The National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) is designed to shift research on psychopathology towards a pathophysiology-based framework (Cuthbert and Insel, 2013), placing a central focus on circuit-behavior relationships. In the two-dimensional RDoC matrix, Domains (in rows) represent fundamental components of behavior instantiated in neural systems that can be investigated using the various Units of Analysis (columns) (Cuthbert, 2014). This allows an in-depth understanding of behaviors along the full range of normative functioning as well as respective disruptions that manifest in psychopathology (Cuthbert and Insel, 2013). As it currently stands, RDoC conceptualizes development as applying to all constructs and units of analysis, but leaves it to the discretion of researchers to define the appropriate developmental timeframe they wish to use to in their investigations (Casey et al., 2014; Cuthbert, 2014). Unfortunately, this can often result in ambiguity on how researchers should incorporate development into an RDoC informed study designs. We believe this is a key missed opportunity. Figure 1 illustrates of how concepts such as development and environment/context can indeed play a role in the RDoC conceptualization.1 This commentary utilizes a neurodevelopmental approach to psychosis as an exemplar for highlighting a more rigorous approach for incorporating developmental considerations within the RDoC framework. To do so, we illustrate the atypicalities in the developmental patterning of motor behaviors across the full clinical sequence of psychosis (i.e., premorbid, prodromal, acute and chronic). We begin by using a traditional RDoC approach, followed by elaboration of developmental considerations to illustrate a developmentally- enriched perspective. This provides a basis for discussing the use of a developmentally-sensitive measurement approach (Wakschlag et al., 2010) into RDoC designs. Finally, the commentary discusses potential future directions, which enhance developmental emphasis in RDoC.
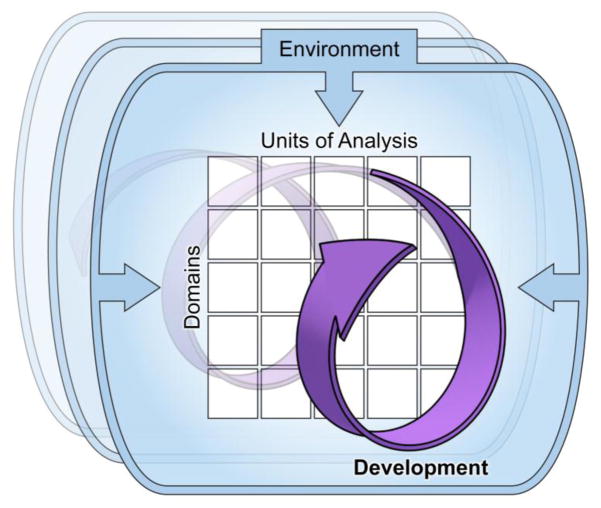
Figure 1.
A Research Domain Criteria (RDoC) framework formally has included and continued to refine Constructs and Domains on one axis and Units of Analysis on the other. Although Development and Environment/Context are also central to the RDoC framework, the current standard is to leave incorporation of these factors up to individual researcher. While this does allow for flexability, there are limitations to the approach- namely, factors such as Development may not receive the same level of careful consideration and integration as Constructs/Domains and Units of Analysis (Factors that are specifically mandated in RDoC funding announcements, and most commonly depicted in RDoC conceptual figures). However, future efforts to conduct research in this innovative system will need to place equal weight on each of the four factors. This four-dimensional conceptual figure provides the traditional Constructs/Domains and Units of Analysis axis, but also prominatly displays the critical Environment/Context and Development factors as well. Developmental influences are depicted as non-linear, dynamic, and highly interactive with other RDoC Dimensions.
Changing Expressions of Motor Dysfunction in the Neurodevelopment of Schizophrenia
Research on neurodevelopment in the prodromal stages of psychotic disorders illustrates the complexities inherent in clinical, developmental applications of RDoC. Although motor behaviors have yet to be incorporated into RDoC (Bernard and Mittal, 2015), efforts are currently underway to explore this possibility (e.g., an RDoC Motor Systems Domain workshop). In this commentary, we explore the link between motor dysfunction and psychosis to illustrate neurodevelopmental aspects of lifecourse psychopathology. Of note, this example illustrates how merging a developmental approach with RDoC and brain-based phenomena (e.g. development of motor control) may more precisely identify individual differences in DSM-defined constructs such as psychosis.
Many patients with adult onset psychotic disorders are believed to show aberrant brain development, with markers evident as early as the first years of life. For example, retrospective observation of infants and young children who develop schizophrenia as adults reveals a pattern of motor deficits, including mild, non-specific, motor perturbations (Cannon et al., 2002; Fish, 1987; Marcus et al., 1985) and possible signs of more specific cortical-subcortical motor dysfunction (Walker, 1994). Analyses of archived home movies reveal perturbed motor development, including delays in motor milestones and difficulties with coordination (Walker, 1994). The long-term predictive utility of these motor abnormalities is limited, as, many young children who exhibit these motor abnormalities do not go on to develop psychosis, and not everyone who develops psychosis exhibits premorbid motor abnormalities. Still, the identification of motor anomalies, that presage later schizophrenia as early as the first years of life, provides critical clues of premorbid vulnerability for psychosis (Mittal and Walker, 2009; Walker et al., 1994). Pinpointing the specific neural substrates for these early premorbid signs (and their potential role as harbingers of psychosis) is a challenge because the same behavioral phenotype may reflect distinct underlying neural processes (Nigg et al., 2005). Predictive utility may be enhanced by quantifying atypical motor control in high-risk populations (e.g. those with a family history of psychosis), by understanding them in relation to perturbations in cortical, striatal, and cerebellar circuitry (i.e., abnormal motor behaviors that reflect a specific form of atypical brain function) (Rogers et al., 2006), and via prospective longitudinal follow up from infancy through the peak age of onset in high risk populations. Studies have shown that the predictive role of motor development aberrations is greater in a child whose parent has a psychotic disorder than in a child whose parents are healthy (Erlenmeyer-Kimling et al., 2000; Rosso et al., 2000; Schiffman et al., 2004), though large-scale prospective population-based cohorts are needed to more precisely define the nature of risk (Franklin et al., 2015; Wakschlag and Hans, 1999).
Most youth with early childhood motor atypicalities resulting in developmental delays-- such as those retrospectively identified in the childhood videos of adults with psychosis -- do eventually attain age appropriate milestones of motor development and no longer show clear motor abnormalities. Because data on these patterns is largely derived from secondary data analysis, it is difficult to know whether these early markers are transient or manifest in other developmental forms not detectable via standard motor milestone assessments not designed for this purpose. Walker (1994) found that after the infant period, videotape analyses of early motor control could not differentiate which children would later develop psychosis and which would not. However, there are limitations to drawing conclusions based on archival home videos alone. In particular, because there are not currently any experimental studies that include developmental neuroimaging and longitudinal follow up of young children with these abnormalities in regard to psychosis development, it is not possible to definitively identify sensitive and specific markers or to differentiate those young children with these patterns who will or will not develop psychosis.
In adolescence, neuromaturational factors and environmental stressors can exacerbate underlying vulnerabilities in the motor system, leading to other outward manifestations in this age group, including spontaneous dyskinesias (i.e., spontaneous jerking and irregular ballistic movements) (Mittal et al., 2010). Indeed, among high-risk groups (i.e., those showing a low level of symptoms) these particular motor behaviors increase in frequency and severity as a function of development and increased disease burden, are associated with increased attenuated positive symptoms (Mittal et al., 2008; Mittal et al., 2007), and strongly predict conversion to psychosis (Mittal and Walker, 2007). As not all individuals in a high-risk group do go on to develop a psychotic disorder, this is highly relevant (Mittal & Walker, 2011). Irrespective of medication (i.e., the motor abnormalities are present in neuroleptic naïve samples), these spontaneous jerking movements in the head, face, lips, and torso can continue to emerge during the adolescent prodromal period, until onset, when they remain a key clinical feature of the illness (Pappa and Dazzan, 2009; Mittal, 2016; Kendler, 2016). At least one cross-sectional study suggests that with advanced age, all patients with schizophrenia will eventually develop these behaviors (Quinn et al., 2001).
Finally, it is important to consider that neurodevelopmental risk is probabilistic. On the one hand, most children with motor delay will mature to become healthy adolescents and adults. A large number of other factors interact with motor delay to shape outcome in complex ways that remain incompletely understood. One the other, analysis of archival data, for example, is highly informative to indicate the presence of abnormality far earlier than frank psychosis or active prodrome; it is relatively sensitive at detecting gross abnormalities but misses more subtle perturbations. In addition, data quality is highly variable and non-standardized. For these reasons, it is important not to depend on a single or small set of factors to determine the risk status of a particular child. To determine whether or not a pathognomonic neurodevelopmental motor trajectory reliably predicts the emergence of psychosis will require careful, developmentally designed, prospective approaches using standardized, developmentally-tuned experimental measures to chart the normal:abnormal spectrum of motor development from early life across developmental periods.
RDoC without an Embedded Developmental Approach
One of the considerable strengths of RDoC is the incorporation of multiple units of analysis. For example, in the present illustration, an RDoC-inspired study might focus on behaviors such as dyskinetic movements in a group of adolescents exhibiting psychosis symptoms and aim to examine associations between these motor signs with cortical-striatal-pallido-thalamic circuits respectively. This type of study could potentially yield important insights into the neural network underlying these motor behaviors and subsequently pinpoint future targets for remediation. Further, integrating multiple Constructs from different Domains would help tease apart overlapping relationships with, for example, constructs within the Cognitive Systems or Positive Valence Systems Domains. As with any research, the questions raised by these studies would guide future research, using yet-to-be defined Constructs or Units of Analysis. Nevertheless, despite the valuable insights gained by using this approach, a significant gap remains. Specifically, it does not include a framework for clarifying how or why among populations at high-risk for psychosis there are general signs of motor dysfunction in infancy, indicators that are more difficult to detect in later childhood, and then eventually, specific emergent motor signs that represent important prognostic indicators adolescence. Addressing such vital questions would provide a unique and integral contribution that would enhance detection of risk at the earliest phases of the clinical sequence.
RDoC and Embedded Developmental Perspectives: Application to Psychosis
When employing an RDoC-informed approach in research, two important considerations must be borne in mind. The first is that different types of neural perturbations may give rise to the same anomalous behavior. The second is that developmental context is key to assessments of brain-behavior relationships because these relationships shift as a function of normative and pathological development (Wakschlag et al., under review). As noted above, risk status is reflected in a trajectory of behavior change across development, rather than in an expression of behavior at one point in time (Wakschlag et al. 2015). Moreover, when risk status is defined only based on measures of behavior, such as motor performance, rather than tightly integrated brain:behavior abnormalities, there is a danger of viewing phenotypic changes in the aberrant motor behavior across development as a change of risk status. On the contrary, these phenotypic changes may simply make it more difficult to detect the underlying vulnerability. Measurement artifact and observer-bias may also explain the apparent behavioral change, as may the use of methods that cannot detect anomalies in early development or their varying expression across development. More specifically, the currently available rater-based scales of clumsiness and approaches focusing on developmental trajectory (i.e., delay in toilet training and walking) provide effective thresholds within early childhood. However, these behaviors are not readily observable, not characterized in a dimensional fashion, non-specific, and not developmentally-sensitive indicators during the school-age period, despite the fact that there is evidence of anomalous motor control in these children. For this reason, rather than focusing on overt motor behaviors in older children, investigators have had some success in following the predictive value of more proximal markers in later childhood such as performance in physical education or woodworking (Isohanni et al., 2004).
A further illustration of this can be seen in the dyskinesias that emerge during adolescence in youth at risk for psychosis. This shift in motor behaviors across different developmental stages of psychosis risk may reflect a heterotypic continuity wherein an underlying latent vulnerability is expressed differently across development. On the other hand, it is possible that the nature of this vulnerability, and not simply its behavioral expression, also changes during adolescence. Evaluating this phenomenon from a developmental perspective may yield important insights. For example, a vulnerable striatal system may be present from very early in development (Walker, 1994), but the altered dopamine (DA) function in this system may come “on-line” in the adolescent prodromal stage immediately preceding onset (Howes et al., 2011), at which time hyperkinetic movements emerge. Interestingly, at this stage the hallmark positive symptoms of psychosis also begin to emerge which are also linked to aberrant dopamine function (Kapur et al., 2005).
Incorporating Developmentally Informative Assessments into RDoC
As noted, many individuals who develop schizophrenia are likely to have exhibited atypical motor behaviors as young children, but it is not until adolescence that these become reliable prognostic indicators of the prodromal phase of schizophrenia. Nevertheless, despite variations in developmentally-based expression, these motor behaviors are likely linked to the same underlying brain circuit vulnerabilities (Mittal et al., 2010; Dean & Mittal 2015). To our knowledge, there are no scales specifically designed to identify aberrant motor behavior in infants at risk for psychosis, which can capture the rapid changes in motoric capacity that occur across this period and characterize the full range of normative variation. However, this may soon change. For example, investigators in other fields including pediatric dysphagia and feeding disorders, are advocating for instrumental approaches to defining physiological swallowing status (Arvedson, 2008). Investigators have also examined isometric force variability and changes with practice in 6 and 10 year old children (Keutsch & Newell, 2004). Further, researchers have examined grip force (using instrumental approaches in young children (Falk, Tam, Schwellnus, Chau, 2010). These studies provide a solid basis for employing validated instrumental approaches in the same individuals preferably across developmental stages. As has been discussed above, secondary data or gross motor scales are not sufficiently sensitive to detect more subtle motor abnormalities since they focus on milestones and clumsiness in childhood. In addition, some traditional scales used to test motor abnormalities are not developmentally appropriate for infants and young children (e.g. several items include developmentally impossible criteria such as standing). This highlights the importance of developing measures with an eye towards both developmental specification within and across developmental periods with a lifespan coherence of brain-behavior patterns in motor development (Wakschlag et al., 2010). These measures could allow the explanatory value of motor dysfunction across various dimensions to be tested. Figure 2 illustrates an approach that utilizes developmentally sensitive assessments to this question. Panels depict assessments that may tap into the same neural circuit dysfunction across several developmental periods. Recent research linking these phenotypes to the underlying frontal-striatal circuit pathology has proven an effective tool in the prodromal period (Dean and Mittal, 2015), and future efforts will need to continue this work in early development.
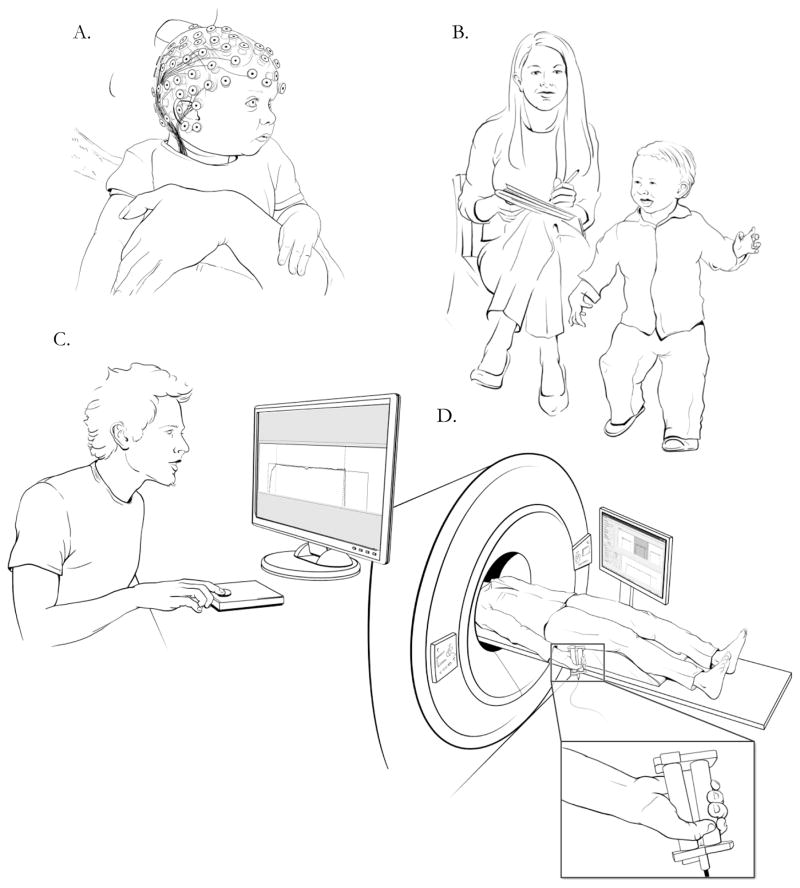
Figure 2.
The concept of heterotypic continuity is an important developmental consideration when conducting research in a Research Domain Criteria-RDoC framework. In this series of panels, investigators employ developmentally appropriate assessment strategies to determine how the same underlying circuit vulnerability may reflect different aberrant behaviors across developmental periods. Panel A depicts a proposed future direction, employing more sophisticated assessment approaches to earlier and earlier ages. One promising direction for incorporating Development into RDoC research is the incorporation of experimental designs that allow for standardized in-depth assessment across developmental periods. However, much more preliminary work is required before investigators can practically implement this strategy. Panel B exhibits traditional rater-based assessment of abnormal hand posturing in a toddler. Panel C illustrates an older child participating in a force variability assessment designed to test irregular patterns of muscle contraction, indicative of frontal-striatal circuit pathology. In Panel D an RDoC approach is employed where the same circuit pathology is being evaluated with multiple levels of analysis. An adolescent is being assessed with a grip force task that is designed for use in the scanner (getting at the same circuit assessed by the force variability task in Panel B), and incoming muscle contraction data will be examined in conjunction with patterns of frontal-striatal neural activation.
Our group has begun to employ strain gauge and handwriting kinematic assessments of motor pathology, which provide continuous variables that are more sensitive than observer-based methods, identifying individuals with overt dyskinesias as well as those who only have subclinical movement pathology (Cortese et al., 2005; Dean and Mittal, 2015; Dean et al., 2016; Dean et al., 2013). Our earlier work combining observer-based ratings of dyskinetic movement abnormalities with cognitive deficits in adolescents with prodromal syndromes showed some promise for predicting psychosis (sensitivity= 60.0%, specificity = 76.0%, PPV = 43.0%, NPV = 86.3%) (Mittal et al., 2010). However, to date, there have been no studies that include a longitudinal approach or accompanying test characteristics for effectiveness of instrumental approaches predicting conversion. Given converging evidence suggesting that these assessments are highly sensitive to detecting explicit and subtle movements, we believe this is an area that holds significant promise. Further, it is now becoming possible to use these and other novel instrumental approaches assessing movement in the scanner (Mosier et al., 2011). With modifications, these instruments might also be able to assess motor control in infants in a developmentally appropriate way. Quantifying motor pathology in this way will significantly impact our understanding of important transitions that may occur in the clinical sequence across development (e.g. premorbid to prodromal to frank psychosis stages). More importantly, by quantifying motor behaviors in this way it will be possible to explore motor system pathophysiology underlying the different types of anomalies that manifest across development in psychosis. For example, while readily observable measures such overt clumsiness (indicative of cerebellar circuit pathology) have been useful in detecting aberrant behaviors in the childhood premorbid period, they may not be effective in adolescents (who have eventually, albeit lately, developed motor proficiency). However, researchers have applied sensitive balance assessments using digital scales, and linked larger sway areas to abnormalities in the cerebellar substructures and connectivity (Bernard et al., 2014), as well as symptom progression (Dean et al., 2015).
Incorporating developmentally sensitive measures of motor behavior/control within the RDoC framework (Bernard & Mittal, 2015) will help to operationalize investigation of the interaction between normative motor development/developmental change and various constructs across multiple RDoC domains. For example, other work from our group shows that dimensional, developmentally-based measurements in early childhood can successfully provide a level of differentiation that better explains variance in prediction of impairment and linkage to neurocognitive atypicalities not currently explained by DSM symptoms (Briggs-Gowan et al., 2014; Wakschlag et al., 2015). This highlights the importance of developmentally specified dimensional phenotypes for quantifying behavioral abnormality - in contrast to current nosologies which largely impose “down-aged” patterns derived from adults and older youth (Wakschlag et al., 2010).
How This May Pertain to Other Disorders
Although illustrated in regard to psychosis, many of the principles we highlight draw upon cross-cutting developmental concepts. In particular, we emphasize four core ideas for consideration in neurodevelopmental study of motor dysfunction as a substrate of psychopathology in childhood/adolescence. (1). Heterogeneity of expression: The manner in which motor behavior atypicalities are developmentally-expressed may vary based on the syndrome. While motor dysfunction in the adolescent prodromal stage is quantitatively/qualitatively different from that seen in infancy and childhood, in other disorders motor dysfunction appears to remain on the same quantitative continuum across development (e.g., motor abnormalities in ADHD that reflect developmental delay) (Shaw et al., 2007). (2) Qualitative differences in the nature of abnormality: some motor anomalies are not found in normal development (i.e. deviations of kind, as in children/youth at-risk for psychosis), while others appear to reflect exaggeration of normatively occurring variability (i.e. deviations in degree, as in, for example, ADHD, DBDs, anxiety and depression) (Garber, 1984; Rutter and Sroufe, 2000; Wakschlag et al., under review). (3) Measurement limitations: the limited predictive value of early behavioral motor atypicalities, perhaps based on variation of expression at different developmental ages (as seen in infants and children at risk for psychosis) may result from measurement artifacts reflecting the use of archival data or proximal markers and no instruments specifically designed for this purpose. (4) Different types of neurobiological abnormalities appear at different developmental stages: while non-specific motor abnormalities are present early in infants who later go on to develop psychosis in adulthood (Walker et al., 1994), changes in the DA system that occur in the adolescent prodromal period (Howes et al., 2011) may contribute to dyskinesias that are specific to striatal dysfunction (Mittal et al., 2010). Mapping changes in neural vulnerability, and its corollary behavioral markers across development in a number of relevant disorders can yield important clues about pathophysiology. We recognize that these are complex issues, and that practical implementation will have its challenges. The goal of this commentary is to provide a framework for a broader and deeper conversation of these four principles and their application to developmentally-informed, RDoC-framed, research approaches used to investigate the unfolding clinical sequence of psychopathology.
Of note, the present commentary has focused on motor abnormalities as an example for how developmental concepts can be integrated more cohesively into RDoC. Nevertheless, multiple clinically significant phenomenological domains, both currently well-represented inside (e.g., error-related negativity; Weinberg, Dieterich, Riesel, 2015) and outside (e.g., negative self referential processing; Mennin & Fresco, 2013) the RDoC Matrix, also have neural correlates that would benefit from a better integrated developmental perspective. Moreover, while the present example centered primarily on motor behaviors commonly associated with psychosis, understanding similar developmental trajectories and clinically significant milestones linked with other traditional diagnostic categories (e.g., autism spectrum disorder, obsessive compulsive disorder, sleep disorders, irritability-related disorders) can serve as a starting place for a truly neurodevelopmental RDoC system aiming to bridge basic and clinical domains.
Where do we go from here?
As noted, developmental influences represent a complex and nuanced series of factors, and careful consideration is required as related concepts are integrated into RDoC. Specifically, it will be important to consider the inherent practicality of any particular direction, and also to ensure that neurodevelopmental aspects of RDoC will have strong empirical grounding. We provide several discussions points below, which are designed to provide a foundational framework for scientific discussion and so inform future studies that will generate this evidence base.
The NIMH has stressed the importance of development to the RDoC project; it will be important to clearly communicate this to the research community using, for example, simple enhancements on the NIMH RDoC website (e.g. having a link to the issue of incorporating development into RDoC on the home page). We also agree, with previous authors (Casey et al., 2014; Franklin et al., 2015; Garvey et al., 2016), that core elements of neurodevelopment should be incorporated into the RDoC matrix. One way to operationalize this is could be to develop recommendations for the formal inclusion of these elements into study designs. For example, this could recommendations to include the study of sensitive periods and trajectories across all stages of life; incorporate designs that adequately examine the non-linear aspects of development (e.g., the dynamic interplay of neural systems during maturation), and their interactions with the environment; and the use of methods to capture individual developmental pathways that can lead to psychopathology (Casey et al., 2014; Franklin et al., 2015; Garvey et al., 2016). Such recommendations in the form of a Guide Notice could act as a stimulus to investigators to include development in RDoC-informed investigator-initiated applications, even though they would not carry as much weight as a statement in a Request for Applications (cf. the stipulation in the group of RFAs on Dimensional Approaches to Research Classification in Psychiatric Disorders “Applications that do not include at least two dependent units of analysis will be considered NOT responsive to this funding opportunity”; see: http://grants.nih.gov/grants/guide/rfa-files/RFA-MH-16-510.html; Retrieved 10.19.16).
It is not clear whether a new RFA would be necessary – or even expedient - to increase the number of RDoC-informed applications that incorporate neurodevelopment in the long term although, in the short term, this type of formal direction might facilitate some critical advances in our knowledge of developmental variation in psychopathology. We acknowledge the need to balance short- and long-term goals when deciding on how to proceed with funding announcements.
The National Advisory Mental Health Council (NAMHC) recently gathered input from experts on valid and reliable tests that tap into the RDoC constructs. It is also critical to prioritize the development of a standard toolkit of valid and reliable dimensional, developmentally-based, measures – with linkage to relevant neural systems - that can be used to quantify the different aspects of neurodevelopment in RDoC oriented studies. Studies using these measures could then establish specific cut-offs/thresholds and that generate parameters for normal:abnormal differentiation of brain:behavior patterns beginning from birth within and across RDoC domains. This could help to characterize early emerging signs of psychopathology prior to the time it becomes clinically apparent.
As the NIMH curates the RDoC project, it might want to consider creating an RDoC archive of crosscutting developmental indicators and trajectories (similar to other recent large public access development efforts (Sporns, Tononi, & Kotter, 2005) that could provide an invaluable resource and serve as a reference point for various studies.
Developmental studies that attempt to delineate trajectories are expensive. In addition, to adequately describe the trajectory from early development through the age of risk, they require longer periods of time than the typical 5-year R01 grant mechanisms. New grant mechanisms that encourage longer follow-up periods (extending the standard 5 year project period) might facilitate these types of projects. One way to determine the added value of a program of this scale would be to first establishing the predictive and incremental utility of this approach. Perhaps an RDoC-informed, developmentally-based, staged grant mechanism (such as the R61/R33 now in use for treatment development and validation studies) could be created by which the longer term (second phase) follow up would be contingent on elucidation of relevant developmental markers in the first phase.
Finally, it is important to note that developmental considerations are relevant outside the context of studies with children, or investigations including a longitudinal approach. For example, important breakthroughs have come from approaches that focus on adult psychosis patients using cross-sectional designs, such as vulnerability markers extracted from retrospective prenatal developmental histories (for a review see Mittal, Ellman & Cannon, 2008). Detailed developmental histories obtained from adults with full-blown psychopathology may also yield important information about etiologic heterogeneity (e.g., trauma induced vs. slow gradual prodromal pattern without apparent environmental spur). In addition, retrospective construction of the unfolding of patients’ clinical sequence and environmental response (based on both patient and other informant reports) may be highly informative for identifying differential patterns that would otherwise appear homogenous cross-sectionally, which may inform tailored treatments. Further, approaches that weigh in developmental markers as units of analysis (e.g., mutations disrupting genes from signaling networks controlling neurodevelopment; Walsh et al., 2008) continue to yield important clues about pathophysiology and treatment targets.
In conclusion, more rigorous integration of developmental constructs will enhance the pathophysiology-based understanding of mental disorders as unfolding neurodevelopmental patterns, which RDoC aims to achieve. This will also actualize the cross-cutting dimensional approach that RDoC is designed to realize.
Highlights.
Neurodevelopment is a central construct in RDoC but it is not fully realized
Research on changing expression of motor development highlights utility of incorporating developmental concepts into RDoC
Advancements for neurodevelopmentally-based RDoC-oriented research are introduced
Acknowledgments
We thank Drs. Marjorie Garvey and Daniel Pine for comments on an earlier version of this manuscript. Dr. Mittal was supported by NIMH R21/33 MH103231 and R01 R01MH094650. Dr. Wakschlag was supported by NIMH 2U01MH082830 and R01MH107652-01A.
Footnotes
A formal discussion of environment/context is outside the scope of this commentary. However, this dimension would also benefit from more detailed operationalization within the RDoC framework. None of the RDoC components can be fully realized without careful consideration of the others. Please see Woody and Gibb 2014 for a more in-depth discussion.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Developmental disabilities research reviews. 2008;14(2):118–127. doi: 10.1002/ddrr.17. [DOI] [PubMed] [Google Scholar]
- Bernard JA, Dean DJ, Kent JS, Orr JM, Pelletier-Baldelli A, Lunsford-Avery JR, Gupta T, Mittal VA. Cerebellar networks in individuals at ultra high-risk of psychosis: impact on postural sway and symptom severity. Hum Br Map. 2014;35:4064–4078. doi: 10.1002/hbm.22458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Mittal VA. Updating the research domain criteria: the utility of a motor dimension. Psychol Med. 2015;45:2685–2689. doi: 10.1017/S0033291715000872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Nichols SR, Voss J, Zobel E, Carter AS, McCarthy KJ, Pine DS, Blair J, Wakschlag LS. Punishment insensitivity and impaired reinforcement learning in preschoolers. J Child Psychol Psychiatry. 2014;55:154–161. doi: 10.1111/jcpp.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, Poulton R. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biol Psychiatry. 2014;76:350–353. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Cortese L, Caligiuri MP, Malla AK, Manchanda R, Takhar J, Haricharan R. Relationship of neuromotor disturbances to psychosis symptoms in first-episode neuroleptic-naive schizophrenia patients. Schizophr Res. 2005;75:65–75. doi: 10.1016/j.schres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN. Translating intermediate phenotypes to psychopathology: the NIMH Research Domain Criteria. Psychophysiol. 2014;51:1205–1206. doi: 10.1111/psyp.12342. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Kent JS, Bernard JA, Orr JM, Gupta T, Pelletier-Baldelli A, Carol EE, Mittal VA. Increased postural sway predicts negative symptom progression in youth at ultrahigh risk for psychosis. Schizophr Res. 2015;162:86–89. doi: 10.1016/j.schres.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Mittal VA. Spontaneous parkinsonisms and striatal impairment in neuroleptic free youth at ultrahigh risk for psychosis. NPJ Schizophrenia. 2015:1. doi: 10.1038/npjschz.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Orr JM, Newberry RE, Mittal VA. Motor behavior reflects reduced hemispheric asymmetry in the psychosis risk period. Schizophr Res. 2016;170:137–142. doi: 10.1016/j.schres.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Teulings HL, Caligiuri M, Mittal VA. Handwriting analysis indicates spontaneous dyskinesias in neuroleptic naive adolescents at high risk for psychosis. Journal of visualized experiments: JoVE. 2013:e50852. doi: 10.3791/50852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch KM, Newell KM. Changes in the structure of children’s isometric force variability with practice. Journal of experimental child psychology. 2004;88(4):319–333. doi: 10.1016/j.jecp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Rock D, Roberts SA, Janal M, Kestenbaum C, Cornblatt B, Adamo UH, Gottesman II. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: the New York High-Risk Project. Am J Psychiatry. 2000;157:1416–1422. doi: 10.1176/appi.ajp.157.9.1416. [DOI] [PubMed] [Google Scholar]
- Falk TH, Tam C, Schwellnus H, Chau T. Grip force variability and its effects on children’s handwriting legibility, form, and strokes. Journal of biomechanical engineering. 2010;132(11):114504. doi: 10.1115/1.4002611. [DOI] [PubMed] [Google Scholar]
- Fish B. Infant predictors of the longitudinal course of schizophrenic development. Schizophr Bull. 1987;13:395–409. doi: 10.1093/schbul/13.3.395. [DOI] [PubMed] [Google Scholar]
- Franklin JC, Jamieson JP, Glenn CR, Nock MK. How developmental psychopathology theory and research can inform the research domain criteria (RDoC) project. J Clin Child Adolesc Psychol. 2015;44:280–290. doi: 10.1080/15374416.2013.873981. [DOI] [PubMed] [Google Scholar]
- Garber J. Classification of childhood psychopathology: a developmental perspective. Child Dev. 1984;55:30–48. [PubMed] [Google Scholar]
- Garvey M, Avenevoli S, Anderson K. The National Institute of Mental Health Research Domain Criteria and Clinical Research in Child and Adolescent Psychiatry. J Am Acad Child Adolesc Psychiatry. 2016;55:93–98. doi: 10.1016/j.jaac.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O, Bose S, Turkheimer F, Valli I, Egerton A, Stahl D, Valmaggia L, Allen P, Murray R, McGuire P. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol Psychiatry. 2011;16:885–886. doi: 10.1038/mp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isohanni M, Murray GK, Jokelainen J, Croudace T, Jones PB. The persistence of developmental markers in childhood and adolescence and risk for schizophrenic psychoses in adult life. A 34-year follow-up of the Northern Finland 1966 birth cohort. Schizophr Res. 2004;71:213–225. doi: 10.1016/j.schres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis--linking biology, pharmacology and phenomenology of psychosis. Schizophr Res. 2005;79:59–68. doi: 10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Phenomenology of Schizophrenia and the Representativeness of Modern Diagnostic Criteria. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2016.1976. Published online September 14, 2016. [DOI] [PubMed] [Google Scholar]
- Marcus J, Hans SL, Lewow E, Wilkinson L, Burack CM. Neurological findings in high-risk children: childhood assessment and 5-year followup. Schizophr Bull. 1985;11:85–100. doi: 10.1093/schbul/11.1.85. [DOI] [PubMed] [Google Scholar]
- Mennin DS, Fresco DM. What, Me Worry and Ruminate About DSM-5 and RDoC? The Importance of Targeting Negative Self-Referential Processing. Clin Psychol Sci Prac. 2013;20(3):258–267. doi: 10.1111/cpsp.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Neumann C, Saczawa M, Walker EF. Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Arch Gen Psychiatry. 2008;65:165–171. doi: 10.1001/archgenpsychiatry.2007.23. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Tessner KD, Trottman HD, Esterberg M, Dhrub SH, Simeonova DI, McMillan AL, Murphy E, Saczawa ME, Walker EF. Movement abnormalities and the progression of prodromal symptomatology in adolescents at risk for psychotic disorders. J Abnorm Psychol. 2007;116:260–267. doi: 10.1037/0021-843X.116.2.260. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Walker EF. Movement abnormalities predict conversion to Axis I psychosis among prodromal adolescents. J Abnorm Psychol. 2007;116:796–803. doi: 10.1037/0021-843X.116.4.796. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schiz Bull. 2008;34(6):1083–1094. doi: 10.1093/schbul/sbn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Walker EF. Movement abnormalities: a putative biomarker of risk for Psychosis. In: Ritsner MSm., editor. The Handbook of Neuropsychiatric Biomarkers, Endophenotypes, and Genes. Springer; New York: 2009. pp. 239–258. [Google Scholar]
- Mittal VA, Walker EF. Minor physical anomalies and vulnerability in prodromal youth. Schiz Res. 2011;129(2):116–121. doi: 10.1016/j.schres.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Walker EF, Bearden CE, Walder D, Trottman H, Daley M, Simone A, Cannon TD. Markers of Basal Ganglia Dysfunction and Conversion to Psychosis: Neurocognitive Deficits and Dyskinesias in the Prodromal Period. Biol Psychiatry. 2010;68:93–99. doi: 10.1016/j.biopsych.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA. Cross-Cutting Advancements Usher in a New Era for Motor Research in Psychosis. Schizophrenia Bulletin. 2016:sbw123. doi: 10.1093/schbul/sbw123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier K, Lau C, Wang Y, Venkadesan M, Valero-Cuevas FJ. Controlling instabilities in manipulation requires specific cortical-striatal-cerebellar networks. J Neurophysiol. 2011;105:1295–1305. doi: 10.1152/jn.00757.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Mental Health. [accessed 01.05.16];RFA Dimensional Approaches to Research Classification in Psychiatric Disorders R01. 2015 http://grants.nih.gov/grants/guide/rfa-files/RFA-MH-16-510.html.
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol Psychiatry. 2005;57:1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Pappa S, Dazzan P. Spontaneous movement disorders in antipsychotic-naive patients with first-episode psychoses: a systematic review. Psychol Med. 2009;39:1065–1076. doi: 10.1017/S0033291708004716. [DOI] [PubMed] [Google Scholar]
- Quinn J, Meagher D, Murphy P, Kinsella A, Mullaney J, Waddington JL. Vulnerability to involuntary movements over a lifetime trajectory of schizophrenia approaches 100%, in association with executive (frontal) dysfunction. Schizophr Res. 2001;49:79–87. doi: 10.1016/s0920-9964(99)00220-0. [DOI] [PubMed] [Google Scholar]
- Rogers J, Viding E, Blair RJ, Frith U, Happe F. Autism spectrum disorder and psychopathy: shared cognitive underpinnings or double hit? Psychol Med. 2006;36:1789–1798. doi: 10.1017/S0033291706008853. [DOI] [PubMed] [Google Scholar]
- Rosso IM, Bearden CE, Hollister JM, Gasperoni TL, Sanchez LE, Hadley T, Cannon TD. Childhood neuromotor dysfunction in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull. 2000;26:367–378. doi: 10.1093/oxfordjournals.schbul.a033459. [DOI] [PubMed] [Google Scholar]
- Rutter M, Sroufe LA. Developmental psychopathology: concepts and challenges. Dev Psychopathol. 2000;12:265–296. doi: 10.1017/s0954579400003023. [DOI] [PubMed] [Google Scholar]
- Schiffman J, Walker E, Ekstrom M, Schulsinger F, Sorensen H, Mednick S. Childhood videotaped social and neuromotor precursors of schizophrenia: a prospective investigation. Am J Psychiatry. 2004;161:2021–2027. doi: 10.1176/appi.ajp.161.11.2021. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Kötter R. The human connectome: a structural description of the human brain. PLoS Comput Biol. 2005;1(4):e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Estabrook R, Petitclerc A, Henry D, Burns JL, Perlman SB, Voss JL, Pine DS, Leibenluft E, Briggs-Gowan ML. Clinical Implications of a Dimensional Approach: The Normal:Abnormal Spectrum of Early Irritability. J Am Acad Child Adolesc Psychiatry. 2015;54:626–634. doi: 10.1016/j.jaac.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Hans SL. Relation of maternal responsiveness during infancy to the development of behavior problems in high-risk youths. Dev Psychol. 1999;35:569–579. doi: 10.1037//0012-1649.35.2.569. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Tolan PH, Leventhal BL. Research Review: ‘Ain’t misbehavin’: Towards a developmentally-specified nosology for preschool disruptive behavior. J Child Psychol Psychiatry. 2010;51:3–22. doi: 10.1111/j.1469-7610.2009.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag L, Perlman S, Leibenluft E, Blair J, Briggs-Gowan M, Pine D. Specifying the neurodevelopmental in early childhood onset mental disorders: Early emerging irritable and callous as exemplars under review. [Google Scholar]
- Walker EF. Developmentally moderated expressions of the neuropathology underlying schizophrenia. Schizophr Bull. 1994;20:453–480. doi: 10.1093/schbul/20.3.453. [DOI] [PubMed] [Google Scholar]
- Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20:441–451. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Sci. 2008;320(5875):539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Dieterich R, Riesel A. Error-related brain activity in the age of RDoC: a review of the literature. Inter J Psychophysiol. 2015;98(2):276–299. doi: 10.1016/j.ijpsycho.2015.02.029. [DOI] [PubMed] [Google Scholar]