Abstract
Background
Clinacanthus nutans Lindau is a well-known plant, native to tropical Asian countries. Reports on this plant that is rich in phenolic compounds have focused on its therapeutic anti-inflammatory, anti-herpes simplex, antioxidant, and anti-cancer characteristics. In this paper, the influence of the extraction parameters—temperatures (60–80 °C), times (80–120 min), and solvent ratios (70:30–90:10) of water:ethanol were investigated using response surface methodology in order to determine the optimum extraction conditions that could produce maximum extraction yields of the phenolic compounds and the highest anti-radical activity of the C. nutans extract.
Results
The optimum conditions suggested by the predicted model were: an extraction temperature of 60 °C, an extraction time of 120 min and a water:ethanol solvent ratio of 90:10 v/v%. The residual standard error of 0.2% indicated that there was no significant difference between the actual and predicted values and it proved that the models were adequate to predict the relevant responses. All the independent variables had a significant effect (p < 0.05) on all the responses which indicated that all extraction parameters employed in this study were important in the optimization process. The R2 values for three responses, extraction yields, DPPH radical scavenging activity and TPC were 0.9999, 0.9999 and 0.9983 respectively, suggesting that the quadratic polynomial models developed were satisfactorily accurate to be used in analyzing the interactions of the parameters (response and independent variables).
Conclusion
This study could be useful in the development of cosmeceutical products containing extracts of C. nutans.
Keywords: C. nutans; Central composite rotatable design (CCRD); Total phenolic content; 1,1-diphenyl-2-picrylhydrazyl (DPPH); Optimization; Anti-radical activity
Background
Clinacanthus nutans Lindau (C. nutans) is a plant that is commonly known in Malaysia as Sabah Snake Grass, and is widely used in folk medicine. Native to tropical Asian countries such as Malaysia, Thailand and Singapore, C. nutans has traditionally been used as an herbal remedy for insect bites [1, 2], detoxification [3, 4], herpes zoster infections [5] and to reduce the progression of cancer [6]. Numerous reports have documented the biological activity of C. nutans, including its anti-viral [7–9], anti-inflammatory [10], antioxidant [11], antinociceptive [12], antiaging [13] and anti-cancer [14, 15] properties. Previous investigations have established the presence of various polyphenols such as vitexin, isovitexin, shaftoside, isomollupentin-7-O-beta-glucopyranoside, orientin, isoorientin, kaempferol, sinapic acid, vanillin, quercetin, rutin trihydrate, syringic acid, protocatechuic acid, 4-vinylphenol and 7-hydroxyflavone in the extracts of C. nutans leaves [14, 16–18]. The ethnomedicinal uses of the C. nutans plant, its chemical constituents and pharmacological properties associated to its therapeutic potential has been of much research focus [19–21]. Plant polyphenols have drawn increasing attention due to their potent antioxidant properties and their marked effects in the prevention of oxidative stresses [22, 23].
As plants survive in environments with massive exposure to ultraviolet radiation, they are perfect antioxidant sources due to their rich endogenous antioxidants [24]. In addition, most quality products formulated from nature-based ingredients have had excellent safety records in the marketplace, which has led to a growing interest in herbal formulations [24]. Due to their relative safety and wide acceptance, plant polyphenols have been incorporated into pharmaceuticals and cosmeceuticals as alternatives to synthetic antioxidants [25]. Moreover, antioxidants can enhance the biological functions of cells by virtue of their radical scavenging activities [26]. About 1.5–5% of our consumed oxygen is converted into reactive oxygen species (ROS). ROS are harmful free radicals that are constantly being produced as by-products in the electron transport chain of aerobic metabolism in the mitochondria [27]. The imbalanced production of ROS and anti-oxidative defense in the body can led to oxidative stress which can result in serious cell damage [28]. Plant polyphenols are an example of non-enzymatic antioxidants. They work by interrupting free radical chain reactions [29]. The antioxidant compounds react by binding to the free radicals, thus preventing them from reaching their biological target [29, 30]. As a result, polyphenols offer protection against various diseases which are caused by oxidative damage due to the harmful effects of ROS to the body [28].
Many factors can influence the efficiency of antioxidant phenolic extractions from the plant matrices. Due to the unstable nature of phenolic compounds, each phenolic source demands an individual approach for extraction and optimization [31]. No universal extraction technique is ideal due to the diversity of polyphenols [32]. Therefore, extraction conditions are important to maximize extraction yields and enrich the phenolic components. Several factors need to be considered when employing extraction techniques including the solvent types and ratios, extraction temperatures, extraction times, and solid to liquid ratios to ensure a complete extraction of the compounds of interest, while avoiding chemical modification [31, 33–35]. In practice, ethanol is often more preferred for food and pharmaceutical processing compared to other solvents due to its safety and affordability [36, 37]. Previous investigations established that extractions with binary solvents or aqueous alcoholic mixtures contributed to high antioxidant capacities [38]. This could be explained by the inability of ethanol to extract 100% of the phenolic compounds, some of which are more water-soluble (hydrophilic). Therefore, the presence of water in the extraction eases the release of hydrophilic antioxidants [38]. Reflux extraction is a simple, rapid, and economical technique for the extraction of antioxidant secondary metabolites from C. nutans which allows a better control of the extraction parameters such as extraction time, temperature and solvent ratio. Furthermore, extraction conditions play a critical role in pharmaceutical productions, especially for extracts that are produced in low yields [39].
Response surface methodology (RSM) is a systematic design for process development and optimization. It helps in evaluating the relative significance of variables that influence the process [40]. RSM is widely used to overcome classical optimization limitations which is time consuming, expensive and lacks data evaluation [41, 42].
There are no known optimization studies on the extraction of antioxidant compounds from C. nutans leaves. The objective of this study is to optimize the extraction conditions (extraction temperature, extraction time, and solvent ratio) needed to extract the phenolic components in C. nutans leaves and to determine the optimum conditions for the maximum extraction yields and the highest anti-radical activity of the extracts.
Methods
Materials
All the chemicals and reagents used were of analytical grade. Ethanol, 1,1-diphenyl-2-picrylhydrazyl (DPPH) and Folin–Ciocalteu phenol reagents were obtained from Sigma-Aldrich (Germany). Sodium carbonate (Na2CO3) was purchased from Merck (Darmstadt, Germany). Distilled water was purified in our laboratory.
Plant material
Fresh leaves of C. nutans were collected from a botanical farm in Jelebu, Negeri Sembilan, Malaysia in January 2014. The plant was authenticated by biologist Associate Prof. Dr. Rusea Go and the specimen voucher (RG5125) was deposited at the Herbarium Unit of Universiti Putra Malaysia.
Extraction
Fresh leaves of C. nutans were air-dried in the shade and ground to a fine powder. The finely-powdered C. nutans (20 g) was placed in a conical flask and mixed with an extraction solution. The extraction was performed at a solid to liquid ratio of 1:10 (w/v) in a reflux system with a magnetic stirrer and a temperature-controlled water bath. All the experiments were performed in triplicate. After the reflux extraction, the samples were filtered, and concentrated using a rotary evaporator (Rotavapor R-210, Buchi, Switzerland) at approximately 60 °C, weighed and stored at −20 °C prior to further analysis.
Free radical scavenging activity (DPPH assay)
Radical scavenging activity was performed according to the protocol by Ramadan et al. [43]. A 0.2 mM methanolic solution of 1,1-diphenyl-2-picrylhydrazyl (DPPH) was freshly prepared. Initially, 0.6 ml of sample (2000 ppm) was mixed with 2.34 mL of DPPH solution. After being vortexed for 20 s, the resulting mixture was allowed to stand for 30 min in the dark. The UV–Visible absorbances of the reaction mixture were recorded at 515 nm using a spectrophotometer (Shimadzu UV-1601). Trolox was used as a standard and the DPPH scavenging activity of C. nutans extracts was expressed as an inhibition percentage. The inhibition percentage was calculated according to the following equation.
| 1 |
Determination of the total phenolic content (TPC)
The TPC of C. nutans extracts was determined according to Negi [44]. 0.5 mL of the sample was prepared in methanol and mixed with 2.5 mL of diluted Folin–Ciocalteu’s reagent (tenfold). 2 mL of 7.5% of Na2CO3 was added. The mixture was allowed to stand for 30 min at room temperature before the absorbance was measured at 760 nm using a UV–Visible spectrometer (Shimadzu UV-1601).
Experimental design for the response surface procedure
A three-factor-five level central composite rotatable design (CCRD) was employed to determine the optimum extraction conditions of the C. nutans leaves. The independent variables selected in this study were extraction temperature (°C), extraction time (min) and solvent ratio (water: ethanol) (v/v%) toward the responses; extraction yield (weight %), DPPH radical scavenging activity (inhibition %) and total phenolic content (mg gallic acid equivalent/g extract). A total of 20 experiments were generated using the Design Expert® software (Version 7, Stat. Ease Inc., Minneapolis, USA). Experiments with three independent variables consisting of eight factorial points, six axial, and six center points were carried out. Experiments were run randomly in order to minimize the effects of unexplained variability in the actual responses due to extraneous factors [45]. A summary of the independent variables and their coded levels are shown in Table 1.
Table 1.
Coded independent variables used in CCRD design
| Symbol | Independent variables | Coded level | ||||
|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | +1 | +1.68 | ||
| A | Extraction temperature (°C) | 53.18 | 60.00 | 70.00 | 80.00 | 86.82 |
| B | Extraction time (min) | 66.36 | 80.00 | 100.00 | 120.00 | 133.64 |
| C | Solvent ratio (water: ethanol), v/v% | 63.18:36.82 | 70:30 | 80:20 | 90:10 | 96.82:3.18 |
Statistical analysis
Analysis of variance (ANOVA) was performed to determine the significant differences between the independent variables. Reduced model (p < 0.05) and multiple regressions were employed in analyzing the experimental data. The design was expressed by polynomial regression as shown in Eq. 2.
| 2 |
where Y is the predicted response, β 0 is constant, β i, β ii and β ij represent the regression coefficients for the response surface model, x i and x j represent the independent variables and ε is the residual associated to the experiments [46]. Only non-significant (p < 0.05) values were involved in constructing a reduced model, while significant (p > 0.05) values were eliminated.
Verification of the models
In order to assess the adequacy of the constructed model, some random extractions were prepared to validate the model predictions. Actual values were compared with the predicted values to check the adequacy of the final reduced models. The percentage of the residual standard error (RSE) was calculated for each response.
Results and discussion
Model fitting and analysis of variance
RSM was employed with CCRD to investigate the effects of extraction temperature, extraction time and solvent ratio on the extraction yield, DPPH radical scavenging activity and total phenolic content (TPC) of the C. nutans leaves. Table 2 presents the design matrices of the actual experiments using CCRD and the predicted data for the response variables. The actual values of the response variables; extraction yields, DPPH scavenging activity, and TPC of C. nutans varied from 14.69–24.50% of dry weight, 46.08–80.22% inhibition and 72.25–136.00 mg GAE/g of the extracts, respectively.
Table 2.
Design matrices of actual and predicted values of extraction temperatures (A), extraction times (B) and solvent ratios (water: ethanol) (C) for the extraction conditions of C. nutans leaves using the CCRD design
| Run | Type | Independent variables | Code level | Response variable | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A (°C) | B (min) | C (v/v%) | A | B | C | Extraction yield (weight %) | DPPH radical scavenging activity (inhibition %) | Total phenolic content (mg GAE/g extract) | |||||
| Act. | Pred. | Act. | Pred. | Act. | Pred. | ||||||||
| 1 | Factorial | 60 | 80 | 70:30 | −1 | −1 | −1 | 16.37 | 16.36 | 72.79 | 72.77 | 116.94 | 116.75 |
| 2 | Factorial | 80 | 80 | 70:30 | 1 | −1 | −1 | 24.50 | 24.48 | 69.87 | 69.86 | 121.63 | 121.99 |
| 3 | Factorial | 60 | 120 | 70:30 | −1 | 1 | −1 | 22.47 | 22.46 | 78.80 | 78.70 | 115.25 | 115.58 |
| 4 | Factorial | 80 | 120 | 70:30 | 1 | 1 | −1 | 17.23 | 17.26 | 54.43 | 54.52 | 99.50 | 102.08 |
| 5 | Factorial | 60 | 80 | 90:10 | −1 | −1 | 1 | 20.68 | 20.64 | 79.43 | 79.32 | 106.13 | 106.28 |
| 6 | Factorial | 80 | 80 | 90:10 | 1 | −1 | 1 | 23.26 | 22.02 | 69.45 | 69.54 | 103.00 | 102.83 |
| 7 | Factorial | 60 | 120 | 90:10 | −1 | 1 | 1 | 23.51 | 23.53 | 72.95 | 72.95 | 129.75 | 129.55 |
| 8 | Factorial | 80 | 120 | 90:10 | 1 | 1 | 1 | 23.18 | 11.60 | 75.77 | 41.90 | 107.00 | 107.35 |
| 9 | Axial | 53.18 | 100 | 80:20 | −1.68 | 0 | 0 | 22.15 | 22.16 | 74.41 | 74.54 | 111.00 | 111.02 |
| 10 | Axial | 86.82 | 100 | 80:20 | 1.68 | 0 | 0 | 18.97 | 18.96 | 46.08 | 45.98 | 97.00 | 96.76 |
| 11 | Axial | 70 | 66.36 | 80:20 | 0 | −1.68 | 0 | 18.25 | 18.29 | 80.22 | 80.24 | 117.00 | 116.98 |
| 12 | Axial | 70 | 133.64 | 80:20 | 0 | 1.68 | 0 | 14.69 | 14.65 | 57.94 | 61.98 | 120.00 | 119.80 |
| 13 | Axial | 70 | 100 | 63.18:36.82 | 0 | 0 | −1.68 | 17.23 | 23.02 | 74.70 | 74.72 | 119.50 | 119.27 |
| 14 | Axial | 70 | 100 | 96.82:3.18 | 0 | 0 | 1.68 | 21.85 | 21.86 | 69.60 | 69.61 | 72.25 | 114.90 |
| 15 | Center | 70 | 100 | 80:20 | 0 | 0 | 0 | 20.29 | 20.33 | 56.88 | 74.41 | 107.13 | 118.39 |
| 16 | Center | 70 | 100 | 80:20 | 0 | 0 | 0 | 21.29 | 20.33 | 74.35 | 74.41 | 117.69 | 118.39 |
| 17 | Center | 70 | 100 | 80:20 | 0 | 0 | 0 | 21.07 | 20.33 | 74.00 | 74.41 | 136.00 | 118.39 |
| 18 | Center | 70 | 100 | 80:20 | 0 | 0 | 0 | 22.44 | 20.33 | 73.53 | 74.41 | 102.75 | 118.39 |
| 19 | Center | 70 | 100 | 80:20 | 0 | 0 | 0 | 19.54 | 20.33 | 74.55 | 74.41 | 109.31 | 118.39 |
| 20 | Center | 70 | 100 | 80:20 | 0 | 0 | 0 | 20.37 | 20.33 | 74.34 | 74.41 | 119.06 | 118.39 |
By applying multiple regression analysis on the actual data, models for each of the three responses were expressed by the following quadratic polynomial model as shown in Eqs. 3–5 (Table 3). The generated equations demonstrated the empirical relationship between the dependent and independent variables for each response. A statistical method based on ANOVA was used to obtain the coefficient of determination (R2) for the extraction yields, DPPH scavenging activity and TPC responses which were 0.9999, 0.9999, and 0.9983, respectively. According to Jumbri et al. [47] and Hamzaoui et al. [48], a good fit with high correlation is achieved if the regression model has an R2 value of above 0.9. The R2 values obtained indicated that more than 99% of the response variables (extraction yields, DPPH scavenging activity and TPC) could be described by the RSM model. The high values of R2 for each response indicated that the CCRD design fitted well into the quadratic polynomial models that were developed. These results confirmed the predictability of the models in determining the optimum conditions needed to obtain the highest antioxidant activity and maximum extraction yields of the C. nutans leaves extracts (Fig. 1).
Table 3.
Quadratic polynomial equations for the three responses in terms of coded factors
| Responses | Equations | |
|---|---|---|
| Extraction yield | (3) | |
| DPPH radical scavenging activity | (4) | |
| TPC | (5) | |
In these equations, Y is the predicted response, A, B and C are the values of the independent variables, extraction temperature (°C), extraction time (min) and solvent ratio (water: ethanol) (v/v%), respectively
Fig. 1.
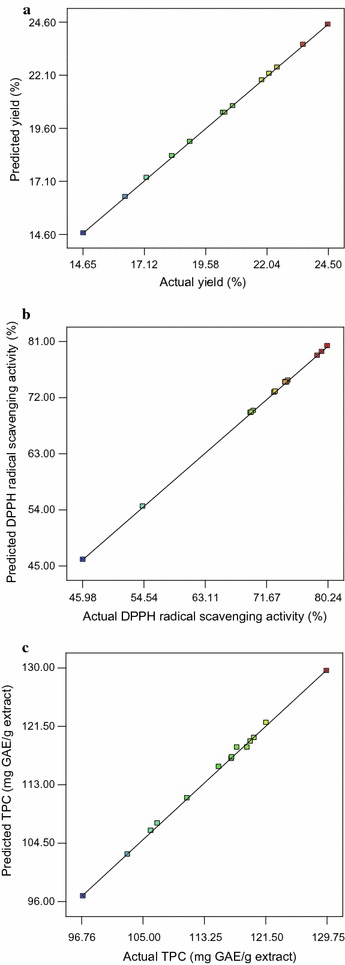
Comparison between predicted and actual values of the response variables a extraction yield b DPPH radical scavenging activity and c TPC of C. nutans leaves
Table 4 represents the regression analysis and ANOVA employed in the model fitting design in order to examine the statistical significance of the terms for all the responses. A number of runs in each response; extraction yields (6, 8, 13, 16, 17, 18 and 19), DPPH scavenging activities (8, 12, 15, 17, and 18) and TPC (4, 14, 15, 17, 18, and 19) were defined as missing independent variables (outliers) and were therefore not applied in the model design. The F values of 2923.40, 7138.07 and 267.02 for extraction yields, DPPH scavenging activity, and TPC respectively, indicated that all the models were significant. There was only a 0.01% chance that the values could be attributed to noise. The probability (p value) was relatively low in all the model responses (<0.0001), which was less than 0.05, indicating the significance of the models. A large F value and small p value is indicative that the independent variables have a significant impact on the respective response variables [49]. ANOVA revealed that all the independent variables had a significant effect (p < 0.05) on all responses. The extraction temperature had the most significant effect on all the responses (p < 0.0001). This was followed by the extraction time which had a significant value of p < 0.0001 towards both extraction yields and DPPH scavenging activity whereas a value of p = 0.0115 was obtained for TPC. Likewise, solvent ratio exhibited significant effects on DPPH scavenging activity (p < 0.0001), extraction yields (p = 0.0008) and TPC (p = 0.0051).
Table 4.
ANOVA for the quadratic polynomial models developed for the response variables; extraction yields, DPPH radical scavenging activity and TPC of C. nutans leaves
| Extraction yield (weight %) | DPPH radical scavenging activity (inhibition %) | TPC (mg GAE/g extract) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | Sum of squares | DF | Mean square | F value | p value | Sum of squares | DF | Mean square | F value | p value | Sum of squares | DF | Mean square | F value | p value |
| Model | 100.54 | 9 | 11.17 | 2923.40 | <0.0001 | 1185.13 | 9 | 131.68 | 7138.07 | <0.0001 | 957.55 | 9 | 106.39 | 267.02 | <0.0001 |
| A-extraction temperature | 8.28 | 1 | 8.28 | 2166.64 | <0.0001 | 750.41 | 1 | 750.41 | 40,677.85 | <0.0001 | 200.12 | 1 | 200.12 | 502.24 | <0.0001 |
| B-extraction time | 11.29 | 1 | 11.29 | 2954.88 | <0.0001 | 156.47 | 1 | 156.47 | 8481.93 | <0.0001 | 7.81 | 1 | 7.81 | 19.60 | 0.0115 |
| C-solvent ratio | 0.72 | 1 | 0.72 | 187.46 | 0.0008 | 24.00 | 1 | 24.00 | 1301.24 | <0.0001 | 12.34 | 1 | 12.34 | 30.97 | 0.0051 |
| AB | 51.89 | 1 | 51.89 | 13,579.03 | <0.0001 | 147.54 | 1 | 147.54 | 7997.49 | <0.0001 | 126.61 | 1 | 126.61 | 317.75 | <0.0001 |
| AC | 12.22 | 1 | 12.22 | 3198.83 | <0.0001 | 15.41 | 1 | 15.41 | 835.51 | <0.0001 | 27.26 | 1 | 27.26 | 68.41 | 0.0012 |
| BC | 3.01 | 1 | 3.01 | 787.41 | <0.0001 | 49.40 | 1 | 49.40 | 2678.06 | <0.0001 | 215.12 | 1 | 215.12 | 539.88 | <0.0001 |
| A2 | 0.055 | 1 | 0.055 | 14.52 | 0.0318 | 269.62 | 1 | 269.62 | 14,615.39 | <0.0001 | 226.83 | 1 | 226.83 | 569.26 | <0.0001 |
| B2 | 15.45 | 1 | 15.45 | 4042.51 | <0.0001 | 7.77 | 1 | 7.77 | 421.14 | <0.0001 | 0.00006 | 1 | 0.00006 | 0.00016 | 0.9905 |
| C2 | 3.59 | 1 | 3.59 | 938.88 | <0.0001 | 6.81 | 1 | 6.81 | 369.24 | <0.0001 | 1.37 | 1 | 1.37 | 3.45 | 0.1368 |
| Residual | 0.011 | 3 | 0.0038 | 0.092 | 5 | 0.018 | 1.59 | 1 | 0.40 | ||||||
| Lack of fit | 0.0083 | 2 | 0.0041 | 1.29 | 0.5283 | 0.064 | 3 | 0.021 | 1.53 | 0.4192 | 0.65 | 3 | 0.22 | 0.23 | 0.8721 |
| Pure error | 0.0032 | 1 | 0.0032 | 0.028 | 2 | 0.014 | 0.94 | 1 | 0.94 | ||||||
| Cor total | 100.55 | 12 | 1185.22 | 14 | 959.15 | 13 | |||||||||
| R2 | 0.9999 | 0.9999 | 0.9983 | ||||||||||||
| Adj. R2 | 0.9995 | 0.9998 | 0.9946 | ||||||||||||
| Pred. R2 | 0.9975 | 0.9991 | 0.9826 | ||||||||||||
| Adequate precision | 181.223 | 308.944 | 61.461 | ||||||||||||
DF is the degree of freedom, A is the extraction temperature, B is the extraction time, C is the solvent ratio (water: ethanol)
The predicted R-square (Pre. R2) value indicates how well a regression model predicts response values; while the adjusted R-square (Adj. R2) indicates the descriptive power of the regression models while including the diverse numbers of variables. Every variable added to a model will increase the R2 value, regardless of statistical significance. Therefore, considering the Adj. R2 value is important to evaluate the adequacy of the model because the value tally only increases if the variables enhance the model beyond what would normally be obtained by probability. According to Koocheki et al. [50], Adj. R2 values above 0.9 may be used to indicate the adequacy of the model. Furthermore, a difference of less than 0.2 between Adj. R2 and Pre. R2 demonstrates the effectiveness of the model. In this study, the Adj. R2 values were found to be 0.9995, 0.9998 and 0.9946 for extraction yields, DPPH scavenging activity, and TPC of C. nutans respectively and thus, the difference in values of Adj. R2 and Pre. R2 for all the responses was less than 0.2.
The validity of the models was also confirmed using the Lack of Fit analysis, where an insignificant p value of more than 0.05 was indicative that the model could accurately fit with the actual data [51]. The results of this study showed that the lack of fit p value for extraction yields, DPPH scavenging activity and TPC were 0.5283, 0.4192 and 0.8721, respectively, indicating that all the developed quadratic polynomial models were reliable and accurate for predicting the relevant responses.
Effects of the parameters
As shown in Fig. 2, extraction times, extraction temperatures and solvent ratios were interpreted in the ranges of 80–120 min, 60–80 °C and 70:30–90:10 (water: ethanol), respectively. The confidence interval for each response was 95% in the mentioned ranges on the plots. At a constant water to ethanol ratio (80:20), the extraction yield was found to be the highest under two conditions; a maximum temperature of 80 °C at a minimum time of 80 min and a minimum temperature of 60 °C at a maximum time of 120 min (Fig. 2a). Theoretically, under high temperatures, plant tissues are softened and the weak interactions affect the cell membranes. As a result, phenolic compounds can be easily extracted into the solvent [52]. However, a prolonged extraction time at 80 °C decreases the extraction yield because the high temperature causes the oxidation and degradation of the desired compounds [53, 54]. Conversely, by keeping the temperature at a minimum level (60 °C) for a maximum extraction time period of 120 min produced the highest yields. Hence, a prolonged exposure of the sample in the solvent, allowed sufficient time for the desired compounds to migrate into the solvent.
Fig. 2.
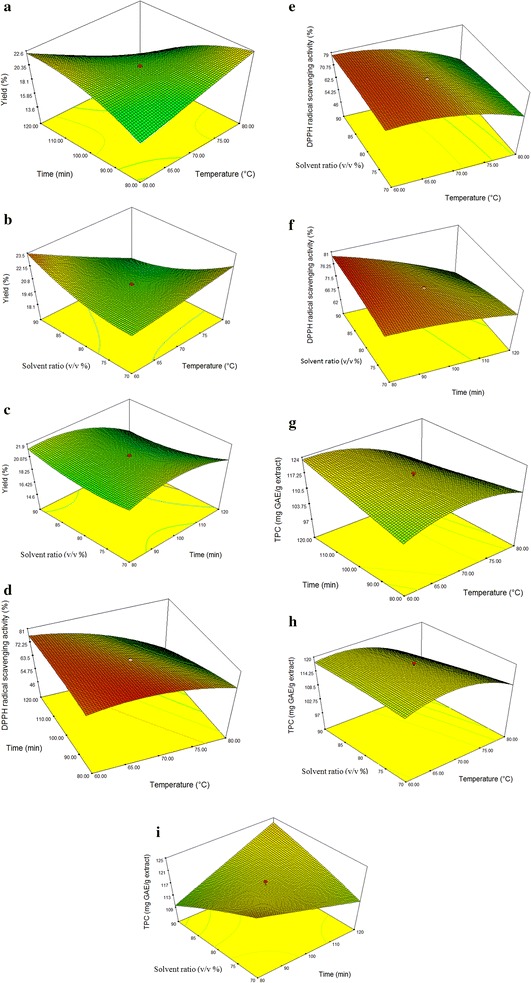
Response surface plots; a–c the interaction effect of extraction yield as a function of extraction temperature, extraction time and solvent ratio, d–f the interaction effect of DPPH radical scavenging activity as a function of extraction temperature, extraction time and solvent ratio and g–i the interaction effect of TPC as a function of extraction temperature, extraction time and solvent ratio
Figure 2b represents the effect of extraction temperatures and solvent ratios on the extraction yields. The response surface plot was generated with an extraction time fixed at 100 min. The highest yield (23.5%) was obtained at a solvent ratio of 90:10 (water: ethanol) at 60 °C. Increasing the water content in the solvent system caused swelling in the plant material which resulted in increased contact between the plant matrix and the solvent, thus contributing to an increased yield [36]. However, increasing the temperature to 80 °C significantly decreased the yield since the compounds are heat-sensitive. In contrast, at a similar temperature (80 °C) using a different solvent system (70:30), greater yields were obtained. Thus, the extracted compounds from C. nutans leaves could be classified into two dominant groups: the polar, water-rich compounds which were heat sensitive, and the less polar compounds that could tolerate high temperatures.
Figure 2c illustrates the effect of solvent ratios and extraction times on the yields. At a fixed temperature of 70 °C, an increase in extraction time slightly decreased the yield. The highest yield was approximately 21.9% at a solvent ratio of 90:10 (water: ethanol) and an extraction time of 80 min. Solvent ratios alone had little effect on the yield.
Figure 2d shows the interaction between extraction times and temperatures on DPPH radical scavenging activity. The lowest percentage of DPPH radical scavenging activity was observed at extraction conditions of 80 °C and 120 min at a fixed solvent ratio of 80:20 (water: ethanol). Similar observations were noted in Fig. 2a, g, where long exposure times of the samples at high temperatures produced lower yields. This could be due to the decomposition of the antioxidant compounds associated with the phenolic compounds. The lowest total phenolic content was attained under high heat (Fig. 2g). Most phenolic compounds are heat-sensitive and easily oxidized [55, 56], hence a upper limit temperature must be observed to preserve its useful components. At a similar extraction time of 120 min but with a minimum extraction temperature of 60 °C, DPPH radical scavenging activity was observed to be greater (72.25%). A decrease in extraction time had little effect on the DPPH radical scavenging activity. A similar trend was observed in Fig. 2e, where DPPH radical scavenging activity was not affected by the solvent ratio if the extraction process was conducted at the same temperature (60 °C).
DPPH radical scavenging activity under different solvent ratios and extraction times at a constant temperature of 70 °C is presented in Fig. 2f. The lowest percentage of DPPH radical scavenging activity was obtained at a solvent ratio of 90:10 (water: ethanol) using a prolonged extraction time of 120 min. As the extraction time decreased, the DPPH radical scavenging activity was greatly increased until the highest activity was reached, at above 76.25% using the same solvent ratio (90:10) but with a minimum extraction time of 80 min. Decreasing the water ratio to 70:30 (water: ethanol) led to a slight decrease in the DPPH radical scavenging activity. According to Saito and Kawabata [57] and Sharma and Bhat [58], in addition to pH and the chemical structure of the radical scavenger, DPPH radical scavenging activity could also be influenced by the polarity of the reaction medium. A water-rich solvent system (90:10) increased the antioxidant activity, which suggested that the samples were rich in antioxidant compounds.
The effect of solvent ratios and temperatures on the TPC is shown in Fig. 2h. In the beginning, lower extraction temperatures of approximately 60–65 °C had little effect on the TPC values when the solvent ratio was increased. However, above 65 °C, the TPC value decreased significantly when using a solvent system with the highest polarity (90:10). Similar observations were recorded in Fig. 2a, g, and this can be attributed to the heat-sensitive properties of some phenolic compounds.
Figure 2i depicts the TPC values with respect to solvent ratios and extraction times at a fixed extraction temperature of 70 °C. An increase in the extraction time slightly decreased the TPC value at a solvent ratio of 70:30 (water: ethanol). However, at a solvent ratio of 90:10 (water: ethanol), the TPC value increased to 121 mg GAE/g extract per time increment. A comparison of DPPH radical scavenging activity and TPC values in Fig. 2f, i for runs conducted using a solvent ratio of 90:10 at 80 min, indicated that DPPH radical scavenging activity was at its highest while TPC value was at its lowest. It is possible that the phenolic groups had no effect on the anti-radical activity measured by the DPPH radical scavenging activity assay in the stated region but other groups of antioxidant contributors had an effect. Previous investigations on C. nutans have established the presence of numerous potential antioxidant constituents such as fatty acids (i.e. linoleic acid, stearic acid, oleic acid, palmitic acid, myristic acid) [14], lupeol, stigmasterol, beta-sitosterol [59], chlorophylls [1] and sulfur-containing glucosides (i.e. Clinacoside A, Clinacoside B, Clinacoside C, Cycloclinacoside A1, Cycloclinacoside A2 and Triacetylcycloclinacoside A2) [60] that could be involved in neutralizing free radical damage.
Verification of the models
In order to determine the adequacy of the final model, three randomized validation sets were performed to verify the models (Table 5). The results were compared to predicted values by calculating the RSE percentages (Eq. 6). RSE values lower than ±5 were considered to be agreement with the predicted values. The RSE values obtained indicated no significant differences between the actual and predicted values, proving that the models were adequate.
| 6 |
Table 5.
Predicted and actual response values for the verification model
| Set | Extraction temperature (°C) | Extraction time (min) | Solvent ratio (water: ethanol) (v/v %) | Extraction yield (%) | DPPH radical scavenging activity (%) | Total phenolic content (mg GAE/g extract) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Act. value | Pred. value | RSE (%) | Act. value | Pred. value | RSE (%) | Act. value | Pred. value | RSE (%) | ||||
| 1 | 75 | 100 | 80:20 | 19.82 | 19.87 | 0.26 | 70.27 | 68.92 | 1.96 | 117.75 | 114.99 | 2.40 |
| 2 | 70 | 100 | 75:25 | 20.69 | 20.69 | 0 | 73.7 | 74.98 | 1.70 | 116.88 | 118.93 | 1.72 |
| 3 | 70 | 100 | 83:17 | 20.18 | 20.29 | 0.55 | 71.28 | 73.89 | 3.53 | 115.25 | 117.69 | 2.30 |
Optimized conditions of the extraction parameters
Optimized conditions for the simultaneous maximum extraction yields, DPPH radical scavenging activity and TPC were determined. From CCRD analysis, the optimized conditions using an extraction temperature of 60 °C, an extraction time of 120 min, and a solvent ratio (water: ethanol) of 90:10 v/v% could produce the optimum extraction yields, DPPH radical scavenging activity and TPC of 23.51, 72.95% and 129.75 mg GAE/g extract, respectively. Table 6 shows the predicted and actual response values for the optimized conditions. Under optimum conditions, the actual responses showed that the models were in good agreement with the predicted values with RSE values of less than 0.2%.
Table 6.
Predicted and actual response values for the optimized extraction parameters
| Parameter no. | Responses | Actual value | Predicted value | RSE (%) |
|---|---|---|---|---|
| 1 | Extraction yield (%) | 23.51 | 23.53 | 0.09 |
| 2 | DPPH radical scavenging activity (%) | 72.95 | 72.95 | 0 |
| 3 | Total phenolic content (mg GAE/g extract) | 129.75 | 129.55 | 0.15 |
The range of parameters was selected based on our preliminary studies (data is not shown). Considering the need to minimize the costs of actual production, it is reasonable to estimate the economic conditions that are required in order to allow minimum energy and solvent consumption but at the same time, achieving the desired output. Thus, the extraction conditions of the C. nutans leaves from this study were obtained by limiting the extraction parameters to a temperature range of 60–80 °C for 80–120 min and a water-rich ratio of water to ethanol 70:30–90:10 v/v%. Water remains the cheapest and safest, eco-friendly solvent to extract bioactive substances such as polyphenols, polysaccharides, proteins and glycosides [61]. Among these water-soluble (hydrophilic) compounds, some have shown good potential as free-radical scavengers and antioxidant agents [61]. The temperature was limited to 80 °C to preserve the useful components in the C. nutans leaves because above this temperature, the phenolic compounds are subject to decomposition. Although, one must bear in mind that the limitations of TPC assay include poor specificity and that antioxidant activity can be influenced by any substance that can be oxidized by the Folin reagent, not only just polyphenols [62]. There are other variations to extraction parameters that can be used for the extraction of plant extracts. Thus, the selection of parameters employed in this study was focused on hydrophilic antioxidants.
Conclusions
This study demonstrated that RSM is an effective tool for optimizing the extraction conditions of C. nutans leaves and allows a better understanding of the relationship between independent variables and response variables. The model was verified statistically with ANOVA. Under the optimum conditions, the actual values were in good agreement with the predicted values as RSE values for the optimum conditions were less than 0.2%. All the independent variables had a significant effect (p < 0.05) on all the responses which indicated that all extraction parameters employed in this study were important in the optimization process. The R2 values for three responses, extraction yields, DPPH radical scavenging activity and TPC were 0.9999, 0.9999 and 0.9983 respectively, suggesting that the quadratic polynomial models developed were satisfactorily accurate to be used in analyzing the interactions of the parameters (response and independent variables). The optimum conditions generated from RSM (an extraction temperature of 60 °C, an extraction time of 120 min, and a solvent ratio (water: ethanol) of 90:10 v/v%) could be used for future upscale extractions of C. nutans leaves by considering the temperature, extraction time, and solvent ratio for economical evaluation. This study could be useful in the development of cosmeceutical products containing extracts of C. nutans.
Authors’ contributions
ISCS and WJC had prominent roles in the implementation of the experimental section and the writing of the manuscript. MB supervised and funded the entire project. HRFM taught and also performed the statistical analysis. SEA and MI assisted in solving problems that arose in the implementation of this work and also in the scientific editing of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We acknowledge financial support from Universiti Putra Malaysia in terms of a GP-IPS research grant (Vote. No GP-IPS/2014/9438735).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- RSM
response surface methodology
- CCRD
central composite rotatable design
- DPPH
1,1-diphenyl-2-picrylhydrazyl
- C. nutans
Clinacanthus nutans
- Na2CO3
sodium carbonate
- ANOVA
analysis of variance
- R2
determined coefficient
- Pre. R2
predicted R-square
- Adj. R2
adjusted R-square
- DF
degrees of freedom
- A
extraction temperature
- B
extraction time
- C
solvent ratio (water: ethanol)
- TPC
total phenolic content
- GAE
gallic acid equivalent
- RSE
residual standard error
Contributor Information
Intan Soraya Che Sulaiman, Email: chesoraya007@yahoo.com.
Mahiran Basri, Email: mahiran@upm.edu.my.
Hamid Reza Fard Masoumi, Email: fardmasoumi@upm.edu.my.
Wei Jian Chee, Email: ac910208@gmail.com.
Siti Efliza Ashari, Email: ctefliza@upm.edu.my.
Maznah Ismail, Email: maznahis@upm.edu.my.
References
- 1.Sakdarat S, Shuyprom A, Pientong C, et al. Bioactive constituents from the leaves of Clinacanthus nutans Lindau. Bioorganic Med Chem. 2009;17:1857–1860. doi: 10.1016/j.bmc.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 2.P’ng X, Akowuah G, Chin J. Acute oral toxicity study of Clinacanthus nutans in mice. Int J Pharm Sci Res. 2012;3:4202–4204. [Google Scholar]
- 3.Siew YY, Zareisedehizadeh S, Seetoh WG, et al. Ethnobotanical survey of usage of fresh medicinal plants in Singapore. J Ethnopharmacol. 2014;155:1450–1466. doi: 10.1016/j.jep.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Uawonggul N, Chaveerach A, Thammasirirak S, et al. Screening of plants acting against Heterometrus laoticus scorpion venom activity on fibroblast cell lysis. J Ethnopharmacol. 2006;103:201–207. doi: 10.1016/j.jep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Charuwichitratana S, Wongrattanapasson N, Timpatanapong P, Bunjob M. Herpes zoster: treatment with Clinacanthus nutans cream. Int J Dermatol. 1996;35:665–666. doi: 10.1111/j.1365-4362.1996.tb03699.x. [DOI] [PubMed] [Google Scholar]
- 6.Farooqui M, Hassali MA, Shatar AKA, et al. Use of complementary and alternative medicines among Malaysian cancer patients: a descriptive study. J Tradit Complement Med. 2016;6(4):321–326. doi: 10.1016/j.jtcme.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunsorn P, Ruangrungsi N, Lilipun V, et al. The identities and anti-herpes simplex virus activity of Clinacanthus nutans and Clinacanthus siamensis. Asian Pac J. 2013;3:284–290. doi: 10.1016/S2221-1691(13)60064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoosook C, Panpisutchai Y, Chaichana S, et al. Evaluation of anti-HSV-2 activities of Barleria lupulina and Clinacanthus nutans. J Ethnopharmacol. 1999;67:179–187. doi: 10.1016/S0378-8741(99)00008-2. [DOI] [PubMed] [Google Scholar]
- 9.Direkbusarakom S, Ruangpan L, Ezura Y, Yoshimizu M. Protective efficacy of Clinacanthus nutans on yellow-head disease in black tiger shrimp (Penaeus monodon) Fish Pathol. 1998;33:401–404. doi: 10.3147/jsfp.33.401. [DOI] [Google Scholar]
- 10.Wanikiat P, Panthong A, Sujayanon P, et al. The anti-inflammatory effects and the inhibition of neutrophil responsiveness by Barleria lupulina and Clinacanthus nutans extracts. J Ethnopharmacol. 2008;116:234–244. doi: 10.1016/j.jep.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 11.Yuann JMP, Wang JS, Jian HL, et al. Effects of Clinacanthus nutans (Burm. f) Lindau leaf extracts on protection of plasmid DNA from riboflavin photoreaction. MC-Trans Biotechnol. 2012;4:45–58. [Google Scholar]
- 12.Abdul Rahim MH, Zakaria ZA, Mohd Sani MH, et al. Methanolic extract of Clinacanthus nutans exerts antinociceptive activity via the opioid/nitric oxide-mediated, but cGMP-independent, pathways. Evid Based Complement Altern Med. 2016;2016:1–11. doi: 10.1155/2016/1494981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Che Sulaiman IS, Basri M, Fard Masoumi HR, et al. Predicting the optimum compositions of a transdermal nanoemulsion system containing an extract of Clinacanthus nutans leaves (L.) for skin antiaging by artificial neural network model. J Chemom. 2017 [Google Scholar]
- 14.Che Sulaiman IS, Basri M, Chan KW, et al. In vitro antioxidant, cytotoxic and phytochemical studies of Clinacanthus nutans Lindau leaf extract. Afr J Pharm Pharmacol. 2015;9:861–874. doi: 10.5897/AJPP2015.4396. [DOI] [Google Scholar]
- 15.Yong YK, Tan JJ, Teh SS, et al. Clinacanthus nutans extracts are antioxidant with antiproliferative effect on cultured human cancer cell lines. Evid Based Complement Altern Med. 2013;2013:1–8. doi: 10.1155/2013/462751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teshima K, Kaneko T, Ohtani K, et al. C-glycosyl flavones from Clinacanthus. Nat Med. 1997;51:557. [Google Scholar]
- 17.Mustapa AN, Martin Á, Mato RBB, Cocero MJ. Extraction of phytocompounds from the medicinal plant Clinacanthus nutans Lindau by microwave-assisted extraction and supercritical carbon dioxide extraction. Ind Crops Prod. 2015;74:83–94. doi: 10.1016/j.indcrop.2015.04.035. [DOI] [Google Scholar]
- 18.Chelyn JL, Omar MH, Mohd Yousof NSA, et al. Analysis of flavone C-glycosides in the leaves of Clinacanthus nutans (Burm. f.) Lindau by HPTLC and HPLC-UV/DAD. Sci World J. 2014;2014:1–6. doi: 10.1155/2014/724267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zulkipli IN, Rajabalaya R, Idris A, et al. Clinacanthus nutans: a review on ethnomedicinal uses, chemical constituents and pharmacological properties. Pharm Biol. 2017;55:1093–1113. doi: 10.1080/13880209.2017.1288749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Da Tsai H, Wu JS, Kao MH, et al. Clinacanthus nutans protects cortical neurons against hypoxia-induced toxicity by downregulating HDAC1/6. Neuromol Med. 2016;18:274–282. doi: 10.1007/s12017-016-8401-2. [DOI] [PubMed] [Google Scholar]
- 21.Huang D, Li Y, Cui F, et al. Purification and characterization of a novel polysaccharide-peptide complex from Clinacanthus nutans Lindau leaves. Carbohydr Polym. 2016;137:701–708. doi: 10.1016/j.carbpol.2015.10.102. [DOI] [PubMed] [Google Scholar]
- 22.Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maestri DM, Nepote V, Lamarque A, Zygadlo J. Natural products as antioxidants. In: Imperato F, editor. Phytochemistry advances in research. Trivandrum: Research Signpost; 2006. pp. 105–135. [Google Scholar]
- 24.Draelos ZD. Cosmeceuticals: undefined, unclassified, and unregulated. Clin Dermatol. 2009;27:431–434. doi: 10.1016/j.clindermatol.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Mota I, Pinto PR. Extraction of polyphenolic compounds from Eucalyptus globulus bark: process optimization and screening for biological activity. Ind Eng Chem Res. 2012;51:6991–7000. doi: 10.1021/ie300103z. [DOI] [Google Scholar]
- 26.Jayaprakasha GK, Girennavar B, Patil BS. Radical scavenging activities of Rio Red grapefruits and sour orange fruit extracts in different in vitro model systems. Bioresour Technol. 2008;99:4484–4494. doi: 10.1016/j.biortech.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 27.Casteilla L, Rigoulet M, Penicaud L. Mitochondrial ROS metabolism: modulation by uncoupling proteins. IUBMB Life. 2001;52:181–188. doi: 10.1080/15216540152845984. [DOI] [PubMed] [Google Scholar]
- 28.Irshad M, Chaudhuri PS. Oxidant-antioxidant system: role and significance in human body. Indian J Exp Biol. 2002;40:1233–1239. [PubMed] [Google Scholar]
- 29.Nimse SB, Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006. doi: 10.1039/C4RA13315C. [DOI] [Google Scholar]
- 30.Poljšak B, Dahmane RG, Godić A. Intrinsic skin aging: the role of oxidative stress. Acta Dermatovenerol Alp Pannonica Adriat. 2012;21:33–36. [PubMed] [Google Scholar]
- 31.Majeed M, Hussain AI, Chatha SAS, et al. Optimization protocol for the extraction of antioxidant components from Origanum vulgare leaves using response surface methodology. Saudi J Biol Sci. 2016;23:389–396. doi: 10.1016/j.sjbs.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva EM, Rogez H, Larondelle Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep Purif Technol. 2007;55:381–387. doi: 10.1016/j.seppur.2007.01.008. [DOI] [Google Scholar]
- 33.Lee JW, Mo EJ, Choi JE, et al. Effect of Korean Red Ginseng extraction conditions on antioxidant activity, extraction yield, and ginsenoside Rg1 and phenolic content: Optimization using response surface methodology. J Ginseng Res. 2016;40(3):229–236. doi: 10.1016/j.jgr.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammi KM, Jdey A, Abdelly C, et al. Optimization of ultrasound-assisted extraction of antioxidant compounds from Tunisian Zizyphus lotus fruits using response surface methodology. Food Chem. 2015;184:80–89. doi: 10.1016/j.foodchem.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 35.Wu X, Yu X, Jing H. Optimization of phenolic antioxidant extraction from wuweizi (Schisandra chinensis) pulp using random-centroid optimazation methodology. Int J Mol Sci. 2011;12:6255–6266. doi: 10.3390/ijms12096255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hemwimon S, Pavasant P, Shotipruk A. Microwave-assisted extraction of antioxidative anthraquinones from roots of Morinda citrifolia. Sep Purif Technol. 2007;54:44–50. doi: 10.1016/j.seppur.2006.08.014. [DOI] [Google Scholar]
- 37.Guo Z, Jin Q, Fan G, et al. Microwave-assisted extraction of effective constituents from a Chinese herbal medicine Radix puerariae. Anal Chim Acta. 2001;436:41–47. doi: 10.1016/S0003-2670(01)00900-X. [DOI] [Google Scholar]
- 38.Thoo YY, Ho SK, Liang JY, et al. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia) Food Chem. 2010;120:290–295. doi: 10.1016/j.foodchem.2009.09.064. [DOI] [Google Scholar]
- 39.Aziz R, Sarmidi M, Kumaresan S, et al. Phytochemical processing: the next emerging field in chemical engineering—aspects and opportunities. J Kejuruter Kim Malays. 2003;3:45–60. [Google Scholar]
- 40.Asfaram A, Ghaedi M, Goudarzi A, et al. Magnetic nanoparticle based dispersive micro-solid-phase extraction for the determination of malachite green in water samples: optimized experimental design. New J Chem. 2015;39:9813–9823. doi: 10.1039/C5NJ01730K. [DOI] [Google Scholar]
- 41.Myers R, Montgomery D. Response surface methodology: a retrospective and literature survey. J Qual Technol. 2004;36:53–77. [Google Scholar]
- 42.Amado IR, Franco D, Sánchez M, et al. Optimisation of antioxidant extraction from Solanum tuberosum potato peel waste by surface response methodology. Food Chem. 2014;165:290–299. doi: 10.1016/j.foodchem.2014.05.103. [DOI] [PubMed] [Google Scholar]
- 43.Ramadan M, Kroh L, Mörsel J. Radical scavenging activity of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.), and niger (Guizotia abyssinica Cass.) crude seed oils and oil. J Agric Food Chem. 2003;51:6961–6969. doi: 10.1021/jf0346713. [DOI] [PubMed] [Google Scholar]
- 44.Negi PS. Plant extracts for the control of bacterial growth: efficacy, stability and safety issues for food application. Int J Food Microbiol. 2012;156:7–17. doi: 10.1016/j.ijfoodmicro.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Ngan CL, Basri M, Lye FF, et al. Comparison of Box-Behnken and central composite designs in optimization of fullerene loaded palm-based nano-emulsions for cosmeceutical application. Ind Crops Prod. 2014;59:309–317. doi: 10.1016/j.indcrop.2014.05.042. [DOI] [Google Scholar]
- 46.Bezerra MA, Santelli RE, Oliveira EP, et al. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76:965–977. doi: 10.1016/j.talanta.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 47.Jumbri K, Al-Haniff Rozy MF, Ashari SE, et al. Optimisation and characterisation of lipase catalysed synthesis of a kojic monooleate ester in a solvent-free system by response surface methodology. PLoS ONE. 2015;10:e0144664. doi: 10.1371/journal.pone.0144664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamzaoui AH, Jamoussi B, M’nif A. Lithium recovery from highly concentrated solutions: response surface methodology (RSM) process parameters optimization. Hydrometallurgy. 2008;90:1–7. doi: 10.1016/j.hydromet.2007.09.005. [DOI] [Google Scholar]
- 49.Tan SF, Masoumi HRF, Karjiban RA, et al. Ultrasonic emulsification of parenteral valproic acid-loaded nanoemulsion with response surface methodology and evaluation of its stability. Ultrason Sonochem. 2016;29:299–308. doi: 10.1016/j.ultsonch.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Koocheki A, Taherian AR, Razavi SMA, Bostan A. Response surface methodology for optimization of extraction yield, viscosity, hue and emulsion stability of mucilage extracted from Lepidium perfoliatum seeds. Food Hydrocoll. 2009;23:2369–2379. doi: 10.1016/j.foodhyd.2009.06.014. [DOI] [Google Scholar]
- 51.Montgomery DC. Response surface methods and design. In: Melhorn A, editor. Design and analysis of experiments. 8. New York: Wiley; 2013. pp. 478–544. [Google Scholar]
- 52.Shi J, Yu J, Pohorly J, et al. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J Food Agric Environ. 2003;1:42–47. [Google Scholar]
- 53.Naczk M, Shahidi F. Extraction and analysis of phenolics in food. J Chromatogr A. 2004;1054:95–111. doi: 10.1016/S0021-9673(04)01409-8. [DOI] [PubMed] [Google Scholar]
- 54.Silva EM, Souza JNS, Rogez H, et al. Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem. 2007;101:1012–1018. doi: 10.1016/j.foodchem.2006.02.055. [DOI] [Google Scholar]
- 55.Robards K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J Chromatogr A. 2003;1000:657–691. doi: 10.1016/S0021-9673(03)00058-X. [DOI] [PubMed] [Google Scholar]
- 56.Khoddami A, Wilkes MA, Roberts TH. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18:2328–2375. doi: 10.3390/molecules18022328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saito S, Kawabata J. Effects of electron-withdrawing substituents on DPPH radical scavenging reactions of protocatechuic acid and its analogues in alcoholic solvents. Tetrahedron. 2005;61:8101–8108. doi: 10.1016/j.tet.2005.06.040. [DOI] [Google Scholar]
- 58.Sharma O, Bhat T. DPPH antioxidant assay revisited. Food Chem. 2009;113:1202–1205. doi: 10.1016/j.foodchem.2008.08.008. [DOI] [Google Scholar]
- 59.Dampawan P, Huntrakul C, Reutrakul V. Constituents of Clinacanthus nutans and the crystal structure of LUP-20 (29)-ene-3-one. J Sci Soc Thail. 1977;3:14–26. doi: 10.2306/scienceasia1513-1874.1977.03.014. [DOI] [Google Scholar]
- 60.Teshima K-I, Kaneko T, Ohtani K, et al. Sulfur-containing glucosides from Clinacanthus nutans. Phytochemistry. 1998;48:831–835. doi: 10.1016/S0031-9422(97)00956-4. [DOI] [Google Scholar]
- 61.Meng L, Lozano Y. Innovative technologies used at pilot plant and industrial scales in water-extraction process. In: Chemat F, Vian MA, editors. Alternative solvents for natural products extraction. Berlin: Springer; 2014. pp. 269–315. [Google Scholar]
- 62.Park JH, Lee M, Park E. Antioxidant activity of orange flesh and peel extracted with various solvents. Prev Nutr Food Sci. 2014;19:291–298. doi: 10.3746/pnf.2014.19.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]


