Abstract
Background
Mood and anxiety disorders are highly heterogeneous and their underlying pathology is complex. The Research Domain Criteria (RDoC) approach seeks to establish dimensionally and neuroscience-based descriptions of psychopathology that may inform better classification and treatment approaches. The current investigation sought to determine the latent variables underlying positive and negative valence processing in terms of symptoms and behavioral units of analysis.
Method
As part of an ongoing study, individuals with mood and anxiety problems were recruited largely from primary care clinics at UCLA (n=62) and UCSD (n=58). These participants underwent a comprehensive symptomatic and behavioral assessment. An implicit approach avoidance task and a modified dot probe detection task were used to measure positive and negative valence processing.
Results
Principal components analysis with varimax rotation identified four symptom components, three behavioral components for the dot probe task, and two behavioral components for the implicit approach avoidance task. These components yielded two meta-components consisting of: negative valence symptoms, negative approach bias, and high sustained, selective attention; and positive valence symptoms, positive approach bias, and slow selective or sustained attention. The components did not differ between males and females, nor by age or medication status.
Limitations
The limitations are: (1) relatively small sample, (2) exploratory analysis strategy, (3) no test/re-test data, (4) no neural circuit analysis, and (5) limited reliability of behavioral data.
Conclusions
These preliminary data show that positive and negative valence processing domains load on independent dimensions. Taken together, multi-level assessment approaches combined with advanced statistical analyses may help to identify distinct positive and negative valence processes within a clinical population that cut across traditional diagnostic categories.
Keywords: Research Domain Criteria, Positive Valence Processing, Negative Valence Processing, Mood Disorders, Anxiety Disorders, Principal Components Analysis
1. Introduction
1.1 Mood and Anxiety Disorders
Mood (Moussavi et al., 2007) and anxiety (Kessler et al., 2010) disorders will account for approximately $16 trillion lost productivity or 25% of global GDP over the next 20 years (Whiteford et al., 2013) and are among the most common and devastating mental health conditions worldwide. Recent epidemiological data estimate the lifetime prevalence of Major Depressive Disorder (MDD) at about 18% and the 12-month prevalence at 7% (Kessler et al., 2012). MDD is phenotypically and etiologically heterogeneous, which has posed a significant challenge to elucidation of the biological mechanisms, creation of objective, non-symptom-based nosological categories that cut across current diagnostic boundaries, and development of novel therapeutics. Recent analyses suggest that current interventions have limited efficacy and help to restore functioning in only a subgroup of individuals (Linde et al., 2015a; Linde et al., 2015b). Anxiety disorders are the most common mental health problem (Kessler et al., 1994) with a lifetime prevalence of approximately 33% (Kessler et al., 2012). Anxiety disorders are the sixth leading cause of disability world-wide and show no signs of reduced burden over recent years (Baxter et al., 2014). As with MDD, extant treatments are only partially effective (e.g., (Loerinc et al., 2015)). Both MDD and anxiety disorders are associated with significant medical comorbidities (Roy-Byrne et al., 2008), which further exacerbate the cost and suffering associated with these disorders. The heterogeneity of mood and anxiety disorders and the limited effectiveness of interventions have provided an impetus to utilize dimensional approaches to help delineate distinct syndromes of mood and anxiety that better reflect the underlying neurobiology.
1.2 Research Domain Criteria Issues
Biological psychiatry is in a crisis (Insel and Cuthbert, 2015) and the fundamental insights into basic neuroscience have not translated into practical and clinical tools or treatment in psychiatry. The development of new therapeutics based on neuroscience approaches to understand the pathophysiology of these illnesses has stalled (Insel, 2012). Despite the development of a newly revised diagnostic classification for mental disorders (APA, 2013), neuroscience has had virtually no impact on the delineation and definition of the disorder categories. There are no clinical tools for prognosis, diagnosis, or treatment monitoring that derive from neuroscience (Prata et al., 2014). The National Institute of Mental Health began the Research Domain Criteria (RDoC) project in 2009 to develop a research classification system for mental disorders based upon neurobiology and observable behavior (Cuthbert and Insel, 2013). The RDoC initiative highlights the need to (1) determine the relationship between different units of analyses, that is, between genes, molecules, cells, circuits, physiology, behavior, self-report, and paradigms and (2) transcend traditional diagnostic groups to adequately capture the variation of domains in clinical populations that can be mapped across units of analyses.
1.3 Positive and Negative Valence Domains
Positive and Negative Valence
Affect, or experience of emotion, can be divided into two domains (James, 1988). Positive affect involves emotions such as happiness, excitement, elation, and enthusiasm. Negative affect involves emotions such as anger, resentment, sadness, anxiety, and fear. Positive and negative affect systems are dimensions of psychopathology identified by the RDoC work groups (Health, 2011a, b). High negative affect is common to anxiety and depression (Brown et al., 1998; Chorpita, 2002; Prenoveau et al., 2010) and comorbid anxiety and depression is associated with more negative affect than each disorder alone (Weinstock and Whisman, 2006). Low positive affect is relatively specific to depression although it does extend to social anxiety and generalized anxiety disorder (Clark and Watson, 1991; Craske et al., 2009; Kashdan, 2007). In addition, psychophysiological and neurobiological data indicate that the negative affect system is closely tied to threat sensitivity whereas the positive affect system is closely tied to reward sensitivity, as described below.
Positive Valence System
A central construct representing the positive valence system is approach motivation, which can be defined as processes that regulate the direction and maintenance of approach behavior, albeit moderated by pre-existing tendencies, learning, memory, stimulus characteristics, and deprivation states. Some have differentiated two separable components of reward-related processing; phasic prediction error signaling and reward sensitivity (Huys et al., 2013). Others have proposed that the construct of reward seeking and reward sensitivity is are components of approach motivation (Shankman et al., 2007). Reward sensitivity refers to the anticipation and receipt of positive stimuli as well as learning about the probabilities of receiving a reward (Romer Thomsen et al., 2015). Dysregulation of reward sensitivity has been observed in depression (Chen et al., 2015; Day et al., 2015). The neural mechanisms of reward sensitivity involve the ventral striatum (VS) and orbitofrontal cortex (OFC). These structures are involved in the processing of primary rewards, such as pleasant tastes (O’Doherty et al., 2002), smells (O’Doherty et al., 2000) or visual stimuli (O’Doherty et al., 2001), as well as secondary (monetary) rewards (Delgado et al., 2005; O’Doherty et al., 2001; Zink et al., 2004). Moreover, there is evidence that reduced reward sensitivity in depression is related to EEG OFC gamma activity (Webb et al., 2016).
Negative Valence System
Responses to acute threat (fear) and potential harm (anxiety) were considered by the RDoC workshop committee to be central constructs within the negative valence system. These responses can be examined with respect to information processing (i.e. biases of attention) or incentive motivational actions (i.e. approach or avoidance behaviors). Attention bias can be quantified using response latency within a modified probe detection task (Rudaizky et al., 2014). Specifically, allocation of attention to the spatial location of affective stimuli (with positive or negative valence) can be determined from response latencies to probes (Mogg et al., 1995). In comparison, implicit approach/avoidance action tendencies are measured by the approach-avoidance task (AAT) (Strack and Deutsch, 2004). The basic premise underlying the AAT is that stimuli from the environment elicit automatic evaluations that activate affectively congruent behavioral schemas of approach and avoidance. These behavioral schemas can be assessed indirectly in terms of arm flexion (approach – i.e., pulling toward oneself) versus extension (avoidance – i.e., pushing away from oneself) through use of a joystick: Positively valenced stimuli are reliably associated with faster arm flexion than arm extension, whereas negatively valenced stimuli are associated with faster arm extension (e.g (Heuer et al., 2007)). By dictating behavioral movements in response to a feature of the task unrelated to the contents of the presented stimuli (e.g., instructing individuals to generate approach vs. avoidance actions according to different colored borders surrounding the target stimuli (Najmi et al., 2010; Taylor and Amir, 2012)), response latency differences in pulling versus pushing a given stimulus category (e.g., positively valenced stimuli) can be interpreted as relatively implicit action-tendencies driven by automatic evaluation of the stimulus contents.
1.3 Units of Analyses
The ultimate goal for RDoC is to derive constructs (i.e., negative valence vs positive valence) that are observable in multiple units of analysis, i.e. that can be observed on a symptom, behavioral, physiological, circuit, molecular, or genetic level. Thus, the RDoC initiative underscores the need to (1) identify measures of multiple units of analysis (e.g., self-report, behavior, physiology) that reliably capture the variation of a given construct, and (2) establish the relationship among different units of analyses, with an emphasis upon linking underlying brain function (e.g., neural circuits) to behavior. Here, we used well-established self-report and behavioral measures as the units of analysis to (1) examine latent constructs of positive and negative valence construct and (2) examine the relationships among those units of analysis. Our choice of measures was guided by empirical data for specific measures heuristically aimed at quantifying each construct, and evidence suggesting that those measures relate to neural circuits governing positive and negative valence system functioning. For example, attentional biases for emotional information are considered a hallmark of anxiety and depression (Bar-Haim et al., 2007; Taylor and Amir, 2010). Attentional bias for threat-relevant stimuli is reliably associated with negative affective states (e.g., anxiety (Bar-Haim et al., 2007)) and has been linked to greater activation in the AMYG during emotion processing (El Khoury-Malhame et al., 2011). In contrast, attentional bias for positive stimuli is associated with positive affective states (Tamir and Robinson, 2007) and neural activity reflecting approach-oriented motivation (Gable and Harmon-Jones, 2010). Moreover, diminished attentional processing of positive information is associated with depression(Joormann et al., 2007), and some anxiety disorders (e.g., social anxiety (Taylor et al., 2010)).
Prior studies using the AAT in specific anxiety disorder or analogue (i.e., elevated symptom) samples found evidence of greater automatic avoidance (or diminished approach) tendencies for anxiety-congruent (negative valence) stimuli relative to non-anxious control participants (social anxiety(Heuer et al., 2007; Roelofs et al., 2010); contamination fears(Najmi et al., 2010); spider fears (Bartoszek and Winer, 2015; Rinck and Becker, 2007); and posttraumatic stress disorder(Fleurkens et al., 2014)). Individuals with elevated social anxiety symptoms also displayed greater automatic avoidance (less approach) for positively valenced social cues(Heuer et al., 2007; Roelofs et al., 2010), a finding consistent with other indices of diminished positive valence system functioning in social anxiety (Alden et al., 2008; Brown et al., 1998; Campbell et al., 2009; Kashdan, 2007; Kashdan et al., 2011; Naragon-Gainey et al., 2009; Taylor et al., 2010, 2011). Studies in individuals with elevated symptoms of depression found evidence for diminished automatic approach tendencies for positively valenced stimuli (Bartoszek and Winer, 2015; Vrijsen et al., 2013) as well as greater avoidance tendencies for negatively valenced stimuli (e.g., angry faces(Seidel et al., 2010); see, however (Derntl et al., 2011; Radke et al., 2014) for no differences in automatic action tendencies for positive or negative emotional stimuli between depressed and non-depressed control). Taken together, previous studies suggest that the AAT may be a valid measure of relatively automatic approach-avoidance action tendencies for positive and negatively valenced stimuli in anxiety and depression, although linkages to neural circuitries are yet to be investigated.
1.4 Goal of this Study
The goal of the current study was to use a clinical population with significant mood and anxiety symptoms but not selected based on DSM disorder criteria in order to determine relationships among variables assessing positive and negative valence domains and to derive latent variables that describe psychopathology across units of analysis (here self-report and behavioral variables). That is, to evaluate whether multiple units of analysis do indeed cohere to become observable indicators of two separate constructs. Moreover, the future goal is to determine whether latent factors can be used to generate clinically meaningful outcome predictions across different aspects of disease severity. This is the first of a set of results from our project that focuses on the baseline (i.e., initial evaluation upon study entry) characteristics of the latent variables, which can have the potential to substantially improve our understanding of how positive and negative valence systems are inter-related in clinically significant and impairing disorders of mood and anxiety.
2. Methods
2.1 Sampling and Participants
We considered various populations based on our prior studies. We opted to identify individuals with anxiety or depression in a primary care setting, which we have used successfully to recruit a significant number of participants (Craske et al., 2011; Roy-Byrne et al., 2010), to maximize generalizability and external validity. This recruitment strategy was augmented using announcements posted in the community and online (e.g., flyers, social media). Medication status was another important consideration. Sample representativeness was enhanced by including medicated individuals (with statistical techniques to account for their effects). Thus, participants on stable doses (6 weeks) of certain psychotropic medications (e.g., SSRIs, SNRIs) were allowed to participate. However, participants on ineligible medications (e.g., benzodiazepines) were excluded if taken more days than not during the past 30 days. We recruited from two clinics: UCSD Primary Care Clinic and the UCLA Family Health Center.
Inclusion criteria: (1) individuals presenting to primary care clinics supplemented by self-identified individuals, who were recruited online, with mood and anxiety problems; (2) score > 10 on the PHQ-9 (Manea et al., 2012) or > 8 on the OASIS (Campbell-Sills et al., 2009); (3) between the ages of 18–55; and (4) able to provide informed, written consent. We expected that inclusion based on elevated anxiety (OASIS) or depression (PHQ-9) scores would yield a sample with the full range of anxiety and depression symptoms and associated functional impairment (see Table 2). Exclusion criteria: (1) Participants with no telephone or easy access to telephone; (2) moderate or severe alcohol or marijuana use disorder according to DSM 5 criteria in the past year; and (3) all other mild substance use disorder in the past year with the exception of alcohol or marijuana use disorder in the past 12 months; (4) Bipolar I or Psychotic disorders; (5) moderate to severe traumatic brain injury with evidence of neurological deficits, neurological disorders, or severe or unstable medical conditions that might be compromised by participation in the study (determined by primary care provider); (6) active suicidal ideation; and (7) inability to speak English.
Table 2.
Symptom Characteristics
| UCSD | UCLA | |||||
|---|---|---|---|---|---|---|
| n | mean | sd | n | mean | sd | |
| OASIS | 58 | 8.53 | 3.47 | 62 | 9.74 | 4.08 |
| GAD7 | 58 | 9.69 | 4.84 | 62 | 9.32 | 5.01 |
| PHQ9 | 58 | 10.47 | 4.81 | 62 | 11.53 | 5.97 |
| PANAS positive | 58 | 25.66 | 8.66 | 62 | 22.05 | 7.24 |
| PANAS negative | 58 | 18.21 | 5.64 | 62 | 17.29 | 7.32 |
| MASQ General Distress | 58 | 26.86 | 7.19 | 62 | 26.35 | 8.81 |
| MASQAnxious Arousal | 58 | 29.93 | 9.48 | 62 | 29.76 | 9.73 |
| MASQ Depressive | 58 | 32.48 | 9.94 | 62 | 34.82 | 10.47 |
| MASQ Anhedonic | 58 | 69.69 | 14.88 | 62 | 75.47 | 14.07 |
| BIS | 58 | 23.93 | 3.37 | 62 | 24 | 3.34 |
| BAS activation | 58 | 11.12 | 3 | 62 | 9.55 | 3.03 |
| BAS funseeking | 58 | 11.45 | 2.52 | 62 | 10.29 | 3.14 |
| BAS reward responsiveness | 58 | 16.93 | 2.23 | 62 | 16.61 | 2.54 |
| Experiential Avoidance | 58 | 39.45 | 7.85 | 62 | 40.24 | 8.08 |
| PID negative affect | 58 | 7.84 | 3.39 | 62 | 7.21 | 3.57 |
| PID detachment | 58 | 4.52 | 3.45 | 62 | 5.69 | 3.34 |
| PID antagonism | 58 | 3.36 | 2.96 | 62 | 2.52 | 2.51 |
| PID disinhibition | 58 | 3.6 | 3.11 | 62 | 3.81 | 3.06 |
| PID psychoticism | 58 | 4.26 | 3.17 | 62 | 3.98 | 2.84 |
| TEPS anticipatory pleasure | 58 | 38.83 | 10.68 | 62 | 36.98 | 10.48 |
| TEPS consumatory pleasure | 58 | 33.31 | 8.29 | 62 | 31.81 | 9.32 |
| SPSRQ reward sensitivity | 58 | 11.72 | 4.89 | 62 | 9.66 | 4.75 |
| SPSRQ punishment sensitivity | 58 | 14.21 | 5.5 | 62 | 15.84 | 5.19 |
| WHODAS 2.0 | 58 | 22 | 7.43 | 62 | 23.32 | 7.12 |
2.2 Self-Report Measures
Positive and Negative Affective Schedule - Trait (PANAS (Watson et al., 1988))
The PANAS is a widely used measure comprising 20-items assessing PA and NA using 5-point scales (1 = very slightly/not at all, 5 = extremely). To assess trait PA and NA, participants were asked to respond according to how they have felt “during the past week”. The PANAS has high internal consistency and temporal stability (trait version), correlational data support its convergent and discriminant validity, and confirmatory factor analyses support the construct validity of the PANAS(Crawford and Henry, 2004).
Mood and Anxiety Symptom Questionnaire - Short Form (MASQ (Clark and Watson, 1991))
The MASQ is a 62-item measure of mood and anxiety symptoms developed to evaluate predictions of the tripartite model of anxiety and depression. The MASQ has four subscales: (1) General Distress Anxious Symptoms (GDA: 11 items), (2) General Distress Depressive Symptoms (GDD: 12 items), (3) Anxious Arousal (AA: 17 items), and (4) Anhedonic Depression (AD: 22 items). Respondents indicated the extent to which they experienced each symptom during the past week from 1=not at all to 5=extremely. The MASQ subscales have been found to have adequate convergent and discriminant validity as well as good internal consistency (Watson et al., 1995a; Watson et al., 1995b) in student, adult volunteer, and clinical samples.
Behavioral Inhibition and Activation Scales (BIS/BAS (Carver and White, 1994))
The behavioral inhibition and activation scales (BIS/BAS) include 20-items assessing dispositional BIS and BAS sensitivities (i.e. avoidance and approach motives), which are hypothesized to reflect the negative and positive valence systems, respectively. Items are rated on four-point scales (1 = strongly disagree; 4 = strongly agree). The BAS has three subscales (Drive, Reward Responsiveness, and Fun Seeking); however, factor analyses support a single higher-order factor. The BIS/BAS has good test-retest reliability. Correlational data support the relative orthogonality and convergent, discriminant, and predictive validity of the subscales(Carver and White, 1994).
Temporal Experience of Pleasure Scale (TEPS)
(Gard et al., 2006) The TEPS is a measure of anticipatory pleasure and consummatory pleasure. It has 18 items, each of which are rated on a 6 point scale (e.g., 1=very false for me; 6=very true for me). This scale differentiates between goal attainment (consummatory pleasure) and the motivated approach of goals (anticipatory pleasure) (Ho et al., 2015).
Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ)
(Torrubia et al., 2001) The SPSRQ is a 48 yes-no response item scale designed to assess trait-like dispositions to respond to aversive and rewarding stimuli or events. Evidence suggests that both scales are orthogonal and display strong psychometric characteristics. The SPSRQ was used to assess threat and reward processing as it relates to positive and negative valence domains.
Personality Inventory for DSM-5 (PID-5)-Adult
(Maples et al., 2015) The PID-5 is a self-rated personality trait assessment scale, assessing 25 personality trait facets. Specific triplets of facets can be combined to yield indices of the 5 broader trait domains of (1) Negative Affect, (2) Detachment, (3) Antagonism, (4) Disinhibition, and (5) Psychoticism. Each item is rated on a 4-point scale, and the participants were asked to rate how well the item describes him or her generally. The PID-5 was used to assess to assess higher order personality domains that cut across anxiety and depressive disorders and may relate to positive and negative valence system functioning (Al-Dajani et al., 2016).
Acceptance, Safety, Escape/Avoidance Scale (AsSEAS)
The AsSEAS is a 12-item measure that assesses behavioral strategies for managing uncomfortable situations and feelings. This scale was developed to fill the gap for a transdiagnostic measure of avoidance. Items are rated on a 5-point Likert scale ranging from 1=not at all typical to 5=very typical. Confirmatory factor analyses reveal that the AsSEAS has three factors: acceptance, escape/avoidance, and safety behaviors. The AsSEAS was used to assess avoidance symptom aspects of the negative valence domain (McEvoy, P. M., LeBeau, R. T., Page, A. C., & Craske, M. G. (in prep). Acceptance, safety behaviours, escape and avoidance scale (AcSEAS): A transdiagnostic assessment.
GAD-7 (Spitzer et al., 2006)
The GAD-7 is a brief self-report measure designed to screen for the presence of GAD. Items assess symptoms of worry (e.g., ‘Not being able to stop or control worrying’) and general somatic tension (e.g., ‘Trouble relaxing’), and are rated on a 4-point Likert-type scale ranging from 0 (not at all sure) to 3 (nearly every day). Higher scores reflect greater symptom severity. The GAD-7 has strong psychometric properties in primary care settings and the general population (Kroenke et al., 2007).
World Health Organization Disability Assessment Schedule (WHODAS)
(World Health Organization, 2010) The WHODAS (12-item version) is a generic assessment instrument for health and disability, and covers 6 domains: (1) Cognition (understanding & communicating), (2) Mobility (moving & getting around), (3) Self-care (hygiene, dressing, eating & staying alone), (4) Getting along (interacting with other people), (5) Life activities (domestic responsibilities, leisure, work & school), and (6) Participation (joining in community activities). The WHODAS produces standardized disability levels and profiles, is applicable across cultures in adult populations, and has a direct conceptual link to the International Classification of Functioning, Disability and Health (ICF).
2.3 Behavioral Measures
Implicit Approach Avoidance Task (AAT)
Participants completed a computer-administered Approach Avoidance Task (AAT(Heuer et al., 2007; Taylor and Amir, 2012)) to assess automatic action tendencies to approach or avoid face stimuli portraying different emotional expressions. The face stimuli were taken from the NimStim Set of Facial Expressions(Tottenham et al., 2009) and comprised 8 actors (4 female, 4 male) displaying positive (happy), negative (anger, disgust), and neutral emotional expressions. The AAT was programmed using E-Prime 2.0 Professional (E-Studio version 2.0.8.74, Psychology Software Tools). Consistent with previous research (Najmi et al., 2010; Taylor and Amir, 2012), colored frames surrounding each picture were used to guide participants’ direction of movement. Participants were seated in front of a computer screen with a joystick situated on the desk. They were instructed that for each picture they should pull the joystick if the border was green and push the joystick if the border was blue. Thus, participants were asked to respond only to the color of the border framing each picture, rather than to the content within the image itself.
Participants completed 12 practice trials. Then, participants completed 128 trials [8 Pictures × 4 Picture Type (happy, neutral, angry, disgust) × 2 Border Color (green, blue) × 2 Repetition]. Trials were presented in a new random order to each participant. To begin each trial, participants were required to press a button on the joystick which resulted in the appearance of a medium-sized picture (400 × 400 pixels) in the center of the screen. The computer program logged the position of the joystick and the images were fluidly resized as the joystick was moved from the central position as follows: The pictures became increasingly larger if the participant pulled the joystick, simulating approach, and increasingly smaller if the participant pushed the joystick, simulating avoidance. Moving the joystick to the right or left did not change the size of the picture. When the joystick reached approximately a 30° position in either direction, the picture disappeared, regardless of whether the participant responded correctly. The next trial began once the joystick was brought fully back to the central position and the participant pressed the trigger button. Response latencies were calculated based on the length of time the image remained on the screen, that is, from the time the picture appeared on the screen to the time it disappeared.
Analysis Approach: Relative approach-avoidance index for each stimulus category
Inaccurate responses were removed prior to the AAT bias computation. Consistent with prior research approach-avoidance bias indices (Heuer et al., 2007) for each stimulus category were computed by subtracting each participant’s median response latency to pull pictures of a given target stimulus from their median response latency to push pictures from that same stimulus category (e.g., median response latency to push positively valenced pictures minus median response latency to pull positively valenced pictures). Higher scores reflect relatively greater automatic approach tendencies for the target emotional stimulus.
Modified Probe Detection Task
(Rudaizky et al., 2014) Attentional bias for positive and negative information was measured using a version of the modified probe detection task (MPDT (MacLeod and Mathews, 1988)). This task is based on two complementary hypotheses. First, the notion that anxiety is related to a tendency for attentionally distal negative stimuli to selectively attract initial attention, which would result in speeded processing of probes in the locus of negative rather than neutral valence images and can be interpreted as an engagement bias. Second, the hypothesis that anxiety is related to a tendency for attentionally proximal negative stimuli to selectively hold attention, which would result in speeded processing of probes in the locus of negative rather than neutral representational images presented in the locus of participants’ original attentional focus, and corresponds to a disengagement bias. Following (Rudaizky et al., 2014), emotional stimuli comprised standardized positive, negative, and neutral images taken from the International Affective Picture System (IAPS, (Lang et al., 2008) ). Each emotional stimulus was paired with an abstract image obtained using a Google Image Search using the search criteria “abstract art” (see (Rudaizky et al., 2014) for details). Participants were presented with 384 trials — comprising all combinations of Emotion (positive, negative, neutral) × Trial type (engagement vs. disengagement) × Stimulus duration (500 vs. 1000ms), and were provided with a self-timed rest period. Each image was presented only once, and the order of stimulus presentation was random across participants.
Each trial began with the presentation of two white square outlines, centrally fixated on alternate sides of the screen, which marked the two locations where images would be shown. A smaller red square outline appeared in either of these white squares with equal frequency, which indicated the area of the screen where the cue probe would briefly appear. Participants were instructed to fixate their attention on the red square, as they would be required to remember the orientation of the cue probe (vertical or horizontal). After 1000ms, the cue probe appeared within the red square outline for 200ms. The cue probe was a 5mm red line presented either vertically or horizontally with equal probability. Immediately after the disappearance of the cue probe, an image pair appeared such that each image filled one of the two white square outlines. One image was emotional (i.e., positive, negative or neutral) and the other image was abstract. The emotional image appeared with equal frequency in the location distal to the subject’s initial attentional focus (attentional engagement trials) or in the same location of the subject’s initial attentional focus (attentional disengagement trials). The images remained on the screen for either 500ms or 1000ms with equal probability. The screen was then cleared and a target probe (red line, 5mm) was presented immediately in either one of the two screen locations (with equal probability) previously occupied by the images. The target probe was presented either vertically or horizontally (with equal probability), and participants were required to indicate whether the orientation of the target probe matched that of the cue probe (50% of trials), or mismatched that of the cue probe (50% of trials) by pressing either the left or right mouse button. Response latencies to make this probe discrimination were recorded and used to compute the attentional bias indices described below. Following the subject’s response, the screen cleared for 1000ms, and the next trial began thereafter.
The following measures were obtained to characterize positive and negative valence processing: Engagement bias index = (Cue probe distal to emotional image (positive or negative) in emotional/abstract image pair: RT for target probe distal to emotional image minus RT for target probe proximal to emotional image) minus (Cue probe distal to neutral image in neutral/abstract image pair: RT for target probe distal to neutral image minus RT for target probe proximal to neutral image). A higher score for the engagement bias index indicates greater selective attention towards initially unattended distal images with positive or negative (rather than neutral) valence. Disengagement bias index = (Cue probe proximal to emotional image in emotional/abstract image pair: RT for target probe distal to emotional image minus RT for target probe proximal to emotional image) minus (Cue probe proximal to neutral image in neutral/abstract image pair: RT for target probe distal to neutral image minus RT for target probe proximal to neutral image). A higher score for the disengagement bias index indicates greater sustained attention towards initially attended proximal images with positive or negative (rather than neutral) valence.
2.4 Procedures
Patients presented to the UCSD and UCLA clinics or responded to flyers and completed the screening measures (OASIS and PHQ-9) for eligibility. Individuals who accepted an invitation to take part in the study were consented by a research assistant and underwent a structured diagnostic interview for DSM-5, Mini International Neuropsychiatric Interview (MINI Version 7.0.0.0, used by permission of David Sheehan, MD). Subsequently, participants returned for a behavioral testing session and a neuroimaging testing session. During the behavioral session participants completed the self-report assessments, modified probe detection task, and the implicit approach/avoidance task.
2.5 Data Analysis
All data analyses were carried out using the statistical package R (2010) and all statistical analyses were computed using the Psych package (Revelle, 2015), which extends many of the basic R functions. The analysis strategy consisted of the following steps. First, the characteristics of all measures were examined for deviation from normality prior to subsequent analyses. Then, each measure was examined for differences across site (the results were corrected for multiple testing). For each unit of analysis (self-report, behavior), separate principal components analyses were computed, and a separate analysis was conducted for each behavioral task to minimize task-specific factors in subsequent analysis steps. Next, the number of components for each analysis was determined using the parallel factors technique, which compares the observed eigenvalues of a correlation matrix with those from random data (Revelle, 2015; Revelle and Wilt, 2013). Each component solution was submitted to a varimax rotation to provide a linearly independent description of the symptoms and task components. In supplemental analyses, the varimax rotated component scores were compared to the obliquely rotated component scores; and split-half analyses were conducted to determine stability of the component solution. Component scores were extracted for each participant and used for a meta-component analysis, i.e. a principal components analysis across the different varimax-rotated symptom and task components. Each solution was examined for differences across gender, site, age, and medication status. Finally, to examine the contribution of the components to disability, component coefficients were used in a multiple linear regression analysis with the overall WHODAS 2.0 score as a dependent measure.
3. Results
3.1 Sample Characteristics
Table 1 summarizes the population characteristics and shows that there were no differences between sites for age, education, or gender. After correction for multiple comparisons there was no difference across race. However, individuals at UCLA were more often single and divorced. The majority of the sample was not taking a psychotropic medication.
Table 1.
Sample Characteristics
| UCSD | n=58 | UCLA | n=62 | ||
|---|---|---|---|---|---|
| mean | SD | mean | SD | p | |
| Age (years) | 35.62 | 12.08 | 33.18 | 12.07 | 0.27 |
| Education (years) | 16.07 | 2.86 | 15.5 | 2.83 | 0.276 |
| n | % | n | % | ||
| Gender = Female (%) | 42 | 72.4 | 45 | 72.6 | 1 |
| Race | 0.038 | ||||
| Black/African-American | 1 | 1.7 | 10 | 16.1 | |
| White/Caucasian | 32 | 55.2 | 21 | 33.9 | |
| Asian/Asian-American | 9 | 15.5 | 12 | 19.4 | |
| Native-American/Alaskan Native | 1 | 1.7 | 0 | ||
| More than one race | 7 | 12.1 | 7 | 11.3 | |
| Other | 8 | 13.8 | 12 | 19.4 | |
| Relation Status | 0.001 | ||||
| Single | 18 | 31.6 | 34 | 54.8 | |
| Married | 17 | 29.8 | 4 | 6.5 | |
| In a committed romantic relationship | 14 | 24.6 | 11 | 17.7 | |
| Divorced/separated | 4 | 7 | 13 | 21 | |
| Widowed | 2 | 3.5 | 0 | 0 | |
| Other | 2 | 3.5 | 0 | 0 | |
| Medication Status | Med.Status | (%) | 0.042 | ||
| Unknown | 0 | 0 | 1 | 1.7 | |
| Unmedicated | 39 | 67.2 | 51 | 86.4 | |
| Benzodiazepine | 5 | 8.6 | 0 | 0 | |
| Serotonin-Selective Reuptake Inhibitor | 8 | 13.8 | 4 | 6.8 | |
| Other | 6 | 10.3 | 3 | 5.1 |
Table 2 summarizes the symptom characteristics of the sample for the UCSD and the UCLA site. After correction for multiple comparisons there were no significant differences across the samples. As expected, the sample showed significant symptoms of anxiety and depression.
3.2 Latent Variable Analysis – Symptoms
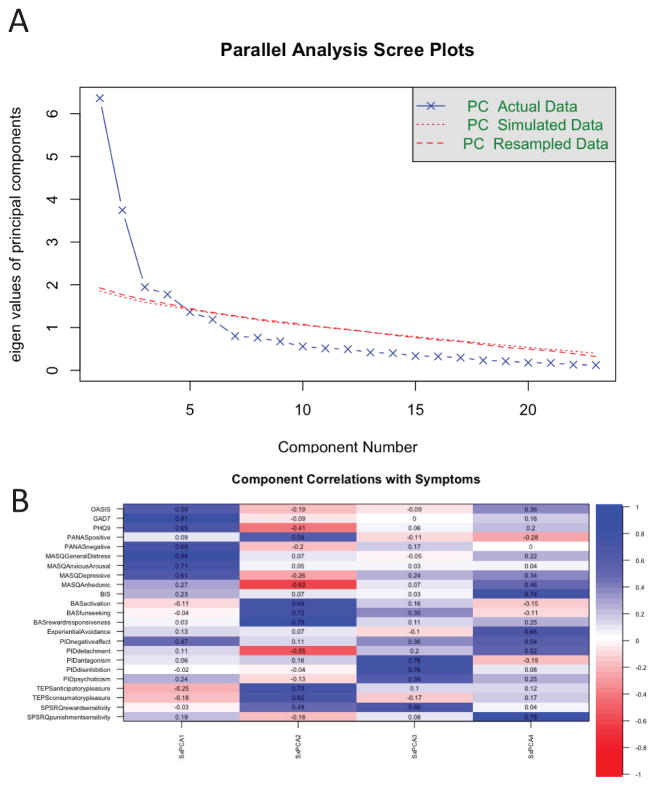
Figure 1 summarizes the principal components analysis of the symptom data of the UCSD and UCLA sample. Specifically, Figure 1B shows a heat map (blue indicating positive correlations, red showing negative correlations) of the correlation matrix between the varimax rotated components and the symptom measures. The parallel analysis (for details see (Revelle, 2015)) revealed that four components optimally describe the underlying correlational structure. Supplemental analyses showed that the varimax-rotated components were highly correlated to the obliquely rotated components and that the components showed good split-half reliability (see supplement). The principal components cumulatively accounted for 60% of the variance, and the Chi-square test (362.74, p < 1.5 × 10−16) on the residuals (0.07) of the correlation matrix showed that 4 components are sufficient. The components were ranked based on the relative variance accounted for after varimax-rotation. Component 1 loaded significantly on the OASIS, the PHQ-9, PANAS negative affect, MASQ “General Distress” and other negative valence scales indicating that this component represented “negative valence processing”. Component 2 included the BAS variables “reward responsiveness, activation, fun-seeking”, the PANAS positive affect measure, as well as the TEPS variables “anticipatory pleasure, consummatory pleasure”. Thus, taken together, this component comprised “positive valence processing”. Component 3 loaded on PID “antagonism, disinhibition, and psychoticism” as well as on SPSR measure of reward sensitivity and could be described as quantifying “antagonistic or irritable disinhibition”. Lastly, component 4 loaded on behavioral inhibition (BIS), “experiential avoidance” (AsSEAS), PID “avoidant, detachment” and on SPSRQ’s measure of punishment sensitivity and could thus be most appropriately described as quantifying “avoidant detachment”.
Figure 1.
Latent Variable Analysis of Symptoms: (a) scree plot of principal component analysis for symptom data; (b) correlation matrix of principal components with symptoms.
Table 3 summarizes the contribution of different socio-demographic variables on the component solution as well as the contribution of the components on a measure of disability. There were no significant effects of age, gender, education or site on components 1–2. Females exhibited significantly lower levels of “antagonistic disinhibition”, which also declined with age. There was a significant effect of age decreasing “avoidant detachment”. In combination, all components, with the possible exception of the “antagonistic disinhibition” component contributed significantly to self-rated levels of disability. Not surprisingly, higher levels of positive valence processing were associated with lower levels of disability.
Table 3.
Symptom component characteristics (*** = p < 0.001, ** = p < 0.01, * = p < 0.05, . = p <0.1)
| Coefficients: | B | SEM | t value | Pr(>|t|) | |
|---|---|---|---|---|---|
| Component 1 | (Intercept) | 0.27 | 1.23 | 0.22 | 0.83 |
| F(7,109) = 0.4887, p = 0.841 | Gender | −0.01 | 0.22 | −0.05 | 0.96 |
| Adj R^2 = 0.031 | Site (1=UCSD,2=UCLA) | −0.19 | 0.20 | −0.95 | 0.35 |
| Negative Valence Appraisal | Age | 0.00 | 0.01 | −0.19 | 0.85 |
| No Medication | 0.14 | 1.03 | 0.13 | 0.90 | |
| Benzodiazepine | −0.07 | 1.14 | −0.06 | 0.95 | |
| SSRI | 0.12 | 1.07 | 0.11 | 0.91 | |
| Other | −0.46 | 1.09 | −0.43 | 0.67 | |
| Component 2 | (Intercept) | −0.04 | 1.18 | −0.04 | 0.97 |
| F(7,109) =1.735, p = 0.1083 | Gender | 0.02 | 0.21 | 0.09 | 0.93 |
| Adj R^2 = 0.04245 | Site (1=UCSD,2=UCLA) | −0.56 | 0.19 | −2.96 | 0.00 |
| Positive Valence Appraisal | Age | −0.01 | 0.01 | −0.88 | 0.38 |
| No Medication | 1.13 | 0.99 | 1.14 | 0.26 | |
| Benzodiazepine | 0.81 | 1.09 | 0.74 | 0.46 | |
| SSRI | 1.12 | 1.03 | 1.08 | 0.28 | |
| Other | 0.71 | 1.04 | 0.68 | 0.50 | |
| Component 3 | (Intercept) | 2.76 | 1.11 | 2.49 | 0.01* |
| F(7,109) = 3.465, p = 0.002176 | Gender | −0.70 | 0.19 | −3.58 | 0.00*** |
| Adj R^2 = 0.1295 | Site (1=UCSD,2=UCLA) | −0.36 | 0.18 | −2.01 | 0.05* |
| Antagonistic Disinhibition | Age | −0.02 | 0.01 | −3.06 | 0.00** |
| No Medication | −0.26 | 0.93 | −0.28 | 0.78 | |
| Benzodiazepine | −0.66 | 1.03 | −0.64 | 0.52 | |
| SSRI | −0.17 | 0.97 | −0.18 | 0.86 | |
| Other | −0.46 | 0.98 | −0.47 | 0.64 | |
| Component 4 | (Intercept) | 0.33 | 1.13 | 0.29 | 0.77 |
| F(4,115) = 5.125, p = 0.0007769 | Gender | 0.35 | 0.20 | 1.77 | 0.08. |
| Adj R^2 = 0.1218 | Site (1=UCSD,2=UCLA) | 0.32 | 0.18 | 1.76 | 0.08. |
| Avoidant Detachment | Age | −0.03 | 0.01 | −3.39 | 0.00*** |
| No Medication | −0.63 | 0.95 | −0.66 | 0.51 | |
| Benzodiazepine | −0.29 | 1.05 | −0.27 | 0.78 | |
| SSRI | −0.19 | 0.99 | −0.19 | 0.85 | |
| Other | −0.40 | 1.00 | −0.40 | 0.69 | |
| WHODAS 2.0 | (Intercept) | 22.68 | 0.52 | 43.98 | < 2e-16*** |
| F(5,114) = 20.52, p <8.806e-13 | Negative Valence Appraisal | 2.95 | 0.52 | 5.70 | 0.00*** |
| Adj R^2 =0.3962 | Positive Valence Appraisal | −2.74 | 0.52 | −5.29 | 0.00*** |
| Antagonistic Disinhibition | 1.41 | 0.52 | 2.72 | 0.01** | |
| Avoidant Detachment | 1.95 | 0.52 | 3.77 | 0.00*** |
3.3 Behavioral Measures
Table 4 summarizes the main dependent measures for the Implicit Approach Avoidance Task and the modified Dot Probe Task across the UCSD and UCLA site. In particular, we opted to utilize a relative measurement approach estimating the strength of positive, neutral, disgust, and anger approach-avoidance bias. In comparison, the modified Probe Detection Task measures engagement/disengagement of attentional allocation from positive or negatively valenced stimuli for short (500 ms) and long (1000 ms) picture presentations, which purportedly engage anxiety and mood-related processes. There were no significant site differences.
Table 4a.
Behavioral Task Measures and Relationships
| Task | Factor 1 | Factor 2 | UCSD | UCLA | ||||
|---|---|---|---|---|---|---|---|---|
| n | mean | sd | n | mean | sd | |||
| IAAT | Avoid - Approach | Positive | 54 | 4.03 | 39.34 | 43 | 36.73 | 72.51 |
| Neutral | 54 | 21.58 | 37.44 | 44 | 14.86 | 72.08 | ||
| Disgust | 54 | 5.31 | 49.69 | 44 | 15.76 | 62.35 | ||
| Anger | 54 | 6.54 | 54.56 | 44 | 17.22 | 68.22 | ||
| mDPT | Engagement (500 ms) | Negative | 55 | −9.15 | 102.86 | 54 | −9.04 | 167.71 |
| Positive | 55 | −21.24 | 114.29 | 54 | −21.49 | 164.35 | ||
| Engagement (1000 ms) | Negative | 55 | −15.63 | 95.95 | 54 | −18.82 | 175.03 | |
| Positive | 55 | 13.67 | 111.5 | 54 | −26.63 | 148.86 | ||
| Disengagement (500 ms) | Negative | 55 | 51.83 | 140.15 | 54 | 14.14 | 226.5 | |
| Positive | 55 | 13.07 | 96.11 | 54 | −20.04 | 182.16 | ||
| Disengagement (1000 ms) | Negative | 55 | 6.59 | 102.85 | 54 | 0.68 | 210.15 | |
| Positive | 55 | −5.29 | 100.39 | 54 | 43.9 | 195.73 | ||
3.2 Latent Variable Analysis – Behavioral Measures
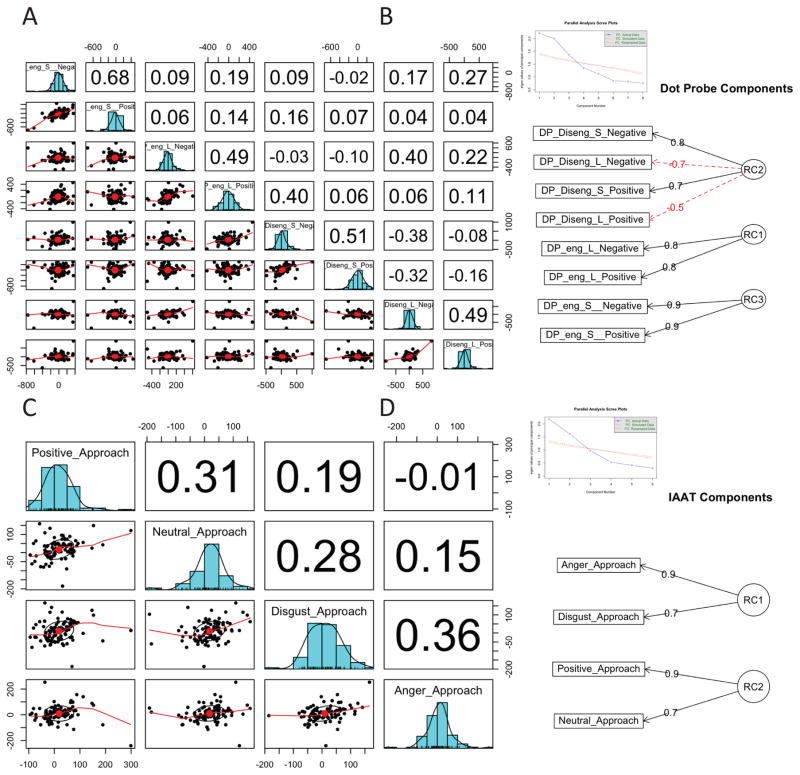
Figure 2 summarizes the dimension reduction results for the modified Probe Detection Task (Figure 2A and B) and the implicit Approach Avoidance Task (Figure 2C and D). The principal components analysis for the modified Probe Detection Task extracted 3 components explaining 70% of the variance, and the Chi-square test (72.11p < 5.5 × 10−13) on the residuals (0.1) of the correlation matrix showed that 3 components are sufficient. In order of the amount of variance explained (see component diagram in Figure 2b), rotated component 1 loaded primarily on engagement biases for long presentations of positive or negatively valenced images, whereas rotated component 3 loaded on engagement biases for short duration images. Therefore, higher scores on these components reflect greater selective attention towards initially unattended distal images with positive or negative valence for short and long duration presentations. Component 2 showed a bivalent component solution for disengagement biases regardless of emotional valence. In particular, a higher score on this component indicates greater sustained attention towards initially attended proximal images with positive or negative valence for short duration presentations but reduced sustained attention for long duration presentations. Table 4B shows that these rotated components were not influenced by age, gender, site or medication status. A principal component solution for the implicit Approach Avoidance Task with 2 components was suggested by the parallel analysis and explained 69% of the variance, and a Chi-square test (48.71 p < 8.1 × 10−10) on the residuals (0.18) of the correlation matrix showed that 2 components are sufficient. The first rotated component comprised loaded on anger approach and disgust approach, the second rotated component involved positive and neutral approach. Thus, these components revealed separate and independent loadings on positive versus negative valence approach biases. Table 4B shows that these rotated components were not influenced by age, gender, site or medication status.
Figure 2.
Latent Variable Analysis of Behavior: (a) distribution and correlation matrix of behavioral variables for the Dot Probe Task; (b) Dot Probe Task components; (c) distribution and correlation matrix of behavioral variables for the Approach Avoidance Task; (d) Approach Avoidance Task components.
Table 4b.
Behavioral Task component characteristics
| mDPT | Coefficients: | B | SEM | t value | p |
|---|---|---|---|---|---|
| Component 1 | (Intercept) | 0.70 | 1.18 | 0.59 | 0.56 |
| F(7,98) = 1.348, p = 0.2362 | Gender | 0.22 | 0.22 | 1.02 | 0.31 |
| Adj R^2 = 0.02268 | Site (1=UCSD,2=UCLA) | −0.27 | 0.20 | −1.36 | 0.18 |
| Heightened sustained attention to proximal images (fast) | Age | 0.01 | 0.01 | 1.16 | 0.25 |
| No Medication | −0.88 | 0.98 | −0.89 | 0.37 | |
| Benzodiazepine | −1.28 | 1.10 | −1.16 | 0.25 | |
| SSRI | −1.55 | 1.02 | −1.52 | 0.13 | |
| Other | −0.66 | 1.03 | −0.64 | 0.53 | |
| Component 2 | (Intercept) | 0.46 | 1.19 | 0.39 | 0.70 |
| F(7,98) = 1.543, p = 0.1618 | Gender | 0.26 | 0.22 | 1.19 | 0.24 |
| Adj R^2 = 0.03494 | Site (1=UCSD,2=UCLA) | −0.19 | 0.20 | −0.96 | 0.34 |
| Heightened selective attention to initially un-attended distal images (slow) | Age | −0.02 | 0.01 | −2.44 | 0.02 |
| No Medication | −0.05 | 0.98 | −0.05 | 0.96 | |
| Benzodiazepine | 0.30 | 1.11 | 0.28 | 0.78 | |
| SSRI | 0.21 | 1.02 | 0.20 | 0.84 | |
| Other | −0.05 | 1.03 | −0.05 | 0.96 | |
| Component 3 | (Intercept) | 1.30 | 1.04 | 1.26 | 0.21 |
| F(7,98) = 1.073, p = 0.3866 | Gender | −0.27 | 0.19 | −1.40 | 0.17 |
| Adj R^2 = 0.004837 | Site (1=UCSD,2=UCLA) | 0.06 | 0.17 | 0.34 | 0.74 |
| Heightened selective attention to initially un-attended distal images (fast) | Age | 0.00 | 0.01 | −0.48 | 0.63 |
| No Medication | −0.70 | 0.86 | −0.81 | 0.42 | |
| Benzodiazepine | −0.48 | 0.96 | −0.50 | 0.62 | |
| SSRI | −0.64 | 0.89 | −0.72 | 0.48 | |
| IAAT | |||||
| Component 1 | (Intercept) | 0.12 | 1.26 | 0.10 | 0.92 |
| F(7,87) = 1.209 p = 0.3064 | Gender | −0.38 | 0.24 | −1.60 | 0.11 |
| Adj R^2 = 0.01534 | Site (1=UCSD,2=UCLA) | 0.00 | 0.22 | −0.01 | 0.99 |
| Negative Valence Avoidance - Approach | Age | −0.01 | 0.01 | −0.88 | 0.38 |
| No Medication | 0.94 | 1.04 | 0.91 | 0.37 | |
| Benzodiazepine | 0.20 | 1.15 | 0.17 | 0.86 | |
| SSRI | 0.69 | 1.08 | 0.64 | 0.53 | |
| Other | 0.54 | 1.10 | 0.50 | 0.62 | |
| Component 2 | (Intercept) | −2.49 | 1.20 | −2.07 | 0.04 |
| F(7,87) = 1.671 p = 0.1266 | Gender | 0.04 | 0.23 | 0.19 | 0.85 |
| Adj R^2 = 0.04761 | Site (1=UCSD,2=UCLA) | 0.39 | 0.21 | 1.85 | 0.07 |
| Positive Valence Avoidance - Approach | Age | −0.01 | 0.01 | −1.10 | 0.27 |
| No Medication | 2.30 | 0.99 | 2.32 | 0.02 | |
| Benzodiazepine | 2.14 | 1.09 | 1.96 | 0.05 | |
| SSRI | 1.91 | 1.03 | 1.86 | 0.07 | |
| Other | 2.24 | 1.05 | 2.14 | 0.04 | |
| Other | −1.32 | 0.90 | −1.46 | 0.15 | |
3.3 Latent Variable Analysis – Symptom and Behavioral Cross-level Analysis
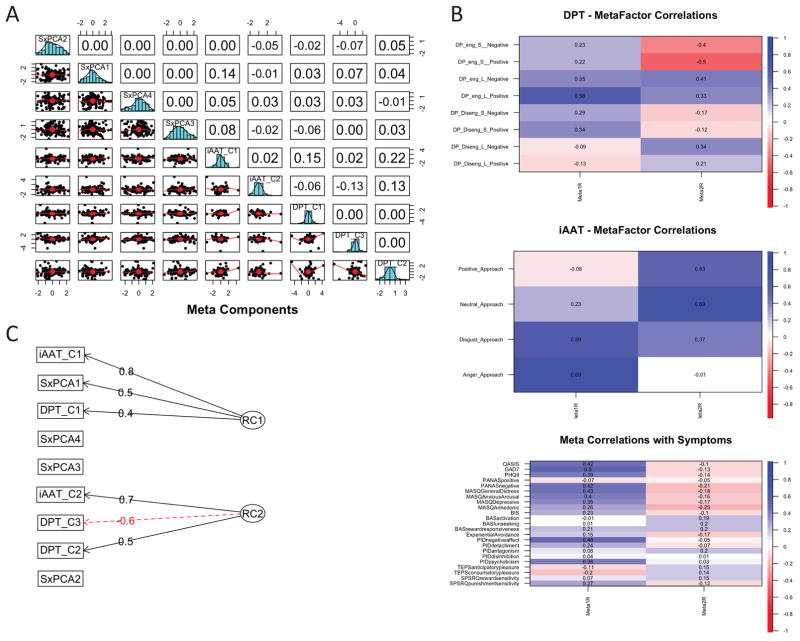
Figure 3 summarizes the meta-component analysis, which included the symptom components, the components extracted from the Implicit Approach Avoidance Task, and the components from the modified Dot Probe task. A schematic of the component diagram is shown in Figure 3C, the correlational pattern of the extracted and rotated meta-components with the dot probe task, the implicit approach avoidance task and the symptom scales are shown in Figure 3B. The principal components analyses retrieved 2 components explaining 28% of the variance, with a Chi-square test (136.92 p < 7.1 × 10−20) on the residuals (0.12) of the correlation matrix showing that 2 components are sufficient. The first rotated meta-component loaded on (1) negative valence symptom scales, (2) disgust and anger approach biases, (3) engagement biases for long presentations of positive valenced images. Thus, this component quantifies negative valence symptoms, negative approach bias, and high sustained, selective attention. The second rotated meta-component loaded on (1) positively valence symptom scales, however relatively weakly, (2) positive and neutral approach biases, (3) both engagement and disengagement biases for long presentations of positive and negatively valenced images. Therefore, this component quantifies positive valence symptoms, positive approach bias, and slow selective or sustained attention.
Figure 3.
Meta Component Analysis – Symptom and Behavioral Level of Analysis: (a) distribution and correlation matrix of symptoms and behavioral components; (b) correlation matrix of meta components with Dot Probe Task, Approach Avoidance Task, and Symptoms; (c) Dot Probe Task components.
Several regression analyses were conducted to examine the effect of age, gender, site and medication status on these meta-components. As shown in Table 5, after corrections for multiple comparisons there was only a minor effect of medication status on the second meta-component. Of all meta-components, only meta-component 1 significantly contributed to the overall levels of disability, i.e. increased levels of engaged negative valence processing was associated with higher self-rated disability. Finally, there was no effect of gender, site, or age on these meta-components (Table 5).
Table 5.
Meta-component characteristics (*** = p < 0.001, ** = p < 0.01, * = p < 0.05, . = p <0.1)
| Coefficients: | B | SEM | t value | Pr(>|t|) | |
|---|---|---|---|---|---|
| Component 1 | (Intercept) | 1.06 | 1.21 | 0.88 | 0.38 |
| F(7,81) = 0.7156, p = 0.6589 | Gender | −0.23 | 0.24 | −0.97 | 0.34 |
| Adj R^2 = 0.02314 | Site (1=UCSD,2=UCLA) | −0.13 | 0.22 | −0.59 | 0.56 |
| Negative valence symptoms, approach bias, high sustained, selective attention | Age | −0.01 | 0.01 | −1.20 | 0.24 |
| No Medication | −0.03 | 0.99 | −0.03 | 0.98 | |
| Benzodiazepine | −0.62 | 1.12 | −0.55 | 0.58 | |
| SSRI | −0.15 | 1.03 | −0.15 | 0.88 | |
| Other | −0.43 | 1.05 | −0.41 | 0.69 | |
| Component 2 | (Intercept) | −2.00 | 1.10 | −1.82 | 0.07. |
| F(7,81) = 0.7156, p = 0.1604 | Gender | 0.09 | 0.21 | 0.44 | 0.67 |
| Adj R^2 = 0.04238 | Site (1=UCSD,2=UCLA) | 0.15 | 0.20 | 0.76 | 0.45 |
| Positive Valence symptoms, approach bias, slow selective, sustained attention | Age | −0.02 | 0.01 | −1.93 | 0.06. |
| No Medication | 2.16 | 0.90 | 2.40 | 0.02* | |
| Benzodiazepine | 2.07 | 1.01 | 2.04 | 0.04* | |
| SSRI | 1.96 | 0.94 | 2.09 | 0.04* | |
| Other | 2.31 | 0.96 | 2.42 | 0.02* | |
| WHODAS 2.0 | (Intercept) | 22.21 | 0.71 | 31.47 | < 2e-16*** |
| F(2,88) = 7.80, p <0.0007591 | Meta-Component 1 | 2.82 | 0.74 | 3.80 | 0.00*** |
| Adj R^2 =0.1313 | Meta-Component 2 | −0.90 | 0.80 | −1.14 | 0.25 |
4. Discussion
Using a mostly primary care clinical population with anxiety or depressive symptoms, selected based on an RDoC approach (i.e. without a threshold of a DSM-5 diagnosis), we aimed to derive latent variables using a principal components analysis approach across two levels of analysis (self-report and behavior) focused on the positive and negative valence constructs as measured by multiple scales and two different behavioral paradigms that assess attentional as well as approach/avoidance biases. There are three main results of this investigation. First, we were able to extract four stable symptom-related components that were comprised of positive valence processing, negative valence processing, avoidant detachment, and antagonistic disinhibition. Second, the combination of symptom and behavioral components yielded two meta-components, which encompass negative valence processing and positive valence processing, respectively. Third, the symptom components more than the behavioral and meta-components were related to self-rated levels of disability. However, none of the components were significantly influenced by socio-demographic variables. Taken together, this investigation provides preliminary evidence that positive and negative valence construct processing are relatively independent on self-report and behavioral levels in a clinical population.
The symptom approach in this analysis was based on an exploratory statistical analysis using primarily a principal components analysis in a clinical sample of individuals with mood and anxiety-related problems. These symptom results are similar to those that have been reported previously. For example, a 3-factor model separating latent dimensions of deficient positive affect, negative affect, and disinhibition was observed in a clinical and non-clinical sample (Ameringer et al., 2015). Clark and Watson (Clark and Watson, 1991) developed a tripartite model of depression and anxiety symptoms such that some symptoms are indicators of general distress and can be considered a negative affect factor. Two other factors represent symptoms that cover unique and distinguishing aspects of anxiety or depression such as somatic hyperarousal or anhedonia, respectively. However, this model was modified (Watson, 2009) into a quadripartite model based on (1) level of specificity and (2) magnitude of the general distress, which combines the tripartite and integrative hierarchical models (Mineka et al., 1998) of symptom structure.
The current symptom components are only partially consistent with the quadripartite model, which may be due to the fact that the sample size was limited. However, correlational patterns across symptom scales were similar across sites and not significantly affected by socio-demographic characteristics, which is consistent with other studies showing that latent variables were relatively invariant across sociodemographic covariates (Blanco et al., 2014). It is important to highlight that the components clearly delineated independent positive and negative valence processing components, which implies that these domains are not opposite ends of the same spectrum but are relatively independent dimensions. This finding may have important implications for both assessment and treatment of individuals with mood and anxiety disorders. Specifically, it suggests that some individuals with mood and anxiety disorders may have relatively intact positive valence processing whereas others may have highly impaired positive valence processing. Moreover, these findings lend support to the notion that positive valence processing in mood and anxiety disorder is an independent contributor to the psychopathology with a distinct genetic (Hess et al., 2015) and neural circuit (Liu et al., 2011) signature. Finally, it will be important to determine whether treatments that are specifically aimed at improving positive valence processing (Coplan et al., 2015) may work best for those individuals who have deficits in positive valence processing or that individuals with positive valence dysfunction are most in need of PVS-targeted interventions. If confirmed, such a finding could contribute to a precision medicine approach to treatment (Zbozinek and Craske, 2016).
The behavioral components highlight the engagement and disengagement biases from affectively valenced information and emphasize the temporal domain of positive/negative valence processing, which is rarely appreciated in self-ratings and may have profound implication for the instantiation of these processes on a neural systems and molecular level. Specifically, excessive engagement with negatively valenced information (Mathews and MacLeod, 2005) or impaired attentional disengagement (Rudaizky et al., 2014) are central for the pathology of mood and anxiety disorders. However, these biases are fundamentally rooted in changes in temporal processing of affectively valenced information. Interestingly, there is evidence for altered neural dynamics of emotional processing in individuals with mood and anxiety disorders, which may be predictive of treatment outcomes (Heller et al., 2015; Heller et al., 2013). More advanced approaches, which aim to characterize causal relations between the underlying network dynamics and the constructs such as symptoms of depression, may help to better characterize the network dynamics (Bielczyk et al., 2015). Altered temporal processing in these individuals will require that one develops more adaptive neural processing models for neuroimaging analyses to adequately capture the neural circuit-based individual differences, which may also be relevant for the molecular level of analysis.
Whereas symptom components were strongly related to self-rated disability, performance on behavioral tasks was relatively weakly related to disability. This finding could be a consequence of shared method variance, wherein assessments within a unit of analysis are more strongly related to one another than across units of analysis. Specifically, both symptoms and disability are measured via the method of self-report and are retrospective in nature, whereas behavioral measures capture the performance of the individual at time of testing. A similar relationship was also observed for the meta-components, which showed that behavioral components and symptom components tended to be only weakly correlated. This finding fundamentally supports the need for a multi-level, dimensional psychopathology approach as suggested by the RDoC initiative. Specifically, the self-report level of analysis may adequately quantify an individual’s self-appraisal of his or her “belief” state of attitudes, symptoms, and propensities to act. However, the appraisal function itself is likely affected by the mental health condition or the individual may simply not be able to adequately assess a particular behavioral process. As an example, self-report of behavioral activity correlates poorly with objectively monitored activity using remote sensors (Prince et al., 2008). On the other hand, mental disorders conceptualized as brain systems dysfunctions may profoundly affect behavioral processes as a consequence of neural circuit abnormalities, as well as appraisals. It will be important for future studies to investigate the degree of correlation of level-based components across levels of analyses. One possibility is to apply network analysis examining the dynamic of changes across units of analyses (van de Leemput et al., 2014). Others have suggested that a distinction between fast defense-behavior processing and slow deliberate and effortful anxiety-related processing may help to differentiate fear-related circuits (LeDoux and Pine, 2016).
This study has several limitations that need to be considered to evaluate findings. First, the size of the sample is relatively small. Correlational techniques such as principal components analyses are sensitive to outliers and these outliers can have more profound effects in small samples. We have conducted reliability analyses (see supplement), which show that although symptom and task principal components analyses are moderately to highly reliable and stable, the meta-components are only weakly stable, i.e., additional components may be necessary to explain the full variance in the data set. Therefore, larger sample sizes will be required to reliably examine the multi-level latent variable structure. The goal for this research project is to double the sample size and to use a confirmatory approach to assess the stability of the components. Second, we focused here on principal components analysis, which is the ideal tool to extract the underlying dimensional structure when attempting to account for the entire variance of the sample. However, there are other techniques that might be useful in the future such as multi-dimensional scaling (Cox and Cox, 2001), clustering approaches (Revelle, 1979) or advanced machine learning tools including unsupervised learning algorithms (Hastie et al., 2001) that would parse the sample differently. Third, this study relied solely on a cross-sectional assessment and thus cannot address the temporal dynamics of these components, i.e., how the symptom and behavioral components change over time. Moreover, it will be important to assess test/re-test reliability to estimate the degree to which within-subject variance is conserved over time, which will be important for planning interventions that aim to modify the components. Fourth, the current analysis strategy was exclusively exploratory, i.e. we did not pose a-priori hypotheses about the underlying multi-level structure of the data. Other latent variable approaches such as linear latent variable models implemented in the lava-package (Holst and Bustz-Joergensen, 2012) as part of the R (2012), may provide a very general modeling framework, which includes structural equation models and mixed models as important special cases. This model class also allows for non-linear effects of covariates and non-linear parameter constraints. Thus, future investigations with larger data sets will be focused on examining specific hypotheses that are derived from this exploratory analysis. Fifth, this analysis did not include neural circuit data, which may further enhance our understanding of the implementation of positive and negative valence processing in the brain. Given the limited number of cases, we will focus on supplementing this analysis approach in future studies and include reward-related processing and fear-learning neural pathways. Sixth, preliminary analyses of the reliability of the reaction time differences yielded low to modest correlations between odd and even trials, which is consistent with a general concern about the reliability of reaction time based measures (see e.g (Kappenman et al., 2014)). These findings suggest that alternative approaches may be necessary to yield measures that more reliably reflect response tendencies. These initial results are part of an ongoing study, which aims to build a latent variable model for positive and negative domain dysfunctions among individuals with mood and anxiety disorders. Thus, we expect to extend our analyses to other levels in future reports.
5. Conclusions
This investigation is the first in a series of studies aimed at delineating the differences between positive and negative valence processing in participants with mood and anxiety disorders. These preliminary data clearly show that these processing domains load on independent dimensions and – thus – are likely to be implemented and modulated by different neural systems. This finding has important consequences for the conceptualization of dimensional psychopathology dysfunction among individuals with mood and anxiety disorders. In particular, modulating positive valence processing may not lead to improvement of negative valence processing and vice versa, and influencing one system or the other may have differential impact on functioning. One may begin to parse sub-populations with mood and anxiety disorders according to positive and/or negative valence processing dysfunctions to select for specific treatments, which is consistent with recent findings that positive valence processing predicts exposure-based treatment success (Dour et al., 2016; Zbozinek and Craske, 2016) and the potential for treatments that target positive valence are most effective for individuals with deficits in positive affect (Craske, Meuret, Ritz, Treanor & Craske, in press). Taken together, multilevel assessment approaches together with sophisticated statistical analyses may help to discern new syndromes that cut across traditional diagnostic categories.
Supplementary Material
Highlights.
Positive and negative valence domain is assessed using two units of analysis (self-report and behavior).
Positive and negative valence domain load on independent components.
Behavioral assessments highlight the importance of the temporal domain of affect processing in individuals with mood and anxiety disorders.
Transdiagnostic dysfunctions of positive and negative valence processing open the possibility for process-specific interventions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: [Google Scholar]
- 2.Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: Vienna, Austria: [Google Scholar]
- 3.Al-Dajani N, Gralnick TM, Bagby RM. A Psychometric Review of the Personality Inventory for DSM-5 (PID-5): Current Status and Future Directions. J Pers Assess. 2016;98:62–81. doi: 10.1080/00223891.2015.1107572. [DOI] [PubMed] [Google Scholar]
- 4.Alden LE, Taylor CT, Mellings TM, Laposa JM. Social anxiety and the interpretation of positive social events. Journal of anxiety disorders. 2008;22:577–590. doi: 10.1016/j.janxdis.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Ameringer KJ, Chou CP, Sussman S, Unger JB, Leventhal AM. Identifying Shared Latent Dimensions of Psychological Symptoms: Implications for the Psychological Correlates of Smoking. J Psychopathol Behav Assess. 2015;37:454–468. doi: 10.1007/s10862-014-9467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.APA, A.P.A. Diagnostic and statistical manual of mental disorders. 5. The American Psychiatric Association; Washington: 2013. [Google Scholar]
- 7.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IMH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Bartoszek G, Winer ES. Spider-fearful individuals hesitantly approach threat, whereas depressed individuals do not persistently approach reward. Journal of behavior therapy and experimental psychiatry. 2015;46:1–7. doi: 10.1016/j.jbtep.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Baxter AJ, Vos T, Scott KM, Ferrari AJ, Whiteford HA. The global burden of anxiety disorders in 2010. Psychol Med. 2014:1–12. doi: 10.1017/S0033291713003243. [DOI] [PubMed] [Google Scholar]
- 10.Bielczyk NZ, Buitelaar JK, Glennon JC, Tiesinga PH. Circuit to construct mapping: a mathematical tool for assisting the diagnosis and treatment in major depressive disorder. Frontiers in psychiatry. 2015;6:29. doi: 10.3389/fpsyt.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanco C, Rubio JM, Wall M, Secades-Villa R, Beesdo-Baum K, Wang S. The latent structure and comorbidity patterns of generalized anxiety disorder and major depressive disorder: a national study. Depress Anxiety. 2014;31:214–222. doi: 10.1002/da.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown TA, Chorpita BF, Barlow DH. Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. J Abnorm Psychol. 1998;107:179–192. doi: 10.1037//0021-843x.107.2.179. [DOI] [PubMed] [Google Scholar]
- 13.Campbell DW, Sareen J, Stein MB, Kravetsky LB, Paulus MP, Hassard ST, Reiss JP. Happy but not so approachable: the social judgments of individuals with generalized social phobia. Depress Anxiety. 2009;26:419–424. doi: 10.1002/da.20474. [DOI] [PubMed] [Google Scholar]
- 14.Campbell-Sills L, Norman SB, Craske MG, Sullivan G, Lang AJ, Chavira DA, Bystritsky A, Sherbourne C, Roy-Byrne P, Stein MB. Validation of a brief measure of anxiety-related severity and impairment: the Overall Anxiety Severity and Impairment Scale (OASIS) J Affect Disord. 2009;112:92–101. doi: 10.1016/j.jad.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of personality and social psychology. 1994;67:319–333. [Google Scholar]
- 16.Chen C, Takahashi T, Nakagawa S, Inoue T, Kusumi I. Reinforcement learning in depression: A review of computational research. Neurosci Biobehav Rev. 2015;55:247–267. doi: 10.1016/j.neubiorev.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Chorpita BF. The tripartite model and dimensions of anxiety and depression: an examination of structure in a large school sample. J Abnorm Child Psychol. 2002;30:177–190. doi: 10.1023/a:1014709417132. [DOI] [PubMed] [Google Scholar]
- 18.Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- 19.Coplan JD, Aaronson CJ, Panthangi V, Kim Y. Treating comorbid anxiety and depression: Psychosocial and pharmacological approaches. World journal of psychiatry. 2015;5:366–378. doi: 10.5498/wjp.v5.i4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox TF, Cox MAA. Multidimensional scaling. Chapman & Hall/CRC; Boca Raton: 2001. [Google Scholar]
- 21.Craske MG, Rauch SL, Ursano R, Prenoveau J, Pine DS, Zinbarg RE. What is an anxiety disorder? Depress Anxiety. 2009;26:1066–1085. doi: 10.1002/da.20633. [DOI] [PubMed] [Google Scholar]
- 22.Craske MG, Stein MB, Sullivan G, Sherbourne C, Bystritsky A, Rose RD, Lang AJ, Welch S, Campbell-Sills L, Golinelli D, Roy-Byrne P. Disorder-specific impact of coordinated anxiety learning and management treatment for anxiety disorders in primary care. Arch Gen Psychiatry. 2011;68:378–388. doi: 10.1001/archgenpsychiatry.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. The British journal of clinical psychology/the British Psychological Society. 2004;43:245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- 24.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day CV, Gatt JM, Etkin A, DeBattista C, Schatzberg AF, Williams LM. Cognitive and emotional biomarkers of melancholic depression: An iSPOT-D report. J Affect Disord. 2015;176C:141–150. doi: 10.1016/j.jad.2015.01.061. [DOI] [PubMed] [Google Scholar]
- 26.Delgado MR, Miller MM, Inati S, Phelps EA. An fMRI study of reward-related probability learning. Neuroimage. 2005;24:862–873. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Derntl B, Seidel EM, Eickhoff SB, Kellermann T, Gur RC, Schneider F, Habel U. Neural correlates of social approach and withdrawal in patients with major depression. Social neuroscience. 2011;6:482–501. doi: 10.1080/17470919.2011.579800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dour HJ, Brown LA, Craske MG. Positive valence reduces susceptibility to return of fear and enhances approach behavior. J Behav Ther Exp Psychiatry. 2016;50:277–282. doi: 10.1016/j.jbtep.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 29.El Khoury-Malhame M, Reynaud E, Soriano A, Michael K, Salgado-Pineda P, Zendjidjian X, Gellato C, Eric F, Lefebvre MN, Rouby F, Samuelian JC, Anton JL, Blin O, Khalfa S. Amygdala activity correlates with attentional bias in PTSD. Neuropsychologia. 2011;49:1969–1973. doi: 10.1016/j.neuropsychologia.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Fleurkens P, Rinck M, van Minnen A. Implicit and explicit avoidance in sexual trauma victims suffering from posttraumatic stress disorder: a pilot study. European journal of psychotraumatology. 2014:5. doi: 10.3402/ejpt.v5.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gable PA, Harmon-Jones E. Late positive potential to appetitive stimuli and local attentional bias. Emotion. 2010;10:441–446. doi: 10.1037/a0018425. [DOI] [PubMed] [Google Scholar]
- 32.Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Research in Personality. 2006;40:1086–1102. [Google Scholar]
- 33.Hastie T, Tibshirani R, Friedman JH. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. Springer; New York: 2001. [Google Scholar]
- 34.Health, N.I.o.M. Negative Valence Systems: Workshop Proceedings. NIMH; Rockville, MD: 2011a. [Google Scholar]
- 35.Health, N.I.o.M. Positive Valence Systems: Workshop Proceedings. NIMH; Rockville, MD: 2011b. [Google Scholar]
- 36.Heller AS, Fox AS, Wing EK, McQuisition KM, Vack NJ, Davidson RJ. The Neurodynamics of Affect in the Laboratory Predicts Persistence of Real-World Emotional Responses. J Neurosci. 2015;35:10503–10509. doi: 10.1523/JNEUROSCI.0569-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heller AS, Johnstone T, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Increased prefrontal cortex activity during negative emotion regulation as a predictor of depression symptom severity trajectory over 6 months. JAMA psychiatry (Chicago, Ill) 2013;70:1181–1189. doi: 10.1001/jamapsychiatry.2013.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hess JL, Kawaguchi DM, Wagner KE, Faraone SV, Glatt SJ. The influence of genes on “positive valence systems” constructs: A systematic review. Am J Med Genet B Neuropsychiatr Genet. 2015 doi: 10.1002/ajmg.b.32382. [DOI] [PubMed] [Google Scholar]
- 39.Heuer K, Rinck M, Becker ES. Avoidance of emotional facial expressions in social anxiety: The Approach-Avoidance Task. Behav Res Ther. 2007;45:2990–3001. doi: 10.1016/j.brat.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Ho PM, Cooper AJ, Hall PJ, Smillie LD. Factor structure and construct validity of the temporal experience of pleasure scales. J Pers Assess. 2015;97:200–208. doi: 10.1080/00223891.2014.940625. [DOI] [PubMed] [Google Scholar]
- 41.Holst K, Bustz-Joergensen E. Linear Latent Variable Models: The lava-package. Computational Statistics 2012 [Google Scholar]
- 42.Huys QJ, Pizzagalli DA, Bogdan R, Dayan P. Mapping anhedonia onto reinforcement learning: a behavioural meta-analysis. Biology of mood & anxiety disorders. 2013;3:12. doi: 10.1186/2045-5380-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Insel TR. Next-generation treatments for mental disorders. Sci Transl Med. 2012;4:155ps119. doi: 10.1126/scitranslmed.3004873. [DOI] [PubMed] [Google Scholar]
- 44.Insel TR, Cuthbert BN. Medicine. Brain disorders? Precisely. Science. 2015;348:499–500. doi: 10.1126/science.aab2358. [DOI] [PubMed] [Google Scholar]
- 45.James W. The principles of psychology. H. Holt and Company; New York: 1988. [Google Scholar]
- 46.Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of abnormal psychology. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- 47.Kappenman ES, Farrens JL, Luck SJ, Proudfit GH. Behavioral and ERP measures of attentional bias to threat in the dot-probe task: Poor reliability and lack of correlation with anxiety. Frontiers in psychology. 2014:5. doi: 10.3389/fpsyg.2014.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kashdan TB. Social anxiety spectrum and diminished positive experiences: theoretical synthesis and meta-analysis. Clin Psychol Rev. 2007;27:348–365. doi: 10.1016/j.cpr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Kashdan TB, Weeks JW, Savostyanova AA. Whether, how, and when social anxiety shapes positive experiences and events: a self-regulatory framework and treatment implications. Clinical psychology review. 2011;31:786–799. doi: 10.1016/j.cpr.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch.Gen. Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 51.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International journal of methods in psychiatric research. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kessler RC, Ruscio AM, Shear K, Wittchen HU. Epidemiology of anxiety disorders. Curr Top Behav Neurosci. 2010;2:21–35. [PubMed] [Google Scholar]
- 53.Kroenke K, Spitzer RL, Williams JB, Monahan PO, Lowe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Annals of internal medicine. 2007;146:317–325. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]
- 54.Lang P, Bradley M, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual 2008 [Google Scholar]
- 55.LeDoux JE, Pine DS. Using Neuroscience to Help Understand Fear and Anxiety: A Two-System Framework. Am J Psychiatry. 2016 doi: 10.1176/appi.ajp.2016.16030353. appiajp201616030353. [DOI] [PubMed] [Google Scholar]
- 56.Linde K, Kriston L, Rucker G, Jamil S, Schumann I, Meissner K, Sigterman K, Schneider A. Efficacy and acceptability of pharmacological treatments for depressive disorders in primary care: systematic review and network meta-analysis. Annals of family medicine. 2015a;13:69–79. doi: 10.1370/afm.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linde K, Sigterman K, Kriston L, Rucker G, Jamil S, Meissner K, Schneider A. Effectiveness of psychological treatments for depressive disorders in primary care: systematic review and meta-analysis. Annals of family medicine. 2015b;13:56–68. doi: 10.1370/afm.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loerinc AG, Meuret AE, Twohig MP, Rosenfield D, Bluett EJ, Craske MG. Response rates for CBT for anxiety disorders: Need for standardized criteria. Clin Psychol Rev. 2015;42:72–82. doi: 10.1016/j.cpr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 60.MacLeod C, Mathews A. Anxiety and the allocation of attention to threat. Q J Exp Psychol A. 1988;40:653–670. doi: 10.1080/14640748808402292. [DOI] [PubMed] [Google Scholar]
- 61.Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. Cmaj. 2012;184:E191–196. doi: 10.1503/cmaj.110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maples JL, Carter NT, Few LR, Crego C, Gore WL, Samuel DB, Williamson RL, Lynam DR, Widiger TA, Markon KE, Krueger RF, Miller JD. Testing Whether the DSM-5 Personality Disorder Trait Model Can Be Measured With a Reduced Set of Items: An Item Response Theory Investigation of the Personality Inventory for DSM-5. Psychol Assess. 2015 doi: 10.1037/pas0000120. [DOI] [PubMed] [Google Scholar]
- 63.Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- 64.Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annu Rev Psychol. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- 65.Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression: the role of awareness. Br J Clin Psychol. 1995;34:17–36. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 66.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 67.Najmi S, Kuckertz JM, Amir N. Automatic avoidance tendencies in individuals with contamination-related obsessive-compulsive symptoms. Behav Res Ther. 2010;48:1058–1062. doi: 10.1016/j.brat.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naragon-Gainey K, Watson D, Markon KE. Differential relations of depression and social anxiety symptoms to the facets of extraversion/positive emotionality. Journal of abnormal psychology. 2009;118:299–310. doi: 10.1037/a0015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Doherty J, Kringelbach ML, Hornak J, Andrews C, Rolls ET. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 70.O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G, Renner B, Ahne G. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex [corrected and republished in Neuroreport 2000 Mar 20;11(4):893–7] Neuroreport. 2000;11:399–403. doi: 10.1097/00001756-200002070-00035. [DOI] [PubMed] [Google Scholar]
- 71.O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural Responses during Anticipation of a Primary Taste Reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- 72.Prata D, Mechelli A, Kapur S. Clinically meaningful biomarkers for psychosis: A systematic and quantitative review. Neurosci Biobehav Rev. 2014 doi: 10.1016/j.neubiorev.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 73.Prenoveau JM, Zinbarg RE, Craske MG, Mineka S, Griffith JW, Epstein AM. Testing a hierarchical model of anxiety and depression in adolescents: a tri-level model. J Anxiety Disord. 2010;24:334–344. doi: 10.1016/j.janxdis.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 74.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. The international journal of behavioral nutrition and physical activity. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Radke S, Guths F, Andre JA, Muller BW, de Bruijn ER. In action or inaction? Social approach-avoidance tendencies in major depression. Psychiatry research. 2014;219:513–517. doi: 10.1016/j.psychres.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 76.Revelle W. Hierarchical cluster analysis and the internal structure of tests. Multivariate Behavioral Research. 1979;14:57–74. doi: 10.1207/s15327906mbr1401_4. [DOI] [PubMed] [Google Scholar]
- 77.Revelle W. psych: Procedures for Psychological, Psychometric, and Personality Research, R package version 1.5.8 ed. Northwestern University; Evanston, Illinois: 2015. [Google Scholar]
- 78.Revelle W, Wilt J. The general factor of personality: A general critique. Journal of Research in Personality. 2013;47:493–504. doi: 10.1016/j.jrp.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rinck M, Becker ES. Approach and avoidance in fear of spiders. J Behav Ther Exp Psychiatry. 2007;38:105–120. doi: 10.1016/j.jbtep.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 80.Roelofs K, Putman P, Schouten S, Lange WG, Volman I, Rinck M. Gaze direction differentially affects avoidance tendencies to happy and angry faces in socially anxious individuals. Behav Res Ther. 2010;48:290–294. doi: 10.1016/j.brat.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 81.Romer Thomsen K, Whybrow PC, Kringelbach ML. Reconceptualizing anhedonia: novel perspectives on balancing the pleasure networks in the human brain. Frontiers in behavioral neuroscience. 2015;9:49. doi: 10.3389/fnbeh.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roy-Byrne P, Craske MG, Sullivan G, Rose RD, Edlund MJ, Lang AJ, Bystritsky A, Welch SS, Chavira DA, Golinelli D, Campbell-Sills L, Sherbourne CD, Stein MB. Delivery of evidence-based treatment for multiple anxiety disorders in primary care: a randomized controlled trial. JAMA. 2010;303:1921–1928. doi: 10.1001/jama.2010.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roy-Byrne PP, Davidson KW, Kessler RC, Asmundson GJ, Goodwin RD, Kubzansky L, Lydiard RB, Massie MJ, Katon W, Laden SK, Stein MB. Anxiety disorders and comorbid medical illness. Gen Hosp Psychiatry. 2008;30:208–225. doi: 10.1016/j.genhosppsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 84.Rudaizky D, Basanovic J, MacLeod C. Biased attentional engagement with, and disengagement from, negative information: independent cognitive pathways to anxiety vulnerability? Cogn Emot. 2014;28:245–259. doi: 10.1080/02699931.2013.815154. [DOI] [PubMed] [Google Scholar]
- 85.Seidel EM, Habel U, Finkelmeyer A, Schneider F, Gur RC, Derntl B. Implicit and explicit behavioral tendencies in male and female depression. Psychiatry research. 2010;177:124–130. doi: 10.1016/j.psychres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 86.Shankman SA, Klein DN, Tenke CE, Bruder GE. Reward sensitivity in depression: a biobehavioral study. J Abnorm Psychol. 2007;116:95–104. doi: 10.1037/0021-843X.116.1.95. [DOI] [PubMed] [Google Scholar]
- 87.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 88.Strack F, Deutsch R. Reflective and impulsive determinants of social behavior. Pers Soc Psychol Rev. 2004;8:220–247. doi: 10.1207/s15327957pspr0803_1. [DOI] [PubMed] [Google Scholar]
- 89.Tamir M, Robinson MD. The happy spotlight: positive mood and selective attention to rewarding information. Personality & social psychology bulletin. 2007;33:1124–1136. doi: 10.1177/0146167207301030. [DOI] [PubMed] [Google Scholar]
- 90.Taylor CT, Amir N. Attention and emotion regulation. Guilford Press; New York: 2010. [Google Scholar]
- 91.Taylor CT, Amir N. Modifying automatic approach action tendencies in individuals with elevated social anxiety symptoms. Behav Res Ther. 2012;50:529–536. doi: 10.1016/j.brat.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taylor CT, Bomyea J, Amir N. Attentional bias away from positive social information mediates the link between social anxiety and anxiety vulnerability to a social stressor. J Anxiety Disord. 2010;24:403–408. doi: 10.1016/j.janxdis.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taylor CT, Bomyea J, Amir N. Malleability of attentional bias for positive emotional information and anxiety vulnerability. Emotion. 2011;11:127–138. doi: 10.1037/a0021301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Torrubia R, Ávila C, Moltó J, Caseras X. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Personality and Individual Differences. 2001;31:837–862. [Google Scholar]
- 95.Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van de Leemput IA, Wichers M, Cramer AO, Borsboom D, Tuerlinckx F, Kuppens P, van Nes EH, Viechtbauer W, Giltay EJ, Aggen SH, Derom C, Jacobs N, Kendler KS, van der Maas HL, Neale MC, Peeters F, Thiery E, Zachar P, Scheffer M. Critical slowing down as early warning for the onset and termination of depression. Proc Natl Acad Sci U S A. 2014;111:87–92. doi: 10.1073/pnas.1312114110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vrijsen JN, van Oostrom I, Speckens A, Becker ES, Rinck M. Approach and Avoidance of Emotional Faces in Happy and Sad Mood. Cognitive therapy and research. 2013;37:1–6. doi: 10.1007/s10608-012-9436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Watson D. Differentiating the mood and anxiety disorders: a quadripartite model. Annu Rev Clin Psychol. 2009;5:221–247. doi: 10.1146/annurev.clinpsy.032408.153510. [DOI] [PubMed] [Google Scholar]
- 99.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 100.Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol. 1995a;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- 101.Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995b;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- 102.Webb CA, Dillon DG, Pechtel P, Goer FK, Murray L, Huys QJ, Fava M, McGrath PJ, Weissman M, Parsey R, Kurian BT, Adams P, Weyandt S, Trombello JM, Grannemann B, Cooper CM, Deldin P, Tenke C, Trivedi M, Bruder G, Pizzagalli DA. Neural Correlates of Three Promising Endophenotypes of Depression: Evidence from the EMBARC Study. Neuropsychopharmacology. 2016;41:454–463. doi: 10.1038/npp.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weinstock LM, Whisman MA. Neuroticism as a common feature of the depressive and anxiety disorders: a test of the revised integrative hierarchical model in a national sample. J Abnorm Psychol. 2006;115:68–74. doi: 10.1037/0021-843X.115.1.68. [DOI] [PubMed] [Google Scholar]
- 104.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 105.World Health Organization. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule (WHODAS 2.0) WHO Press; Geneva, Switzerland: 2010. [Google Scholar]
- 106.Zbozinek TD, Craske MG. Positive affect predicts less reacquisition of fear: relevance for long-term outcomes of exposure therapy. Cogn Emot. 2016:1–14. doi: 10.1080/02699931.2016.1142428. [DOI] [PubMed] [Google Scholar]
- 107.Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.