Abstract
Lactobacilli constitute a large genus of Gram-positive lactic acid bacteria which have widespread roles ranging from gut commensals to starters in fermented foods. A combination of in silico and laboratory-based screening allowed us to determine the overall bacteriocin producing potential of representative strains of each species of the genus. The genomes of 175 lactobacilli and 38 associated species were screened for the presence of antimicrobial producing genes and combined with screening for antimicrobial activity against a range of indicators. There also appears to be a link between the strains’ environment and bacteriocin production, with those from the animal and human microbiota encoding over twice as many bacteriocins as those from other sources. Five novel bacteriocins were identified belonging to differing bacteriocin classes, including two-peptide bacteriocins (muricidin and acidocin X) and circular bacteriocins (paracyclicin). In addition, there was a clear clustering of helveticin type bacteriolysins in the Lactobacillus acidophilus group of species. This combined in silico and in vitro approach to screening has demonstrated the true diversity and complexity of bacteriocins across the genus. It also highlights their biological importance in terms of communication and competition between closely related strains in diverse complex microbial environments.
Introduction
Bacteriocins are ribosomally-synthesised antimicrobial peptides which generally act by inducing pore formation or inhibiting cell wall synthesis in target cells1. Some bacteriocins such as nisin have found widespread applicability as bio preservatives in food systems where they have been used for decades. Moreover, bacteriocin production can also be a key probiotic trait2, 3, and bacteriocins have been suggested as potential alternatives to antibiotics in the future4. The Lactobacillus genus has a long association with bacteriocin production, with numerous bacteriocins isolated from such species5–7. Originally bacteriocin producers were isolated from functional screens against selected target strains, but many studies now rely on prior in silico screening, using tools such as BAGEL8, 9. BAGEL scans the bacterial genome for putative bacteriocin open reading frames (ORFs) and also analyses surrounding ORFs to search for possible biosynthetic genes, immunity genes and transporters10. Whilst the areas of interest identified by BAGEL represent potential bacteriocin operons, this does not always translate into functional bacteriocin production for many reasons including problems with mutation, regulation or target specificity.
There are varying accounts on the extent of bacteriocin production in the environment. While numerous accounts assume ubiquity in production11, 12, a definitive analysis has yet to focus on clarifying the actual extent of bacteriocin production. In this study, we elucidate the bacteriocinogenic potential of representative species of the Lactobacillus genus and some related genera; i.e. the Lactobacillus Genus Complex. Previously Sun et al.13 analysed the genomes of 175 Lactobacillus species and 38 closely related species, carrying out a screen for putative bacteriocin operons using the BAGEL bacteriocin mining tool. Despite no longer formally being considered as bacteriocins, large (>30 kDa) helveticin-like antimicrobial proteins were also included in the study. Based on those results, we analysed strains which were identified as encoding putative bacteriocin operons for in vitro production using well diffusion assays (WDAs) and MALDI TOF MS. Well diffusion assays were used to detect antimicrobial production whilst MALDI TOF MS and SDS PAGE were used to identify the masses of the bacteriocins. Peptide masses identified by MS were correlated with the theoretical masses of bacteriocins identified by BAGEL to confirm the identity of the antimicrobial. We reinforced the BAGEL results with BLAST searches for key lantibiotic and sactibiotic enzymes using specific sequences employed in previous studies against this new dataset of Lactobacillus genomes8, 14, 15. This redundancy allows for a more comprehensive analysis of bacteriocin gene clusters in the sequenced strains.
Results
Distribution of Bacteriocin Operons
Several studies have completed bacteriocin screens on diverse and unrelated species of bacteria8, 16, 17. The aim of this study was to focus primarily on the lactobacilli and investigate the distribution of bacteriocin genes across this single large important genus. From the information identified by BAGEL, we used a phylogenetic tree to visualise the distribution of bacteriocin operons within the genus (Fig. 1). Historically the Lactobacillus genus has a long association with bacteriocin production. While this study focuses on the type strain of each Lactobacillus species, Table 1 identifies those bacteriocins which have been previously identified and characterised from all strains in the Lactobacillus Genus Complex. In all, 66 bacteriocins have been characterised from lactobacilli previously, which would suggest a high degree of production within the genus. It is notable that the production of these unique bacteriocins is, in fact, restricted to 16 different species.
Figure 1.
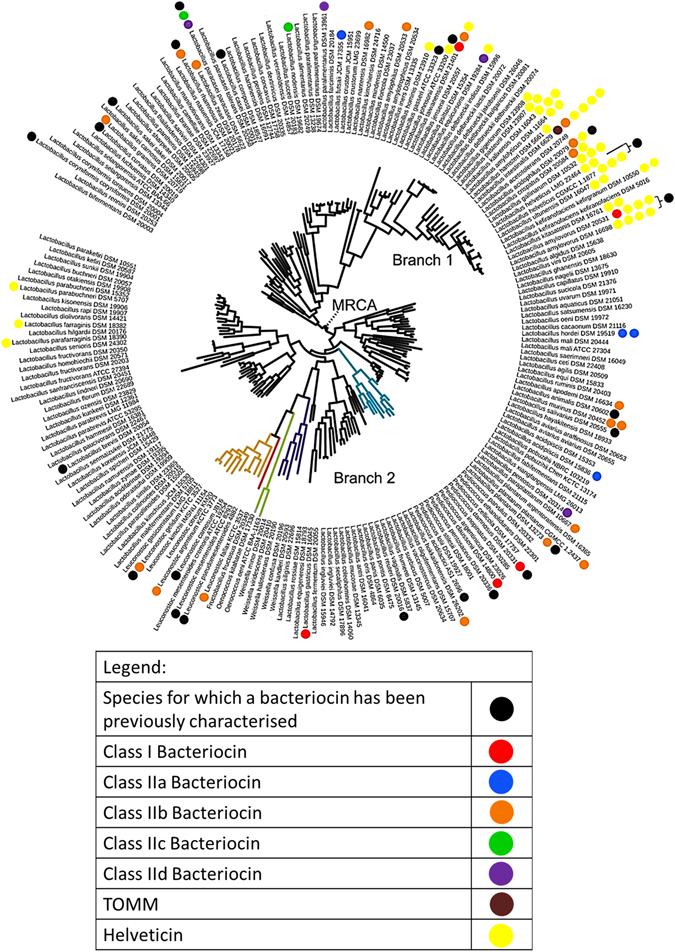
Distribution of complete bacteriocin operons amongst the Lactobacillus Genus Complex (Adapted from Sun et al. 12 596).
Table 1.
Bacteriocins characterised from species within the Lactobacillus Genus Complex.
| Bacteriocin | Subclass | Producing strain | Origin |
|---|---|---|---|
| Class I | |||
| Plantaricin W (α and β) | II | Lactobacillus plantarum LMG 2379 | Wine |
| Plantaricin C | II | L. plantarum LL441 | Cabrales cheese |
| Lactocin Sa | II | L. sakei L45 | Sausages |
| Pediocin PD-1 | II | Pediococcus damnosus NCFB1832 | Lager Beer |
| Glycocin F | Glycocin | L. plantarum KW30 | Fermented corn |
| Class II | |||
| Acidocin A | IIa | L. acidophilus TK9201 | Fermented milk (starter) |
| Curvaticin L442 | IIa | L. curvatus L442 | Greek fermented sausage |
| Curvaticin 13 | IIa | L. curvatus SB13 | Sausages |
| Sakacin P (variant)b | IIa | L. curvatus LTH1174 | Fermented meat |
| Plantaricin BM-1 | IIa | L. plantarum BM-1 | Fermented meat |
| Plantaricin C19 | IIa | L. plantarum C19 | Fermented cucumber |
| Plantaricin 423 | IIa | L. plantarum 423 | Sorghum (beer) |
| Sakacin Pc | IIa | L. sakei LTH673 | Cured meat |
| Sakacin Ad | IIa | L. sakei Lb706 | Meat |
| Sakacin Ge | IIa | L. sakei 2512 | Food origin |
| Sakacin Xf | IIa | L. sakei 5 | Malt |
| Bavaricin A | IIa | L. sakei MI1401 | Sourdough |
| Bavaricin MN | IIa | L. sakei MN | Meat (bovine) |
| Bacteriocin L-1077 | IIa | L. salivarius L-1077 | Intestine (broilers) |
| Leucocin Agh | IIa | Leuconostoc geldium UAL 187 | Vacuum-packed meat |
| Leucocin C | IIa | Leuc. mesenteroides TA33a | Spoiled vacuum-packed meat |
| Leucocin 10Ch | IIa | Leuc. mesenteroides 10 | Malted barley |
| Leucocin 683Y | IIa | Leuc. mesenteroides 683 | Malted barley |
| Mesentericin Y105 | IIa | Leuc. mesenteroides subsp. mesenteroides Y105 | Goats milk |
| Pediocin PA-1 (ACH)i | IIa | P. acidilactici PAC1.0 | Meat |
| Pediocin SA-1 | IIa | P. acidilactici NRRL B5627 | Meat |
| Penocin A | IIa | P. pentosaceus ATCC 25745 | Plants |
| Pediocin SM-1 | IIa | P. pentosaceus Mees 1934 | Meat |
| Weissellin A | IIa | Weissella paramesenteroides DX | Sausage |
| Lactobin Aj | IIb | L. amylovorus LMG P-13139 | Corn liquor |
| Brevicin 174 (breB and breC) | IIb | L. brevis 174A | Iyokan (fruit) |
| Lactocin 705 (Lac705α and Lac705β) | IIb | L. casei CRL 705 | Meat |
| Acidocin LF221 (LF221A and LF221B)k | IIb | L. gasseri LF221 | Faeces (child) |
| Gassericin T (GatA and GatX) | IIb | L. gasseri SBT2055 | Faeces (human) |
| Lactacin F (LafA and LafX)l | IIb | L. johnsonii VPI11088 | Intestine (human) |
| Bacteriocin | Type/Subclass | Producing strain | Origin |
| Sakacin T (SakTα and SakTβ)m | IIb | L. sakei CTC372 | Sausages |
| Plantaricin E/F (PlnE and PlnF) | IIb | L. plantarum C11 | Fermented cucumber |
| Plantaricin J/K (PlnJ and PlnK) | IIb | L. plantarum C11 | Fermented cucumber |
| Plantaricin S (Plsα and Plsβ)n | IIb | L. plantarum LPCO10 | Green olives |
| Plantaricin NC8 (PLNC8α and PLNC8β) | IIb | L. plantarum NC8 | Ensilage |
| Salivaricin ABP-118 (Abp118α and Abp118β) | IIb | L. salivarius UCC118 | Intestine (human probiotic) |
| Salivaricin CLR 1328 (Salα and Salβ) | IIb | L. salivarius CLR1328 | Vagina (human) |
| Salivaricin P (Sln1 and Sln2) | IIb | L. salivarius DPC6005 | Intestine (pig) |
| Salivaricin T (SalTα and SalTβ) | IIb | L. salivarius DPC6488 | Intestine (neonate) |
| Acidocin B | IIc | L. acidophilus M46 | Food origin |
| Gassericin A° | IIc | L. gasseri LA39 | Faeces (child) |
| Leucocyclicin Q | IIc | Leuc. mesenteroides TK41401 | Japanese pickles |
| Acidocin 8912 | IId | L. acidophilus TK8912 | Dairy origin |
| Brevicin 27 | IId | L. brevis SB27 | Sausages |
| Lactocin MXJ 32 A | IId | L. coryniformis MXJ 32 | Fermented vegetables |
| Curvalicin BAP2 | IId | L. curvatus CWBI-B28 | Meat |
| Curvaticin FS47 | IId | L. curvatus FS47 | Meat |
| Sakacin Q (variant)p | IId | L. curvatus LTH1174 | Fermented meat |
| Bacteriocin SJ2-8 | IId | L. paracasei BGSJ2-8 | Home-made cheese |
| Paracin C | IId | L. paracasei CICC 20241 | Probiotic |
| Plantaricin 1.25 α | IId | L. plantarum TMW1.25 | Fermented sausages |
| Plantaricin 1.25 β | IId | L. plantarum TMW1.25 | Fermented sausages |
| Plantaricin 149 | IId | L. plantarum NRIC 149 | Pineapple |
| Plantaricin 163 | IId | L. plantarum 163 | Fermented vegetables |
| Plantaricin A | IId | L. plantarum C11 | Fermented cucumber |
| Plantaricin ASM1 | IId | L. plantarum A-1 | Corn bread |
| Plantaricin JLA-9 | IId | L. plantarum JLA-9 | Suan-Tsai (Chinese fermented cabbage) |
| Plantaricin ST31 | IId | L. plantarum ST31 | Sourdough |
| Sakacin Qq | IId | L. sakei LTH673 | Fermented dry sausage |
| Salivaricin L | IId | L. salivarius DPC6488 | Intestine (neonate) |
| Bacteriocin | Type/ Subclass | Producing strain | Origin |
| Plantaricin Y | IId | L. plantarum 510 | Koshu vineyard |
| Rhamnosin A | IId | L. rhamnosus 68 | Intestinal microbiota (human) |
| Bactofencin A | IId | L. salivarius DPC6502 | Intestine (porcine) |
| Bacteriocin LS2 | IId | L. salivarius BGHO1 | Oral (human) |
| Leucocin B | IId | Leuc. mesenteroides TA33a | Spoiled vacuum-packed meat |
| Mesentericin 52Br | IId | Leuc. mesenteroides FR52 | Raw Milk |
| Leucocin N | IId | Leuc. pseudomesenteroides QU 15 | Nukadoko |
| Leucocin Q | IId | Leuc. pseudomesenteroides QU 15 | Nukadoko |
| Weissellicin 110 | IId | Weissella cibaria 110 | Plaa-Som |
| Weissellicin L | IId | W. hellenica 4–7 | Sian-sianzih |
| Weissellicin M | IId | W. hellenica QU 13 | Pickel barrel |
| Weissellicin Y | IId | W. hellenica QU 13 | Pickel barrel |
| Lactacin Bs | — | L. acidophilus N2 | Food origin |
| Bacteriocin TSU4 | — | L. animalis TSU4 | Intestine (fish) |
| Curvalicin BAP3 | — | L. curvatus CWBI-B28 | Meat |
| Gassericin E | — | L. gasseri EV1461 | Healthy vagina (human) |
| Plantacin B | — | L. plantarum NCDO1193 | Dairy origin |
| Plantaricin F | — | L. plantarum BF001 | Spoiled cat fish filets |
| Plantaricn T | — | L. plantarum LPCO10 | Green olives |
| Bacteriocin SMXD51 | — | L. salivarius SMXD51 | Faeces (chicken) |
| Salivaricin B | — | L. salivarius M7 | Food origin |
| Bacteriolysin | |||
| Helveticin J | L. helveticus NCDO481 | Dairy origin | |
Characterised bacteriocins with identical amino acid sequences: aSakacin M/lactocin S from L. sakei 148. bVariant of sakacin P from L. curvatus L442. cSakacin 674 from L. sakei 674. dCurvacin A from L. curvatus LTH1174 and sakacin K from L. sakei CTC 494 . eBacteriocin R1333 from Lb. sakei R1333. fSakacin X from L. curvatus 2711 and L. curvatus CRL705. gLeucocin A-TA33a from Leuonostoc mesenteroides TA33a, and Leucocin B-Ta11a from Leuc. carnosum Ta11a. hLeucocin A-4010 and Lecucocin B-4010 from Leuc. carnosum 4010. iAlso produced by L. plantarum WHE92. jAmilovorin L471 from L. amylovorus DCE471. kGassericin K7 (K7A y K7B) from L. gasseri K7. lLactacin F from L. acidophilus 30SC. mSakacin T (SakTα and SakTβ) from L. sakei 5, L. curvatus 2711 and L. curvatus CRL705. nAlso produced by L. pentosus B96. pReutericin 6 from L. reuteri LA6. pVarient of sakacin Q from L. curvatus L442 and L. curvatus CRL705. qSakacin Q from L. sakei Lb674 and sakacin Q from L. curvatus CRL705. rMesentericin B105 from Leuc. mesenteroides subsp. mesenteroides Y105. sAcidocin J1132 from L. acidophilus JCM1132.
Visualisation of the distribution of bacteriocins throughout the Lactobacillus Genus Complex shows that there is a clear clustering of helveticin-like operons amongst the L. acidophilus branch of species, indicating that such genes have been retained from a common ancestor (Fig. 1). Despite being previously classified as class III bacteriocins, these proteins are now termed bacteriolysins and are considered a distinct group of antimicrobials. Whilst these proteins are ribosomally synthesised, they are much larger than classical bacteriocins (~30 kDa) and are heat labile. Helveticin J is the only member previously characterised18, but here we show that these genes are actually highly prevalent in the lactobacilli, with 43 potential homologs identified from 23 strains (for alignment results see Supplementary Figure 1). Of the 18 strains in the L. acidophilus group, 36 helveticin homologs were distributed amongst 16 of these strains. While certain strains can encode up to four helveticin homologs, there is insufficient homology between those to suggest recent gene duplications. The high degree of homology (in some cases greater than 99%) between some structural genes encoded by different strains does indicate that horizontal gene transfer of helveticin homologs has occurred; such a mechanism may also explain the presence of these genes in the six strains outside of the L. acidophilus group (Fig. 1).
The environment from which these strains have been isolated also seems to correlate with their bacteriocinogenic potential (Supplementary Table 1). For example, of the strains isolated from an animal or human origin 37.5% were identified as encoding a complete bacteriocin or helveticin like operon in BAGEL or BLAST screens (21 of 56 strains). This value for strains isolated from non-animal source (food, plants, environmental and alcohol/wine products) displays an over two-fold reduction at 16.67% (25 of 150 strains). This result suggests that the bacteriocin production may prove to be a competitive advantage for strains from complex environments such as the microbiota of humans and animals.
Diversity of Bacteriocins Identified
Bacteriocins are a diverse and varied group of antimicrobials, which use different systems for bacteriocin modification, transport and immunity. In silico analysis allows us to determine which types of bacteriocins the lactobacilli can synthesise. To analyse the diversity of the bacteriocins encoded by lactobacilli an in silico screen was first carried out on the genome of each strain followed by in vitro screening of each bacteriocin encoding strain to identify antimicrobial activity against a range of indicators (Table 2). MALDI TOF MS and SDS PAGE allowed us to determine the mass and subsequently the identity of the bacteriocins produced by the strains (Supplementary Figure 2). The bacteriocin classification scheme devised by Cotter et al.1, 4 was used to distinguish between the different classes of bacteriocins.
Table 2.
Spectrum of inhibition of bacteriocin producing strains against a range of indicator strains.
| Bacteriocin Producers | Strain (DSM) | Activity of Bacteriocin Producers vs. Indicator Organisms* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L. delbrueckii subsp. bulgaricus | L. delbrueckii subsp. lactis | L. amylovorus | L. casei | L. plantarum | L. rhamnosus | Listeria innocua | Enterococcus saccharolyticus | E. mundtii | ||
| L. paralimentarius | 13961 | ++ | +++ | ++ | + | +++ | ||||
| L. murinus | 20452 | + | ||||||||
| L. hordei | 19519 | ++ | ++ | ++ | +++ | +++ | +++ | |||
| L. intestinalis | 6629 | +++ | + | |||||||
| L. paracasei subsp. paracasei | 5622 | + | + | |||||||
| L. acidophilus | 20079 | ++ | ++ | + | + | |||||
| L. agilis | 20509 | + | ||||||||
| L. crispatus | 20584 | ++ | + | + | ||||||
| L. equicursoris | 19284 | ++ | ||||||||
| L. pentosus | 20314 | + | ||||||||
| L. kalixensis | 16043 | + | ||||||||
| L. amylovorus | 20531 | + | ||||||||
| L. kitasatonis | 16761 | ++ | ||||||||
| P. damnosus | 20331 | +++ | ||||||||
| C. maltaromaticum | 20342 | + | + | |||||||
| C. maltaromaticum | 20722 | ++ | + | + | ++ | |||||
Activity of pH neutralised cell free supernatants from bacteriocin producers in agar well diffusion assay. Inhibition of indicators is described in radius (mm) of the zone of inhibition in WDA, scores are as follows: + = 0.5–2 mm, ++ = 2.5–5mm, +++ = >5 mm.
Class I
Class I bacteriocins are comprised of ribosomally synthesised, post-translationally modified bacteriocins (RiPPs)4. Originally restricted to lantibiotics, this class has now been extended to include other post-translationally modified bacteriocins such as sactibiotics.
Lantibiotics
Lantibiotics are a group of bacteriocins characterised by the presence of lanthionine and methyllanthionine bridges. Here, serine and threonine residues are converted to 2,3-didehydroalanine (Dha) and 2,3-didehydrobutyrine (Dhb), respectively, which then react with the thiol group found in cysteine residues, forming lanthionine or methyllanthionine thioether cross-links19. Currently three lantibiotics have been attributed to the Lactobacillus genus; lactocin S20, plantaricin C21 and the two peptide lantibiotic plantaricin W6.
The BAGEL screen of the Lactobacillus dataset identified three further lactobacilli encoding lantibiotic structural peptides (Table 3, Supplementary Table 2 displays these genes with the associated leader sequence). Of these, potential production was only identified in L. taiwanensis DSM 21401 which encodes a type I lantipeptide (a lantibiotic which doesn’t display antimicrobial activity), characterised by the presence of LanB and LanC modification enzymes. What is unusual about this peptide is the fact the structural gene is small compared to other lantipeptides, with the mature peptide predicted to contain only 14 amino acids. Despite a lack of demonstrated antibacterial activity against the range of indicators tested, MALDI TOF MS did identify a mass which correlates with the predicted mass of the mature lantipeptide. The lack of antimicrobial activity may simply imply that the indicator organisms tested were not sensitive, or that the putative lantipeptide has a signalling rather than a bacteriocidal role.
Table 3.
Potential Lantibiotic/Lantipeptide Structural Peptides.
| Species | Strain | Potential Unmodified Lantibiotic/Lantipeptide Sequence |
|---|---|---|
| L. taiwanensis | DSM 21401 | TSTGCCNGPSKLQG |
| L. amylovorus | DSM 20531 | AKSYSAYSSCSCVNPPCPIATMD |
| L. gastricus | DSM 16045 | GTETAQSTPAISRVTLSIARKSSAKCISWISFSAGGLNSYKSKC |
| P. damnosus (Pediocin PD-1) | DSM 20331 | KKIKKSSSGDICTLTSECDHLATWVCC |
A further type I lantibiotic operon was identified by BAGEL in the strain L. amylovorus DSM 20531. This strain appears to encode a complete lantibiotic operon which contains the required modification enzymes and an ABC transporter. L. gastricus DSM 16045 was found to encode a Lan C homolog but a LanB homolog was absent from the operon which is necessary for initial dehydration of serine and threonine residues. The production of either of these bacteriocins was not detected in vitro.
Lantibiotic operons were also identified in some of the other genera studied. Pediococcus damnosus DSM 20331 was found to encode a class II lantibiotic. This strain has previously been found to produce the partially characterised lantibiotic pediocin PD-122. From genomic data used in this study, the sequence of the pediocin PD-1 gene has now been elucidated, showing a high similarity to the lantibiotic plantaricin C (PlnC)23. Due to the similarity between the two bacteriocins, pediocin PD-1 likely shares a common mode of action with PlnC whose activity has been shown to be as a result of the combination of pore formation and inhibition of lipid II synthesis24. P. claussenii DSM 14800 was also shown to encode pediocin PD-1, however, this strain failed to display bacteriocin production. The Carnobacterium maltaromaticum strains DSM 20722 and DSM 20730 were also both found to encode the two-component lantibiotic carnolysin, however the in vitro production of this bacteriocin was not seen in either strain25.
To supplement the results of BAGEL searches, previous in silico lantibiotic screens were repeated on the new Lactobacillus dataset. We used the modification enzymes NisC, LtnM1 and VenL as drivers in the BLAST search for novel lantibiotics8, 14, 15. L. gallinarum DSM 10532, L. crispatus DSM 20584 and P. cellicola DSM 17757 were all found to harbour a NisC homolog, despite not being identified by BAGEL. However, upon examination of the surrounding genes, no potential structural genes were identified. Strains identified in BLAST searches as encoding LanM homologs had also been identified by BAGEL. No homolog of the novel lanthionine synthase VenL was identified in the BLAST screen.
Sactibiotics
The sactibiotics are a growing class of bacteriocins characterised by the presence of unusual sulphur to α-carbon linkages. These modifications are carried out by radical S-adenosylmethionine (SAM) proteins which catalyse the formation of these thioether bonds26, 27. To analyse the prevalence of potential sactibiotic operons within the lactobacilli, the sequences for the radical SAMs associated with a two-component sactibiotic thuricin CD (TrnC and TrnD) were used as drivers in a BLAST analysis of the genomes available17, 28. Only two radical SAMs were found resembling those associated with thuricin CD. L. mali DSM 20444 was found to encode one such SAM, however, analysis of the operon failed to identify a potential structural gene. Kandleria vitulina DSM 20405 appears to encode a complete sactibiotic operon, encompassing a structural gene, transporter and associated radical SAM, however, no biological activity could be attributed to this strain with the panel of indicators tested. BAGEL further identified two potential sactibiotic related radical SAM proteins in C. maltaromaticum DSM 20342 and DSM 20722 but no potential structural gene for these enzymes was apparent.
TOMMs
Thiazole/oxazole modified microcins (TOMMs) are a class of RiPPs which are now included with the class I bacteriocins. These peptides undergo extensive post-translational modification, with the conversion of cysteine, serine and threonine residues into the corresponding heterocycles; thiazole, oxazole and methyloxazole, respectively29. TOMMs exist in gene clusters encoding several factors involved in transport, modification and immunity. Using streptolysin as an example, the modification of the structural peptide is the result of the activity of the SagBCD enzyme complex, encompassing a cyclodehydratase (SagC), a dehydrogenase (SagB) and a docking protein (SagD)30. Whilst SagBCD clusters are described as being relatively widespread amongst prokaryotes, no TOMM has yet been identified from a Lactobacillus species30. In our study L. crispatus DSM 20584, L. intestinalis DSM 6629 and Oenococcus kitaharae DSM 17330 were identified by BAGEL as encoding homologs of the SagBCD gene cluster. Whilst the operons in O. kitaharae DSM 17330 and L. intestinalis DSM 6629 appear to be complete, the L. crispatus DSM 20584 TOMM operon appears to lack a structural gene, however, the structural gene for similar operons has been found to be some distance from the SagBCD homologs previously31. Of these three strains, L. crispatus DSM 20584 was the only one found to display antimicrobial activity; the source of such activity, however, remains unclear.
Class II
Class II bacteriocins are small heat stable peptides which are not subject to extensive post translational modification, most of which act to permeabilize the membrane of target cells1. This class of bacteriocins is further subdivided based on the structure and activity of the peptides.
Class IIa
Class IIa or ‘pediocin-like’ bacteriocins display a narrow range of antimicrobial activity, particularly displaying strong anti-listerial activity. Such bacteriocins encompass a highly conserved YGNGV/L N-terminal motif followed by cysteine residues which can form a disulphide bridge. Unlike the N-terminus, the C-terminus is less conserved and is likely involved in membrane insertion and pore formation32. These bacteriocins likely act by using the mannose-phosphotransferase system on sensitive cells as a receptor33.
Despite having a long association with this class of bacteriocins, surprisingly only 3 Lactobacillus strains were found to encode what appear to be complete class IIa bacteriocin operons, containing structural, immunity and transport genes (Table 4a, Supplementary Table 2). Of these, L. hordei DSM 19519 displayed bacteriocin production against six of the indicators tested. From MALDI TOF MS and BAGEL results, the production of coagulin was confirmed. This 44 amino acid bacteriocin was originally isolated from Bacillus coagulans and closely resembles the bacteriocin pediocin PA-1, differing by a single amino acid due to a N41T substitution34, 35. The presence of a further pediocin-like operon was noted within the L. hordei genome, encoding a structural peptide displaying 74% amino acid identity to plantaricin 423. Production of this bacteriocin however was not seen.
Table 4.
Structural genes for complete (a) and incomplete (b) Class IIa operons.
| Species | Strain | Structural Peptide | Homolog (%Amino Acid Identity) |
|---|---|---|---|
| (a) Structural Genes for Complete Class IIa Operons | |||
| L. hordei | DSM 19519 | KYYGNGVTCGKHSCSVDWGKATTCIINNGAMAWATGGHQGTHKC | Coagulin (100%) |
| KYYGNGVSCTKKHGCKVNWGQAFTCSVNRFANFGHGNC | Plantaricin 423 (74%) | ||
| L. acidipiscis | DSM 15836 | KYYGNGLHIPKHGKPYINWGQAIQSIGKISYHGWVNGITSGAAGVGRH | Hiracin JM79 (44%) |
| L. futsaii | JCM 17355 | KYYGNGVSCGKHTCKVNWGQAWNESVNRWGNSWVNGLTGLRQH | Plantaricin 423 (57%) |
| C. maltaromaticum | DSM 20722 | AISYGNGVYCNKEKCWVNKAENKQAITGIVIGGWASSLAGMGH | Carnobacteriocin cbn BM1 (100%) |
| VYYGNGVSCSKTKCSVNWGQAFQERYTAGINSFVSGVASGAGSIGRRP | Carnobacteriocin cbn B2 (98%) | ||
| (b) Structural Genes for Incomplete Class IIa Operons | |||
| L. agilis | DSM 20509 | SRYYGNGITCGKHKCTVNWGQAWTCGVNRLANFGHGNC | Plantaricin 423 (73%) |
| L. aquaticus | DSM 21051 | KNYGNGVYCTKKHGYKVDWGQAWSIIGNNSAANSTTRGAAGWKSK | Avicin A (74%) |
| L. rennini | DSM 20253 | KYYGNGVSCSKHSCSVDWGKALTCTINNGAMAWTTGGHQGNHKC | Pediocin Ach/PA-1 (89%) |
| L. ruminis | DSM 20403 | KYYGNGVYCGKHKCRVDWGQAWGCSVNRWGAAVGTGGKATIGHC | Pediocin Ach/PA-1 (55%) |
| P. pentosaceus | DSM 20336 | KYYGNGLYCGKHSCSVDWGKATTCIINNGAMAWATGGHQGTHKC | Pediocin Ach/PA-1 (93%) |
| C. maltaromaticum | DSM 20342 | AISYGNGVYCNKEKCWVNKAENKQAITGIVIGGWASSLAGMGH | Carnobacteriocin cbn BM1 (100%) |
Numerous lactobacilli identified in this study were found to carry partial pediocin-like operons, often containing the bacteriocin structural gene and associated immunity protein but lacking the appropriate transporters (Table 4b, Supplementary Table 2). One potential explanation is that when a strain acquired the gene for pediocin resistance that the neighbouring small bacteriocin structural gene was also transferred, whilst the larger transporters were not.
Although not included in the Lactobacillaceae family, several Carnobacterium strains were included in the genomic study carried out by Sun et al.13. Numerous bacteriocins have been attributed to this genus previously25, 36. While the source of antimicrobial activity from C. maltaromaticum DSM20342 is unclear, C. maltaromaticum DSM 20722 was found to produce the class IIa bacteriocin cbnB2 and cbnBM1, the class IId bacteriocin cbnX was also produced by the strain25. CbnB2 contains an N2Y mutation which was also previously seen by Tulini et al.25.
Class IIb
Class IIb are comprised of unmodified two peptide bacteriocins, whose activity is dependent on the synergistic activity of both peptides which interact to form a single antimicrobial unit37. These bacteriocins are likely to act by forming membrane spanning pores which result in the leakage of small molecules from the cell. Such bacteriocins tend to contain conserved GxxxG or AxxxA motifs which are responsible for close helix interactions between each bacteriocin peptide37. A wide range of class IIb bacteriocins were identified by BAGEL in this study (Table 5, Supplementary Table 2).
Table 5.
Potential Class IIb Structural Genes.
| Species | Strain | Structural Peptide | ||
|---|---|---|---|---|
| L. murinus | DSM 20452 | YNRLAGQIGHYTGKAVIVGATVLGIASLF | Produced in vitro | (Muricidin) |
| KRGLGYHIVDAVVSFGKGFLDAF | ||||
| YDIEKALWGGYGYQLGWRNKWNLSHRYFKI | ||||
| GVPGWYYGMLWKIGVSGYKHRKDIMNGFDRGFNNYPK | ||||
| L. acidophilus | DSM 20079 | SNNIFWTRVGVGWAAEARCMIKPSLGNWTTKAVSCGAKGLYAAVRG | Produced in vitro | (Acidocin X) |
| VAPIVYPIAGYVMKQMFEHSDQIIKGFKRGWKKYK | ||||
| L. taiwanensis | DSM 21401 | NRWGDTVLSAASGAGTGIKACKSFGPWGMAICGSNRRLFWLYS | ||
| RNNWQTNVGGAVGSAMIGATVGGTICGPACAVAGAHYLPILWTGVTAATGGFGKIRK | ||||
| L. crispatus | DSM 20584 | NRWTNAYSAALGCAVPGVKYGKKLGGVWGAVIGGVGGAAVCGLAGYVRKG | ||
| SKGKGRNNWAGNTIGIVSSAATGAALGSAICGPGCGFVGAHWGAVGWTAVASFSGAFGKIRK | ||||
| L. nantensis | DSM 16982 | SFKGFVQGFINGLTGKKH | ||
| KGPWNYKTGYNLGKWISKRF | ||||
| L. apodemi | DSM 16634 | YDIEKALWKGYGYQLGWRSKWNLSHRYFKI | ||
| GVPGWYYSMLWKIGVSGYKHRKDIMSGFDKGFNNYPK | ||||
| L. plantarum | DSM 13273 | RRSRKNGIGYAIGYAFGAVERAVLGGSRDYNK | ||
| GAWKNFWSSLRKGFYDGEAGRAIRR | ||||
| FNRGGYNFGKSVRHVVDAIGSVAGIRGILKSIR | ||||
| VFHAYSARGVRNNYKSAVGPADWVISAVRGFIHG | ||||
| L. plantarum subsp. plantarum | CGMCC 1.2437 | RRSRKNGIGYAIGYAFGAVERAVLGGSRDYNK | ||
| GAWKNFWSSLRKGFYDGEAGRAIRR | ||||
| FNRGGYNFGKSVRHVVDAIGSVAGIRGILKSIR | ||||
| VFHAYSARGVRNNYKSAVGPADWVISAVRGFIHG | ||||
| L. paraplantarum | DSM 10667 | FNRGGYNFGKSVRHVVDAIGSVAGIRGILKSIR | ||
| VFHAYSARGVRNNYKSAVGPADWVISAVRGFIHG | ||||
| L. intestinalis | DSM 6629 | RHSVPYSYGYQSGRGFKGAAAAYNIIKTVASFFE | ||
| KRKKHHPWYWSIQEFGRGFLAGLASKYNL | ||||
| L. rhamnosus | DSM 20021 | IGPLAIPVAAILGFLATDAWSHADELVAGVKQGWERS | ||
| DNGNLWTFIGKAIGSTARSWAEGAMFAPAIGPAKEIVDKLNGN | ||||
| L. zeae | DSM 20178 | NAWGNAVNGALNGAATGARFGKNLGPWGMIGGMALGAGIGGYFGYNG | ||
| RNTWQQNVSGVAGAAAGGAALGAVVGGPAGAFLGAHYGPILWTAVTGFTGGF | ||||
| Leuc. fallax | KCTC 3537 | CPLLPIVVTVAASGAHFVAKDGWNHLDQIRSGWRKSGNSKW | ||
| STDGSWEDFGAGLHKTVNTVIYAGTTVARAHTRSHQRCFTGNKW | ||||
L. murinus DSM 20452 was one of the strains which demonstrated bacteriocin production. MALDI TOF MS identified masses which correlate with a two-peptide bacteriocin identified by BAGEL (muricidin). Both peptides of muricidin display homology to the class IIa bacteriocin plantaricin S, with the α peptide displaying 41% amino acid identity to pln Sα and the β peptide 48% to pln Sβ. The β peptide found here however lacks the AxxxA motif found in pln Sβ, a sequence which has been shown to be important for helix-helix interactions in pln S38.
Another potential two-peptide bacteriocin (acidocin X) was also identified from L. acidophilus DSM 20079. Correlation between the bacteriocins identified by BAGEL and the results of MALDI TOF MS led to the identification of two, bacteriocin like, peptides. The first of these was a 35 amino acid peptide displaying 53% identity with the enterocin X β peptide. The second peptide was not identified in BAGEL and was found by manual analysis of the bacteriocin operon, this displays 25% identity to the enterocin X α peptide.
Class IIc
Class IIc bacteriocins are also known as circular bacteriocins due to the covalent linkage of the N- to C-termini. The compact circular structure of these bacteriocins can contribute to their temperature and pH stability39. These circular bacteriocins permeabilize the target cell membrane, resulting in a loss of membrane potential which leads to cell death40. Despite having similar modes of action, this class of bacteriocins are further broken down into two subgroups, based on the isoelectric point of the peptides and the conservation seen amongst the groups41. Currently, there are two examples of class IIc bacteriocins produced from lactobacilli, both of which belong to subgroup II. Originally identified as two separate class IIc bacteriocins, Gassericin A (L. gasseri LA39) and reutericin 6 (L. reuteri LA6) have now been shown to be identical42, 43. Acidocin B (L. acidophilus M46), originally thought to be linear, has also been recently reclassified as a circular bacteriocin. Leuconostoc mesenteroides TK41401 has also been shown to produce leucocyclicin Q, a subgroup I circular bacteriocin.
From the analysis carried out in this study, L. paracasei subsp. paracasei DSM 5622 was found to produce a potential class IIc bacteriocin (paracyclicin), with a structural gene displaying 64% amino acid identity to butyrivibriocin AR1044. The operon contains a putative ABC permease, ATPase and a protein belonging to the DUF 95 protein family, all of which have been associated with the gene clusters of circular bacteriocins39. Upon purification of the bacterial supernatant, a mass of 5905.75 Da was identified as the causative agent of antimicrobial activity. This mass correlates closely with the predicted mass of the mature bacteriocin structural peptide which is calculated as 5906.87 Da. It is clear that paracyclicin belongs to the subgroup II circular bacteriocins, due to a high level of conservation found within the group (Table 6). Despite this conservation, this novel bacteriocin does display variation in certain conserved regions which is not seen in the rest of the class. L. nodensis DSM 19682 was also found to encode one such potential bacteriocin, however, no antimicrobial activity was observed with this strain.
Table 6.
Alignment of Class IIc Subgroup II Bacteriocins.
| Bacteriocin | Structural Peptide |
|---|---|
| Gassericin A | IYWIADQFGIHLATGTARKLLDAMASGASLGTAFAAILGVTLPAWALAAAGALGATAA |
| Acidocin B | IYWIADQFGIHLATGTARKLLDAVASGASLGTAFAAILGVTLPAWALAAAGALGATAA |
| Butyrivibriocin AR10 | IYFIADKMGIQLAPAWYQDIVNWVSAGGTLTTGFAIIVGVTVPAWIAEAAAAFGIASA |
| L. paracesei subsp. paracasei DSM 5622 (Paracyclicin) | IYFIANKLGIHLAPGWYQDMVNYVSAGGSLAGAFSVVAGVTLPAWIVPIATAFGAVSA |
| L. nodensis DSM 19682 | -IWIAGLFGIHLDNSLESKLVSGILNGGSAAGVFAAMLGITLPAWAAAAATAMGATAA |
| :**:**: *..::.:*.:*::*:*:*****:*.:* |
* = Positions with a single conserved residue. : = Conservation between groups with strongly similar properties, scoring >0.5 in the Gonnet PAM 250 matrix. . = Conservation between groups with weakly similar properties, scoring ≤0.5 in the Gonnet PAM 250 matrix.
Class IId
Class IId bacteriocins are single peptide, linear bacteriocins which do not display homology to the pediocin like bacteriocins4. This class of bacteriocins displays a high degree of diversity and numerous class IId bacteriocins have been characterised from lactobacilli previously (Table 1). In silico analysis of the Lactobacillus dataset identified numerous novel structural genes (Table 7, Supplementary Table 2) with several shown to be produced.
Table 7.
Potential Class IId structural proteins (a) and Class IId lactococcin 972 homologs (b).
| Species | Strain | Structural Protein |
|---|---|---|
| (a) Potential Class IId Structural proteins | ||
| L. paralimentarius | DSM 13961 | NFFGGSNGYSWRDKKGHWHYTVTSGVSSTVAQIIGNGWGSAGAPGVGQR |
| L. pentosus | DSM 20314 | KSNTYSLQMGSVVRTATKIFKKMEW |
| L. hokkaidonensis | DSM 26202 | VTLSVATHSKNGLKKFFKWVRKL |
| L. xiangfangensis | LMG 26013 | KLVKLYTAEPYTFYRDTRTKKIVMRQTTGYSAHLQHVIADGWVRSAHL |
| L. paracasei | DSM 5622 | DSIRDVSPTFNKIRRWFDGLFK |
| L. murinus | DSM 20452 | YDIEKALWGGYGYQLGWRNKWNLSHRYFKI |
| Leuc. kimchii | IMSNU 11154 | KSFWSWASDASSWLSGPQQPNSPLLKKKR |
| Leuc. geldium | KCTC 3527 | KRVYIPNGNGAWLDSNTGKGGVDWNVAVPALGSIMVNGWAQNGPLAHLHP |
| (b) Potential Class IId Lactococcin 972 Homologs | ||
| L. equicursoris (equicursorin) | DSM 19284 | GGTWNYGVGSKYVWSYYSHNSKTHKASVEGKYYVTSGWIKEKTQARASAAKAAAGNQSYYDVK |
| L. amylophilus | DSM 20533 | GGTWNYGVGLTGTFGYSDYLHNSKTHSASVGRTKSDCNKVTKTKGVWAQSKYTKIPPTGLNYWWSVS |
| L. graminis | DSM 20719 | GGTWYSGFSGTKVYSQYYHGSKKHSATAKNGWGAGVRNTQKAGIWAYSSVNSTLTGNKTYWAVY |
| L. hamsteri | DSM 5661 | GGVWNYGVGKKYVWSYYSHHRLTHKSSVEGKYYSSSGWVSPGTEARASAEKAQHGNKSYFDVE |
| Leuc. argentinum | KCTC 3773 | GGDWRHGVGSYYVWSYYFHNYRNHSSSVSGQYFASSGRTSPGYDAQASAPKSLFGNKAYYDFW |
L. paralimentarius DSM 13961 was one such strain to display the production of a class IId bacteriocin (paralimenterocin). The paralimenterocin structural gene identified encodes a 44 amino acid single peptide bacteriocin whose closest homolog appears to be the relatively uncharacterised bacteriocin BacSJ2-8 to which it has 77% identity45. The mode of action of both of these bacteriocins remains unclear.
L. equicursoris DSM 19284 is also highly likely to produce a novel class IId bacteriocin (equicursorin). The strain displayed antimicrobial production upon analysis, but MALDI TOF MS did not identify an associated mass. In silico BAGEL analysis identified three putative bacteriocin operons, two of which encoded larger bacteriolysins of approximately 30 kDa, the remaining operon encodes a homolog of lactococcin 972. SDS PAGE analysis of the concentrated culture supernatant identified a mass between the 5 kDa and 10 kDa markers which displayed antimicrobial activity once overlaid with L. delbrueckii subsp. bulgaricus LMG 6901 (Supplementary Figure 2). This mass correlates well with the predicted mass (approximately 7 kDa) of the lactococcin 972 homolog ‘equicursorin’. Lactococcin 972 is unique with respect to its activity in comparison to other class II bacteriocins. These bacteriocins do not induce pore formation in the cells but instead act by binding to lipid II and inhibiting septum formation. Lactococcin 972 is also unusual in that it’s biologically active form is as a homodimer46, 47. Given that only two such bacteriocins have been identified, it was surprising that four further lactococcin 972-like operons were identified in the genomic dataset screened in this study (Table 7(b), Supplementary Table 2). An in silico screen carried out by Letzel et al.48 identified 9 further Lactococcin 972 operons in anaerobic bacteria, thus due to the expansion of this group, these bacteriocins may warrant a separate classification, given their unique mode of action when compared to other class II bacteriocins.
Bacteriolysins (Formerly Class III Bacteriocins)
In the Lactobacillus dataset, a number of homologs of the bacteriolysin helveticin18 were found to be encoded, with several displaying in vitro antimicrobial activity. The approximate size of these proteins was determined using SDS PAGE overlay assays, as MALDI TOF MS was not used to determine the size of these larger proteins. Several strains encoded numerous helveticin homologs, however, SDS PAGE overlays were not able to identify which of these homologs was actually produced as all had masses of approximately 37 kDa (Supplementary Figure 3).
L. intestinalis DSM 6629 was shown to produce one of these helveticin homologs, with four potential structural genes found within the genome ranging from 38% to 67% amino acid identity to helveticin J. L. kitasatonis DSM16761 also produced a helveticin like peptide, the strain encodes two such proteins displaying 35% and 41% identity to helveticin J. Two L. amylovorus strains (DSM 16698 and DSM 20531) were shown to produce a helveticin homolog. L amylovovrus DSM 16698 encodes four of such proteins, whilst L. amylovorus DSM 20531 encodes three. Both share a single identical helveticin homolog but it is unclear whether this is the protein produced by both strains. L. kalixensis DSM 16043 also produces a helveticin-like protein, with 3 homologs encoded within the genome displaying, 34%, 49% and 50% amino acid identity to helveticin J.
BAGEL also identified a helveticin homolog (77% identity to helveticin J) from L. crispatus DSM20584. Interestingly, analysis of the results of an exoproteomic study carried out by Johnson et al.49 identified the secretion of this protein previously. The antimicrobial activity of the strain in this study was determined to be due to a small peptide by an SDS PAGE overlay assay, this is most likely a lactacin F homolog50 or else a novel TOMM like peptide.
Discussion
This study gives the first complete assessment of bacteriocin production across the Lactobacillus Genus Complex, combining both in silico and laboratory based screening methods. This combination of approaches allows for a more representative estimation of bacteriocin production to be calculated. Well-diffusion assays and MALDI TOF MS allows for the confirmation of in vitro bacteriocin production by cells. Bacteriocin production however can be a highly regulated process, with strains requiring specific conditions and environments to induce production of these antimicrobials51, 52. Such regulations would make it extremely difficult to identify the bacteriocins found here using in silico screens if we were to rely on in vitro screening methods alone. Thus, the use of BAGEL and BLAST bacteriocin screens allows us to identify these bacteriocin operons from the Lactobacillus Genus Complex without the shortcomings and restrictions of laboratory based screens.
In silico analysis has allowed us to determine the overall bacteriocinogenic potential of the Lactobacillus genus. Of the 213 strains analysed, 51 were identified by BAGEL or in BLAST screens as harbouring what appears to be a complete bacteriocin or helveticin like operon, a prevalence of 23.94%. If we focus on the lactobacilli, of the 175 strains analysed only 25 were found to encode bacteriocin operons (14%). If helveticin operons and those of previously characterised bacteriocins are included, of the Lactobacillus species analysed 30% were found to encode at least one antimicrobial. This figure of 30% is surprisingly high given that lactobacilli are not associated with the production of more traditional antibiotics formed by non-ribosomal peptide synthetases (NRPS) and polyketide synthases (PKS). Given the extent of bacteriocin production within the genus, the production of antimicrobials by these means may be unnecessary, especially given the size of such NRPS and PKS operons and the subsequent energy it would take to produce them. Thus, bacteriocin production may supplant the need for NRPS and PKS enzyme complexes in certain genera.
There was a high degree of novelty within the bacteriocins identified by BAGEL in this study and of all the structural genes identified, 73% had not previously been characterised. Screening of these strains identified five novel functional bacteriocins (muricidin, acidocin X, paracyclicin, paralimenterocin and equicursorin) from a range of bacteriocin classes. In addition, five novel producers of helveticin-like peptides were also identified. The abundance of homologs of helveticin-like bacteriolysins encoded by lactobacilli is surprising given how little these proteins have been characterised to date. The observation that most strains in the L. acidophilus group encode helveticins with significant homology suggests that this trait was derived from a common ancestor and then disseminated by horizontal transfer. Apart from narrow spectrum antimicrobial activity, no other function has been ascribed to these proteins. The role these proteins play in the life cycle of this narrow branch of strains warrants further study.
The variety and distribution of bacteriocins throughout the genus is interesting when compared to the results of other in silico screens which were carried out. Letzel et al.48 used BAGEL and other tools to screen the genomes of 211 anaerobes for bacteriocin encoding genes (no lactobacilli were included in the screen, and helveticin like proteins were excluded). Of these 211 strains, just over 25% were found to encode a bacteriocin like peptide. Thus despite the differences in the make-up of the datasets, there is a similar level of bacteriocin encoding genes found in both groups. While the overall levels may be similar, the diversity of the bacteriocins encoded differs greatly. Of the bacteriocins encoded in the anaerobic dataset, 78% were found to be class I modified bacteriocins, while in the Lactobacillus Genus Complex this value is only 17%. One similarity between these sets of results, however, is the presence of lactococcin 972 like bacteriocins. 9 novel homologs were identified in the anaerobic bacteria, this result taken with the number of novel homologs identified from the lactobacilli suggests that this group of unique bacteriocins merit their own class of bacteriocins in the future given their unique mode of action and increasing prevalence.
In a bioinformatic screen of Bacillus species for bacteriocin operons53, the overall level of bacteriocins encoded by such strains was much higher, with 583 putative bacteriocin operons encoded in the genomes of 328 strains. 89% of these strains, covering 50 different species encode a bacteriocin, a much higher level than seen in the anaerobic bacteria and the lactobacilli. The diversity of encoded bacteriocins again differs to that of the lactobacilli with 66% of operons identified here encoding class I bacteriocins. This difference suggests that there is not an even distribution in the types of bacteriocins across genera, with the lactobacilli in particular relying on the production of class II bacteriocins in comparison to other groups. A similar high prevalence of bacteriocin operons can be found in the cyanobacteria, with 145 putative bacteriocin gene clusters being identified in 43 of the 58 complete and partial genomes screened54. It must be remembered, however, that in both studies these operons were not manually analysed so, in reality, overall levels may be lower.
The inter-species diversity of bacteriocin production can be seen in a screen carried out by Liu et al.55 whereby, the genomes of 169 Streptococcus mutans strains were screened by BAGEL for bacteriocin operons. 211 bacteriocin operons were found distributed amongst 157 strains, of which 32 were lantibiotic operons. These results show that despite carrying out a comprehensive analysis of bacteriocin production in lactobacilli, a high level of diversity within each species can still result in novel bacteriocins being identified.
The environment from which strains are isolated may also influence their bacteriocinogenic potential. 37.5% of strains isolated from human and animal microbiomes encoded bacteriocins or bacteriolysins, this is over twice the value for strains isolated from food, wine and beer, plants and the environment at 16.67%. The microbiota of animals is a complex community with microbes under constant competition for nutrients and resources56. Bacteriocin production can provide a competitive advantage for strains, allowing them to inhibit sensitive strains thus reducing competition and allowing them to establish themselves in these environments2, 3, 57. This may suggest why a greater proportion of lactobacilli from these environments encode bacteriocins. Environments such as fermented foods would provide a much narrower niche for the growth of microbes. Less competition here may negate the need for these bacteria to expend energy on bacteriocin production.
Given the association of lactobacilli with probiotics and food production, the knowledge of their potential to produce antimicrobials is of great value58. Bacteriocin production may increase their ability to establish themselves in a community such as the gut, or provide a natural mechanism to inhibit the growth of food spoilage microorganisms3, 59. Thus bacteriocin production can prove a useful trait for an industrially important group of bacteria. Previously, the isolation of bacteriocins from lactobacilli relied on intensive laboratory screens of individual cultures. The use of tools such a BAGEL and BLAST however now allow for the rapid identification of bacteriocin operons within strains, and with the increasing availability of genomic data, these tools are becoming more relevant.
Materials and Methods
Bacteriocin Identification
The bacteriocin mining tool BAGEL2 was used to identify putative bacteriocin operons10 and the genome visualisation tool ARTEMIS was subsequently used for manual analysis of the bacterial genomes60. To determine the degree of novelty in the bacteriocins identified by BAGEL2, BLASTP searches were done for each putative bacteriocin peptide against those identified in the BAGEL screen. The levels of identity described in this study are derived from Clustal Omega. For bacteriocin analysis using specific “driver” sequences, the BLASTP program was used using default parameters. The driver sequences used were NisC (GenBank Accession no. CAA79470.1), LtnM1 (GenBank Accession no. NP_047321.1), VenL (GenBank Accession no. AEA03262.1), TrnC and TrnD from Bacillus thuringiensis DPC 6431.
Bacterial strains
The bacterial strains screened for bacteriocin production and the conditions for growth are listed in Supplementary Table 3. Anaerocult A gas packs (Merck, Darmstadt, Germany) were used to generate anaerobic conditions.
Bacteriocin Assays
Bacteriocin activity was analysed via well diffusion assays against the indicator organisms listed in Supplementary Table 4. Briefly, each strain screened was grown in broth under the appropriate conditions. The cell free supernatant of each culture was prepared by centrifugation of the fully grown culture at 4000 RCF for 20 minutes, the pH was adjusted to pH 7 using sodium hydroxide to negate any antimicrobial activity which may be caused by the acidity of the cell free supernatants. 50 μl of an overnight culture of each indicator was then added to 20 ml of the appropriate media containing 1.5% agar. Plates were allowed cool and the 7 mm wide wells were bored into the agar. 50 μl of the cell free supernatant of the strains being tested was then placed in a well. These indicator plates were refrigerated for two hours prior to incubation.
Mass Spectrometry (MS)
MALDI TOF colony mass spectroscopy was carried out on each of the strains as described by Field et al.61 to identify masses of putative bacteriocins. Here colonies were first mixed with a 70% propan-2-ol 0.1% TFA solution to elute bacteriocin from the cell. Following centrifugation, the subsequent supernatant was spotted on the target pre-coated with CHCA matrix solution. A further layer of matrix solution was then added on top of this supernatant. An Axima TOF2 MALDI TOF mass spectrometer (Shimadzu Biotech, Manchester, UK) was used to identify the peptide masses using positive-ion reflectron mode.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
SDS PAGE was used for the identification of higher molecular weight antimicrobial proteins (bacteriolysins). Cultures were grown overnight in broth and the cell free supernatants were prepared as described above. The proteins from the bacterial supernatant were precipitated by the addition of ammonium sulphate salts up to a concentration of 50%. The precipitate was collected by centrifugation and resuspended in water. Supernatants were then incubated with TruPAGETM LDS sample buffer (Sigma-Aldrich, Wicklow, Ireland) for 10 minutes at 70°C. Samples were run on 12% acrylamide gels at 30 mA, together with Precision Plus Protein™ Dual Xtra prestained protein standards (Bio-Rad, Hertfordshire, UK) which were used to estimate molecular mass with a range of 2–250 kDa. The completed gels were divided in two, one half was stained using the EZBlueTM staining reagent (Sigma-Aldrich). The other half was washed with 1% tween-80 (Sigma-Aldrich) for 45 minutes, followed by three 5 minute washes in distilled water. This gel was overlaid with soft MRS agar (0.8% agar), seeded with 0.25% of an overnight culture of L. delbrueckii subsp. bulgaricus LMG 6901. The plate was incubated overnight to determine the mass of any antimicrobial proteins produced.
Bacteriocin Purification
Carnobacteriocins CbnB2, CbnBM1 and CbnX
Carnobacterium maltaromaticum DSM 20722 was grown overnight in TSA broth, 100 ml of the supernatant was passed through a 5 g, 20 ml Strata C18-E solid-phase extraction (SPE) column (Phenomenex, Cheshire, UK). The column was washed with 20 ml of 30% ethanol and 20 ml of 70% 2-propanol (IPA) 0.1% TFA. The 70% IPA eluent was concentrated and applied to a Semi Prep Proteo Jupiter RP-HPLC column (10 × 250 mm, 90 Å, 4 µ) (Phenomenex, Cheshire, UK) running a 20–55% gradient whereby buffer B was 90% acetonitrile. MALDI TOF MS was carried out on fractions to identify the presence of the peptides of interest.
Paracyclicin
L. paracasei subsp. paracasei DSM 5622 was grown overnight in MRS broth. Culture supernatant was passed through a column containing 60 g Amberlite XAD beads and washed with 400 ml of 50% ethanol and the antimicrobial peptide eluted with 400 ml of 70% IPA 0.1% TFA. The IPA was removed and the eluent passed through a 5 g, 20 ml C18 SPE column pre-equilibrated with methanol and water. The column was washed with 30 ml of 50% ethanol and activity eluted with 30 ml of IPA. The IPA was removed from the C18 SPE IPA eluent and the sample applied to a semi preparative Vydac C4 Mass Spec (10 × 250 mm, 300 Å, 5 µ) RP-HPLC column (Grace, Columbia, USA) running an acetonitrile and propan-2-ol gradient described as follows: 5–55% buffer B and 0–5% buffer C over 25 minutes followed by and 55–19% buffer B and 5–81% buffer C over 60 minutes, 19–5% buffer B and 81–95% buffer C over 5 minutes where buffer A is Milli Q water containing 0.1% TFA, buffer B is 90% acetonitrile 0.1% TFA and buffer C is 90% propan-2-ol 0.1% TFA. Eluent was monitored at 214 nm and fractions were collected at 1 minute intervals. Fractions were assayed using well diffusion assays against L. delbrueckii subsp. bulgaricus LMG 6901. MALDI TOF MS was used to determine the mass of the antimicrobial peptide.
Electronic supplementary material
Acknowledgements
This publication has emanated from research conducted with the financial support of Science Foundation Ireland (SFI) under Grant Number SFI/12/RC/227. Orla O’Sullivan is funded by Science Foundation Ireland through a Starting Investigator Research Grant award (13/SIRG/2160).
Author Contributions
R.P.R., C.H. and M.C.R. conceived the study and designed the project. F.C. carried out bacteriocin screens and analysis. Peptide purification and MALDI-TOF M.S. was carried out by P.M.O.C. O.O.S. carried out genomic analysis. F.C., B.G.S., M.C.R., C.H. and R.P.R. contributed in the preparation of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-03339-y
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cotter PD, Hill C, Ross RP. Bacteriocins: developing innate immunity for food. Nature reviews. Microbiology. 2005;3:777–788. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 2.Dobson A, Cotter PD, Ross RP, Hill C. Bacteriocin production: a probiotic trait? Applied and environmental microbiology. 2012;78:1–6. doi: 10.1128/AEM.05576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh MC, et al. Predominance of a bacteriocin-producing Lactobacillus salivarius component of a five-strain probiotic in the porcine ileum and effects on host immune phenotype. FEMS microbiology ecology. 2008;64:317–327. doi: 10.1111/j.1574-6941.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- 4.Cotter PD, Ross RP, Hill C. Bacteriocins - a viable alternative to antibiotics? Nature reviews. Microbiology. 2013;11:95–105. doi: 10.1038/nrmicro2937. [DOI] [PubMed] [Google Scholar]
- 5.O’Shea EF, et al. Bactofencin A, a new type of cationic bacteriocin with unusual immunity. MBio. 2013;4:e00498–00413. doi: 10.1128/mBio.00498-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holo H, Jeknic Z, Daeschel M, Stevanovic S, Nes IF. Plantaricin W from Lactobacillus plantarum belongs to a new family of two-peptide lantibiotics. Microbiology. 2001;147:643–651. doi: 10.1099/00221287-147-3-643. [DOI] [PubMed] [Google Scholar]
- 7.Ryan MP, Rea MC, Hill C, Ross RP. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Applied and Environmental Microbiology. 1996;62:612–619. doi: 10.1128/aem.62.2.612-619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Begley M, Cotter PD, Hill C, Ross RP. Identification of a Novel Two-Peptide Lantibiotic, Lichenicidin, following Rational Genome Mining for LanM Proteins. Applied and Environmental Microbiology. 2009;75:5451–5460. doi: 10.1128/AEM.00730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClerren AL, et al. Discovery and in vitro biosynthesis of haloduracin, a two-component lantibiotic. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17243–17248. doi: 10.1073/pnas.0606088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jong A, van Heel AJ, Kok J, Kuipers OP. BAGEL2: mining for bacteriocins in genomic data. Nucleic Acids Res. 2010;38:W647–651. doi: 10.1093/nar/gkq365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillor O, Etzion A, Riley M. The dual role of bacteriocins as anti-and probiotics. Applied microbiology and biotechnology. 2008;81:591–606. doi: 10.1007/s00253-008-1726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inglis RF, Bayramoglu B, Gillor O, Ackermann M. The role of bacteriocins as selfish genetic elements. Biology letters. 2013;9:20121173. doi: 10.1098/rsbl.2012.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun, Z. et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. 6, 8322, doi:10.1038/ncomms9322 (2015). [DOI] [PMC free article] [PubMed]
- 14.Marsh AJ, O’Sullivan O, Ross RP, Cotter PD, Hill C. In silico analysis highlights the frequency and diversity of type 1 lantibiotic gene clusters in genome sequenced bacteria. BMC genomics. 2010;11:679. doi: 10.1186/1471-2164-11-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goto Y, et al. Discovery of unique lanthionine synthetases reveals new mechanistic and evolutionary insights. PLoS Biol. 2010;8:e1000339. doi: 10.1371/journal.pbio.1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh CJ, et al. In silico identification of bacteriocin gene clusters in the gastrointestinal tract, based on the Human Microbiome Project’s reference genome database. BMC microbiology. 2015;15:183. doi: 10.1186/s12866-015-0515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy K, et al. Genome mining for radical SAM protein determinants reveals multiple sactibiotic-like gene clusters. PloS one. 2011;6:e20852. doi: 10.1371/journal.pone.0020852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joerger MC, Klaenhammer TR. Characterization and purification of helveticin J and evidence for a chromosomally determined bacteriocin produced by Lactobacillus helveticus 481. Journal of bacteriology. 1986;167:439–446. doi: 10.1128/jb.167.2.439-446.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McAuliffe O, Ross RP, Hill C. Lantibiotics: structure, biosynthesis and mode of action. FEMS microbiology reviews. 2001;25:285–308. doi: 10.1111/j.1574-6976.2001.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 20.Mortvedt CI, Nissen-Meyer J, Sletten K, Nes IF. Purification and amino acid sequence of lactocin S, a bacteriocin produced by Lactobacillus sake L45. Appl Environ Microbiol. 1991;57:1829–1834. doi: 10.1128/aem.57.6.1829-1834.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez B, Arca P, Mayo B, Suarez JE. Detection, purification, and partial characterization of plantaricin C, a bacteriocin produced by a Lactobacillus plantarum strain of dairy origin. Appl Environ Microbiol. 1994;60:2158–2163. doi: 10.1128/aem.60.6.2158-2163.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer R, Chikindas ML, Dicks LM. Purification, partial amino acid sequence and mode of action of pediocin PD-1, a bacteriocin produced by Pediococcus damnosus NCFB 1832. Int J Food Microbiol. 2005;101:17–27. doi: 10.1016/j.ijfoodmicro.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 23.Turner DL, et al. Solution structure of plantaricin C, a novel lantibiotic. European journal of biochemistry / FEBS. 1999;264:833–839. doi: 10.1046/j.1432-1327.1999.00674.x. [DOI] [PubMed] [Google Scholar]
- 24.Wiedemann I, et al. Lipid II-based antimicrobial activity of the lantibiotic plantaricin C. Appl Environ Microbiol. 2006;72:2809–2814. doi: 10.1128/AEM.72.4.2809-2814.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tulini FL, et al. Purification and characterization of antimicrobial peptides from fish isolate Carnobacterium maltaromaticum C2: Carnobacteriocin X and carnolysins A1 and A2. Int J Food Microbiol. 2014;173:81–88. doi: 10.1016/j.ijfoodmicro.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Fluhe L, et al. The radical SAM enzyme AlbA catalyzes thioether bond formation in subtilosin A. Nature chemical biology. 2012;8:350–357. doi: 10.1038/nchembio.798. [DOI] [PubMed] [Google Scholar]
- 27.Mathur, H., Rea, M. C., Cotter, P. D., Hill, C. & Ross, R. P. The sactibiotic subclass of bacteriocins: an update. Current Protein & Peptide Science16 (2015). [DOI] [PubMed]
- 28.Rea MC, et al. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9352–9357. doi: 10.1073/pnas.0913554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molloy EM, Cotter PD, Hill C, Mitchell DA, Ross RP. Streptolysin S-like virulence factors: the continuing sagA. Nature reviews. Microbiology. 2011;9:670–681. doi: 10.1038/nrmicro2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SW, et al. Discovery of a widely distributed toxin biosynthetic gene cluster. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5879–5884. doi: 10.1073/pnas.0801338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haft DH. A strain-variable bacteriocin in Bacillus anthracis and Bacillus cereus with repeated Cys-Xaa-Xaa motifs. Biology direct. 2009;4:15. doi: 10.1186/1745-6150-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fimland G, Johnsen L, Dalhus B, Nissen-Meyer J. Pediocin-like antimicrobial peptides (class IIa bacteriocins) and their immunity proteins: biosynthesis, structure, and mode of action. Journal of peptide science: an official publication of the European Peptide Society. 2005;11:688–696. doi: 10.1002/psc.699. [DOI] [PubMed] [Google Scholar]
- 33.Ramnath M, Beukes M, Tamura K, Hastings JW. Absence of a putative mannose-specific phosphotransferase system enzyme IIAB component in a leucocin A-resistant strain of Listeria monocytogenes, as shown by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Appl Environ Microbiol. 2000;66:3098–3101. doi: 10.1128/AEM.66.7.3098-3101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyronimus B, Le Marrec C, Urdaci MC. Coagulin, a bacteriocin-like inhibitory substance produced by Bacillus coagulans I4. Journal of applied microbiology. 1998;85:42–50. doi: 10.1046/j.1365-2672.1998.00466.x. [DOI] [PubMed] [Google Scholar]
- 35.Le Marrec C, Hyronimus B, Bressollier P, Verneuil B, Urdaci MC. Biochemical and genetic characterization of coagulin, a new antilisterial bacteriocin in the pediocin family of bacteriocins, produced by Bacillus coagulans I(4) Appl Environ Microbiol. 2000;66:5213–5220. doi: 10.1128/AEM.66.12.5213-5220.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quadri LE, Sailer M, Roy KL, Vederas JC, Stiles ME. Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. The Journal of biological chemistry. 1994;269:12204–12211. [PubMed] [Google Scholar]
- 37.Nissen-Meyer J, Oppegard C, Rogne P, Haugen HS, Kristiansen PE. Structure and Mode-of-Action of the Two-Peptide (Class-IIb) Bacteriocins. Probiotics and antimicrobial proteins. 2010;2:52–60. doi: 10.1007/s12602-009-9021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soliman W, Wang L, Bhattacharjee S, Kaur K. Structure-activity relationships of an antimicrobial peptide plantaricin s from two-peptide class IIb bacteriocins. Journal of medicinal chemistry. 2011;54:2399–2408. doi: 10.1021/jm101540e. [DOI] [PubMed] [Google Scholar]
- 39.Gabrielsen C, Brede DA, Nes IF, Diep DB. Circular bacteriocins: biosynthesis and mode of action. Appl Environ Microbiol. 2014;80:6854–6862. doi: 10.1128/AEM.02284-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Belkum MJ, Martin-Visscher LA, Vederas JC. Structure and genetics of circular bacteriocins. Trends in microbiology. 2011;19:411–418. doi: 10.1016/j.tim.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Acedo JZ, et al. Solution structure of acidocin B, a circular bacteriocin produced by Lactobacillus acidophilus M46. Applied and environmental microbiology. 2015;81:2910–2918. doi: 10.1128/AEM.04265-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawai Y, et al. Lactobacillus reuteri LA6 and Lactobacillus gasseri LA39 isolated from faeces of the same human infant produce identical cyclic bacteriocin. Food microbiology. 2001;18:407–415. doi: 10.1006/fmic.2001.0412. [DOI] [Google Scholar]
- 43.Arakawa K, et al. HPLC purification and re‐evaluation of chemical identity of two circular bacteriocins, gassericin A and reutericin 6. Letters in applied microbiology. 2010;50:406–411. doi: 10.1111/j.1472-765X.2010.02810.x. [DOI] [PubMed] [Google Scholar]
- 44.Kalmokoff ML, Cyr TD, Hefford MA, Whitford MF, Teather RM. Butyrivibriocin AR10, a new cyclic bacteriocin produced by the ruminal anaerobe Butyrivibrio fibrisolvens AR10: characterization of the gene and peptide. Canadian journal of microbiology. 2003;49:763–773. doi: 10.1139/w03-101. [DOI] [PubMed] [Google Scholar]
- 45.Kojic M, et al. Construction of a new shuttle vector and its use for cloning and expression of two plasmid-encoded bacteriocins from Lactobacillus paracasei subsp. paracasei BGSJ2-8. Int J Food Microbiol. 2010;140:117–124. doi: 10.1016/j.ijfoodmicro.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Martinez B, Rodriguez A, Suarez JE. Lactococcin 972, a bacteriocin that inhibits septum formation in lactococci. Microbiology. 2000;146((Pt 4)):949–955. doi: 10.1099/00221287-146-4-949. [DOI] [PubMed] [Google Scholar]
- 47.Martinez B, et al. Specific interaction of the unmodified bacteriocin Lactococcin 972 with the cell wall precursor lipid II. Appl Environ Microbiol. 2008;74:4666–4670. doi: 10.1128/AEM.00092-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Letzel A-C, Pidot SJ, Hertweck C. Genome mining for ribosomally synthesized and post-translationally modified peptides (RiPPs) in anaerobic bacteria. BMC genomics. 2014;15:1. doi: 10.1186/1471-2164-15-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson BR, et al. Conserved S-Layer-Associated Proteins Revealed by Exoproteomic Survey of S-Layer-Forming Lactobacilli. Appl Environ Microbiol. 2016;82:134–145. doi: 10.1128/AEM.01968-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fremaux C, Ahn C, Klaenhammer TR. Molecular analysis of the lactacin F operon. Appl Environ Microbiol. 1993;59:3906–3915. doi: 10.1128/aem.59.11.3906-3915.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diep DB, Axelsson L, Grefsli C, Nes IF. The synthesis of the bacteriocin sakacin A is a temperature-sensitive process regulated by a pheromone peptide through a three-component regulatory system. Microbiology. 2000;146((Pt 9)):2155–2160. doi: 10.1099/00221287-146-9-2155. [DOI] [PubMed] [Google Scholar]
- 52.Maldonado-Barragan A, Caballero-Guerrero B, Lucena-Padros H, Ruiz-Barba JL. Induction of bacteriocin production by coculture is widespread among plantaricin-producing Lactobacillus plantarum strains with different regulatory operons. Food microbiology. 2013;33:40–47. doi: 10.1016/j.fm.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Zhao X, Kuipers OP. Identification and classification of known and putative antimicrobial compounds produced by a wide variety of Bacillales species. BMC Genomics. 2016;17:882. doi: 10.1186/s12864-016-3224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang H, Fewer DP, Sivonen K. Genome mining demonstrates the widespread occurrence of gene clusters encoding bacteriocins in cyanobacteria. PloS one. 2011;6:e22384. doi: 10.1371/journal.pone.0022384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu, L., Hao, T., Xie, Z., Horsman, G. P. & Chen, Y. Genome mining unveils widespread natural product biosynthetic capacity in human oral microbe Streptococcus mutans. Scientific Reports6 (2016). [DOI] [PMC free article] [PubMed]
- 56.Kostic AD, Howitt MR, Garrett WS. Exploring host–microbiota interactions in animal models and humans. Genes & development. 2013;27:701–718. doi: 10.1101/gad.212522.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gillor O, Giladi I, Riley MA. Persistence of colicinogenic Escherichia coli in the mouse gastrointestinal tract. BMC microbiology. 2009;9:165. doi: 10.1186/1471-2180-9-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanders M, Klaenhammer T. Invited review: the scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. Journal of dairy science. 2001;84:319–331. doi: 10.3168/jds.S0022-0302(01)74481-5. [DOI] [PubMed] [Google Scholar]
- 59.Yang S-C, Lin C-H, Sung CT, Fang J-Y. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Frontiers in microbiology. 2014;5:241. doi: 10.3389/fmicb.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rutherford K, et al. Artemis: sequence visualization and annotation. Bioinformatics (Oxford, England) 2000;16:944–945. doi: 10.1093/bioinformatics/16.10.944. [DOI] [PubMed] [Google Scholar]
- 61.Field D, et al. Studies with bioengineered Nisin peptides highlight the broad-spectrum potency of Nisin V. Microbial biotechnology. 2010;3:473–486. doi: 10.1111/j.1751-7915.2010.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


