Abstract
Colorectal mucinous adenocarcinoma (MAC) and serrated adenocarcinoma (SAC) share many characteristics, including right-side colon location, frequent mucin production, and various molecular features. This study examined the frequency of SAC morphology in MACs. We assessed the correlation of SAC morphology with clinicopathological parameters, molecular characteristics, and patient prognosis. Eighty-eight colorectal MACs were collected and reviewed for SAC morphology according to Makinen's criteria. We sequenced KRAS and BRAF, assessed CpG island methylator phenotype (CIMP) frequency, and analyzed DNA mismatch repair enzyme levels using immunohistochemistry in tumor samples. SAC morphology was observed in 38% of MACs, and was associated with proximal location (P=0.001), BRAF mutation (P=0.042), CIMP-positive status (P=0.023), and contiguous traditional serrated adenoma (P=0.019). Multivariate analysis revealed that MACs without both SAC morphology and CIMP-positive status exhibited 3.955 times greater risk of cancer relapse than MACs having both characteristics or either one (P=0.035). Our results show that two MAC groups with distinct features can be identified using Makinen's criteria, and suggest a favorable prognostic role for the serrated neoplastic pathway in colorectal MAC.
Keywords: colorectal cancer, mucinous adenocarcinoma, serrated adenocarcinoma, CIMP, BRAF
INTRODUCTION
Mucinous adenocarcinoma (MAC) of the colorectum accounts for approximately 10% of colorectal cancers (CRCs) and is diagnosed when >50% of the tumor is composed of extracellular mucin pools containing malignant epithelia [1, 2]. MAC is more common in women; it presents in the right colon and is generally detected at more advanced stages than non-MAC colorectal cancers [3]. The prognostic significance of colorectal MAC is controversial [2–5], but a recent meta-analysis associated MACs with slightly poorer prognosis [3]. MACs exhibit a higher frequency of DNA mismatch repair (MMR) defects, microsatellite instability (MSI) and RAS/RAF/MAPK pathway mutations than non-MACs, and a lower incidence of p53 mutations [6–9]. MACs also more frequently exhibit a CpG island methylator phenotype (CIMP). This is characterized by CpG island hypermethylation in the promoter regions of carcinogenesis-related genes, resulting in epigenetic silencing [6, 8, 10, 11].
Serrated adenocarcinoma (SAC) of the colorectum was first described by Jass in 1992 as having a close structural and histochemical resemblance to hyperplastic polyps with glandular serration [12], and was defined as a CRC subtype in the 2010 WHO classification [1]. SAC is associated with malignant transformation of serrated polyps, including sessile serrated adenomas and traditional serrated adenomas, which constitute the so-called “serrated neoplastic pathway” [13–16]. Makinen, et al. refined the SAC histological criteria to include epithelial serrations, clear or eosinophilic cytoplasm, abundant cytoplasm, vesicular nuclei, absence of or <10% necrosis of the total surface area, mucin production, and cell balls and papillary rods in the mucin [15, 17]. A previous study using these criteria found that SACs constituted 9.1% of the CRC cohort (n=927), and approximately half of these SACs contained a residual serrated adenoma precursor [18]. SACs are more often located in the proximal colon than conventional adenocarcinomas, and are associated with a lower 5-year survival [18, 19].
SAC histological features have been validated by mRNA expression profiling, which demonstrated that SACs differ from conventional adenocarcinomas both morphologically and molecularly [20]. In contrast with conventional colorectal adenocarcinoma resulting from a conventional adenoma-carcinoma sequence, SACs have a higher frequency of BRAF or KRAS mutations and higher levels of CIMP [15, 21–23]. The cause or mechanism of CIMP in CRC is not yet known. BRAF mutation might be an early event in CIMP tumors [24] and might direct CIMP development [25].
CIMP and BRAF mutations are associated with serrated CRC development, and these mutations occur frequently in MACs and SACs [7, 8, 10, 11, 15, 21–23, 26]. MACs and SACs also share other features, including right-side colon location and frequent mucin production. Whether MACs with and without SAC morphology represent two distinguishable CRC types with different behaviors is unknown. This study examined the occurrence of SAC in MACs, the correlation with clinicopathological parameters and molecular characteristics, and the prognostic implications.
RESULTS
Clinicopathological characteristics
Colorectal MACs from 88 patients (50 men and 38 women) were included in our study. Mean patient age at surgery was 66 years. Tumors were located as follows: 10 (11%) in the cecum, 21 (24%) in the ascending colon, 12 (14%) in the transverse colon, 12 (14%) in the descending colon, 19 (21%) in the sigmoid colon, and 14 (16%) in the rectum.
Diagnostic concordance between pathologists for SAC morphology evaluation
There was 100% agreement among all three diagnosing pathologists in 61/88 cases (69%) separating MACs with or without SAC morphology (Figure 1). Inter-observer variation between the first investigator (Lee C. T.) and the two other observers showed moderate agreement (mean κ=0.578; range 0.462–0.693). The mean κ value for all pathologists was 0.579 (P<0.001), demonstrating moderate agreement between their diagnoses. Four cases without consensus were excluded from the SAC morphology analysis.
Figure 1. Representative MACs with and without SAC morphology.
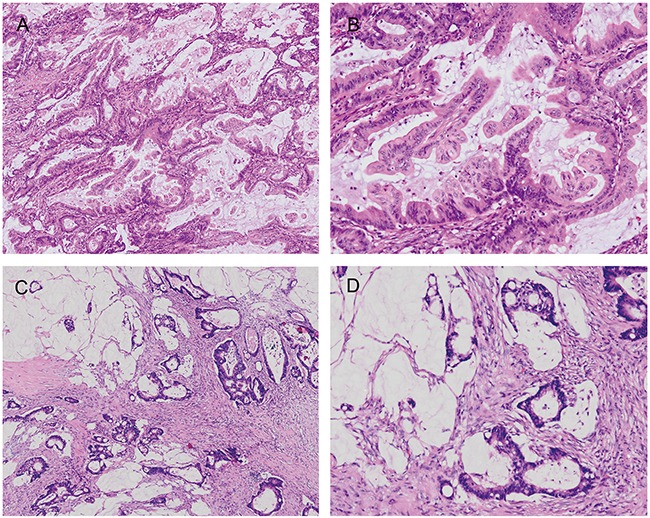
MACs with SAC morphology (A & B) show prominent epithelial serration and typical cytology with eosinophilic cytoplasms and vesicular nuclei, in contrast with MACs without SAC morphology (C & D).
SAC morphology correlation with clinicopathological and molecular characteristics
Thirty-two (38%) of the 84 cases that reached a consensus were diagnosed as MAC with SAC morphology (Table 1). MACs with SAC morphology were associated with proximal location (P=0.001), BRAF mutation (P=0.042), and CIMP-positive status (P=0.023) (Table 1).
Table 1. Clinicopathologic and molecular characteristics in 84 colorectal MACs with and without SAC morphology.
| Characteristic | MAC withoutSAC morphology(n=52)a | MAC with SACmorphology(n=32)b | P-value |
|---|---|---|---|
| Gender | 0.255 | ||
| Male | 31 (59.6) | 15 (46.9) | |
| Female | 21 (40.4) | 17 (53.1) | |
| Age | 0.157 | ||
| ≤ 70 years | 31 (59.6) | 14 (43.8) | |
| > 70 years | 21 (40.4) | 18 (56.3) | |
| Location | 0.001 | ||
| Proximal colon | 18 (34.6) | 23 (71.9) | |
| Distal colon or rectum | 34 (65.4) | 9 (28.1) | |
| Differentiation | 0.952 | ||
| Well | 9 (17.3) | 5 (15.6) | |
| Moderate | 36 (69.2) | 22 (68.8) | |
| Poor | 7 (13.5) | 5 (15.6) | |
| AJCC TNM stage | 0.889 | ||
| Stage I | 2 (3.8) | 1 (3.1) | |
| Stage II | 22 (42.3) | 14 (43.8) | |
| Stage III | 21 (40.4) | 11 (34.4) | |
| Stage IV | 7 (13.5) | 6 (18.8) | |
| KRAS mutation (n=75) | 19 (42.2) | 13 (43.3) | 0.924 |
| BRAF mutation (n=73) | 3 (7) | 8 (26.7) | 0.042 |
| CIMP positive (n=77) | 3 (6.5) | 8 (25.8) | 0.023 |
| Defective mismatch repair protein | 12 (23.1) | 7 (21.9) | 0.898 |
AJCC, American Joint Committee on Cancer; CIMP, CpG island methylator phenotype; MAC, mucinous adenocarcinoma; SAC, serrated adenocarcinoma.
a Values are calculated for 52 MACs without SAC morphology unless specified otherwise, and are presented as number and percentage of patients.
b Values are calculated for 32 MACs with SAC morphology unless specified otherwise, and are presented as number and percentage of patients.
Histological SAC features were observed in both mucinous and non-mucinous areas, but their distribution was not diffuse in most MACs with SAC morphology. Only two MACs with SAC morphology showed diffuse epithelial serration (>50% of the tumor volume). The most common contiguous precursor lesions were tubulovillous adenomas (Figure 2A–2B). Contiguous traditional serrated adenomas (Figure 2C–2D) were observed in 4 (12.5%) MACs with SAC morphology, but were not seen in any MACs without SAC morphology (P=0.019) (Supplementary Table 1). Three of these four tumors were located in the proximal colon. Contiguous sessile serrated adenomas were not observed; however, a few serrated glands around the malignant tumor (Figure 2E–2F) were noted in three (9.4%) MACs with SAC morphology.
Figure 2. Lesions contiguous with the malignant tumor.
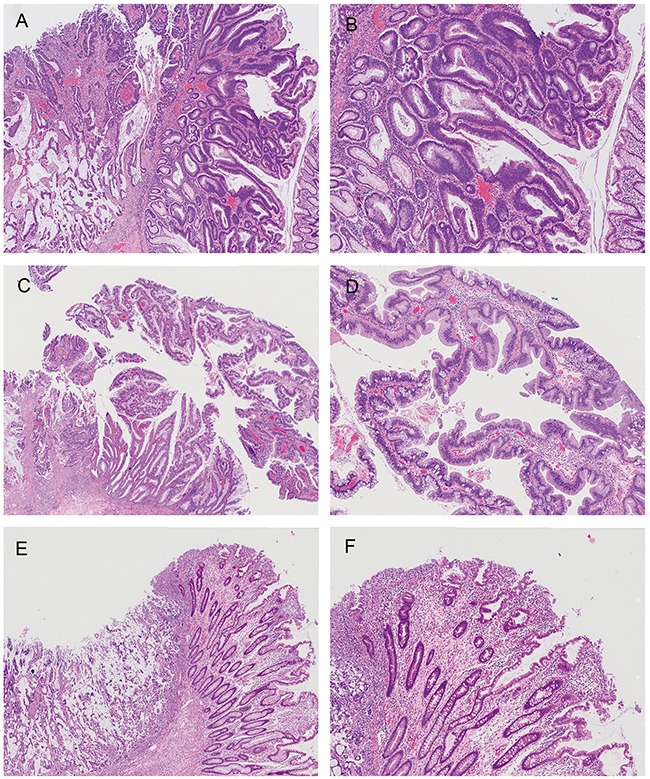
MAC without SAC morphology arising in a tubulovillous adenoma, showing a mixture of tubular and villous structures and dysplastic epithelium with elongated and hyperchromatic nuclei (A & B) MAC with SAC morphology arising in a traditional serrated adenoma showing ectopic crypts, crypt serration, and abundant eosinophilic cytoplasm (C & D) There were a few serrated glands around MACs with SAC morphology, but the size was not large enough to be diagnosed as a serrated polyp (E & F) Histological SAC features of the cancer were focally seen, and were not demonstrated in the figure.
KRAS and BRAF mutations and CIMP status in colorectal MACs
Mutation analysis of KRAS codons 12 and 13 in exon 2 was performed successfully in 79 colorectal MACs; 34 (43%) of these had KRAS mutations. The most common KRAS mutation in our samples was G12D (16.5%, 13/79); other mutations included G13D (13.9%, 11/79), G12V (6.3%, 5/79), G12A (2.5%, 2/79), G12C (1.3%, 1/79), G12R (1.3%, 1/79), and A18D (1.3%, 1/79) (Supplementary Table 2).
Analysis of BRAF mutations in codon 600 of exon 15 was successfully performed in 77 colorectal MACs, and 12 (15.6%) exhibited BRAF mutations. The most common BRAF mutation in our series was V600E (13%, 10/77). Other BRAF mutations included D594G (1.3%, 1/77) and D594N (1.3%, 1/77) in two MACs without SAC morphology. BRAF mutation was associated with SAC morphology (P=0.042), poorer tumor differentiation (P=0.002), and CIMP-positive status (P=0.001) in colorectal MACs (Supplementary Table 3). No KRAS and BRAF mutations were identified in the same tumor.
CIMP analyses were performed successfully in 81 colorectal MACs. Fifteen percent (12/81) of these MACs had at least three aberrantly methylated genes and were CIMP-positive. CIMP-positive tumors were associated with SAC morphology (P=0.023), BRAF mutation (P=0.001), and wild-type KRAS (P=0.037) (Supplementary Table 4). BRAF mutation analysis was successfully performed in three of the four MACs with contiguous traditional serrated adenoma; all harbored BRAF mutations and were CIMP positive.
MMR expression in colorectal MACs
Absence of MLH1 and MSH2 expression was observed in 15/88 (17%) and 5/88 (5.7%) colorectal MACs, respectively (Supplementary Table 5). All four MACs with contiguous traditional serrated adenoma were MMR proficient (pMMR), and three were located in the proximal colon.
Association of clinicopathological and molecular characteristics with patient survival
Sixty patients with AJCC stage I–III MACs were included in relapse-free survival analyses. Seventeen patients had relapsed at the last follow-up (mean relapse-free survival after surgery, 14.51 months; range, 5.33–32.25 months). Mean follow-up duration for the 43 patients without relapse was 60.57 months (range, 9.24–106.82 months). In AJCC stage I–III MACs, tumor location, stage, and presence of SAC morphology or CIMP-positive status were associated with relapse-free survival (P<0.05 for all comparisons; Table 2). In AJCC stage III MACs, tumor location, pT status, SAC morphology (Figure 3A), CIMP status (Figure 3B), and presence of SAC morphology or CIMP-positive status were associated with relapse-free survival (P<0.05 for all comparisons; Table 2). In univariate analysis, AJCC stage III MACs without both SAC morphology and CIMP-positive status exhibited 10.567 times greater risk of cancer relapse than those having both characteristics or either one (P=0.025).
Table 2. Associations of MAC clinicopathologic and molecular characteristics with cancer relapse.
| Characteristic | Stage I to III | Stage III | ||||
|---|---|---|---|---|---|---|
| No. of patientsa | Relapse-free survival (%) | P Value | No. of patientsb | Relapse-free survival (%) | P-value | |
| Gender | 0.605 | 0.255 | ||||
| Female | 29 | 75.9 | 17 | 70.6 | ||
| Male | 31 | 67.7 | 12 | 41.7 | ||
| Age | 0.172 | 0.335 | ||||
| ≤ 70 years | 33 | 63.6 | 18 | 50 | ||
| > 70 years | 27 | 81.5 | 11 | 72.7 | ||
| Location | 0.001 | < 0.001 | ||||
| Proximal colon | 33 | 90.9 | 13 | 100 | ||
| Distal colon or rectum | 27 | 48.1 | 16 | 25 | ||
| Differentiation | 0.78 | 0.307 | ||||
| Well | 10 | 80 | 5 | 80 | ||
| Moderate | 43 | 69.8 | 19 | 47.4 | ||
| Poor | 7 | 71.4 | 5 | 80 | ||
| pT status | 0.225 | 0.043 | ||||
| pT1 or pT2 or pT3 | 51 | 74.5 | 26 | 65.4 | ||
| pT4 | 9 | 55.6 | 3 | 0 | ||
| pN status | 0.091 | 0.578 | ||||
| pN0 | 31 | 83.9 | - | - | ||
| pN1 | 20 | 60 | 20 | 60 | ||
| pN2 | 9 | 55.6 | 9 | 55.6 | ||
| AJCC TNM stage | 0.035 | - | - | - | ||
| Stage I or II | 31 | 83.9 | ||||
| Stage III | 29 | 58.6 | ||||
| SAC morphology | (n=56) | 0.144 | (n=27) | 0.035 | ||
| Absent | 34 | 67.6 | 17 | 47.1 | ||
| Present | 22 | 86.4 | 10 | 90 | ||
| CIMP status | (n=56) | 0.091 | (n=26) | 0.009 | ||
| Negative | 44 | 63.6 | 18 | 33.3 | ||
| Positive | 12 | 91.7 | 8 | 100 | ||
| SAC morphology and CIMP-positive status | (n=54) | 0.035 | (n=25) | 0.005 | ||
| Presence of both or either one | 26 | 88.5 | 12 | 91.7 | ||
| Absence of both | 28 | 60.7 | 13 | 30.8 | 0.488 | |
| KRAS status | (n=55) | 0.467 | (n=28) | |||
| Wild type | 33 | 72.7 | 16 | 62.5 | ||
| Mutant | 22 | 63.6 | 12 | 50 | ||
| BRAF status | (n=55) | 0.643 | (n=28) | 0.214 | ||
| Wild type | 46 | 69.6 | 22 | 50 | ||
| Mutant | 9 | 77.8 | 6 | 83.3 | ||
| MMR status | 0.093 | 0.241 | ||||
| Proficient | 44 | 65.9 | 21 | 52.4 | ||
| Defective | 16 | 87.5 | 8 | 75 | ||
AJCC, American Joint Committee on Cancer; CIMP, CpG island methylator phenotype; MAC, mucinous adenocarcinoma; MMR, DNA mismatch repair; HR, hazard ratio; SAC, serrated adenocarcinoma.
a Values are calculated for 60 patients unless specified otherwise.
b Values are calculated for 29 patients unless specified otherwise.
Figure 3. Relapse-free survival in AJCC stage III MAC patients.
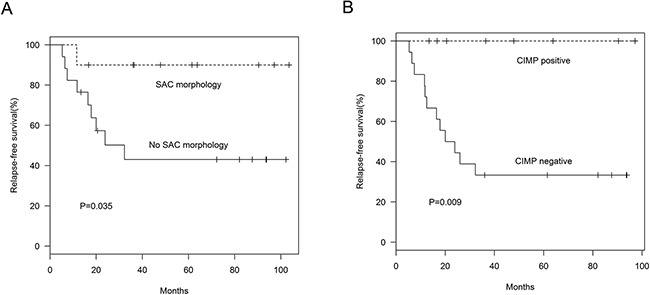
SAC morphology (A) (P=0.035) and CIMP status (B) (P=0.009) were associated with relapse-free survival in AJCC stage III MAC patients.
In multivariate analysis, AJCC stage I–III MACs without both SAC morphology and CIMP-positive status exhibited 3.955 times greater risk of cancer relapse than those having both characteristics or either one (P=0.035; Table 3) after adjusting for cancer stage. To avoid the effect of multicollinearity, we did not include the covariate “location” in this analysis. SAC morphology or CIMP status singly did not influence survival in the multivariate model (data not shown).
Table 3. Multivariable associations with cancer relapse in colorectal MAC patients.
| Characteristic | Stage I to III | Stage III | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| SAC morphology and CIMP-positive status | ||||
| Presence of both or either one | 1.0 (reference) | 1.0 (reference) | 0.036 | |
| Absence of both | 3.955 (1.101-14.214) | 0.035 | 9.416 (1.157-76.642) | |
| AJCC TNM stage | ||||
| Stage I or II | 1.0 (reference) | - | - | |
| Stage III | 3.719 (1.159-11.927) | 0.027 | ||
| pT status | ||||
| pT1 or pT2 or pT3 | - | - | 1.0 (reference) | |
| pT4 | 2.033 (0.410-10.071) | 0.385 | ||
AJCC, American Joint Committee on Cancer; HR, hazard ratio; MAC, mucinous adenocarcinoma; SAC, serrated adenocarcinoma.
DISCUSSION
The histological criteria proposed by Makinen, et al. describe a colorectal cancer subtype with distinct serrated architecture associated with serrated polyps. Previous studies and ours confirmed that these criteria are feasible, with satisfactory interobserver reproducibility [18]. SAC incidence, and its association with various molecular features, had not previously been surveyed in colorectal MAC. We showed that MAC with SAC morphology tended to grow in the proximal colon with a higher frequency of “serrated pathway carcinoma” molecular features, including BRAF mutations and CIMP-positivity than MACs without SAC morphology. SAC morphology was noted in 38% of MACs in our study, a result higher than previously reported (9.1%) for SAC in colorectal cancer [18], which might explain the high frequency of BRAF mutations and CIMP-positive status in MACs.
Contiguous serrated precursor lesions have previously been observed in 52% of reported colorectal SACs [18]. We observed serrated precursor lesions in only four (13%) MACs with SAC morphology. These lesions were not observed in MACs without SAC morphology, which further suggests an association between serrated polyps and SACs. Notably, all serrated precursor lesions in our series were traditional serrated adenomas, which are the least frequently occurring serrated polyps. However, this was consistent with the findings of Bettington, et al., who observed that serrated morphology carcinomas most often arise from traditional serrated adenomas and tubulovillous adenomas with serrated features [13]. García-Solano, et al. postulated that precursor sessile serrated adenoma frequency is probably underestimated, because sessile serrated adenomas can undergo complete histological dysplasia and high-grade transformation, leading to a traditional serrated adenoma or in situ adenocarcinoma appearance [18, 27].
In contrast with previous conventional CRC findings (most are non-MAC) [18, 19], patients in our study who had colorectal MACs with SAC morphology exhibited improved relapse-free survival, and this was significant in AJCC stage III MAC patients. SAC morphology was associated with proximal cancer location and CIMP-positive status, which were favorable prognostic factors in our analyses.
Prior studies of CIMP status for predicting CRC patient prognosis have been controversial [28]. Discrepancies between studies might be explained by differences in the study populations or in the methodologies and panels used to determine CIMP status. The CIMP panel we used was identified by Weisenberger and colleagues, and was validated in CRC in a large, population-based cohort [29]. CIMP panels used in many previous studies were modified from the Weisenberger panel, with new markers added. In previous studies using the Weisenberger panel, CIMP-positive status suggested good patient outcome in KRAS wild-type or MSI CRC [28, 30], but a poor prognosis in rectal cancer or CRC with chromosomal instability [30, 31].
Our study had several limitations. First, we did not include non-MACs for comparison, as our findings on the prognostic implications of SAC morphology contradicted results from studies of conventional, mostly non-MAC, CRC [18, 19]. Second, to avoid the effect of neoadjuvant therapy in histological analyses, we excluded low rectal cancer cases, which had often undergone neoadjuvant concurrent chemoradiotherapy. Third, PMS2 and MSH6 were not analyzed via IHC. However, as cases with isolated loss of PMS2 or MSH6 expression were uncommon [32], it is unlikely that this exclusion affected our main findings.
In conclusion, our results showed that two MAC groups with distinct clinicopathological and molecular features could be identified using Makinen's criteria, and suggested a favorable prognostic role for the serrated neoplastic pathway in colorectal MAC. The relatively high SAC incidence in MACs might explain the high frequency of BRAF mutations and CIMP-positive status in MACs. A more complete understanding of the prognostic role of the serrated neoplastic pathway in colorectal MAC and conventional CRC (mostly non-MAC) will require further research.
MATERIALS AND METHODS
Patients and materials
The Institutional Review Board of the National Cheng Kung University Hospital approved this study. We identified 88 surgically resected colorectal MACs without neoadjuvant therapy by searching our electronic pathology database for records dated between 2007 and 2014. Signet-ring cell carcinomas were excluded. Tumor status was recorded according to the AJCC cancer-staging manual (7th ed.). Adjuvant chemotherapy with 5-FU was administered to patients with AJCC stage II–IV cancers following standard schedules and doses. Histology was reviewed for all cases, and diagnoses were confirmed. Six patients had one or two synchronous MACs or non-MACs, and in these cases we analyzed the largest MAC. No patient had a family history of Lynch syndrome. Proximal cancers were defined as those in the cecum, ascending, and transverse colon.
Histological evaluation of SAC morphology
Three pathologists with >10 years of experience (Lee C. T., Tsai H. W., and Ho C. L.) reviewed the sections. MAC was diagnosed when more than half of the tumor volume was composed of extracellular mucin-containing tumor cells [1, 2]. “SAC morphology” was defined according to established Makinen's criteria [13, 15, 17], including epithelial serrations, clear or eosinophilic cytoplasm, abundant cytoplasm, vesicular nuclei, distinct nucleoli, scarceness (<10%) of necrosis, mucin production, and cell balls or papillary rods in the mucin. Histologic evaluation details are provided in the Supplementary Methods.
“SAC morphology” for a well or moderately differentiated MAC was considered positive when the cancer met at least six of the first seven features listed above [13, 18]. We omitted the “epithelial serration” criterion for poorly differentiated MACs without glandular architecture, and those that met at least five of the first seven features were categorized as having SAC morphology. Differences in opinion for SAC morphology diagnosis were resolved by re-evaluating the sections to reach a consensus.
Any lesions contiguous with the cancer were also evaluated. Conventional (tubular, tubulovillous, and villous) adenoma, sessile serrated adenoma, and traditional serrated adenoma were classified according to WHO classifications [1].
DNA extraction and BRAF and KRAS mutation analysis
Ten-micrometer-thick sections were cut from paraffin-embedded tumor samples, placed on slides, and the tumor area was scraped off. DNA was extracted using standard phenol chloroform methods. Mutations covering KRAS codons 12 and 13, and BRAF codon 600 were assessed with direct sequencing of PCR-amplified DNA (Supplementary Methods).
CIMP analysis
CIMP status was assessed using the Weisenberger panel (CACNA1G, IGF2, NEUROG1, RUNX3, and SOCS1) with an automated real-time, PCR-based MethyLight system as previously described (Supplementary Methods) [33]. All primer sequences were published previously [33]. Tumor samples were categorized as CIMP positive if methylation was detected in ≥3/5 genes, and CIMP negative if methylation was detected in ≤2/5 genes.
Immunohistochemistry (IHC)
IHC for MLH1 and MSH2 was performed using an avidin-biotin complex-peroxidase procedure with an automated stainer (Supplementary Methods). A pathologist (Lee C. T.) assessed all IHC stains. MLH1 or MSH2 expression was defined as abnormal when nuclear staining of tumor cells was absent despite positive staining in surrounding stromal cells. Defective MMR (dMMR) was defined as loss of MLH1 or MSH2 expression in tumor cells. Proficient MMR (pMMR) was defined by the presence of MLH1 and MSH2 expression in tumor cells [34].
Statistical analysis
Relationships between categorical characteristics were analyzed via χ2 test or Fisher's exact test. κ values were calculated to assess observer agreement for SAC morphology diagnosis. κ<0.2 indicated poor agreement, 0.21–0.40 was fair, 0.41–0.60 was moderate, 0.61–0.80 was good and >0.8 was very good [35].
Relapse-free survival was calculated from the date of surgery. Events were defined as any disease recurrence shown histologically or via imaging. Survival analyses included only patients with AJCC stage I–III colorectal MACs. Patients who were followed-up for <3 years were excluded unless an event occurred or the patient had died. Patients who died within one month of surgery and patients who had more than one cancer were also excluded from survival analyses. The significance of various covariates was assessed using univariate analysis with the log-rank test. Survival curves were calculated using the Kaplan-Meier method. A multivariate model was constructed through stepwise selection with P set at 0.05 for a characteristic to be included or excluded from the model. All tests were 2-sided, and P<0.05 was considered statistically significant.
SUPPLEMENTARY MATERIALS TABLES
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
GRANT SUPPORT
This work was supported by National Cheng Kung University Hospital (NCKUH-10203005, NCKUH-10305016, and NCKUH-10604037).
REFERENCES
- 1.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. Lyon, France: IARC; 2010. 4th ed. [Google Scholar]
- 2.Symonds DA, Vickery AL. Mucinous carcinoma of the colon and rectum. Cancer. 1976;37:1891–1900. doi: 10.1002/1097-0142(197604)37:4<1891::aid-cncr2820370439>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Verhulst J, Ferdinande L, Demetter P, Ceelen W. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol. 2012;65:381–388. doi: 10.1136/jclinpath-2011-200340. [DOI] [PubMed] [Google Scholar]
- 4.Nozoe T, Anai H, Nasu S, Sugimachi K. Clinicopathological characteristics of mucinous carcinoma of the colon and rectum. J Surg Oncol. 2000;75:103–107. doi: 10.1002/1096-9098(200010)75:2<103::aid-jso6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 5.Purdie CA, Piris J. Histopathological grade, mucinous differentiation and DNA ploidy in relation to prognosis in colorectal carcinoma. Histopathology. 2000;36:121–126. [PubMed] [Google Scholar]
- 6.Hugen N, Simons M, Halilovic A, van der Post RS, Bogers AJ, Marijnissen-van Zanten MA, de Wilt JH, Nagtegaal ID. The molecular background of mucinous carcinoma beyond MUC2. J Pathol Clin Res. 2015;1:3–17. doi: 10.1002/cjp2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogino S, Brahmandam M, Cantor M, Namgyal C, Kawasaki T, Kirkner G, Meyerhardt JA, Loda M, Fuchs CS. Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod Pathol. 2006;19:59–68. doi: 10.1038/modpathol.3800482. [DOI] [PubMed] [Google Scholar]
- 8.Song GA, Deng G, Bell I, Kakar S, Sleisenger MH, Kim YS. Mucinous carcinomas of the colorectum have distinct molecular genetic characteristics. Int J Oncol. 2005;26:745–750. [PubMed] [Google Scholar]
- 9.Zhang H, Evertsson S, Sun X. Clinicopathological and genetic characteristics of mucinous carcinomas in the colorectum. Int J Oncol. 1999;14:1057–1061. doi: 10.3892/ijo.14.6.1057. [DOI] [PubMed] [Google Scholar]
- 10.Hawkins N, Norrie M, Cheong K, Mokany E, Ku SL, Meagher A, O’Connor T, Ward R. CpG island methylation in sporadic colorectal cancers and its relationship to microsatellite instability. Gastroenterology. 2002;122:1376–1387. doi: 10.1053/gast.2002.32997. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka H, Deng G, Matsuzaki K, Kakar S, Kim GE, Miura S, Sleisenger MH, Kim YS. BRAF mutation, CpG island methylator phenotype and microsatellite instability occur more frequently and concordantly in mucinous than non-mucinous colorectal cancer. Int J Cancer. 2006;118:2765–2771. doi: 10.1002/ijc.21701. [DOI] [PubMed] [Google Scholar]
- 12.Jass JR, Smith M. Sialic acid and epithelial differentiation in colorectal polyps and cancer--a morphological, mucin and lectin histochemical study. Pathology. 1992;24:233–242. doi: 10.3109/00313029209068874. [DOI] [PubMed] [Google Scholar]
- 13.Bettington M, Walker N, Clouston A, Brown I, Leggett B, Whitehall V. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367–386. doi: 10.1111/his.12055. [DOI] [PubMed] [Google Scholar]
- 14.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 15.Makinen MJ. Colorectal serrated adenocarcinoma. Histopathology. 2007;50:131–150. doi: 10.1111/j.1365-2559.2006.02548.x. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien MJ, Zhao Q, Yang S. Colorectal serrated pathway cancers and precursors. Histopathology. 2015;66:49–65. doi: 10.1111/his.12564. [DOI] [PubMed] [Google Scholar]
- 17.Tuppurainen K, Makinen JM, Junttila O, Liakka A, Kyllonen AP, Tuominen H, Karttunen TJ, Makinen MJ. Morphology and microsatellite instability in sporadic serrated and non-serrated colorectal cancer. J Pathol. 2005;207:285–294. doi: 10.1002/path.1850. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Solano J, Perez-Guillermo M, Conesa-Zamora P, Acosta-Ortega J, Trujillo-Santos J, Cerezuela-Fuentes P, Makinen MJ. Clinicopathologic study of 85 colorectal serrated adenocarcinomas: further insights into the full recognition of a new subset of colorectal carcinoma. Hum Pathol. 2010;41:1359–1368. doi: 10.1016/j.humpath.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Shida Y, Fujimori T, Tanaka H, Fujimori Y, Kimura R, Ueda H, Ichikawa K, Tomita S, Nagata H, Kubota K, Tsubaki M, Kato H, Yao T, et al. Clinicopathological features of serrated adenocarcinoma defined by Makinen in dukes’ B colorectal carcinoma. Pathobiology. 2012;79:169–174. doi: 10.1159/000334837. [DOI] [PubMed] [Google Scholar]
- 20.Laiho P, Kokko A, Vanharanta S, Salovaara R, Sammalkorpi H, Jarvinen H, Mecklin JP, Karttunen TJ, Tuppurainen K, Davalos V, Schwartz S, Jr, Arango D, Makinen MJ, Aaltonen LA. Serrated carcinomas form a subclass of colorectal cancer with distinct molecular basis. Oncogene. 2007;26:312–320. doi: 10.1038/sj.onc.1209778. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Solano J, Conesa-Zamora P, Carbonell P, Trujillo-Santos J, Torres-Moreno DD, Pagan-Gomez I, Rodriguez-Braun E, Perez-Guillermo M. Colorectal serrated adenocarcinoma shows a different profile of oncogene mutations, MSI status and DNA repair protein expression compared to conventional and sporadic MSI-H carcinomas. Int J Cancer. 2012;131:1790–1799. doi: 10.1002/ijc.27454. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien MJ, Yang S, Mack C, Xu H, Huang CS, Mulcahy E, Amorosino M, Farraye FA. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 2006;30:1491–1501. doi: 10.1097/01.pas.0000213313.36306.85. [DOI] [PubMed] [Google Scholar]
- 23.Stefanius K, Ylitalo L, Tuomisto A, Kuivila R, Kantola T, Sirnio P, Karttunen TJ, Makinen MJ. Frequent mutations of KRAS in addition to BRAF in colorectal serrated adenocarcinoma. Histopathology. 2011;58:679–692. doi: 10.1111/j.1365-2559.2011.03821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42:1–10. doi: 10.1016/j.humpath.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Fang M, Ou J, Hutchinson L, Green MR. The BRAF oncoprotein functions through the transcriptional repressor MAFG to mediate the CpG Island Methylator phenotype. Mol cell. 2014;55:904–915. doi: 10.1016/j.molcel.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kambara T, Simms LA, Whitehall VL, Spring KJ, Wynter CV, Walsh MD, Barker MA, Arnold S, McGivern A, Matsubara N, Tanaka N, Higuchi T, Young J, Jass JR, Leggett BA. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53:1137–1144. doi: 10.1136/gut.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheridan TB, Fenton H, Lewin MR, Burkart AL, Iacobuzio-Donahue CA, Frankel WL, Montgomery E. Sessile serrated adenomas with low- and high-grade dysplasia and early carcinomas: an immunohistochemical study of serrated lesions “caught in the act”. Am J Clin Pathol. 2006;126:564–571. doi: 10.1309/C7JE8BVL8420V5VT. [DOI] [PubMed] [Google Scholar]
- 28.Juo YY, Johnston FM, Zhang DY, Juo HH, Wang H, Pappou EP, Yu T, Easwaran H, Baylin S, van Engeland M, Ahuja N. Prognostic value of CpG island methylator phenotype among colorectal cancer patients: a systematic review and meta-analysis. Ann Oncol. 2014;25:2314–2327. doi: 10.1093/annonc/mdu149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogino S, Kawasaki T, Kirkner GJ, Kraft P, Loda M, Fuchs CS. Evaluation of markers for CpG island methylator phenotype (CIMP) in colorectal cancer by a large population-based sample. J Mol Diagn. 2007(9):305–314. doi: 10.2353/jmoldx.2007.060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simons CC, Hughes LA, Smits KM, Khalid-de Bakker CA, de Bruine AP, Carvalho B, Meijer GA, Schouten LJ, van den Brandt PA, Weijenberg MP, van Engeland M. A novel classification of colorectal tumors based on microsatellite instability, the CpG island methylator phenotype and chromosomal instability: implications for prognosis. Ann Oncol. 2013;24:2048–2056. doi: 10.1093/annonc/mdt076. [DOI] [PubMed] [Google Scholar]
- 31.Jo P, Jung K, Grade M, Conradi LC, Wolff HA, Kitz J, Becker H, Ruschoff J, Hartmann A, Beissbarth T, Muller-Dornieden A, Ghadimi M, Schneider-Stock R, Gaedcke J. CpG island methylator phenotype infers a poor disease-free survival in locally advanced rectal cancer. Surgery. 2012;151:564–570. doi: 10.1016/j.surg.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 32.South CD, Yearsley M, Martin E, Arnold M, Frankel W, Hampel H. Immunohistochemistry staining for the mismatch repair proteins in the clinical care of patients with colorectal cancer. Genet Med. 2009;11:812–817. doi: 10.1097/GIM.0b013e3181b99b75. [DOI] [PubMed] [Google Scholar]
- 33.Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, Kang GH, Widschwendter M, Weener D, Buchanan D, Koh H, Simms L, Barker M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 34.Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, French AJ, Kabat B, Foster NR, Torri V, Ribic C, Grothey A, Moore M, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong NA, Hunt LP, Novelli MR, Shepherd NA, Warren BF. Observer agreement in the diagnosis of serrated polyps of the large bowel. Histopathology. 2009;55:63–66. doi: 10.1111/j.1365-2559.2009.03329.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


