Abstract
A dominant selectable marker for Candida albicans and other Candida species, which confers resistance to nourseothricin, was characterized. In a heterologous promoter system and a recyclable cassette, the marker efficiently permitted deletion and complementation of C. albicans genes. Neither growth nor filamentous development was affected in strains expressing this marker.
Candida albicans is the most commonly isolated invasive fungal pathogen. Molecular genetic analysis of this fungus to date is principally based on the marker URA3, a biosynthetic gene that complements uridine auxotrophy (5), and on a dominant selectable marker, MPAr or IMH3r, developed from a mutant C. albicans gene (2, 9).
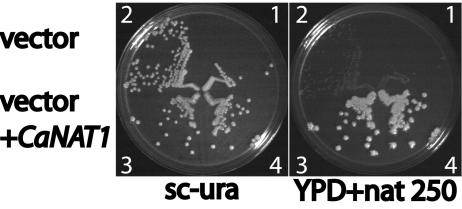
We developed a heterologous dominant marker to investigate morphogenesis of C. albicans. We chose a gene conferring resistance to nourseothricin, since wild-type C. albicans is susceptible to moderate nourseothricin concentrations (250 to 450 μg/ml). Codon usage of the Streptomyces noursei nat1 gene, encoding nourseothricin acetyltransferase, was adapted to that of C. albicans to generate CaNAT1 (6, 8). De novo synthesis was performed by Bionexus (Oakland, Calif.). CaNAT1 was placed under the control of the ACT1 promoter in vector pAU34, which contains URA3 as a selectable marker (15), generating pJK850. We transformed strain CAI4 (ura3/ura3) (5) with pJK850 to be uridine prototrophic. The resulting strains JKC435 and JKC436 were re sistant to 250 μg of nourseothricin/ml, as shown in Fig. 1. CAI4 was also transformed with the empty vector pAU34 to be uridine prototrophic (15). The resulting control strains JKC437 and JKC438 were sensitive to nourseothricin.
FIG. 1.
CaNAT1 confers nourseothricin resistance on C. albicans. The ura3/ura3 strain CAI4 was transformed with the empty vector pAU34, which bears the selectable marker URA3, giving rise to JKC347 and JKC348, and with pAU34 containing CaNAT1, giving rise to JKC345 and JKC346. Transformants were streaked onto medium to select for uridine prototrophy (sc-ura). They were then replica plated onto YPD containing 250 μg of nourseothricin/ml (YPD+nat250). 1, JKC437; 2, JKC438; 3, JKC436; 4, JKC435.
Mutant phenotype analysis requires that the marker used to generate the mutation have minimal effects on cell growth and development. To determine the effect of CaNAT1 expression on growth, we used competition experiments (1, 7). Specifically, with the use of pJK799, CaNAT1 was integrated at the locus of a gene, DLH1, whose Saccharomyces cerevisiae homolog is expressed only during meiosis (4). We expressed CaNAT1 from the Ashbya gossypii TEF1 promoter and terminator (16) in order to avoid C. albicans promoter and terminator sequences that might misdirect homologous recombination of constructs. The C. albicans SC5314 wild type and its DLH1/dlh1::p-AgTEF1 CaNAT1 t-AgTEF1 daughter strain JKC336 were subjected to competition essentially as described in reference 7, in 2× yeast extract-peptone-dextrose (YPD) with 8% glucose to maintain the cultures in the yeast form. In control experiments, strains CAF2-1 (URA3/ura3) and CAI4 (ura3/ura3) (5) were subjected to identical competition conditions, but they were initially inoculated at a ratio of 1:20. As shown in Fig. 2, CaNAT1 expression was phenotypically neutral with respect to growth.
FIG. 2.
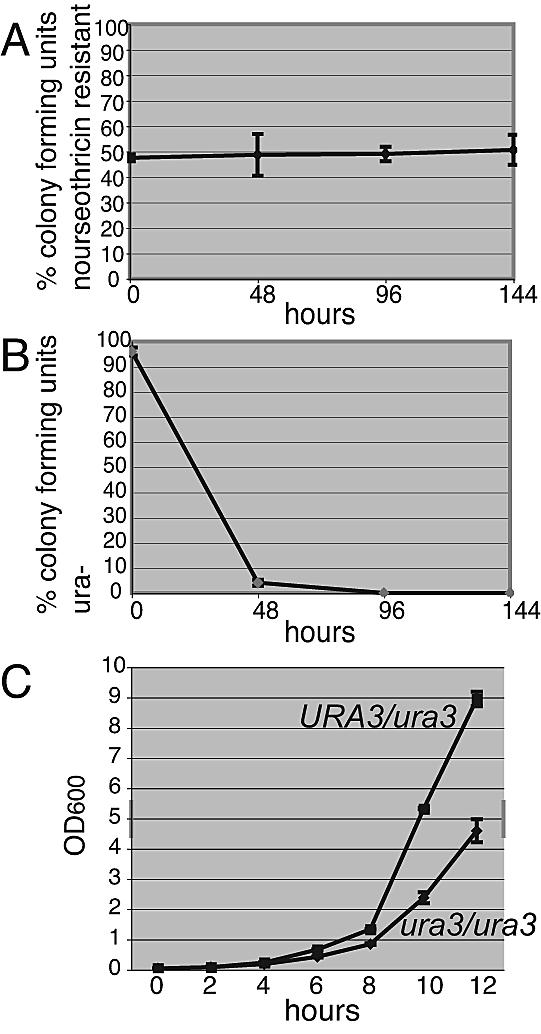
Cells expressing CaNAT1 do not exhibit a growth disadvantage. (A) Competition between wild-type SC5314 and the DLH1/dlh1::CaNAT1 strain JKC336. Fresh cultures of the two strains were inoculated in equal parts into rich medium at a density of ca. 1 cell/ml. The numbers of total colonies and nourseothricin-resistant colonies were determined at 48 h, and the proportion of CaNAT1-expressing cells to wild-type cells was calculated. The cultures were diluted in fresh medium after 48 h to the starting density of ca. 1 cell/ml. This process was repeated three times. Two separate experiments were performed. Per time point, three samples were taken to determine numbers of CFU. Bars represent standard deviations. (B) Competition between strains CAF2-1 (URA3/ura3) and CAI4 (ura3/ura3). To control for the sensitivity of the competition experiment protocol, the ura3/ura3 homozygote CAI4 was tested in competition with the URA3/ura3 CAF2-1 heterozygote. For colony counts, cultures were plated onto YPD. They were replica plated onto medium to select for uridine prototrophy to determine the percentage of uridine-prototrophic and uridine-auxotrophic (ura−) colonies. Two separate experiments were performed. Per time point, three samples were taken to determine numbers of CFU. Bars represent standard deviations. (C) Growth rates of CAF2-1 (URA3/ura3) and CAI4 (ura3/ura3). Strains were diluted from an overnight culture in 2× yeast extract-peptone-8% dextrose to an optical density at 600 nm (OD600) of 0.04 in 50 ml of 2× yeast extract-peptone-8% dextrose. Growth conditions were the same as those for the competition experiments. The OD600 was measured every 2 h. Results of two separate experiments are shown.
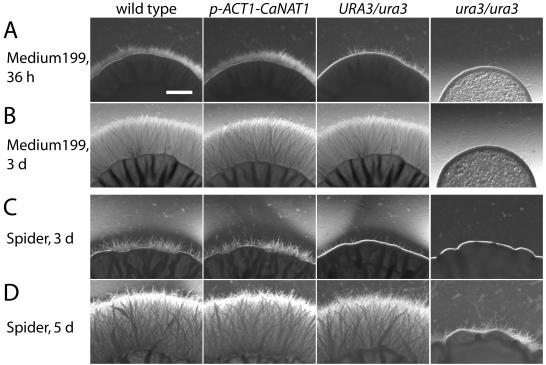
In C. albicans, the expression level of URA3 affects filament development and virulence (3) and is apparently influenced by position effects (2). We wished to determine the effect of CaNAT1 expression on filamentous growth. We transformed the wild-type strain SC5314 with pJK881, in which CaNAT1 is driven by the ACT1 promoter (p-ACT1). pJK881 was derived from pJK850 by deleting a portion of the URA3 gene so that URA3 was no longer functional in order to avoid possible confounding effects of an extra allele of URA3. We compared four nourseothricin-resistant transformants with the wild-type parent. Filamentous-growth phenotypes of these five strains were indistinguishable. Figure 3 shows filamentous growth of a p-ACT1 CaNAT1 strain and of the wild type. The URA3/ura3 and ura3/ura3 strains derived from SC5314 (5) served as controls for decreased filamentous growth.
FIG. 3.
Expression of CaNAT1 does not affect filamentous growth. Filamentous growth of SC5314 (URA3/URA3) (5) was compared with that of its daughter strain JKC439 (p-ACT1 CaNAT1 URA3/URA3). CAF2-1 (URA3/ura3) and its daughter strain CAI4 (ura3/ura3) (5) were used as controls for decreased filamentous growth. Fresh cultures of the strains were diluted to an OD600 of 0.1. Three microliters of each strain's cell suspension was spotted at equal distances from the others around the diameter of an agar plate, with six spots per plate. Spots on medium 199, pH 7, and Spider medium after various periods of incubation are shown. d, days. Bar, 1 mm.
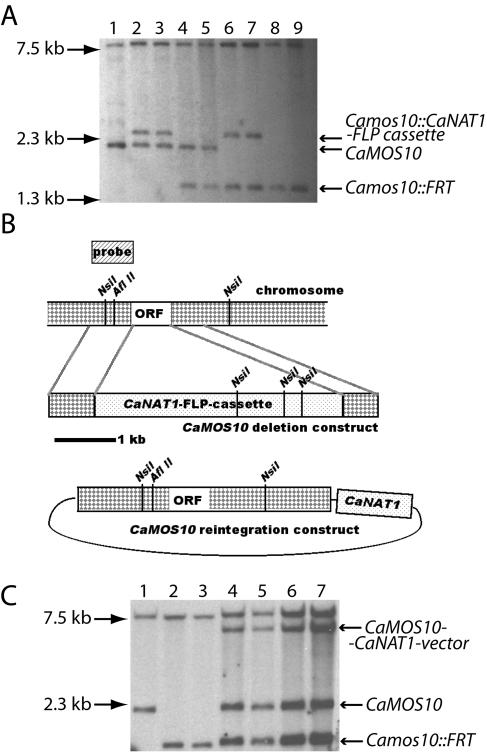
In order to delete both alleles of a gene with a single selectable marker, it must be possible to eliminate the marker from the genome. Use of the FLP recombinase to recycle a Candida selectable marker was pioneered by Morschhäuser and colleagues (11, 13, 17). We replaced URA3 with CaNAT1 in the FLP cassette (11, 13). We used the CaNAT1-FLP cassette to delete both alleles of CaMOS10, a gene whose homolog is involved in yeast growth on filaments in S. cerevisiae (10). The final products of this procedure were strains in which both open reading frames of CaMOS10 were replaced with the 34-bp FLP recombinase recognition target (FRT). CaNAT1 was then used to reintegrate an intact CaMOS10 allele by a single crossover event at the AflII site in the CaMOS10 promoter, at which the reintegration construct was cut. Southern blots of the resulting heterozygotes and homozygotes, as well as those of reintegrants, showed genomic fragments which corresponded to the predicted CaMOS10 alleles of each strain (Fig. 4). CaNAT1 can thus function as the only selectable marker for gene deletion studies of C. albicans. A related marker, SAT1, was used in a large-scale gene deletion study of C. albicans (12). However, the investigators in that study utilized uridine and histidine auxotrophy markers in addition to the dominant marker to construct their strains. We find that recycling the CaNAT1 mar ker can obviate the need for auxotrophic markers.
FIG. 4.
CaNAT1 can mark a recyclable cassette for deletion of both alleles of a C. albicans gene. (A) Genomic DNA of the following strains was digested with NsiI: lane 1, wild-type SC5314; lane 2, JKC377, and lane 3, JKC378 (CaMOS10/Camos10::CaNAT1-FLP cassette); lane 4, JKC380, and lane 5, JKC382 (CaMOS10/Camos10::FRT); lane 6, JKC390, and lane 7, JKC392 (Camos10::FRT/Camos10::CaNAT1-FLP cassette); and lane 8, JKC393, and lane 9, JKC395 (Camos10::FRT/Camos10::FRT). The CaNAT1-FLP cassette is FRT-p-SAP2-FLP-t-CaACT1-p-AgTEF1-CaNAT1-t-AgTEF1-FRT. Left arrows show locations of molecular weight markers. Right arrows show genomic fragments. (B) Cartoon of the CaMOS10 locus, the deletion construct, the construct to reintegrate an intact CaMOS10 allele, and the probe used in Southern blotting. ORF, open reading frame. (C) Genomic DNA of the following strains was digested with NsiI: lane 1, wild-type SC5314; lane 2, JKC393, and lane 3, JKC395 (Camos10::FRT/Camos10::FRT); and lanes 4 and 5, transformants of JKC393 (lane 4, JKC504, and lane 5, JKC505), and lanes 6 and 7, transformants of JKC395 (lane 6, JKC506, and lane 7, JKC507) (Camos10::FRT/Camos10::FRT/CaMOS10 CaNAT1).
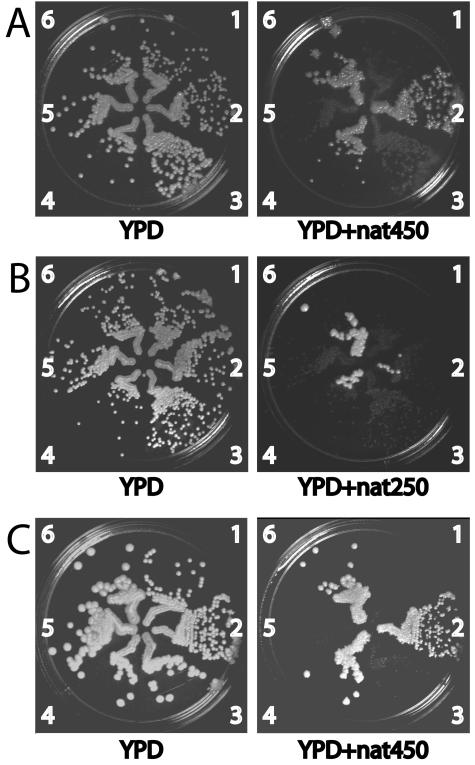
To test whether CaNAT1 could be used as a selectable marker in clinical Candida isolates, we obtained three C. albicans bloodstream isolates and seven clinical isolates of other Candida spp. from the Boston Children's Hospital Microbiology Laboratory. None of these strains were intrinsically resistant to nourseothricin. The clinical isolates were transformed with CaNAT1 to be nourseothricin resistant. Transformants appeared after 1 to 2 days on YPD plus 450 μg of nourseothricin/ml. At 3 days, small colonies that did not contain the CaNAT1 sequence as detected by PCR appeared. The transformants were streaked onto YPD and replica plated onto YPD plus 450 μg of nourseothricin/ml to confirm that they were nourseothricin resistant. Their growth phenotypes are shown in Fig. 5. In C. parapsilosis transformants, nourseothricin resistance was not clonally stable, as shown in Fig. 5B. We did not examine whether the CaNAT1-carrying plasmid was maintained episomally in this species, with loss of the plasmid under nonselective conditions.
FIG. 5.
Nourseothricin resistance phenotypes of clinical isolates of C. albicans and other Candida species. Clinical Candida isolates and their CaNAT1-containing transformants were streaked next to each other on YPD. They were then replica plated onto YPD with 250 (YPD+nat250) or 450 (YPD+nat450) μg of nourseothricin/ml. (A) C. albicans bloodstream isolates. 1, JKC8 (wild-type bloodstream isolate); 2, JKC410 (JKC8 transformed with pJK850); 3, JKC9 (wild-type bloodstream isolate); 4, JKC412 (JKC9 transformed with pJK799); 5, JKC7 (wild-type bloodstream isolate); and 6, JKC408 (JKC7 transformed with pJK850). (B) C. parapsilosis clinical isolates. 1, JKC355 (wild-type clinical isolate); 2, JKC414 (JKC355 transformed with pJK850); 3, JKC357 (wild-type clinical isolate); 4, JKC416 (JKC357 transformed with pJK799); 5, JKC358 (wild-type clinical isolate); and 6, JKC419 (JKC358 transformed with pJK850). (C) Candida species clinical isolates. 1, JKC356 (C. lusitaniae wild type); 2, JKC420 (JKC356 transformed with pJK799); 3, JKC361 (C. glabrata wild type); 4, JKC424 (JKC361 transformed with pJK850); 5, JKC359 (C. kefyr wild type); and 6, JKC426 (JKC359 transformed with pJK850).
PCR was used to confirm that the nourseothricin-resistant strains which grew after transformation with CaNAT1 were actually transformed with CaNAT1 and were not spontaneous nourseothricin-resistant mutants. The PCRs for all nourseothricin-resistant transformants yielded a product of the expected size, while none of the reactions for the untransformed parents yielded this product (data not shown). In addition, we subjected 22 nourseothricin-resistant Candida transformants and their 11 untransformed parent strains to slot blotting to detect the CaNAT1 gene. As shown in Fig. 6, all tested transformants were found to contain a sequence that hybridized to a portion of the CaNAT1 open reading frame. DNA of a C. parapsilosis clinical isolate appeared to hybridize with the CaNAT1 probe in the slot blot. Southern blotting of this strain and its CaNAT1-transformed daughters showed no CaNAT1-sized band in the clinical isolate and showed the expected band in the transformants; in addition, a faint cross-hybridizing band of higher molecular weight than the CaNAT1-sized band was seen in these strains and in the positive and negative controls (data not shown). Our results demonstrated that transformation with CaNAT1 conferred nourseothricin resistance on clinical isolates of several Candida species, as well as on S. cerevisiae (data not shown). C. lusitaniae and S. cerevisiae are the most distantly related hemiascomycetes that we transformed with CaNAT1 to be nourseothricin resistant (14). CaNAT1 is expected to function in other ascomycetous species within this span of evolutionary distance.
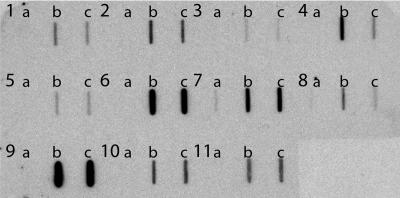
FIG. 6.
Nourseothricin-resistant strains transformed with CaNAT1 contain the CaNAT1 sequence. Genomic DNA from untransformed parent strains (a) and from two each of their nourseothricin-resistant transformants (b and c) was slot blotted and probed with a portion of the CaNAT1 open reading frame. The strains were as follows: 1a, SC5314; 1b, JKC406; 1c, JKC336; 2, 3, and 4, C. albicans bloodstream isolates (2a, JKC7; 2b, JKC406; 2c, JKC375; 3a, JKC8; 3b, JKC410; 3c, JKC411; 4a, JKC9; 4b, JKC412; and 4c, JKC413); 5, 6, and 7, C. parapsilosis clinical isolates (5a, JKC355; 5b, JKC414; 5c, JKC415; 6a, JKC357; 6b, JKC416; 6c, JKC417; 7a, JKC358; 7b, JKC418; and 7c, JKC419); 8 and 9, C. lusitaniae clinical isolates (8a, JKC356; 8b, JKC420; 8c, JKC421; 9a, JKC360; 9b, JKC422; and 9c, JKC423); 10, C. glabrata clinical isolate (10a, JKC361; 10b, JKC424; and 10c, JKC425); and 11, C. kefyr clinical isolate (11a, JKC359; 11b, JKC426; and 11c, JKC407).
We have shown that CaNAT1 can function as a selectable marker in C. albicans and in several other Candida species. It is phenotypically neutral with respect to growth and filament development under our experimental conditions. We anticipate that the use of CaNAT1 will expand the scope of molecular genetic analysis to clinical isolates of many pathogenic Candida species.
Nucleotide sequence accession number.
The nucleotide sequence of CaNAT1 has been deposited in GenBank under accession number AY854370.
Acknowledgments
This work was supported by a Charles A. Janeway Child Health Research Center Award to J.R.K.
We thank Bill Fonzi and Joachim Morschhäuser for generous gifts of strains and plasmids. We thank Jeanne Latourneau, Eileen Gorss, Alex McAdam, and Oscar Torres for clinical Candida isolates and information on identification procedures. We are grateful to Simon Dove, Qinghua Feng, Steffen Rupp, Bob Husson, and Horst Schroten for critical comments on the manuscript.
Editor: V. J. DiRita
REFERENCES
- 1.Baganz, F., A. Hayes, D. Marren, D. C. Gardner, and S. G. Oliver. 1997. Suitability of replacement markers for functional analysis studies in Saccharomyces cerevisiae. Yeast 13:1563-1573. [DOI] [PubMed] [Google Scholar]
- 2.Beckerman, J., H. Chibana, J. Turner, and P. T. Magee. 2001. Single-copy IMH3 allele is sufficient to confer resistance to mycophenolic acid in Candida albicans and to mediate transformation of clinical Candida species. Infect. Immun. 69:108-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng, S., M. H. Nguyen, Z. Zhang, H. Jia, M. Handfield, and C. J. Clancy. 2003. Evaluation of the roles of four Candida albicans genes in virulence by using gene disruption strains that express URA3 from the native locus. Infect. Immun. 71:6101-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diener, A. C., and G. R. Fink. 1996. DLH1 is a functional Candida albicans homologue of the meiosis-specific gene DMC1. Genetics 143:769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerami-Nejad, M., J. Berman, and C. A. Gale. 2001. Cassettes for PCR-mediated construction of green, yellow, and cyan fluorescent protein fusions in Candida albicans. Yeast 18:859-864. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein, A. L., and J. H. McCusker. 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15:1541-1553. [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi, Y., H. Honda, J. Taniguchi-Morimura, and S. Iwasaki. 1989. The codon CUG is read as serine in an asporogenic yeast Candida cylindracea. Nature 341:164-166. [DOI] [PubMed] [Google Scholar]
- 9.Köhler, G. A., T. C. White, and N. Agabian. 1997. Overexpression of a cloned IMP dehydrogenase gene of Candida albicans confers resistance to the specific inhibitor mycophenolic acid. J. Bacteriol. 179:2331-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Köhler, J. R. 2003. Mos10 (Vps60) is required for normal filament maturation in Saccharomyces cerevisiae. Mol. Microbiol. 49:1267-1285. [DOI] [PubMed] [Google Scholar]
- 11.Morschhäuser, J., S. Michel, and P. Staib. 1999. Sequential gene disruption in Candida albicans by FLP-mediated site-specific recombination. Mol. Microbiol. 32:547-556. [DOI] [PubMed] [Google Scholar]
- 12.Roemer, T., B. Jiang, J. Davison, T. Ketela, K. Veillette, A. Breton, F. Tandia, A. Linteau, S. Sillaots, C. Marta, N. Martel, S. Veronneau, S. Lemieux, S. Kauffman, J. Becker, R. Storms, C. Boone, and H. Bussey. 2003. Large-scale essential gene identification in Candida albicans and applications to antifungal drug discovery. Mol. Microbiol. 50:167-181. [DOI] [PubMed] [Google Scholar]
- 13.Staib, P., M. Kretschmar, T. Nichterlein, G. Kohler, S. Michel, H. Hof, J. Hacker, and J. Morschhäuser. 1999. Host-induced, stage-specific virulence gene activation in Candida albicans during infection. Mol. Microbiol. 32:533-546. [DOI] [PubMed] [Google Scholar]
- 14.Sugita, T., and T. Nakase. 1999. Non-universal usage of the leucine CUG codon and the molecular phylogeny of the genus Candida. Syst. Appl. Microbiol. 22:79-86. [DOI] [PubMed] [Google Scholar]
- 15.Uhl, M. A., and A. D. Johnson. 2001. Development of Streptococcus thermophilus lacZ as a reporter gene for Candida albicans. Microbiology 147:1189-1195. [DOI] [PubMed] [Google Scholar]
- 16.Wach, A., A. Brachat, R. Pohlmann, and P. Philippsen. 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10:1793-1808. [DOI] [PubMed] [Google Scholar]
- 17.Wirsching, S., S. Michel, and J. Morschhäuser. 2000. Targeted gene disruption in Candida albicans wild-type strains: the role of the MDR1 gene in fluconazole resistance of clinical Candida albicans isolates. Mol. Microbiol. 36:856-865. [DOI] [PubMed] [Google Scholar]