Abstract
Galactosemia is an inborn error of galactose metabolism caused by mutations in the GALT gene. Though early detection and galactose restriction prevent severe liver disease, affected individuals have persistently elevated biomarkers and often neuro-developmental symptoms. We present a teenage compound heterozygote for a known pathogenic mutation (H132Q) and a novel variant of unknown significance (S222N), with nearly absent erythrocyte GALT enzyme activity but normal biomarkers and only mild anxiety despite diet non-adherence. This case is similar to a previously reported S135L mutation. In this report we investigate the novel S222N variant and critically evaluate a clinically puzzling case.
Keywords: Galactosemia, Pathogenesis, Diagnosis
1. Introduction
1.1. Clinical background information
Galactosemia (OMIM# 230400) is an autosomal recessive inborn error of metabolism due to decreased or absent galactose-1-phosphate uridyltransferase (GALT) enzyme activity, secondary to mutations in the GALT gene [1], [2], [3]. The disease occurs in approximately 1 in 14,000 to 1 in 80,000 live births, varying widely in incidence and mutation type among populations and ethnic groups [4], [5]. Galactosemia typically presents in the neonatal period, following ingestion of dietary galactose, with jaundice, hepatosplenomegaly, poor feeding, failure to thrive, sepsis, cataracts, renal tubular dysfunction, and hypotonia [1], [2]. Restriction of dietary galactose is the mainstay of treatment, though it does not appear to prevent long term complications, including intellectual and motor developmental delays, verbal dyspraxia, tremor, decreased IQ, hypogonadic hypogonadism in females, and anxiety found in most, but not all patients [6], [7], [8], [9]. These complications are believed to be the result of endogenous galactose production [10]. In people with galactosemia, by-products of abnormal galactose metabolism accumulate and can be measured in urine in the form of galactitol or in serum as galactose-1-phosphate. With appropriate treatment these levels decrease but remain above the upper limit of normal for non-galactosemics [10], [11].
The atypical galactosemic phenotype presents with loss of GALT activity in erythrocytes but approximately 10% activity in the liver and small intestine, as first reported in cases of the S135L mutation [12], [13], [14], [15], [16]. While the original brothers reported with this mutation were not galactose-restricted in the first three weeks of life and suffered neurocognitive deficits, other patients treated from birth are reportedly free of the lifelong sequelae seen in patients with typical galactosemia [13], [16], [17].
1.2. Biochemical description
GALT is located on chromosome 9p13.3, spans 4.3 kb of DNA, and contains 11 exons [18]. The protein product catalyzes the second step of galactose metabolism [19]. The GALT enzyme is a homodimer with a well-studied mechanism. The structure of GALT was solved in 1995, and subsequent analyses correlated abnormal enzyme structure with disease-causing mutations [20], [21], [22], [23]. A large number of GALT mutations have been reported, with 266 variants published in the ARUP GALT database to date [24]. The majority of these variants are missense mutations (69%). The remaining variants consist of nearly equal proportions of deletions, insertions, silent, splice site and nonsense mutations (3–8% each).
2. Case report
Our patient is a 19-year-old male who, shortly after birth, had a positive newborn screen for galactosemia. Follow-up testing revealed undetectable GALT enzyme activity. DNA studies performed at that time did not identify any copies of the common Q188R, N314D or S135L GALT mutations. He was treated with a galactose free diet starting in his first week of life and had regular biochemical testing of galactose-1-phosphate, which remained in the normal range throughout his childhood. At the age of 3, his family slowly moved away from a strict galactose restriction, with no increase in biochemical markers. Due to the family frequently relocating he was lost to clinical follow-up, and around age 10 his diet was liberalized completely. He had no history of cataracts, liver disease, or developmental delay. Growth was normal, with weight at the 30%, height at the 40% and BMI at the 50% for age. He has been a high achiever academically. His main medical concern has been anxiety, for which he only recently decided to seek treatment.
This patient presented to our metabolic clinic at the age of 17, wanting to better understand his disease before entering college. His labs at this time were notable for normal transaminases, a normal galactose-1-phosphate level of 0.18 mg (Normal: < 1.0 mg; Galactosemics on lactose-free diet: 2–5 mg), and urine galactitol of 19 mmol/mol Cr, which is at the upper limit of the normal range for non-galactosemics (Non-galactosemic controls > 6 years old: < 2–19 mmol/mol Cr; Galactosemics > 6 years old: < 98–222 mmol/mol Cr). GALT enzyme activity in erythrocytes was 0.3 U/g Hb (normal > 18.5 U/g Hb), consistent with a diagnosis of classic galactosemia. GALT gene sequencing revealed one classic deleterious mutation (c.396C > A; p.H132Q) and one variant of unknown significant (c.665G > A; p.S222N). His father, who is of mixed European ancestry, was found to carry the p.H132Q mutation, with an expected ~ 50% decrease in GALT enzyme activity (9.7 U/g Hb). His mother, of Mexican and Filipino ancestry, remains unavailable for testing.
Given the discrepancy between our patient's biochemical testing and clinical history, further investigational studies were performed. We hypothesized that the c.665G > A variant, located in exon 7, might result in a splicing defect. We noted that, although the c.665G > A variant did not create or destroy a predicted splice site, it did eliminate a cluster of predicted exon splice enhancers (ESEs) (Data in Brief).
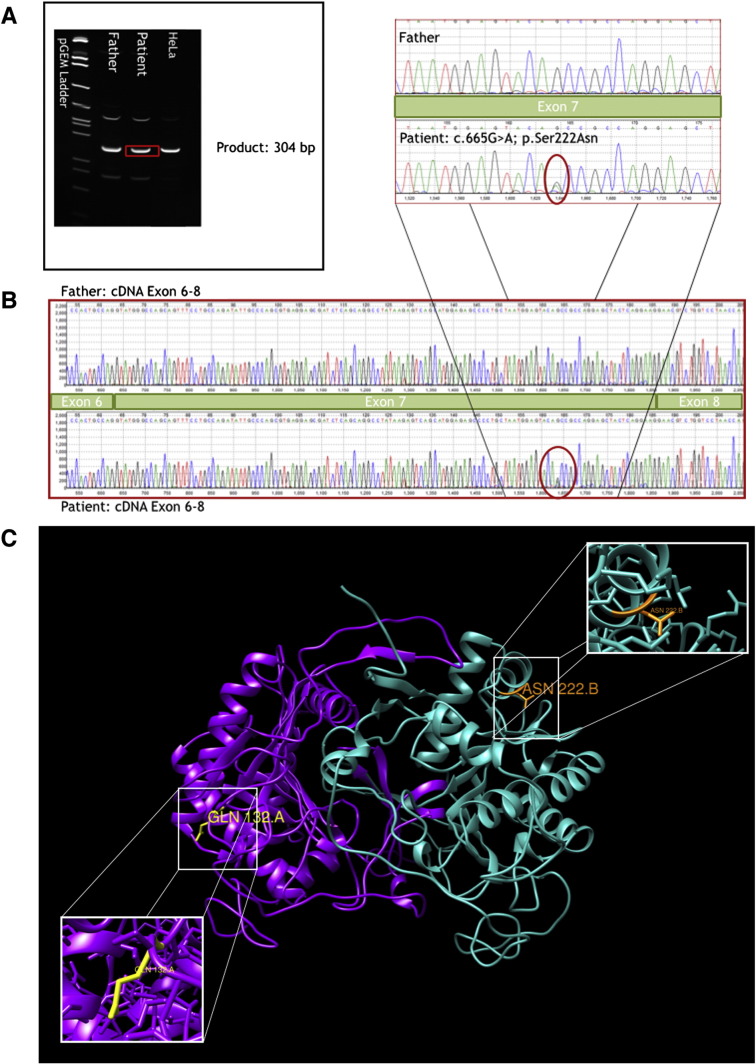
In order to test this hypothesis, RNA was isolated from the whole blood sample, a cDNA library was created, and PCR was used to amplify the entire gene in several overlapping pieces. No shift in cDNA size was seen, which would be expected in the case of interrupted or aberrant splicing (Fig. 1a). The cDNA was sequenced to look for splicing defects that may not be apparent on gel electrophoresis, but there were no changes in exon structure from the reference or the father's cDNA sequence (Fig. 1b). The only base pair change identified was the c.665G > A variant itself.
Fig. 1.
Molecular and in silico analyses of GALT variant. (A) RT-PCR electrophoresis shows no shift in GALT cDNA size between father, patient, and control (HeLa) cells. (B) cDNA sequencing shows that exons 6, 7, and 8 are intact with no exon skipping event. The S222N variant of uncertain significant (VUS) was detected. (C) View of S222N/H132Q heterodimer with mutation locations annotated. Chain A is depicted in purple with GLN 132 highlighted in yellow. Chain B is depicted in cyan with ASN 222 highlighted in orange. Magnified views of each variant residue of interest are provided. Modeled using UCSF Chimera Software [16], [27]. Animation of model available in data in brief.
An in silico analysis of the variants present in our patient was performed (Fig. 1c). Given his genetic background, there are three potential dimeric structures; (i) an H132Q homodimer, (ii) an S222N homodimer, and (iii) an H132Q/S222N heterodimer. Simple side chain replacement and structural optimization were performed with the Modeller program using the “Mutate model” script for each [21], [22], [25]. The H132Q homodimer is a known pathogenic mutation that destabilizes the protein structure. The S222N homodimer is predicted to destabilize the protein according to our model. The H132Q/S222N heterodimer, however is not predicted to destabilize the protein or interrupt any of the active sites. In addition, residues 132 and 222 appear too far from one another in the folded structure to interact directly.
3. Discussion
Here we describe a patient with nearly absent erythrocyte GALT activity but without any clinical or biochemical evidence for disease, other than anxiety, who is a compound heterozygote for a classic GALT mutation (H132Q) and a novel S222N variant. Though enzyme activity levels were not directly measured in tissues other than erythrocytes in our patient, it appears that the S222N variant may result in tissue-specific GALT enzyme levels, similar to patients with the S135L mutation reported first by Segal and colleagues in the 1960s and later sequenced in the lab of Elsas in 1996 [12], [13], [16]. The S135L mutation is found almost exclusively in patients with African ancestry and leads to loss of GALT activity in erythrocytes without loss of whole body galactose oxidation [16], [17].
A recent report of an Irish infant of African origin with the S135L mutation highlights the importance of early identification and treatment of patients with this mutation [28]. This infant was a false negative on the Irish newborn screen. The test assayed for free galactose and not enzyme activity, which picks up the highly prevalent Q188R mutation phenotype found in the Irish Traveller population but does not identify the S135L mutation. The infant was diagnosed at 16-months-old when he presented with developmental delay and failure to thrive. Following galactose restriction, the infant has made a near complete recovery, barring neurocognitive deficits which, at the time of publication, were still concerning. In contrast, patients with the S135L mutation identified by newborn screen and treated from birth have very good outcomes with no stigmata of galactosemia [16], [17]. The neonatal period appears to be the most critical time for galactose restriction in this population.
Our initial hypothesis was that the S222N variant results in tissue-specific splicing that could explain the loss of GALT activity in erythrocytes but lack of symptoms in our patient. This hypothesis, however, contradicts our experimental data. The in silico models of our patient's GALT enzyme structure revealed that he is able to produce two homodimers and one heterodimer. Both homodimers are predicted to destabilize the protein structure [21], [22]. Thus, it is reasonable to assume that the primary enzyme responsible for GALT activity in his case is the H132Q/S222N heterodimer (Animation 1, Data in Brief). It is possible that the mutation combination leads to a protein that is unstable in the oxygen rich environment of the erythrocyte, but not in hepatocytes. It is also possible that the turnover of the GALT protein in hepatocytes is significantly higher than in erythrocytes, which are enucleated. Regardless, this unique protein variant would be an interesting candidate for further investigation. While we did not feel it ethical to perform a liver biopsy on an otherwise healthy young man, analysis of hepatocytes would clearly contribute to our understanding of this variant on both the molecular and clinical levels.
It is noteworthy that our patient reports anxiety. In a recent study of the adult galactosemic phenotype, 67% of patients reported anxiety, which is a 4-fold increase over the yearly prevalence of 16.4% in the general population [9], [26]. If our patient's anxiety is related to his GALT mutation, it is possible that the S222N/H132Q combination contributes to the neuropsychiatric phenotype. This is, of course, speculative at this point, especially given the large prevalence of anxiety in the general population. Identification and analysis of additional patients with this variant are needed to validate the phenotypic expression. If this is the case, galactose restriction may be of therapeutic benefit for our patient in reducing anxiety.
Further classification is important for complete elucidation of the mechanism underlying atypical galactosemia as seen previously in the S135L mutation and now in our H132Q/S222N variant. It is unclear whether our patient required urgent treatment from birth, as is beneficial for patients with the S135L mutation, or if residual GALT activity is sufficient for protection in the neonatal period. For the sake of future patients with this mutation, dietary restriction of galactose in the neonatal period and early childhood is recommended, though, lifelong dietary restriction may not be necessary. The biochemical nature of this variant remains unclear, though this report provides fertile ground for future experimentation into the nature of mutations underlying the atypical galactosemic phenotype.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ymgmr.2014.12.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Supplementary video 1.
Supplementary video 2.
References
- 1.Waggoner D.D., Buist N.R., Donnell G.N. Long-term prognosis in galactosaemia: results of a survey of 350 cases. J. Inherit. Metab. Dis. 1990;13(6):802–818. doi: 10.1007/BF01800204. [DOI] [PubMed] [Google Scholar]
- 2.Schweitzer S., Shin Y., Jakobs C., Brodehl J. Long-term outcome in 134 patients with galactosaemia. Eur. J. Pediatr. 1993;152(1):36–43. doi: 10.1007/BF02072514. [DOI] [PubMed] [Google Scholar]
- 3.Fridovich-Keil J.L., Walter J.H. Galactosaemia chapter 72. In: Valle D., Vogelstein B., Kinzler K.W., Antonarakis S.E., Ballabio A., editors. The Online Metabolic and Molecular Bases of Inherited Disease, OMMBID. McGraw Hill; New York: 2008. (Part 7: Carbohydrates). [Google Scholar]
- 4.Bosch A.M., Ijlst L., Oostheim W., Mulders J., Bakker H.D., Wijburg F.A., Wanders R.J., Waterham H.R. Identification of novel mutations in classical galactosemia. Hum. Mutat. 2005;25(5):502. doi: 10.1002/humu.9330. [DOI] [PubMed] [Google Scholar]
- 5.Jumbo-Lucioni P.P., Garber K., Kiel J., Baric I., Berry G.T., Bosch A., Burlina A., Chiesa A., Pico M.L., Estrada S.C., Henderson H., Leslie N., Longo N., Morris A.A., Ramirez-Farias C., Schweitzer-Krantz S., Silao C.L., Vela-Amieva M., Waisbren S., Fridovich-Keil J.L. Diversity of approaches to classic galactosemia around the world: a comparison of diagnosis, intervention, and outcomes. J. Inherit. Metab. Dis. 2012;35(6):1037–1049. doi: 10.1007/s10545-012-9477-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufman F.R., Kogut M.D., Donnell G.N., Goebelsmann U., March C., Koch R. Hypergonadotropic hypogonadism in female patients with galactosemia. N. Engl. J. Med. 1981;304:994–998. doi: 10.1056/NEJM198104233041702. [DOI] [PubMed] [Google Scholar]
- 7.Rubio-Gozalbo M.E., Gubbels C.S., Bakker J.A., Menheere P.P., Wodzig W.K., Land J.A. Gonadal function in male and female patients with classic galactosemia. Hum. Reprod. Update. 2010;16:177–188. doi: 10.1093/humupd/dmp038. [DOI] [PubMed] [Google Scholar]
- 8.Berry G.T., Walter J.H. Disorders of galactose metabolism. In: Fernandes J., van der Berghe G., Walter J.H., editors. Inborn Metabolic Diseases — Diagnosis and Treatment (5th edn) Springer; New York, NY: 2011. [Google Scholar]
- 9.Waisbren S.E., Potter N.L., Gordon C.M., Green R.C., Greenstein P., Gubbels C.S., Rubio-Gozalbo E., Schomer D., Welt C., Anatasoaie V. The adult galactosemic phenotype. J. Inherit. Metab. Dis. 2012;35(2):279–286. doi: 10.1007/s10545-011-9372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry G.T., Nissim I., Zhiping L., Mazur A.T., Gibson J.B., Segal S. Endogenous synthesis of galactose in normal men and patients with hereditary galactosaemia. Lancet. 1995;346:1073–1074. doi: 10.1016/s0140-6736(95)91745-4. [DOI] [PubMed] [Google Scholar]
- 11.Jakobs C., Schweitzer S., Dorland B. Galactitol in galactosemia. Eur. J. Pediatr. 1995;154(Suppl. 2):s50–s52. doi: 10.1007/BF02143804. [DOI] [PubMed] [Google Scholar]
- 12.Segal S., Blair A., Roth H. The metabolism of galactose by patients with congenital galactosemia. Am. J. Med. 1965;38:62. doi: 10.1016/0002-9343(65)90160-9. [DOI] [PubMed] [Google Scholar]
- 13.Baker L., Mellman W.J., Tedesco T.A., Segal S. Galactosemia: symptomatic and asymptomatic homozygotes in one Negro sibship. J. Pediatr. 1966;68:551–558. (1966) [Google Scholar]
- 14.Segal S., Cuatrecasas P. The oxidation of C 14 galactose by patients with congenital galactosemia. Am. J. Med. 1968;44:340–347. [Google Scholar]
- 15.Segal S., Rogers S., Holtzapple P.G. Liver galactose-1-phosphate uridyl transferase: activity in normal and galactosemic subjects. J. Clin. Invest. 1971;50:500–506. doi: 10.1172/JCI106518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai K., Langley S.D., Singh R.H., Dembure P.P., Hjelm L.N., Elsas L.J. A prevalent mutation for galactosemia among black Americans. J. Pediatr. 1996;128(1):89–95. doi: 10.1016/s0022-3476(96)70432-8. [DOI] [PubMed] [Google Scholar]
- 17.Manga N., Jenking T., Jackson H., Whittaker D.A., Lane A.B. The molecular basis of transferase galactosaemia in South African negroids. J. Inherit. Metab. Dis. 1999;22(1):37–42. doi: 10.1023/a:1005491014280. [DOI] [PubMed] [Google Scholar]
- 18.Bosch A.M. Classical galactosaemia revisited. J. Inherit. Metab. Dis. 2006;29(4):516–525. doi: 10.1007/s10545-006-0382-0. (Aug) [DOI] [PubMed] [Google Scholar]
- 19.Leloir L. Two decades of research on the biosynthesis of saccharides. Science. 1971;172:1299–1303. doi: 10.1126/science.172.3990.1299. [DOI] [PubMed] [Google Scholar]
- 20.Wedekind J.E., Frey P.A., Rayment I. Three-dimensional structure of galactose-1-phosphate uridylyltransferase from Escherichia coli at 1.8 A resolution. Biochemistry. 1995;34:11049–11061. doi: 10.1021/bi00035a010. (1 1049) [DOI] [PubMed] [Google Scholar]
- 21.Marabotti A., Facchiano A.M. Homology modeling studies on human galactose-1-phosphate uridylyltransferase and on its galactosemia-related mutant Q188R provide an explanation of molecular effects of the mutation on homo- and heterodimers. J. Med. Chem. 2005;48:773–779. doi: 10.1021/jm049731q. [DOI] [PubMed] [Google Scholar]
- 22.d'Acierno A., Facchiano A., Marabotti A. GALT protein database: querying structural and functional features of GALT enzyme. Hum. Mutat. 2014;35:1060–1067. doi: 10.1002/humu.22613. [DOI] [PubMed] [Google Scholar]
- 23.McCorvie T.J., Gleason T.J., Fridovich-Keil J.L., Timson D.J. Misfolding of galactose 1-phosphate uridylyltransferase can result in type I galactosemia. Biochim. Biophys. Acta. 2013;1832:1279–1293. doi: 10.1016/j.bbadis.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calderon F.R., Phansalkar A.R., Crockett D.K., Miller M., Mao R. Mutation database for the galactose-1-phosphate uridyltransferase (GALT) gene. Hum. Mutat. 2007;28(10):939–943. doi: 10.1002/humu.20544. (Oct) [DOI] [PubMed] [Google Scholar]
- 25.Eswar N., Marti-Renom M.A., Webb B., Madhusudhan M.S., Eramian D., Shen M., Pieper U., Sali A. Comparative protein structure modeling with MODELLER. Curr. Protoc. Bioinforma. 2006;(Suppl. 15) doi: 10.1002/0471250953.bi0506s15. (John Wiley & Sons, Inc., 5.6.1-5.6.30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surgeon General . U.S. Department of Health and Human Services; Washington, DC.: 2009. Mental Health: A Report of the Surgeon General — Chapter 4. [Google Scholar]
- 27.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera — a visualization system for exploratory research and analysis. J. Comput. Chem. Oct 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 28.Crushell E., Chukwu J., Mayne P., Blatny J., Treacy E. Negative screening tests in classical galactosaemia caused by S135L homozygosity. J. Inherit. Metab. Dis. 2009;32(3):412–415. doi: 10.1007/s10545-009-1081-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary video 1.
Supplementary video 2.