Abstract
We report on two novel patients with ALG11-CDG. The phenotype was characterized by severe psychomotor disability, progressive microcephaly, sensorineural hearing loss, therapy-resistant epilepsy with burst suppression EEG, cerebral atrophy with, in one of them, neuronal heterotopia, and early lethality. Analysis of ALG11 revealed compound heterozygosity involving three novel mutations: the splice site mutation c.45-2A > T, the c.36dupG duplication, and the missense mutation c.479G > T (p.G160V) that was present in both.
Keywords: ALG11-CDG, Burst suppression EEG, Neuronal heterotopia
1. Introduction
N-linked glycosylation is an important posttranslational modification of proteins. N-linked glycans are assembled in the endoplasmic reticulum (ER) and subsequently remodeled in the ER and the Golgi apparatus. The assembly of N-glycans starts at the cytosolic side of the ER with the addition of GlcNAc monophosphate to the lipid carrier dolichol phosphate (Dol-P) and leads to the formation of a 14 monosaccharides-containing dolichol-linked oligosaccharide (DLO), Dol-PP-GlcNAc2Man9Glc3. At the cytosolic side of the ER the intermediate DLO, Dol-PP-GlcNAc2Man5, is formed and then flipped by support of the RFT1 protein to the luminal side of the ER where the assembly is completed. Genetic defects in the N-glycan assembly belong to the large family of congenital disorders of glycosylation (CDG) (review in [1]). Recently, five patients (from four families) have been reported with ALG11-CDG [2], [3]. ALG11 encodes the mannosyltransferase that adds the fourth and the fifth of the nine mannoses in the completed DLO. We report on two novel patients. The characterization of their disease broadens the clinical and mutational spectrum of ALG11-CDG.
2. Patients
2.1. Patient 1
He was born as the first child of unrelated, Belgian parents at a gestational age of 37.5 weeks. At birth weight was 2360 g, length 46 cm and head circumference 30 cm. He had micrognathia, a sloping forehead with a salmon patch, absent midpalmar creases, thick feet and an umbilical hernia. He had polypnea with inspiratory stridor. There was striking axial hypotonia with absent head control during pull-to-sit, only brief head elevation lying prone, and slipping through in axillary suspension, even at the age of 13 months. Peripheral tone was increased, as were tendon reflexes. Moro reflex was absent and glabella reflex was normal. Plantar reflexes were extensor, with trembling of the lateral toes. He had little spontaneous movements. At 18 months, he did not follow with the eyes, and there was no consistent eye contact. Ophthalmologic evaluation showed papillary atrophy and retinal dystrophy with low amplitudes on flash ERG. Because of severe sensorineural hearing loss (no response at 80 dB), he received hearing aids. From the first week there were frequent epileptic seizures each day, at first with elevation and extension of the arms, elevation of the eyes and followed by crying, later in the course of the illness evolving to lateralized clonic movements with the head and left arm, lasting several minutes. EEG showed a burst suppression pattern, which persisted despite treatment with phenobarbitone, valproic acid, levetiracetam and vigabatrin. After the introduction of topiramate, improvement in seizure frequency and EEG characteristics was seen. There were feeding problems with gastroesophageal reflux, which responded to conservative treatment. An incarcerated inguinal hernia was surgically corrected at age 5 months. The fontanel was nearly closed at that time. Absolute and relative microcephaly was progressive, with a standard deviation score (SDS) of — 6.7 for head circumference, SDS for length being — 2.4 and for weight — 2.7 at 18 months.
Biochemical investigation showed a normal blood count, and normal levels of blood lactate and serum albumin, cholesterol, transaminases, creatine kinase, lactate dehydrogenase, amino acids, arylsulphatase A, thyroid stimulating hormone, total T4, thyroxine binding globulin and prolactin. Blood coagulation factor XI (27%; normal range for age: 38–164%), and protein C (25%; normal range for age: 37–81%) were decreased. Capillary zone electrophoresis of serum transferrin revealed a type 1 pattern.
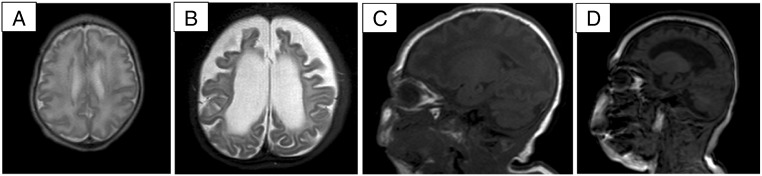
Ultrasonography of heart, liver, spleen and kidneys was normal. Radiology of the skeleton revealed a mildly broadened aspect of the distal metaphysis of the long bones of the limbs. Brain magnetic resonance imaging (MRI) showed symmetrical anteriorly predominant subcortical heterotopias and a simplified gyral pattern. Repeated MRI at the age of 1 year revealed a striking atrophy of white matter and cortex and secondary dilatation of the ventricles, with relative sparing of cerebellum and brainstem. Myelination was severely retarded (Fig. 1). Epilepsy was never satisfactory controlled. He died at the age of 3 1.5/12 years from respiratory insufficiency due to a pulmonary infection.
Fig. 1.
A–D: Axial T2-weighted (A, B) and sagittal T1-weighted (C, D) cerebral MRI in patient 1 at 1 month (A, C) and at 1 year (B, D), showing anteriorly predominant subcortical heterotopias (A), a simplified gyral pattern (A, C), and severe progressive cerebral atrophy and delay in myelination at 1 year (B, D).
Analysis of dolichol-linked oligosaccharides in patient-derived fibroblasts (according to Rind et al. [2]) revealed an accumulation of Man3GlcNAc2-PP-Dol and Man4GlcNAc2-PP-Dol, the substrates of ALG11. These compounds are normally undetectable.
Genetic testing showed two variants in ALG11: c.479G > T (p.G160V) and the splice site variant c.45-2A > T. The parents are carriers of these variants.
2.2. Patient 2
This boy was admitted at the age of 3 months with feeding problems and failure to thrive. He was the first child of unrelated, Dutch parents and born after a full term uncomplicated pregnancy. Neonatal hearing examination had revealed cochlear hearing loss for which he received hearing aids. During the first month of life he was otherwise doing well. He made eye contact and was fixating objects. After the first month, he started to cry inconsolably in between feeds. His parents noted the loss of eye contact. Progressive microcephaly was noted with SDS for head circumference decreasing from − 2 SDS at one month to − 3.2 SDS at 4 months of age. There were no dysmorphic features. The boy was awake and showed wandering eye movements. There was no visual reaction on light. He showed axial hypotonia and peripheral hypertonia. Reflexes were brisk. Tonic seizures were seen with opening of the eyes and freezing, as well as infantile spasms. EEG showed an abnormal background with high amplitude, bursts of spikes and waves and periods of suppression consistent with epileptic encephalopathy. Brain MRI at 3.5 months showed frontally a large extracerebral fluid collection probably due to cerebral atrophy. Ophthalmological examination was normal except for a mild intermittent esotropia. On follow-up examination hearing loss had worsened (70–90 dB loss) with the involvement of the cochlea as well as the nerve and the brainstem. Biochemical investigation showed a slight increase of serum creatine kinase (218 U/L, nl < 170 U/L), an increased lactate (3.6, 3.9 mmol/L), slightly decreased copper (0.53, nl > 0.70 mg/L) and ceruloplasmin (0.10, nl > 0.20 g/L) levels, normal plasma aminoacids (except for an elevated threonine), acylcarnitine profile, very long chain fatty acids, phytanic acid, pristanic acid, chitotriosidase activity, antithrombin, protein C and factor XI, and normal urinary analysis of organic acids, amino acids, purines and pyrimidines, glycosaminoglycans, polyols and sialic acid. Cerebral spinal fluid showed normal glucose, lactate, neurotransmitters, amino acids and pipecolic acid. Capillary zone electrophoresis of serum transferrin repeatedly showed a type 1 pattern. The treatment for epileptic seizures was started with ACTH and pyridoxal phosphate. Vigabatrin was added because of increasing seizure frequency. Continuous intravenous midazolam and phenobarbital were added because of ongoing seizures, followed by propofol infusion, without significant effect on seizure activity. In spite of adequate blood levels of anti-epileptic drugs, seizures continued and were associated with respiratory distress. The refractory epilepsy resulted in respiratory insufficiency. Because of the poor prognosis further intensive care treatment was discontinued. He died at 4 months due to respiratory insufficiency secondary to epilepsy.
Genetic testing showed two variants in ALG11: c.479G > T (p.G160V) and c.36dupG. The parents are carriers of these variants.
3. Discussion
The type 1 serum sialotransferrin pattern in these patients pointed to a defect in the N-glycan assembly pathway. The result of the dolichol-linked analysis in fibroblasts of patient 1 strongly suggested a defect in ALG11. Mutation analysis of ALG11 showed compound heterozygosity in both patients. Three novel variants were found. Two of these variants are certainly deleterious: c.36dupG causes a frame shift for translation; c.45-2A > T hits a consensus splice site. One missense mutation was shared by both patients. The following arguments are in favor of the pathogenicity of p.G160V: i. glycine at position 160 is strongly conserved among species; ii. this variant was not found in the 1000G (www.1000genomes.org), Genome of the Netherlands (GoNL; www.nlgenome.nl) or Exome Variant Server (http://evs.gs.washington.edu/EVS/) databases; and iii. the programs SIFT and POLYPHEN predict a pathogenic effect.
Five patients have been described with ALG11-CDG [2], [3]. Table 1 summarizes the clinical findings of the reported and of our patients. Common to all seven patients are an important psychomotor disability, severe epilepsy (including infantile spasms in one), and axial hypotonia. The following symptoms were present in a majority of patients: feeding problems (4/7), microcephaly (3/5), deafness (4/7), poor eye contact (4/6), and peripheral hypertonia (4/7). A minority of patients showed convergent strabism, oscillations of body temperature, ataxia, various dysmorphisms, papillary atrophy and retinal dystrophy, cerebral atrophy and white matter abnormalities. Our patients as well as the first patient of Rind et al. [2] died at an early age. Novel ALG11-CDG features in our patients were a burst suppression pattern on EEG (in both), and subcortical heterotopias in one. In the previously reported siblings with ALG11-CDG, generalized epileptic activity on EEG is described without further details. A burst suppression EEG pattern has to our knowledge not been reported in CDG. While the epilepsy was clearly therapy-resistant, therapy with topiramate appeared to be beneficial. Also, heterotopia is not known as a feature of CDG-I. Follow-up cerebral MRI at the age of 1 year in patient 1 revealed rapidly progressive cerebral atrophy, explaining the progressive microcephaly and the poor developmental outcome in this patient. Microcephaly was also progressive in patient 2. Comparable progressive atrophy has been reported in mitochondrial disorders, but seems not to have been reported in CDG. Sensorineural deafness was a prominent feature in four of the seven ALG11-CDG patients. Sensorineural deafness is highly unusual in other types of CDG, except RFT1-CDG [4], [5]. Interestingly, RFT1 is responsible for the translocation of the DLO Dol-PP-Man5GlcNAc2 from the cytosolic side of the ER membrane to its luminal side. This provides support for the hypothesis that the cytoplasmic accumulation of one or more of the substrates of ALG11 and RFT1 has an otoneurotoxic effect [5], or maybe an even more general deleterious effect on the brain.
Table 1.
Summary of clinical features and mutations in ALG11-CDG patients.
| Rind et al. 2010 (pt A) | Rind et al. 2010 (pt B) | Thiel et al. 2012 (pt A) | Thiel et al. 2012 (pt B) | Thiel et al. 2012 (pt C) | Present report (pt 1) | Present report (pt 2) | |
|---|---|---|---|---|---|---|---|
| Age | Died at 2 years | NA | 7 years at publication | 4.5 years at publication | 8.5 years at publication | Died at 3 years | Died at 4 months |
| Gender | Female | Male | Female | Female | Male | Male | Male |
| Ethnic origin | Turkish | Turkish | French–Canadian | Caucasian | Turkish | Belgian | Dutch |
| Microcephaly | + | NA | + | NA | − | + | + |
| Psychomotor disability | + | + | + | + | + | + | + |
| Hypotonia | Axial | + | Axial | Axial | Axial | Axial | Axial |
| Hypertonia | − | − | Peripheral | Peripheral | − | Peripheral | Peripheral |
| Hyperreflexia | NA | NA | NA | NA | NA | + | + |
| Epilepsy | + | + | + | + | + | + | + |
| Deafness | + | + | Not mentioned | Not mentioned | Not mentioned | + | + |
| Eye/visual problems | Poor visual tracking | NA | Strabismus convergens | No visual tracking, strabismus convergens | Strabismus convergens | No consistent eye contact, papillary atrophy and retinal dystrophy | Loss of eye contact |
| Feeding problems | + | + | − | − | + | + | − |
| Dysmorphism | Fat pads, inverted nipples | − | − | Plagiocephaly, nasal anteversion, long filtrum, mild retrognathia, fat pads, inverted nipples, poorly developed teeth, scoliosis, bilateral hip dislocation, | − | Sloping forehead with salmon patch, micrognathia, absent midpalmar creases, umbilical hernia, thick feet | − |
| Other features | Oscillations of body temperature | NA | Cerebral atrophy and abnormal white matter | Oscillations of body temperature | Atactic movement disorder | Hypokinesia, burst suppression pattern on EEG, atrophy of white matter and cortex, subcortical heterotopia, retarded myelination | Burst suppression pattern on EEG, cerebral atrophy |
| Mutation(s): cDNA/protein | Homozygous c.T257C/p.L86S | Homozygous c.T257C/p.L86S | c.623_642del/frame shift; c836A > C/p.Y279S | c.1142 T > C/p.L381S; c.1192G > A/p.E398K | Homozygous c.953A > C/p.Q318P | c.479G > T/p.G160V; c.45-2A > T | c.479G > T/p.G160V; c.36dupG |
NA: not available.
References
- 1.Krasnewich D. Human glycosylation disorders. Cancer Biomark. 2014;14:3–16. doi: 10.3233/CBM-130374. [DOI] [PubMed] [Google Scholar]
- 2.Rind N., Schmeiser V., Thiel C., Absmanner B., Lübbehusen J., Hocks J., Apeshiotis N., Wilichowski E., Lehle L., Körner C. A severe human metabolic disease caused by deficiency of the endoplasmic mannosyltransferase hALG11 leads to congenital disorder of glycosylation-Ip. Hum. Mol. Genet. 2010;19:1413–1424. doi: 10.1093/hmg/ddq016. [DOI] [PubMed] [Google Scholar]
- 3.Thiel C., Rind N., Popovici D., Hoffmann G.F., Hanson K., Conway R.L., Adamski C.R., Butler E., Scanion R., Lambert M., Apeshiotis N., Thiel C., Matthijs G., Körner C. Improved diagnostics lead to identification of three new patients with congenital disorder of glycosylation-Ip. Hum. Mutat. 2012;33:485–487. doi: 10.1002/humu.22019. [DOI] [PubMed] [Google Scholar]
- 4.Haeuptle M.A., Pujol F.M., Neupert C., Winchester B., Kastaniotis A.J., Aebi M., Hennet T. Human RFT1 deficiency leads to a disorder of N-linked glycosylation. Am. J. Hum. Genet. 2008;82:600–606. doi: 10.1016/j.ajhg.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaeken J., Vleugels W., Régal L., Corchia C., Goemans N., Haeuptle M.A., Foulquier F., Hennet T., Matthijs G., Dionisi-Vici C. RFT1-CDG: deafness as a novel feature of congenital disorders of glycosylation (CDG) J. Inherit. Metab. Dis. 2009;32:756–757. doi: 10.1007/s10545-009-1297-3. [DOI] [PubMed] [Google Scholar]