supported by grant R01 AI44102 from the National Institutes of Allergy and Infectious Diseases.
We gratefully acknowledge the technical contributions of Jen Walls and Luke Pfannensteil.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Akkoyunlu, M., S. E. Malawista, J. Anguita, and E. Fikrig. 2001. Exploitation of interleukin-8-induced neutrophil chemotaxis by the agent of human granulocytic ehrlichiosis. Infect. Immun. 69:5577-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakken, J. S., and J. S. Dumler. 2000. Human granulocytic ehrlichiosis. Clin. Infect. Dis. 31:554-560. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee, R., J. Anguita, D. Roos, and E. Fikrig. 2000. Cutting edge: infection by the agent of human granulocytic ehrlichiosis prevents the respiratory burst by down-regulating gp91phox. J. Immunol. 164:3946-3949. [DOI] [PubMed] [Google Scholar]
- 4.Batungbacal, M. R. 1995. Effect of Cytoecetes phagocytophila on phagocytosis by neutrophils in sheep. Philipp. J. Vet. Med. 32:70-76. [Google Scholar]
- 5.Brodie, T. A., P. H. Holmes, and G. M. Urquhart. 1986. Some aspects of tick-borne diseases of British sheep. Vet. Rec. 118:415-418. [DOI] [PubMed] [Google Scholar]
- 6.Carlyon, J. A., W.-T. Chan, J. Galán, D. Roos, and E. Fikrig. 2002. Repression of rac2 mRNA expression by Anaplasma phagocytophila is essential to the inhibition of superoxide production and bacterial proliferation. J. Immunol. 169:7009-7018. [DOI] [PubMed] [Google Scholar]
- 7.Carlyon, J. A., and E. Fikrig. 2003. Invasion and survival strategies of Anaplasma phagocytophilum. Cell. Microbiol. 5:743-754. [DOI] [PubMed] [Google Scholar]
- 8.Choi, K.-S., and J. S. Dumler. 2003. Early induction and late abrogation of respiratory burst in A. phagocytophilum-infected neutrophils. Ann. N. Y. Acad. Sci. 990:488-493. [DOI] [PubMed] [Google Scholar]
- 9.Choi, K.-S., J. Garyu, J. Park, and J. S. Dumler. 2003. Diminished adhesion of Anaplasma phagocytophilum-infected neutrophils to endothelial cells is associated with reduced expression of leukocyte surface selectin. Infect. Immun. 71:4586-4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi, K.-S., D. J. Grab, and J. S. Dumler. 2004. Anaplasma phagocytophilum infection induces protracted neutrophil degranulation. Infect Immun. 72:3680-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Fuente, J., J. C. Garcia-Garcia, E. F. Blouin, J. T. Saliki, and K. M. Kocan. 2002. Infection of tick cells and bovine erythrocytes with one genotype of the intracellular ehrlichia Anaplasma marginale excludes infection with other genotypes. Clin. Diagn. Lab. Immunol. 9:658-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Fuente, J., E. F. Blouin, and K. M. Kocan. 2003. Infection exclusion of the rickettsial pathogen Anaplasma marginale in the tick vector Dermacentor variabilis. Clin. Diagn. Lab. Immunol. 10:182-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foggie, A. 1956. The effect of tick-borne fever on the resistance of lambs to staphylococci. J. Comp. Pathol. 66:278-285. [DOI] [PubMed] [Google Scholar]
- 14.Goodman, J., C. Nelson, B. Vitale, J. E. Madigan, J. S. Dumler, T. Kurtti, and U. Munderloh. 1996. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N. Engl. J. Med. 334:209-215. [DOI] [PubMed] [Google Scholar]
- 15.Greig, B., K. M. Asanovich, P. J. Armstrong, and J. S. Dumler. 1996. Geographic, clinical, serologic, and molecular evidence of granulocytic ehrlichiosis, a likely zoonotic disease, in Minnesota and Wisconsin dogs. J. Clin. Microbiol. 34:44-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein, M. B., S. Hu, C. C. Chao, and J. L. Goodman. 2000. The agent of human granulocytic ehrlichiosis induces the production of myelosuppressing chemokines without induction of proinflammatory cytokines. J. Infect. Dis. 182:200-205. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi, S. D., J. M. Voyich, and F. R. DeLeo. 2003. Regulation of the neutrophil-mediated inflammatory response to infection. Microbes Infect. 5:1337-1344. [DOI] [PubMed] [Google Scholar]
- 18.Lee, W. L., R. E. Harrison, and S. Grinstein. 2003. Phagocytosis by neutrophils. Microbes Infect. 5:1299-1306. [DOI] [PubMed] [Google Scholar]
- 19.Lepidi, H., J. E. Bunnell, M. E. Martin, J. E. Madigan, S. Stuen, and J. S. Dumler. 2000. Comparative pathology, and immunohistology associated with clinical illness after Ehrlichia phagocytophila-group infections. Am. J. Trop. Med. Hyg. 62:29-37. [DOI] [PubMed] [Google Scholar]
- 20.Lilliehöök, I., A. Johannisson, U. Magnusson, A. Egenvall, G. Trowald-Wigh, and L. Hakansson. 1999. Granulocyte function in dogs experimentally infected with a Swedish granulocytic Ehrlichia species. Vet. Immunol. Immunopathol. 67:141-152. [DOI] [PubMed] [Google Scholar]
- 21.Ogden, N. H., Z. Woldehiwet, and C. A. Hart. 1998. Granulocytic ehrlichiosis: an emerging or rediscovered tick-borne disease? J. Med. Microbiol. 47:475-482. [DOI] [PubMed] [Google Scholar]
- 22.Park, J., K.-S. Choi, D. J. Grab, and J. S. Dumler. 2003. Divergent interactions of Ehrlichia chaffeensis- and Anaplasma phagocytophilum-infected leukocytes with endothelial cell barriers. Infect. Immun. 71:6728-6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scorpio, D. G., M. Akkoyunlu, E. Fikrig, and J. S. Dumler. 2004. CXCR2 blockade influences Anaplasma phagocytophilum propagation but not histopathology in the mouse model of human granulocytic anaplasmosis. Clin. Diagn. Lab. Immunol. 11:963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker, D. H., and J. S. Dumler. 1997. Human monocytic and granulocytic ehrlichioses. Discovery and diagnosis of emerging tick-borne infections and the critical role of the pathologist. Arch. Pathol. Lab. Med. 121:785-791. [PubMed] [Google Scholar]
- 25.Webster, P., J. W. Ijdo, L. M. Chicoine, and E. Fikrig. 1998. The agent of human granulocytic ehrlichiosis resides in an endosomal compartment. J. Clin. Investig. 101:1932-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whist, S. K., A. K. Storset, and H. J. Larsen. 2002. Functions of neutrophils in sheep experimentally infected with Ehrlichia phagocytophila. Vet. Immunol. Immunopathol. 86:183-193. [DOI] [PubMed] [Google Scholar]
- 27.Woldehiwet, Z. 1987. The effects of tick-borne fever on some functions of polymorphonuclear cells of sheep. J. Comp. Pathol. 97:481-485. [DOI] [PubMed] [Google Scholar]
- 28.Yoshiie, K., H.-Y. Kim, J. Mott, and Y. Rikihisa. 2000. Intracellular infection by the human granulocytic ehrlichiosis agent inhibits human neutrophil apoptosis. Infect. Immun. 68:1125-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]