Abstract
We report on a 6 year old boy with severe MPS II undergoing immune modulation therapy due to high IgG antibody titers to IV idursulfase and no significant decline in urinary GAG levels since initiating enzyme replacement therapy. He has complete deficiency of iduronate-2-sulfatase activity due to a submicroscopic deletion of the X chromosome involving the entire I2S gene but not including in the fragile X locus. At 19 months of age, IV idursulfase therapy at the recommended dose of 0.5 mg/kg/week was initiated and then increased to 1.0 mg/kg/week after no observed clinical improvement and no decline in urine GAG level. After one year of ERT at the increased dose, he had no significant decline in urinary GAG excretion and increase of anti-idursulfase IgG antibody titers to 102,000 with complete neutralizing antibodies. In light of the evidence of lack of efficacy of idursulfase therapy, the patient was started on an immune modulation regimen consisting of ofatumumab, bortezomib, methotrexate and IVIG for a 12 week period. Only a slight decrease in IgG titers and urine GAG levels was observed, leading to increased intensity of bortezomib administration and addition of dexamethasone to the regimen, while continuing with the current schedule ofatumumab, IVIG and methotrexate. Over 18 month period of immune modulation therapy, we observed a significant reduction in anti-idursulfase IgG titers and a moderate reduction in urine GAG levels compared to baseline. Modest clinical improvements were observed. Our experience suggests that future MPS II patients with a complete gene deletion may be likely to develop persistent anti-idursulfase antibody titers and may benefit from immune modulation therapy prior to the development of high titer levels.
Keywords: MPS II, Immune modulation, Anti-idursulfase antibody titer, Enzyme replacement therapy, Treatment
1. Introduction
Mucopolysaccharidosis type II (MPS II), also referred to as Hunter syndrome, is an X-linked recessive lysosomal storage disorder caused by a deficiency of the iduronate 2-sulfatase (I2S) enzyme. The incidence is estimated to range from 1/140,000–1/320,000 live births (1/72,000–1/165,000 live male births) in various populations [1], [2], [3], [4]. The diagnosis can be established by demonstrating elevated heparan sulfate and dermatan sulfate levels on urine glycosaminoglycan (GAG) analysis and deficient I2S activity in leukocytes or cultured fibroblasts or detection of a pathogenic mutation in the IDS gene. The condition is characterized by macrocephaly, coarse facial features, hepatosplenomegaly, dysostosis multiplex, short stature, multiple joint restrictions, central nervous system abnormalities, hearing loss, progressive airway disease, and cardiac disease [5], [6], [7], [8]. In MPS II, severely affected patients are distinguished from those with the attenuated form of the disorder based on the presence of CNS involvement with neurological regression. Patients with partial or complete IDS gene deletions have no residual enzyme activity and a severe phenotype [9]. Regardless of the absence or presence of CNS disease, the systemic health issues are progressive in all patients and are a significant cause of morbidity.
In July 2006, idursulfase was approved by the US Food and Drug Administration as enzyme replacement therapy for MPS II and an EU marketing authorization was issued in January 2007. Weekly peripheral intravenous administration of idursulfase improves some of the systemic manifestations of disease, most notably cardiopulmonary function [10], [11]. Patients receiving therapy often have significant reduction in urine GAG levels [10], [11], [22], some to normal levels. Up to 50% of patients receiving idursulfase test positive for IgG antibodies to idursulfase [10], [11]. In the long-term, open-label extension study of idursulfase treatment, approximately 23% of patients were positive for neutralizing antibodies which seemed to be a significant factor in drug response, with slightly less improvement in pulmonary function tests compared to neutralizing antibody negative patients [11]. The authors however, reported that neutralizing antibodies did not appear to affect urinary GAG levels, reduction of liver and spleen size, and performance on the 6 minute walk test.
We report our experience with use of an immune modulation protocol to significantly reduce anti-idursulfase antibodies in a severe MPS II patient with sustained high antibody titers and limited clinical efficacy of idursulfase treatment. A 19 month old boy presenting with coarse facial features, obstructive sleep apnea, hepatomegaly, and delayed motor milestones was initially diagnosed with MPS II by elevated urine GAG levels and deficient iduronate-2-sulfatase activity. He was subsequently found by IDS gene duplication/deletion testing to have a complete gene deletion. Chromosome microarray analysis further revealed a 680 kb deletion at Xq28, encompassing both IDS and IDS2 genes but excluding the FMR1 gene for fragile X syndrome. He was started on IV idursulfase therapy at the recommended dose of 0.5 mg/kg/week. No reduction in urinary GAG excretion was observed after two years of therapy and rising titers of IgG antibodies to idursulfase were detected in plasma. At 3 years of age, the dose of idursulfase was increased to 1.0 mg/kg/week. This was accompanied by mild infusion reactions, easily managed with diphenhydramine. After one year of therapy at the increased dose, there was no significant decline in urinary GAG excretion and the titer of IgG antibodies to idursulfase was further increased to 1:102,000 with complete neutralizing antibody activity. IgE antibodies were also detected at a titer of 1:160 on a few occasions. Examination at 4 years 9 months of age revealed typical MPS facies, hepatomegaly with liver edge palpable 4 cm below right costal margin, generalized joint restriction, stiff, incurving fingers, and tight Achilles tendons with toe walking. He had previously undergone VP shunt placement for asymptomatic elevated intracranial pressure and surgical repair of bilateral carpal tunnel syndrome at 3 years of age. He wore hearing aids for bilateral sensorineural hearing loss. Echocardiograms were essentially unremarkable. The patient was developmentally delayed but still gaining skills. He had several signs and spoke several single words. He was walking independently and climbing stairs.
Based on the high anti-idursulfase IgG titers, mild infusion related reactions, lack of reduction of urinary GAG levels, and minimal clinical response to therapy, we initiated an immune tolerance induction regimen similar to that used in severe infantile Pompe disease patients with negative cross reactive immunologic material (CRIM) [12], [13], [14] in our patient at 4 years 11 months of age. Patients with infantile onset Pompe disease and CRIM negative status have been reported to have reduced clinical response to enzyme replacement therapy with human recombinant acid α glucosidase (rhGAA) due to the development of sustained high antibody titers to the drug. Messinger et al. [14] reported successful reduction and/or elimination of antibody titers in 4 CRIM negative Pompe patients after treatment with rituximab, methotrexate and intravenous immunoglobulin (IVIG) and improved clinical response. Immune tolerance induction (ITI) was initiated in 2 patients shortly after antibody titers were detected while 2 patients underwent ITI at time of initiation of ERT. Negative antibody titers were sustained in all patients several years after completion of ITI and B-cell recovery. Subsequently, Banugaria et al. [18] reported successful use of bortezomib along with a similar immune modulation protocol in 3 infantile Pompe patients with high sustained antibody titers and a decline in clinical response to enzyme replacement therapy.
2. Methods
2.1. Immune modulation protocol
The anti-CD20 humanized monoclonal antibody ofatumumab (Table 1a) was administered at a dose of 173 mg/m2 at week 1 and then at 1160 mg/m2 for all other treatment weeks (2, 3, 4, and then every 4 weeks). Bortezomib was initially administered at a dose of 1.3 mg/m2 weekly at weeks 1–4 and 9–12. Methotrexate 0.5 mg/kg was administered by mouth weekly. IVIG infusions were administered every 4 weeks at 500 mg/kg. Since the titer of anti-idursulfase antibodies remained too high, between 1:102,400 and 1:51,200, the regimen was intensified (Table 1b). Bortezomib 1.3 mg/m2 was administered twice weekly at weeks 15–16, 18–20, 34–35, 38–39, 69–70, and 73–74. Dexamethasone was added for days 1–4 of treatment weeks 15, 18, 34, 38, 69 and 73. Following the decline of antibody titers, the patient now continues on maintenance therapy of weekly methotrexate, monthly IVIG, and quarterly ofatumumab infusions (Table 1c). Maintenance therapy for one year was recommended to prevent redevelopment of antibodies since the patient never achieved complete elimination of anti-idursulfase antibodies after induction and intensified treatment regimens.
Table 1.
Immune modulation regimens.
|
A. Induction | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Ofatumumab (Ofa) IV 1160 mg/m2/dose | Ofa | Ofa | Ofa | Ofa | Ofa | Every 4 weeks | |||||||
| Bortezomib (BTZ) IV 1.3 mg/m2 days weekly | BTZ | BTZ | BTZ | BTZ | BTZ | BTZ | BTZ | BTZ | |||||
| Immunoglobulin (IVIG) 500 mg/kg | IVIG every 4 weeks
|
||||||||||||
| Methotrexate (Mtx) 0.5 mg/kg PO | Mtx weekly
|
||||||||||||
| B. Intensification | |||||||||||||
| Day | 1 | 2 | 3 | 4 | 8 | 11 | // | 22 | 23 | 24 | 25 | 29 | 32 |
| Bortezomib (BTZ) IV 1.3 mg/m2 days 1,4, 8 and 11 | BTZ | BTZ | BTZ | BTZ | BTZ | BTZ | BTZ | BTZ | |||||
| Dexamethasone (Dex) PO 25 mg/m2/day days 1–4; 21 day cycle | Dex | Dex | Dex | Dex | Dex | Dex | Dex | Dex | |||||
| Ofatumumab (Ofa) IV 1160 mg/m2/dose | Ofat every 4 weeks
|
||||||||||||
| Immune globulin (IVIG) 500 mg/kg | IVIG every 4 weeks
|
||||||||||||
| Methotrexate (Mtx) 0.5 mg/kg PO | Mtx weekly
|
||||||||||||
| C. Maintenance | |||||||||||||
| Ofatumumab (Ofa) IV 1160 mg/m2/dose | every 12 weeks for 52 weeks | ||||||||||||
| Immune globulin (IVIG) 500 mg/kg | every 4 weeks for 52 weeks | ||||||||||||
| Methotrexate (Mtx) 0.5 mg/kg PO | weekly for 52 weeks | ||||||||||||
The patient continued to receive weekly infusions of idursulfase at 1 mg/kg. Premedications included corticosteroids, anti-pyretic, and anti-histamines. Weekly antibiotic prophylaxis with sulfamethoxazole–trimethoprim was administered. Monthly laboratory studies to monitor treatment response included CBC with differential, comprehensive metabolic panel, serum IgG, IgA, IgM and IgE levels, serum IgG and IgE titers to idursulfase, and urine GAG levels. Peripheral B-cell numbers were determined from the number of CD20 lymphocytes at the flow cytometry laboratory of Mayo Medical Laboratories, Rochester, MN using standard techniques.
2.2. Detection and characterization of anti-idursulfase IgG and IgE antibodies
Serum samples were collected prior to idursulfase IV infusion and sent to Shire HGT for analysis. A tier-based approach was used for detection and characterization of anti-idursulfase antibodies (Ab) as described previously [15]. Samples were first screened for the presence of anti-idursulfase Ab at the minimum required dilution (MRD) either by a conformation specific antibody assay or enzyme-linked immunoabsorbent assay. The samples with results below assay cut point were reported as Ab negative. The Ab status for samples with results equal to or above the assay cut point was confirmed by a radioimmunoprecipitation (RIP) assay. If the RIP result was also below the confirmatory assay cut point, the sample was reported as Ab negative. If the results were equal to or above the confirmatory assay cut point, the samples were reported as Ab positive, and retested with the same assay format by serial dilution to determine Ab titer. The Ab titers were reported as the reciprocal of the highest sample dilution factor with a positive Ab response.
All confirmed Ab positive samples were further evaluated for the presence of neutralizing antibodies (NAb) using an enzymatic activity assay with 4-methylumbelliferyl-sulfate as a substrate. Samples were reported as NAb negative when percent inhibition was below the NAb assay cut point.
2.3. Urine GAG quantitation
Urine GAG concentration was determined by the dimethylmethylene blue (DMB) dye-binding method with the addition of Tris base to the DMB solution to prevent interference from urinary proteins [16], [17]. The urine GAG concentration was reported relative to the urine creatinine concentration as μg GAG/mg creatinine. Analysis was provided by Shire HGT.
3. Results
3.1. Anti-idursulfase antibody levels
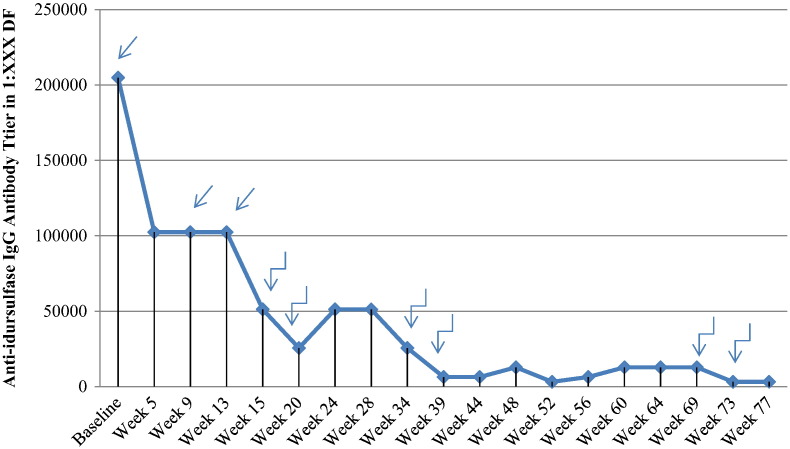
B cell lymphocytes were eliminated after 1 month (absolute CD 20 count of 0 cell/μL). Serum anti-idursulfase IgG titer at the start of immune modulation therapy was 1:204,800 with complete neutralizing activity. The IgE titer level was negative. After the initial 12 week immune modulation treatment course, our patient had a minimal decline in the serum anti-idursulfase IgG titer level to 1:102,400 and therefore, an intensified bortezomib and dexamethasone treatment was initiated at week 15. After 73 weeks of treatment, a sustained significantly reduced anti-idursulfase IgG titer of 1:3200 was achieved allowing reduction of immune modulation therapy (Fig. 1).
Fig. 1.
Anti-idursulfase IgG titer levels in DF.
 Induction regimen.
Induction regimen.  Intensification regimen.
Intensification regimen.
3.2. Urine GAG levels
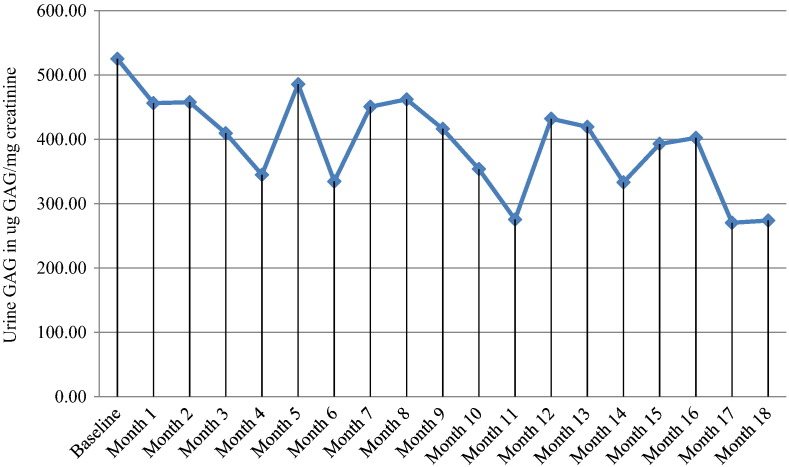
The patient's baseline urinary GAG level was 525.20 μg GAG/mg creatinine (normal < 127). Urinary GAG levels fluctuated during the immune modulation course with modest reduction of levels at treatment month 11 of 275.40 μg GAG/mg creatinine and treatment month 17 of 270.40 μg GAG/mg creatinine (Fig. 2).
Fig. 2.
Urinary GAG levels (μg GAG/mg creatinine).
3.3. Clinical response
Our patient demonstrated some modest clinical improvement during the immune modulation course. An abdominal ultrasound to characterize the liver size was performed prior to the start of treatment and at 6 months and 15 months. Liver size appeared smaller on the 15 month examination as compared to baseline. Reduction in liver size was also noted on physical examination. The patient was also observed to have a decreased number of respiratory and ear infections and increased energy level; he regained the ability to climb stairs.
4. Discussion
We report on our experience with immune modulation therapy in a severe MPS II patient with minimal clinical response to weekly peripheral IV idursulfase treatment and very high anti-idursulfase neutralizing antibody. Given the successful elimination of antibody titers in CRIM negative infantile Pompe patients using an immune tolerance induction protocol, we decided to follow a similar treatment course with our patient. A backbone of anti-CD20 monoclonal antibody, methotrexate and IVIG were used as previously described for patients with established antibody levels [14]. Ofatumumab (humanized anti-CD20 monoclonal antibody) was chosen over rituximab for several reasons. 1) It is a completely humanized anti-CD20 antibody, less likely to induce anti-monoclonal antibodies. 2) It binds a different epitope on the CD20 molecule and thus has a slower off rate and is more potent in inducing complement. 3) It has more potent NK-cell mediated ADCC and thus is independent of the FcγRIIIa 158V and FcγRIIIa 158F allotypes [19]. It has shown marked activity in lymphoma patients and is approved for the treatment of chronic lymphoblastic lymphoma (CLL). It was found to be highly effective in autoimmune diseases such as rheumatoid arthritis [20]. Bortezomib, a proteasome inhibitor, was added to the treatment regimen due to its ability to eliminate long lasting plasma cells. Banugaria et al. [18] reported successful use of bortezomib along with their standard immune modulation protocol in 3 infantile Pompe patients with high sustained antibody titers and decline in clinical response to enzyme replacement therapy. Bortezomib was felt to be safe and the authors suggested addition of bortezomib in patients with high antibody titers (≥ 1:51,200) at or beyond 6 months on enzyme replacement therapy and/or when an increasing trend or persistence of antibody titers is associated with clinical decline. We chose to intensify the bortezomib regimen with dexamethasone to enhance more rapid plasma cell death, and the intensification was modeled on established myeloma regimens (such as bortezomib–thalidomide–dexamethasone — VTD) [21]. An additional 1 year maintenance regimen was recommended given that the patient did not have complete elimination of his anti-idursulfase antibodies. Additional therapy was warranted to prevent redevelopment of antibody and to further eliminate or sustain low titer levels for improved clinical benefit.
The immune modulation protocol was generally well tolerated. Mild hypokalemia was observed at week 4 and potassium chloride supplementation was initiated, returning potassium levels to within normal limits. The patient experienced intermittent diarrhea following his weekly prophylactic antibiotic administration. He had increased appetite and weight gain likely attributable to corticosteroid use in pre-medication regimens to prevent infusion related reactions. Increased irritability, fatigue and nausea were also noted following ofatumumab infusions. During weeks of bortezomib administration, the patient was observed chewing his fingers and having decreased weight bearing, which could be symptoms of peripheral neuropathy, a known side effect of the drug.
Weekly peripheral IV administration of idursulfase was not interrupted during the immune modulation treatment with the exception of times of patient illness or hospitalization for shunt malfunction. Midway through treatment, the patient began to experience mild idursulfase associated reactions, mainly in the form of urticaria, itching and increased agitation, during the flush portion of the infusion. The symptoms quickly resolved after administration of IV antihistamine or steroids and infusions were completed. Additional antihistamines and corticosteroids were incorporated into the pre-medication regimen and the infusion rate slowed. Idursulfase dose was temporarily decreased to 0.7 mg/kg. After several months without infusion related reactions, idursulfase dose was again increased to 1.0 mg/kg.
In a study of anti-idursulfase antibody status and safety and efficacy of idursulfase treatment in attenuated MPS II patients, Barbier et al. [15] observed a correlation between genotype and the likelihood of developing persistent antibody levels. Patients with frameshift or nonsense mutations were more likely to develop anti-idursulfase antibodies compared to missense mutation patients and had less significant reduction in urine GAG levels after two years of enzyme replacement therapy. The authors suspected that this observation may be due to the fact that patients with nonsense and frameshift mutations are more likely to produce little to no endogenous enzyme. Our patient with MPS II and complete IDS gene deletion clearly produces no endogenous enzyme and developed persistent high anti-idursulfase IgG titers with no reduction of urine GAG levels during three years of IV idursulfase therapy.
Over the course of 18 months, immune modulation therapy with ofatumumab, bortezomib, methotrexate, short term dexamethasone, and IVIG resulted in a significant reduction of neutralizing anti-idursulfase IgG titers and a moderate reduction in urine GAG levels compared to baseline. Modest clinical improvements were observed. While treatment is ongoing, our experience suggests that future MPS II patients with a complete gene deletion may be more likely to develop persistent anti-idursulfase antibodies and may benefit from immune modulation therapy. Immune modulation therapy is likely to be most effective if begun at the initiation of enzyme replacement therapy or shortly after sustained antibody levels are noted [14]. Further investigation is needed to determine the role of this type of therapy in the treatment of patients with MPS II and other lysosomal storage disorders in which enzyme replacement therapy is used.
Acknowledgment
We would like to thank Dr. Ann Barbier, Senior Director of Clinic Research at Shire for her insight into the production of this manuscript. We would also like to thank Ms. Kelly Hilton and Dr. Thomas McCauley at Shire for providing the laboratory analysis and technical assistance for the production of this manuscript. Their assistance and cooperation have been invaluable.
References
- 1.Nelson J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum. Genet. 1997;101:335–358. doi: 10.1007/s004390050641. [DOI] [PubMed] [Google Scholar]
- 2.Poorthius B.J.H.M., Wevers R.A., Kleijer W.J. The frequency of lysosomal storage diseases in The Netherlands. Hum. Genet. 1999;105:151–156. doi: 10.1007/s004399900075. [DOI] [PubMed] [Google Scholar]
- 3.Nelson J., Crowhurst J., Carey B., Greed L. Incidence of the mucopolysaccharidoses in Western Australia. Am. J. Med. Genet. 2003;123A:310–313. doi: 10.1002/ajmg.a.20314. [DOI] [PubMed] [Google Scholar]
- 4.Baehner F., Schmiedeskamp C., Krummenauer F. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J. Inherit. Metab. Dis. 2005;28:1011–1017. doi: 10.1007/s10545-005-0112-z. [DOI] [PubMed] [Google Scholar]
- 5.Young I.D., Harper P.S., Archer I.M., Newcombe R.G. A clinical and genetic study of Hunter's syndrome. 1 Heterogeneity. J. Med. Genet. 1982;19:401–407. doi: 10.1136/jmg.19.6.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz I.V.D., Ribeiro M.G., Mota J.G. A clinical study of 77 patients with mucopolysaccharidosis type II. Acta Paediatr. 2007;96:63–70. doi: 10.1111/j.1651-2227.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 7.Martin R., Beck M., Eng C. Recognition and diagnosis of mucopolysaccharidosis II (Hunter syndrome) Pediatrics. 2008;121:e377–e3386. doi: 10.1542/peds.2007-1350. [DOI] [PubMed] [Google Scholar]
- 8.Matheus M.G., Castillo M., Smith J.K. Brain MRI findings in patients with mucopolysaccharidosis types I and II and mild clinical presentation. Neuroradiology. 2004;46:666–672. doi: 10.1007/s00234-004-1215-1. [DOI] [PubMed] [Google Scholar]
- 9.Froissart R., Moreira Da Silva I., Maire I. Mucopolysaccharidosis type II: an update on mutation spectrum. Acta Paediatr. 2007;96:71–77. doi: 10.1111/j.1651-2227.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 10.Muenzer J., Wraith J.E., Beck M. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 11.Muenzer J., Beck M., Eng C. Long-term, open-labeled extension study of idursulfase in the treatment of Hunter syndrome. Genet Med. 2011;13:95–101. doi: 10.1097/GIM.0b013e3181fea459. [DOI] [PubMed] [Google Scholar]
- 12.Mendelsohn N.J., Messinger Y.H., Rosenberg A.S., Kishnani P.S. Elimination of antibodies to recombinant enzyme in Pompe's disease. N. Engl. J. Med. 2009;360:194–195. doi: 10.1056/NEJMc0806809. [DOI] [PubMed] [Google Scholar]
- 13.Kishnani P.S., Goldenberg P.C., DeArmey S.L. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol. Genet. Metab. 2010;99:26–33. doi: 10.1016/j.ymgme.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messinger Y.H., Mendelsohn N.J., Rhead W. Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease. Genet Med. 2012;14:135–142. doi: 10.1038/gim.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbier A.J., Bielefeld B., Whiteman S.A.H. The relationship between anti-idursulfase antibody status and safety and efficacy outcomes in attenuated mucopolysaccharidosis II patients aged 5 years and older treated with intravenous idursulfase. Mol. Genet. Metab. 2013;110:303–310. doi: 10.1016/j.ymgme.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 16.De Jong J.G.N., Wevers R.A., Liebrand van Sambeek R. Measuring urinary glycosaminoglycans in the presence of protein: an improved screening procedure for mucopolysaccharidoses based on dimethylmethylene blue. Clin. Chem. 1992;38:803–807. [PubMed] [Google Scholar]
- 17.Stone J.E., Akhtar N., Botchway S., Pennock C.A. Interaction of 1,9-dimethylmethylene blue with glycosaminoglycans. Ann. Clin. Biochem. 1994;31:147–152. doi: 10.1177/000456329403100206. [DOI] [PubMed] [Google Scholar]
- 18.Banugaria S.G., Prater S.N., McGann J.K. Bortezomib in the rapid reduction of high sustained antibody titers in disorders treated with therapeutic protein: lessons learned from Pompe disease. Genet Med. 2013;15:123–131. doi: 10.1038/gim.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Meertena T., Hagenbeekb A. CD20-targeted therapy: the next generation of antibodies. Semin. Hematol. 2010;47:199–210. doi: 10.1053/j.seminhematol.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Østergaard M., Baslund B., Rigby W. Ofatumumab, a human anti-CD20 monoclonal antibody, for treatment of rheumatoid arthritis with an inadequate response to one or more disease-modifying antirheumatic drugs: results of a randomized, double-blind, placebo-controlled, phase I/II study. Arthritis Rheum. 2010;62:2227–2238. doi: 10.1002/art.27524. [DOI] [PubMed] [Google Scholar]
- 21.Kapoor P., Ramakrishnan V., Rajkumar S.V. Bortezomib combination therapy in multiple myeloma. Semin. Hematol. 2012;49:228-142. doi: 10.1053/j.seminhematol.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomanin R., Zanetti A., D'Avanzo F. Clinical efficacy of enzyme replacement therapy in paediatric Hunter patients, an independent study of 3.5 years. Orphanet J. Rare Dis. 2014;9:1–16. doi: 10.1186/s13023-014-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]