Abstract
The cell membrane-coated nanoparticle is a biomimetic platform consisting of a nanoparticulate core coated with membrane derived from a cell, such as a red blood cell, platelet, or cancer cell. The cell membrane “disguise” allows the particles to be perceived by the body as the source cell by interacting with its surroundings using the translocated surface membrane components. The newly bestowed characteristics of the membrane-coated nanoparticle can be utilized for biological interfacing in the body, providing natural solutions to many biomedical issues. This Topical Review will cover the interactions of these cell membrane-coated nanoparticles and their applications within three biomedical areas of interest, including (i) drug delivery, (ii) detoxification, and (iii) immune modulation.
Keywords: nanomedicine, biomimetic nanoparticle, cell membrane coating, drug delivery, detoxification, vaccination
Toc Image
INTRODUCTION
The recent use of nanotechnology has offered new ways to address old problems in the biomedical field due to the novel properties of nanoparticles, including high drug loading and controlled release,1 delivery of hydrophobic drugs,2 access to multiple endocytic routes,3 and passive accumulation in certain organs based on size, shape, or surface charge.4–5 These combined characteristics have been used for many impactful therapeutic applications, including drug delivery,6–7 vaccination,8–10 gene delivery,11 and antimicrobial.12 Another main benefit of nanoparticles is the ability to functionalize the surface with moieties such as targeting ligands,13 polymers,14 imaging dyes,15 enzymes,16 and other biomolecules.17–18 The external decoration of nanoparticles can increase the bioavailability of the encapsulated drugs without directly modifying them, and the high surface area to volume ratio allows the nanoparticles to be more reactive to their environment. Surface functionalization is particularly taken advantage of for actively targeting particles to specific disease sites,19–20 tumor drug delivery,21–22 toxin removal,23–25 and vaccination.26–28
There has been increasing interest in creating biomimetic nanoparticles to facilitate the development of therapeutics for poorly-understood or biologically complex applications. Surface functionalization is commonly a bottom-up technique, where moieties are individually incorporated onto the particle surface via chemical conjugation or non-covalent binding.29 These strategies are often singular and only exploit known biological interactions. However, in reality, efficient biological interfacing is oftentimes multifactorial and involves mechanisms not yet fully elucidated. For challenging applications, including highly specific targeted drug delivery, antibiotic-resistant bacterial infections secreting unknown toxins, autoimmune diseases, and cancer immunotherapy, nanoparticle platforms that look beyond traditional paradigms and instead directly harness the carefully evolved, multivalent, and multi-specific interfaces found in nature are highly desirable.
A recent and exciting development in biomimetic nanoengineering is the cell membrane-coated nanoparticle, which consists of a core material coated with membrane derived from a source cell. The first cell membrane-coated nanoparticle used red blood cells (RBCs) as the source cell, with the RBC membrane derived by hypotonic treatment and coated onto negatively charged polymeric nanoparticles by extrusion.30 Cell membrane coating technology has now expanded to include particles coated with the membrane of nucleated cells, which can be separated from nuclear and mitochondrial components through a sucrose gradient31 or differential centrifugation32 with very little contamination from those intracellular contents. Particles have also been coated using a sonication method33 or formed inside of cell vesicles using a cell membrane-templated gelation technique.34 These methods have each been found to fully coat the particles and retain cell surface proteins in a right-side-out manner.35–36 This is believed to be due to the asymmetric charge of the cell membranes, which causes them to coat around the particle cores in a manner that minimizes charge repulsion. With these coating techniques, it has been shown that nanoparticle cores ranging from 65 nm to 340 nm in diameter can be successfully coated. Depending on the application, the size of the cell membrane-coated nanoparticles can be tuned to have certain properties such as high cargo loading, long circulation, or deep tumor penetration. In addition, the coated nanoparticles remain stable under high shear stress conditions, making them a suitable platform for in vivo therapeutic use.37
Being a top-down method, cell membrane coating is able to retain the complex makeup of the entire cell surface on the nanoparticle, bypassing the need for complicated chemistry and the identification of individual membrane components. These particles have demonstrated the ability to function well within the body, appearing as the source cell and using the multiple interactions occurring between the cell membrane and its substrates. This Topical Review will examine the use of cell membrane-coated nanoparticles for (i) drug delivery, (ii) detoxification, and (iii) immune modulation (Figure 1).
Figure 1.
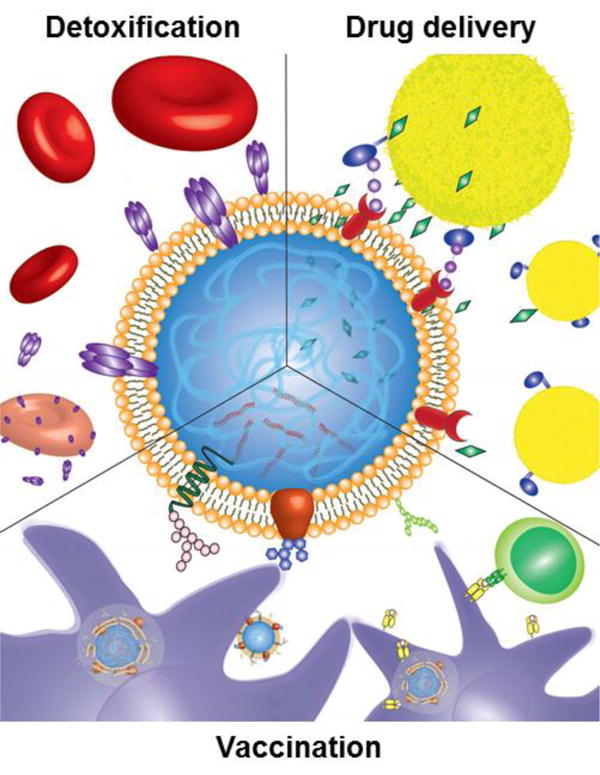
Illustration showing three applications of cell membrane-coated nanoparticles. The particles can be used for targeted drug delivery to tumors, sites of inflammation, or pathogens via translocated surface markers (top right). They can also act as a decoy for toxins that damage cells through membrane interactions, safely detaining them and sparing their intended targets (top left). Finally, cell membrane-coated nanoparticles faithfully present antigens from their source cells and can be used for improved anticancer or antibacterial vaccination (bottom).
DRUG DELIVERY
Stealth Coating and Non-Specific Drug Delivery
A major hurdle for many in vivo applications of nanoparticles is rapid immune system clearance. The immune system is trained to capture and excrete foreign substances introduced into the body and further train the body for quick clearance of the foreign material upon reintroduction. This phenomenon can necessitate high dosages to compensate for the large amounts cleared from the body, leading to an increased risk of side effects. One widely accepted solution is the functionalization of nanoparticles with polyethylene glycol (PEG), often termed PEGylation. PEG works to form a flexible polymer brush layer on the surface of the nanoparticle, which prevents opsonization due to steric hindrance. Without the adsorption of opsonins on the surface, the PEGylated nanoparticles are able to escape uptake by phagocytic cells, increasing the circulation time within the body dramatically.38–39 However, there have been recent concerns regarding the immunocompatibility of PEG; some patients can have preexisting anti-PEG titers, or develop immune responses after repeated injections.40
RBC membrane-coated nanoparticles have emerged as a novel strategy to improve the circulation half-life of nanoparticles. RBCs naturally have a long circulation life in the body, with a lifetime of 100–120 days before immune clearance. This is in part due to the interaction between CD47, the “marker-of-self” protein found on the RBC surface, and signal-regulatory protein alpha, SIRPα, expressed by phagocytic cells. The SIRPα glycoprotein recognizes CD47 as a “don’t eat me” signal and then inhibits phagocytosis of RBCs by immune cells.41 RBC membrane coating onto nanoparticles preserves the CD47 marker, taking advantage of the CD47-SIRPα interaction to allow for long circulation of the particles. Hu et al. have shown that, by coating poly(lactic-co-glycolic acid) (PLGA) nanoparticles with RBC membrane, surface CD47 is retained at a density similar to native RBCs with the correct orientation, which lends a 64% reduction in macrophage engulfment in vitro (Figure 2).42 Further, these RBC membrane-coated nanoparticles have been shown to have an elimination half-life of 39.6 hours, compared to 15.8 hours for comparable PEGylated nanoparticles.30 Other works have corroborated these results and utilized RBC membrane coating to extend the circulation of various nanostructures.43–44 Rao et al. in particular have also shown that RBC membrane-coated nanoparticles have reduced reticuloendothelial system uptake and no obvious in vivo toxicity.45–46 Further, upon repeated administration the particles elicited no cellular or humoral immune response.46
Figure 2.
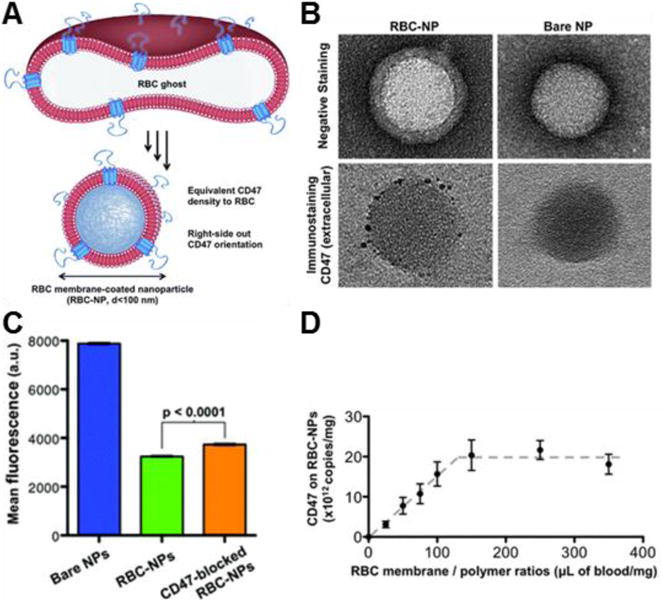
Stealth properties of RBC membrane-coated nanoparticles (RBC-NPs). (A) Schematic demonstrating transfer of CD47 onto RBC-NPs. (B) Transmission electron microscopy image of an RBC-NP and a bare NP stained with uranyl acetate (top) or immunostained for the extracellular domain of CD47 (bottom). (C) Quantitative flow cytometry of particle uptake into murine macrophage cells after a ten-minute incubation period. (D) CD47 density on RBC-NPs with different RBC membrane to polymer ratios. Adapted with permission from reference 42. Copyright 2013, Royal Society of Chemistry.
Cell membrane coating for immune evasion has been expanded by using other cell types for coating materials. Leukocyte membrane-coated porous silica particles have been developed by Parodi et al., with the leukocyte membrane retention of sialic acid and N-acetylglucosamine glycans important in cellular self-recognition, serving to reduce binding to similar immune cells. For particles coated with J774 macrophage-like cells, there was a 75% decrease in uptake by J774 cells, and for particles coated with THP-1 cells, there was a 50% decrease in THP-1 uptake.47 Macrophage cells have also been used to coat particles to extend circulation time for improved photothermal therapy.48 Platelets, which circulate through the body to survey for damage, can also impart nanoparticles with immune evasion through mechanisms such as CD47-dependent reduction of macrophage uptake and prevention of complement activation via CD55 and CD59.49–50
These stealth properties have been particularly useful for non-specific cancer drug delivery by taking advantage of the enhanced retention and permeation (EPR) effect. Tumor growth is characterized by angiogenesis, which is the rapid growth of new blood vessels to provide adequate oxygen and nutrients to the growing cancer cells. The new vessels are often abnormally formed and leaky, so nanoparticles can preferentially be taken up into the tumor site via extravasion, and stay at the tumor site for an extended period of time.51 Stealth coating improves the circulation time and therefore increases the chance of the particulate drug getting into the tumor via the EPR effect. For this reason, RBC membrane-coated nanoparticles can accumulate in tumors and enhance delivery of chemotherapeutics such as docetaxel, doxorubicin (DOX), and paclitaxel either alone or in combinations.52 Once inside the cell, cargo can be slowly released by natural diffusion through the membrane as the polymer matrix degrades53 or rapidly released through external manipulations such as ultraviolet triggered membrane degradation.54 These biomimetic nanocarriers are also immunocompatible and can improve treatment safety. Luk et al. found that RBC membrane-coated nanoparticles delivering DOX to established lymphoma tumors could double survival time of mice compared to control mice, with no systemic release of inflammatory cytokines and no myelosuppressing indications like low white blood cells count that is typical of free DOX administration.55
Targeted Drug Delivery to Tumors
Besides angiogenesis, tumor growth is also dependent on interactions between individual cancer cells. Homotypic binding occurs when cancer cells adhere to one another, allowing for the growth of tumor masses. Fang et al. took advantage of these cancer cell surface adhesion domains by coating polymeric nanoparticles with membrane derived from MDA-MB-435 breast cancer cells. The cancer cell membrane-coated nanoparticles (CCNPs) had a 20-fold increase in uptake by source cells as compared to RBC membrane-coated nanoparticles.56 Nanoparticles coated with the membrane of 4T1 breast cancer cells have been found to retain other adhesion molecules such as Thomsen-Friedenreich antigen, E-cadherin, CD44, and CD326, and facilitate the delivery of paclitaxel to primary as well as metastatic tumors.57 Similar results were found with cancer cell membrane-coated, DOX-loaded magnetic nanoparticles. In mice burdened with two tumors of different cell line sources, particles were taken up into their autologous tumor 3-fold higher than into the competitive tumor of a different cancer source. DOX delivery by these particle clusters also allowed for reduced growth of autologous tumors.58
Solid tumors require an increased recruitment of various cell types to promote rapid cell growth. Stem cells are often recruited to the tumor site due to the increased demand for connective stromal cells in the area. This demand is met by engraftment of mesenchymal stem cells in tumors, mediated by integrin-ICAM and integrin-VCAM interactions.59–60 Taking advantage of this recruitment, DOX-loaded gelatin nanoparticles coated with mesenchymal stem cell membrane preferentially accumulate in tumor sites and can enhance tumor destruction with the release of DOX.61 Macrophages and their monocyte precursors are also drawn to tumor sites, partially due to surface expression of CD49d, a heterodimeric integrin that binds to VCAM1 on target cells. DOX-loaded nanoparticles coated with membrane derived from these cells have been shown to increase drug uptake into MCF-7 breast cancer cells and also reduce 4T1 tumor growth.62–63
Platelets serve a special function for circulating cancer cells. Circulating tumor cells can induce thrombus formation, which attracts local platelets to form a “shield” around the cancer cells to aid immune evasion and enable extravasation.64 This binding is likely due to the formation GPIIb-IIIa-fibrinogen bridges between the two cells, as well as interactions between P-selectin, expressed on platelets, and CD44, which is overexpressed in many cancer cells.65–66 Taking advantage of this relationship, platelet membrane-coated nanovehicles functionalized with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) cytokine and loaded with DOX cargo have been used to treat primary tumors and were shown to kill circulating tumor cells. After intravenous administration, the particles accumulate at tumor sites, significantly inhibiting primary tumor growth nodules in an established breast cancer model. Treatment with the particles also decreased the number of lung metastases in a circulating tumor cell model (Figure 3).50 Similarly, silica particles coated with platelet membrane and functionalized with TRAIL showed significant efficacy in reducing lung metastases formation when administered after an intravenous injection of luciferase-expressing MDA-MB-231 cells.67
Figure 3.
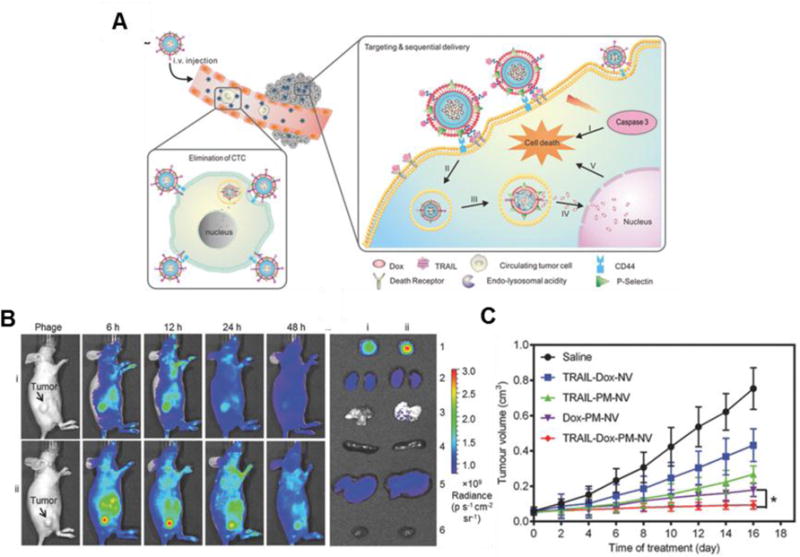
Platelet-mimicking nanovehicles (PM-NVs) for targeted cancer drug delivery. (A) Schematic of PM-NV targeting mechanisms to both circulating and primary tumor cells. (B) In vivo fluorescence imaging of PM-NV biodistribution in MDA-MB-231 tumor-bearing nude mice for (i) Cy5.5-labeled TRAIL-DOX-NVs (ii) and Cy5.5-labeled TRAIL-DOX-PM-NVs. (C) MDA-MB-231 tumor growth curves after intravenous injection of different TRAIL/DOX formulations. Error bars indicate s.d. (n = 5). Adapted with permission from reference 50. Copyright 2015, Wiley.
Targeted Drug Delivery to Bacteria
There has been an increased interest in targeted drug delivery to bacteria due to the growing concern of antibiotic-resistant bacteria, such as methicillin-resistant staphylococcus aureus (MRSA). Similar to targeting of circulating tumor cells, platelet membrane-coated nanoparticles can also target to opportunistic bacteria, as bacteria also exploit platelets as a way to shield themselves from the immune system and localize to certain vulnerable tissues.68 Binding between platelets and bacteria is varied and complex, occurring through either direct adhesion via bacterial surface proteins or involving plasma bridging molecules. Hu et al. fabricated platelet membrane-coated nanoparticles (PNPs) capable of multiple biological interactions (Figure 4A), and they have shown that coating vancomycin-loaded PNPs can improve binding to MRSA 12-fold compared to bare nanoparticles. This efficient binding greatly improves bacteria killing efficacy, decreasing overall bacterial load in the organs of mice better than free vancomycin at just one-sixth the clinical dose in a systemic MRSA challenge mouse model (Figure 4B).49
Figure 4.
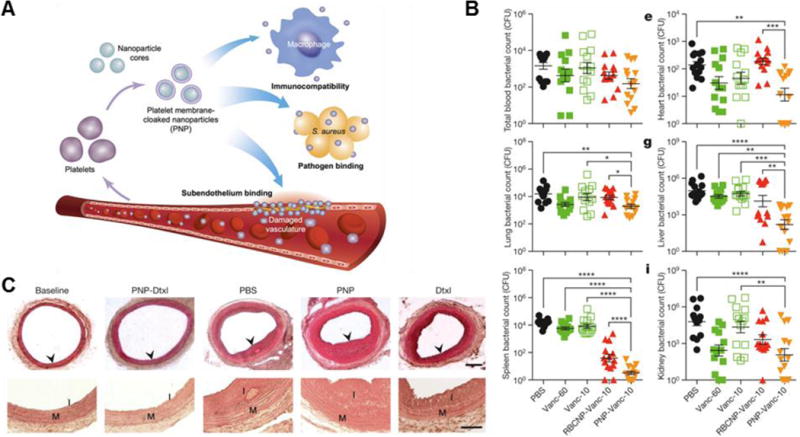
Platelet membrane-coated nanoparticles (PNPs) for biointerfacing. (A) Illustration demonstrating how PNPs are capable of multiple interactions in the body. (B) In vivo bacterial load in selected organs after treatment with free vancomycin at 10 mg/kg (Vanc-10), RBC-NP Vanc-10, PNP-Vanc-10, or free vancomycin at 60 mg/kg times the dosing (Vanc-60) in a systemic MRSA challenge model. (C) Histological cross-sections of rat arteries from a coronary restenosis model after different treatments (top; scale bar = 200 μm). Zoomed in images of arterial sections to highlight intima (I) to media (M) ratio (bottom; scale bar = 100 μm). Dtxl, docetaxel. Adapted with permission from reference 49. Copyright 2015, Macmillan Publishers Limited.
Targeted Drug Delivery to Inflamed Injuries
Delivery of drugs to sites of inflammation is important for proper wound healing and prevention of post-injury complications. Platelets and leukocytes home to sites of injury and inflammation in order to clot bleeding and facilitate formation of extracellular matrices, making these cells a natural choice for membrane coating. Leukocytes are able to traverse the endothelium, and leukocyte-coated nanoparticles have been found to also possess this function, enabling nanoparticles like porous silica to traffic through inflamed endothelium due to the retention of CD45, CD3z, LFA-1 and CD11a, showing potential for trans-endothelium drug delivery.47 Platelets, on the other hand, bind to collagen in the subendothelium, which is exposed when the upper endothelium layer is damaged. This property has been used for the treatment of coronary restenosis, which occurs when the intima overgrows in response to injury, narrowing the artery and restricting blood flow. The retention of glycoprotein IV allows PNPs to also bind to exposed collagen, such as that in a damaged artery that could be susceptible to coronary restenosis. When loaded with docetaxel, the PNPs have been shown to bind to the exposed collagen in denuded rat artery and almost completely prevent overgrowth of the intima (Figure 4C).49
DETOXIFICATION
Pore Forming Toxins
Pore forming toxins (PFTs) are virulence factors secreted by bacteria that can cause considerable damage to the host cells and can facilitate pathogenesis by disrupting the membranes of target cells. For example, α-hemolysin (Hla) released from Staphylococcus aureus targets to RBCs and a number of nucleated cells, oligomerizes into a transmembrane structure on the cell surface, and lyses the cell. Without treatment, intoxication with PFTs like Hla, streptolysin-O, or melittin can cause skin necrosis and, in severe cases, death.69 As PFTs all use this general mechanism of membrane insertion, RBC-coated nanoparticles, which mimic the entire surface of an RBC, can serve as a valuable therapeutic for broad-spectrum toxin neutralization. Termed “nanosponges”, these particles act as decoys, retaining toxins that insert into the RBC membrane and preventing them from lysing healthy RBCs. Hu et al. have shown that RBC nanosponges prevent skin lesions when premixed with Hla prior to subcutaneous injection. Further, during lethal intravenous Hla challenges, nanosponge administration can improve survival rate to 44% in a therapeutic setting, and 89% in a prophylactic setting.70
The nanosponge technology can be further improved via integration with other biomedical platforms. Coating RBC membrane on Janus microparticles or synthetic motors incorporates dynamic mixing for improved interaction between decoy membrane and PFTs, which is especially useful for blood or water supply detoxification.71–72 Conversely, hydrogel encapsulation of RBC-coated nanoparticles can localize the detoxifying particles to the area where bacteria are colonizing and releasing the most toxins, for example near a MRSA-induced skin lesion. The improved localization and slow release of the nanosponges can synergistically provide impressive antivirulence efficacy.73 The exotoxin absorption property of RBC membranes can prove valuable in multi-step antibiotics systems as well. RBC-coated gelatin particles have been used to absorb various different toxins released by six different bacteria species, drastically reducing hemolysis in all cases. After protecting the RBCs, the particles then degrade in the presence of gelatinase-positive bacteria, releasing loaded vancomycin and killing the bacteria (Figure 5).74
Figure 5.
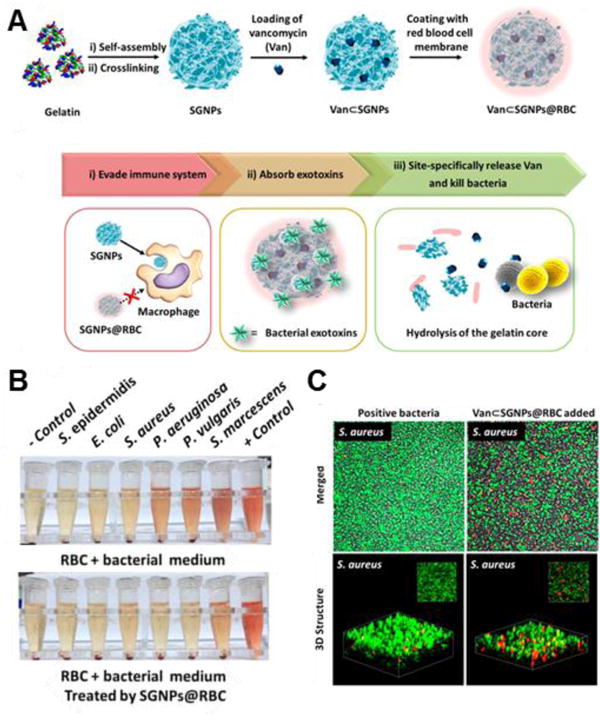
RBC membrane-coated supramolecular gelatin nanoparticles (SGNPs@RBC) for three-step antibiotic treatment. (A) Schematic showing nanoparticle formulation and antibiotic treatment steps. (B) Hemolysis activity by toxins from six different bacteria before (top) and after (bottom) treatment with SGNPs@RBC. (C) 2D (top) and 3D (bottom) confocal microscopy images of bacteria killing by vancomycin-loaded SGNPs@RBC when exposed to gelatinase-positive bacteria (S. aureus). Adapted with permission from reference 74. Copyright 2014, American Chemical Society.
Organophosphates
Organophosphate poisoning occurs when the molecule irreversibly phosphorylates acetylcholinesterase, inactivating the enzyme and preventing breakdown of the neurotransmitter acetylcholine. The subsequent accumulation of acetylcholine can cause acute neuromuscular disorders and can be fatal.75 Organophosphate exposure is generally due to pesticide and insecticide use, but has also been implicated as a warfare agent. RBC nanosponges can be used to spare synaptic acetylcholinesterases and prevent symptoms by presenting decoy acetylcholinesterase on the membrane coating for the organophosphates to target. In the event of acute exposure to organophosphates, RBC nanosponges can circulate in the body for a long time, binding to the organophosphates until they can be safely metabolized in the liver. Pang et al. showed that in a lethal challenge with an intravenous injection of dichlorvos, an insecticide and organophosphate, untreated mice died in seven minutes, while impressively all RBC nanosponge-treated mice survived. To mimic a real-world organophosphate exposure, mice were orally administered with a lethal dose of dichlorvos and then treated with RBC nanosponges. All treated mice survived the challenge, while 90% of untreated mice did not survive.33
IMMUNE MODULATION
Therapy for Autoimmune Disease
Autoimmune diseases manifest when the body attacks its own normal cells and is often characterized by the opsonization of target cells by pathological autoantibodies produced by B cells.76 Current treatments for such autoimmune disease include immune suppression via cytotoxic drugs, administration of other types of antibodies, and systemic glucocorticoids.77 There are side effects to such broad immune suppression therapies, but the treatments are still clinically used as the underlying cause of many autoimmune diseases remains unknown. One such disease, autoimmune hemolytic anemia (AIHA) is characterized by the production of antibodies against the body’s own RBCs.78 RBC nanosponges have been used to act as an absorbent for autoantibodies against RBCs. These nanoparticles can sequester antibodies and protect the healthy RBCs from self-destruction by the body. In a mouse model of antibody-induced anemia, mice treated with RBC nanosponges were able to preserve their baseline RBC counts and saw no elevation in autoimmune antibodies.79
Similar to AIHA, immune thrombocytopenia purpura (ITP) is characterized by the production of anti-platelet antibodies and a reduction of platelet counts in the blood. In the case of injury, the lack of sufficient platelets can lead to uncontrolled bleeding, which may be fatal. To treat ITP, PNPs have been used to neutralize the pathological anti-platelet antibodies. The platelet cell membrane coating serves as an alternate target for the autoimmune antibodies, sparing platelets from destruction. In an established mouse model of ITP, treatment with PNPs significantly preserved platelet counts, which resulted in much shorter bleeding times after tail tip excision when compared to no treatment or treatment with control PEGylated nanoparticles.37
Antibacterial Vaccination
Current vaccinations against bacteria utilize denatured toxins or bacteria as vaccines to generate a humoral response against these pathogens. However, a balance must be struck between safety and efficacy, as denaturing the pathogenic material eliminates virulence but can also alter the native structure of the antigen, training the body against non-authentic representations of the bacteria or toxins that it will need to identify and destroy. RBC membrane-coated nanoparticles have been shown to retain toxins in the membrane after absorption.70 Toxin-bearing RBC nanoparticles, or “nanotoxoids” safely neutralize the toxins while keeping them structurally intact, providing an ideal means for antitoxin and antibacterial vaccination without compromising safety. Nanotoxoids have been shown to detain Hla indefinitely and cause a significant increase in anti-Hla titers after a prime and two boost injections of the formulation (nanotoxoid(Hla)), over 15-fold higher than the titers induced by equivalent treatment with heat-treated Hla. In addition, the anti-Hla titers were sustained at similar levels for at least 150 days. After a lethal bolus dose of Hla toxin, 100% of the nanotoxoid-immunized mice survived (Figure 6).80 Wang et al. went further to show that the nanotoxoid(Hla) induced a large production of B cells with a germinal center phenotype, and when used to vaccinate against live, toxin-producing bacteria, could cause a 14.7-fold decrease in overall bacterial burden.81
Figure 6.
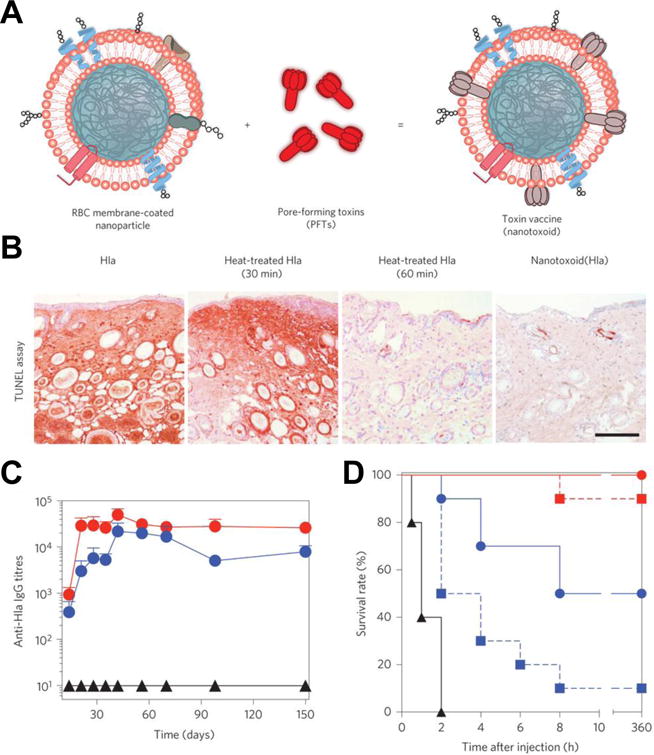
Nanoparticle-detained toxins (nanotoxoid) for antivirulence vaccination. (A) Illustration of the nanotoxoid platform. (B) TUNEL assay on mouse skin 24 hours after injection with free α-hemolysin (Hla), heat-treated Hla (30 minutes), heat-treated Hla (60 minutes), or nanotoxoid(Hla). Scale bar = 400 μm. (C) Anti-Hla IgG titers over time for unvaccinated mice (black triangles) and mice immunized by nanotoxoid(Hla) with a single prime dose (blue circles) or a prime + boost (red circles). (D) Mouse survival rate over 15 days after bolus intravenous injection of Hla on day 21 after vaccination. Groups included no vaccination (black triangles), a single prime dose of heat-treated Hla (blue squares), a single prime dose of nanotoxoid(Hla) (blue circles), a prime + boost of heat-treated Hla (red squares), and a prime + boost of nanotoxoid(Hla) (red circles). Adapted with permission from reference 80. Copyright 2013, Macmillan Publishers Limited.
Alternatively, bacteria outer membrane vesicles (OMVs), which contain many of the same surface antigens as their source bacteria, can be directly coated onto gold nanoparticles to serve as an antibacterial vaccine. For example, E. coli OMV-coated nanoparticles can recruit dendritic cells to lymph nodes, and cause maturation as indicated by overexpression of CD40, CD80, and CD86 surface markers. After administration with the bacteria membrane-coated nanoparticles, significantly higher E. coli-specific antibody titers were induced in mice and sustained for about 150 days; it was also determined that both cellular and humoral immune responses were activated in the process.82
Anticancer Vaccination
Cancer immunotherapy has garnered significant interest in the cancer research world. Although there have been some clinically approved cancer vaccines such as the short-lived Provenge, anticancer vaccination has not had a major breakthrough in terms of long lasting clinical success.83 The challenge of selecting the best source of cancer antigens to be included in the vaccine and managing the co-delivery of immune-stimulating adjuvants alongside the antigens can often hamper cancer vaccine development.84 Current vaccinations focus on either whole cell antigen sources, where lysed cancer cells are used as the target, or single antigen formulations, where individual cancer-specific peptides or proteins are used as the target. Whole cell vaccines present many antigens to the immune system, which is good for destroying a heterogeneous population of cells, but often results in weak immune responses due to the inclusion of a large amount of housekeeping protein.85 Single antigen formulations, on the other hand, can elicit a strong and focused immune response, but are limited by the identification of specific tumor-associated antigens, and even then the heterogeneity of tumors can still result in eventual immune escape.86 Cancer cell membrane-coated nanoparticles hold promise as a cancer vaccine platform. Cancer cell membrane coatings can serve as an ideal source of antigen, providing a complete repertoire of the tumor surface antigens while minimizing inclusion of extraneous proteins that can dilute immune responses. Fang et al. explored a B16-F10 cancer cell membrane-coated nanoparticle, which retained multiple antigens on the particle surface. When the adjuvant monophosphoryl lipid A (MPLA) was inserted into the membrane coating, incubation of the particles with dendritic cells induced maturation. These particle-pulsed dendritic cells were shown to physically interact with and stimulate antigen-specific T-lymphocytes, indicating potential for generating strong and specific antitumor immune responses (Figure 7).56 The long-term immunomodulatory effect of administering these particles in vivo is the subject of current investigation.
Figure 7.
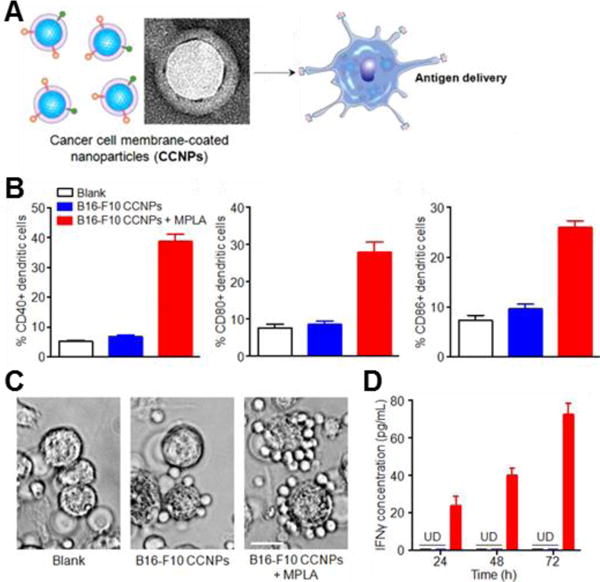
Cancer cell membrane-coated nanoparticles (CCNPs) for anticancer vaccination. (A) Depiction of CCNPs for antigen delivery to dendritic cells. (B) Quantitative flow cytometry data of dendritic cell maturation when incubated for 48 hours with CCNPs coated with membrane from B16-F10 mouse melanoma cancer cells (B16-F10 CCNPs), with or without the adjuvant MPLA. (C) Phase contrast microscopy images of splenocytes derived from pmel-1 transgenic mice when incubated with dendritic cells pulsed with B16-F10 CCNPs, with or without MPLA. Scale bar = 25 μm. (D) IFNγ ELISA of supernatant collected from co-culture at 24, 48, and 72 hours. UD, undetectable by ELISA. Adapted with permission from reference 56. Copyright 2014, American Chemical Society.
CONCLUSIONS
Nanoparticles coated with the membrane of a cell can take on the functions and properties inherent to the source cell due to the faithful translocation of many membrane proteins, glycans, and lipids to the surface of the nanoparticles. These cell membrane-coated nanoparticles can interact with other cells and pathogenic materials using their cell surface coating, which can be leveraged for a wide variety of therapeutic purposes. The multivalency of interactions that can occur simultaneously allows these particles to interface with diseases at more than one stage, exhibit stronger binding to sites of interest, or train the immune system against many antigens for a strong and broad response. For other applications, like detoxification, the multi-specificity can be advantageous as a broad spectrum therapeutic.
So far, this nascent technology has been used mainly for applications involving RBCs, platelets, cancer cells, and bacteria. However, due to the expansive options of core materials and cell types that can be used for this platform, it can be envisioned that there will be rapid development of new cell membrane-coated nanoparticles for other novel biomedical applications, like disease detection, combination therapies, or organotropic cargo delivery using exosomes as a potential membrane source.87–88 Looking forward, cell membrane-coated nanoparticles can also be endowed with additional properties not native to the cell membrane through additional surface functionalization, adding another degree of freedom to this already modular biomimetic platform.89–90
Acknowledgments
This work is supported by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense under Grant Number HDTRA1-14-1-0064 and by the National Institutes of Health under Award Numbers R01CA200574.
References
- 1.Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem Rev. 2016;116:2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snipstad S, Westrom S, Morch Y, Afadzi M, Aslund AK, de Lange Davies C. Contact-Mediated Intracellular Delivery of Hydrophobic Drugs from Polymeric Nanoparticles. Cancer Nanotechnol. 2014;5:8. doi: 10.1186/s12645-014-0008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang S, Gao H, Bao G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano. 2015;9:8655–8671. doi: 10.1021/acsnano.5b03184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco E, Shen H, Ferrari M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazak R, Houri M, Achy SE, Hussein W, Refaat T. Passive Targeting of Nanoparticles to Cancer: A Comprehensive Review of the Literature. Mol Clin Oncol. 2014;2:904–908. doi: 10.3892/mco.2014.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assanhou AG, Li W, Zhang L, Xue L, Kong L, Sun H, Mo R, Zhang C. Reversal of Multidrug Resistance by Co-Delivery of Paclitaxel and Lonidamine Using a TPGS and Hyaluronic Acid Dual-Functionalized Liposome for Cancer Treatment. Biomaterials. 2015;73:284–295. doi: 10.1016/j.biomaterials.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Zhou H, Fan Z, Deng J, Lemons PK, Arhontoulis DC, Bowne WB, Cheng H. Hyaluronidase Embedded in Nanocarrier PEG Shell for Enhanced Tumor Penetration and Highly Efficient Antitumor Efficacy. Nano Lett. 2016;16:3268–3277. doi: 10.1021/acs.nanolett.6b00820. [DOI] [PubMed] [Google Scholar]
- 8.Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H, et al. Systemic RNA Delivery to Dendritic Cells Exploits Antiviral Defence for Cancer Immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 9.Siefert AL, Caplan MJ, Fahmy TM. Artificial Bacterial Biomimetic Nanoparticles Synergize Pathogen-Associated Molecular Patterns for Vaccine Efficacy. Biomaterials. 2016;97:85–96. doi: 10.1016/j.biomaterials.2016.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrillo-Conde B, Song EH, Chavez-Santoscoy A, Phanse Y, Ramer-Tait AE, Pohl NL, Wannemuehler MJ, Bellaire BH, Narasimhan B. Mannose-Functionalized “Pathogen-Like” Polyanhydride Nanoparticles Target C-Type Lectin Receptors on Dendritic Cells. Mol Pharm. 2011;8:1877–1886. doi: 10.1021/mp200213r. [DOI] [PubMed] [Google Scholar]
- 11.Giljohann DA, Seferos DS, Prigodich AE, Patel PC, Mirkin CA. Gene Regulation with Polyvalent siRNA-Nanoparticle Conjugates. J Am Chem Soc. 2009;131:2072–2073. doi: 10.1021/ja808719p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Ouay B, Stellacci F. Antibacterial Activity of Silver Nanoparticles: A Surface Science Insight. Nano Today. 2015;10:339–354. [Google Scholar]
- 13.Steichen SD, Caldorera-Moore M, Peppas NA. A Review of Current Nanoparticle and Targeting Moieties for the Delivery of Cancer Therapeutics. Eur J Pharm Sci. 2013;48:416–427. doi: 10.1016/j.ejps.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia H, Titmuss S. Polymer-Functionalized Nanoparticles: From Stealth Viruses to Biocompatible Quantum Dots. Nanomedicine (Lond) 2009;4:951–966. doi: 10.2217/nnm.09.81. [DOI] [PubMed] [Google Scholar]
- 15.Yen SK, Janczewski D, Lakshmi JL, Dolmanan SB, Tripathy S, Ho VH, Vijayaragavan V, Hariharan A, Padmanabhan P, Bhakoo KK, et al. Design and Synthesis of Polymer-Functionalized NIR Fluorescent Dyes--Magnetic Nanoparticles for Bioimaging. ACS Nano. 2013;7:6796–6805. doi: 10.1021/nn401734t. [DOI] [PubMed] [Google Scholar]
- 16.Vertegel AA, Reukov V, Maximov V. Enzyme-Nanoparticle Conjugates for Biomedical Applications. Methods Mol Biol. 2011;679:165–182. doi: 10.1007/978-1-60761-895-9_14. [DOI] [PubMed] [Google Scholar]
- 17.Arruebo M, Valladares M, Gonzalez-Fernandez A. Antibody-Conjugated Nanoparticles for Biomedical Applications. J Nanomater. 2009;2009:439389. [Google Scholar]
- 18.Mout R, Moyano DF, Rana S, Rotello VM. Surface Functionalization of Nanoparticles for Nanomedicine. Chem Soc Rev. 2012;41:2539–2544. doi: 10.1039/c2cs15294k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan JM, Monaco C, Wylezinska-Arridge M, Tremoleda JL, Gibbs RG. Imaging of the Vulnerable Carotid Plaque: Biological Targeting of Inflammation in Atherosclerosis Using Iron Oxide Particles and MRI. Eur J Vasc Endovasc Surg. 2014;47:462–469. doi: 10.1016/j.ejvs.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Kwon HJ, Cha MY, Kim D, Kim DK, Soh M, Shin K, Hyeon T, Mook-Jung I. Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimer’s Disease. ACS Nano. 2016;10:2860–2870. doi: 10.1021/acsnano.5b08045. [DOI] [PubMed] [Google Scholar]
- 21.Yeh CY, Hsiao JK, Wang YP, Lan CH, Wu HC. Peptide-Conjugated Nanoparticles for Targeted Imaging and Therapy of Prostate Cancer. Biomaterials. 2016;99:1–15. doi: 10.1016/j.biomaterials.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Hu CM, Kaushal S, Tran Cao HS, Aryal S, Sartor M, Esener S, Bouvet M, Zhang L. Half-Antibody Functionalized Lipid-Polymer Hybrid Nanoparticles for Targeted Drug Delivery to Carcinoembryonic Antigen Presenting Pancreatic Cancer Cells. Mol Pharm. 2010;7:914–920. doi: 10.1021/mp900316a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrmann IK, Urner M, Graf S, Schumacher CM, Roth-Z’graggen B, Hasler M, Stark WJ, Beck-Schimmer B. Endotoxin Removal by Magnetic Separation-Based Blood Purification. Adv Healthc Mater. 2013;2:829–835. doi: 10.1002/adhm.201200358. [DOI] [PubMed] [Google Scholar]
- 24.Herrmann IK, Urner M, Koehler FM, Hasler M, Roth-Z’graggen B, Grass RN, Ziegler U, Beck-Schimmer B, Stark WJ. Blood Purification Using Functionalized Core/Shell Nanomagnets. Small. 2010;6:1388–1392. doi: 10.1002/smll.201000438. [DOI] [PubMed] [Google Scholar]
- 25.Hoshino Y, Koide H, Urakami T, Kanazawa H, Kodama T, Oku N, Shea KJ. Recognition, Neutralization, and Clearance of Target Peptides in the Bloodstream of Living Mice by Molecularly Imprinted Polymer Nanoparticles: A Plastic Antibody. J Am Chem Soc. 2010;132:6644–6645. doi: 10.1021/ja102148f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Li Y, Jiao J, Hu HM. Alpha-Alumina Nanoparticles Induce Efficient Autophagy-Dependent Cross-Presentation and Potent Antitumour Response. Nat Nanotechnol. 2011;6:645–650. doi: 10.1038/nnano.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin AY, Lunsford J, Bear AS, Young JK, Eckels P, Luo L, Foster AE, Drezek RA. High-Density Sub-100-nm Peptide-Gold Nanoparticle Complexes Improve Vaccine Presentation by Dendritic Cells In Vitro. Nanoscale Res Lett. 2013;8:72. doi: 10.1186/1556-276X-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, Tsai S, Wang J, Garabatos N, Izquierdo C, et al. Expanding Antigen-Specific Regulatory Networks to Treat Autoimmunity. Nature. 2016;530:434–440. doi: 10.1038/nature16962. [DOI] [PubMed] [Google Scholar]
- 29.Pandey A, Roy MK, Pandey A, Zanella M, Sperling RA, Parak WJ, Samaddar AB, Verma HC. Chloroform- and Water-Soluble Sol-Gel Derived Eu+++/Y2o3 (Red) and Tb+++/Y2o3 (Green) Nanophosphors: Synthesis, Characterization, and Surface Modification. IEEE Trans Nanobioscience. 2009;8:43–50. doi: 10.1109/TNB.2009.2017316. [DOI] [PubMed] [Google Scholar]
- 30.Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte Membrane-Camouflaged Polymeric Nanoparticles as a Biomimetic Delivery Platform. Proc Natl Acad Sci USA. 2011;108:10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boone CW, Ford LE, Bond HE, Stuart DC, Lorenz D. Isolation of Plasma Membrane Fragments from HeLa Cells. J Cell Biol. 1969;41:378–392. doi: 10.1083/jcb.41.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suski JM, Lebiedzinska M, Wojtala A, Duszynski J, Giorgi C, Pinton P, Wieckowski MR. Isolation of Plasma Membrane-Associated Membranes from Rat Liver. Nat Protoc. 2014;9:312–322. doi: 10.1038/nprot.2014.016. [DOI] [PubMed] [Google Scholar]
- 33.Pang Z, Hu CM, Fang RH, Luk BT, Gao W, Wang F, Chuluun E, Angsantikul P, Thamphiwatana S, Lu W, et al. Detoxification of Organophosphate Poisoning Using Nanoparticle Bioscavengers. ACS Nano. 2015;9:6450–6458. doi: 10.1021/acsnano.5b02132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Gao W, Fang RH, Dong A, Zhang L. Synthesis of Nanogels Via Cell Membrane-Templated Polymerization. Small. 2015;11:4309–4313. doi: 10.1002/smll.201500987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao W, Hu CM, Fang RH, Luk BT, Su J, Zhang L. Surface Functionalization of Gold Nanoparticles with Red Blood Cell Membranes. Adv Mater. 2013;25:3549–3553. doi: 10.1002/adma.201300638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luk BT, Hu CM, Fang RH, Dehaini D, Carpenter C, Gao W, Zhang L. Interfacial Interactions between Natural RBC Membranes and Synthetic Polymeric Nanoparticles. Nanoscale. 2014;6:2730–2737. doi: 10.1039/c3nr06371b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei X, Gao J, Fang RH, Luk BT, Kroll AV, Dehaini D, Zhou J, Kim HW, Gao W, Lu W, et al. Nanoparticles Camouflaged in Platelet Membrane Coating as an Antibody Decoy for the Treatment of Immune Thrombocytopenia. Biomaterials. 2016;111:116–123. doi: 10.1016/j.biomaterials.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis FF. The Origin of Pegnology. Adv Drug Deliv Rev. 2002;54:457–458. doi: 10.1016/s0169-409x(02)00021-2. [DOI] [PubMed] [Google Scholar]
- 39.Veronese FM, Pasut G. PEGylation, Successful Approach to Drug Delivery. Drug Discov Today. 2005;10:1451–1458. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- 40.Verhoef JJ, Anchordoquy TJ. Questioning the Use of PEGylation for Drug Delivery. Drug Deliv Transl Res. 2013;3:499–503. doi: 10.1007/s13346-013-0176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barclay AN, Van den Berg TK. The Interaction between Signal Regulatory Protein Alpha (SIRPα) and CD47: Structure, Function, and Therapeutic Target. Annu Rev Immunol. 2014;32:25–50. doi: 10.1146/annurev-immunol-032713-120142. [DOI] [PubMed] [Google Scholar]
- 42.Hu CM, Fang RH, Luk BT, Chen KN, Carpenter C, Gao W, Zhang K, Zhang L. ‘Marker-of-Self’ Functionalization of Nanoscale Particles through a Top-Down Cellular Membrane Coating Approach. Nanoscale. 2013;5:2664–2668. doi: 10.1039/c3nr00015j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piao JG, Wang L, Gao F, You YZ, Xiong Y, Yang L. Erythrocyte Membrane Is an Alternative Coating to Polyethylene Glycol for Prolonging the Circulation Lifetime of Gold Nanocages for Photothermal Therapy. ACS Nano. 2014;8:10414–10425. doi: 10.1021/nn503779d. [DOI] [PubMed] [Google Scholar]
- 44.Su JH, Sun HP, Meng QS, Yin Q, Tang S, Zhang PC, Chen Y, Zhang ZW, Yu HJ, Li YP. Long Circulation Red-Blood-Cell-Mimetic Nanoparticles with Peptide-Enhanced Tumor Penetration for Simultaneously Inhibiting Growth and Lung Metastasis of Breast Cancer. Adv Funct Mater. 2016;26:1243–1252. [Google Scholar]
- 45.Rao L, Xu JH, Cai B, Liu H, Li M, Jia Y, Xiao L, Guo SS, Liu W, Zhao XZ. Synthetic Nanoparticles Camouflaged with Biomimetic Erythrocyte Membranes for Reduced Reticuloendothelial System Uptake. Nanotechnology. 2016;27:085106. doi: 10.1088/0957-4484/27/8/085106. [DOI] [PubMed] [Google Scholar]
- 46.Rao L, Bu LL, Xu JH, Cai B, Yu GT, Yu X, He Z, Huang Q, Li A, Guo SS, et al. Red Blood Cell Membrane as a Biomimetic Nanocoating for Prolonged Circulation Time and Reduced Accelerated Blood Clearance. Small. 2015;11:6225–6236. doi: 10.1002/smll.201502388. [DOI] [PubMed] [Google Scholar]
- 47.Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Enzo MV, et al. Synthetic Nanoparticles Functionalized with Biomimetic Leukocyte Membranes Possess Cell-Like Functions. Nat Nanotechnol. 2013;8:61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xuan M, Shao J, Dai L, Li J, He Q. Macrophage Cell Membrane Camouflaged Au Nanoshells for In Vivo Prolonged Circulation Life and Enhanced Cancer Photothermal Therapy. ACS Appl Mater Interfaces. 2016;8:9610–9618. doi: 10.1021/acsami.6b00853. [DOI] [PubMed] [Google Scholar]
- 49.Hu CM, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, et al. Nanoparticle Biointerfacing by Platelet Membrane Cloaking. Nature. 2015;526:118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu Q, Sun W, Qian C, Wang C, Bomba HN, Gu Z. Anticancer Platelet-Mimicking Nanovehicles. Adv Mater. 2015;27:7043–7050. doi: 10.1002/adma.201503323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greish K, Fang J, Inutsuka T, Nagamitsu A, Maeda H. Macromolecular Therapeutics: Advantages and Prospects with Special Emphasis on Solid Tumour Targeting. Clin Pharmacokinet. 2003;42:1089–1105. doi: 10.2165/00003088-200342130-00002. [DOI] [PubMed] [Google Scholar]
- 52.Fu Q, Lv P, Chen Z, Ni D, Zhang L, Yue H, Yue Z, Wei W, Ma G. Programmed Co-Delivery of Paclitaxel and Doxorubicin Boosted by Camouflaging with Erythrocyte Membrane. Nanoscale. 2015;7:4020–4030. doi: 10.1039/c4nr07027e. [DOI] [PubMed] [Google Scholar]
- 53.Aryal S, Hu CM, Fang RH, Dehaini D, Carpenter C, Zhang DE, Zhang L. Erythrocyte Membrane-Cloaked Polymeric Nanoparticles for Controlled Drug Loading and Release. Nanomedicine (Lond) 2013;8:1271–1280. doi: 10.2217/nnm.12.153. [DOI] [PubMed] [Google Scholar]
- 54.Rao L, Meng QF, Huang Q, Liu P, Bu LL, Kondamareddy KK, Guo SS, Liu W, Zhao XZ. Photocatalytic Degradation of Cell Membrane Coatings for Controlled Drug Release. Adv Healthc Mater. 2016;5:1420–1427. doi: 10.1002/adhm.201600303. [DOI] [PubMed] [Google Scholar]
- 55.Luk BT, Fang RH, Hu CM, Copp JA, Thamphiwatana S, Dehaini D, Gao W, Zhang K, Li S, Zhang L. Safe and Immunocompatible Nanocarriers Cloaked in RBC Membranes for Drug Delivery to Treat Solid Tumors. Theranostics. 2016;6:1004–1011. doi: 10.7150/thno.14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fang RH, Hu CM, Luk BT, Gao W, Copp JA, Tai Y, O’Connor DE, Zhang L. Cancer Cell Membrane-Coated Nanoparticles for Anticancer Vaccination and Drug Delivery. Nano Lett. 2014;14:2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun H, Su J, Meng Q, Yin Q, Chen L, Gu W, Zhang P, Zhang Z, Yu H, Wang S, et al. Cancer-Cell-Biomimetic Nanoparticles for Targeted Therapy of Homotypic Tumors. Adv Mater. 2016 doi: 10.1002/adma.201602173. Advanced online publication. [DOI] [PubMed] [Google Scholar]
- 58.Zhu JY, Zheng DW, Zhang MK, Yu WY, Qiu WX, Hu JJ, Feng J, Zhang XZ. Preferential Cancer Cell Self-Recognition and Tumor Self-Targeting by Coating Nanoparticles with Homotypic Cancer Cell Membranes. Nano Lett. 2016 doi: 10.1021/acs.nanolett.6b02786. Advanced online publication. [DOI] [PubMed] [Google Scholar]
- 59.Cuiffo BG, Karnoub AE. Mesenchymal Stem Cells in Tumor Development: Emerging Roles and Concepts. Cell Adh Migr. 2012;6:220–230. doi: 10.4161/cam.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leibacher J, Henschler R. Biodistribution, Migration and Homing of Systemically Applied Mesenchymal Stem/Stromal Cells. Stem Cell Res Ther. 2016;7:7. doi: 10.1186/s13287-015-0271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao C, Lin Z, Jurado-Sanchez B, Lin X, Wu Z, He Q. Stem Cell Membrane-Coated Nanogels for Highly Efficient In Vivo Tumor Targeted Drug Delivery. Small. 2016;12:4056–4062. doi: 10.1002/smll.201600624. [DOI] [PubMed] [Google Scholar]
- 62.Krishnamurthy S, Gnanasammandhan MK, Xie C, Huang K, Cui MY, Chan JM. Monocyte Cell Membrane-Derived Nanoghosts for Targeted Cancer Therapy. Nanoscale. 2016;8:6981–6985. doi: 10.1039/c5nr07588b. [DOI] [PubMed] [Google Scholar]
- 63.Xuan M, Shao J, Dai L, He Q, Li J. Macrophage Cell Membrane Camouflaged Mesoporous Silica Nanocapsules for In Vivo Cancer Therapy. Adv Healthc Mater. 2015;4:1645–1652. doi: 10.1002/adhm.201500129. [DOI] [PubMed] [Google Scholar]
- 64.Gay LJ, Felding-Habermann B. Contribution of Platelets to Tumour Metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lou XL, Sun J, Gong SQ, Yu XF, Gong R, Deng H. Interaction between Circulating Cancer Cells and Platelets: Clinical Implication. Chin J Cancer Res. 2015;27:450–460. doi: 10.3978/j.issn.1000-9604.2015.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amo L, Tamayo-Orbegozo E, Maruri N, Eguizabal C, Zenarruzabeitia O, Rinon M, Arrieta A, Santos S, Monge J, Vesga MA, et al. Involvement of Platelet-Tumor Cell Interaction in Immune Evasion. Potential Role of Podocalyxin-Like Protein 1. Front Oncol. 2014;4:245. doi: 10.3389/fonc.2014.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J, Ai Y, Wang L, Bu P, Sharkey CC, Wu Q, Wun B, Roy S, Shen X, King MR. Targeted Drug Delivery to Circulating Tumor Cells Via Platelet Membrane-Functionalized Particles. Biomaterials. 2016;76:52–65. doi: 10.1016/j.biomaterials.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fitzgerald JR, Foster TJ, Cox D. The Interaction of Bacterial Pathogens with Platelets. Nat Rev Microbiol. 2006;4:445–457. doi: 10.1038/nrmicro1425. [DOI] [PubMed] [Google Scholar]
- 69.Berube BJ, Bubeck Wardenburg J. Staphylococcus Aureus Alpha-Toxin: Nearly a Century of Intrigue. Toxins (Basel) 2013;5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu CM, Fang RH, Copp J, Luk BT, Zhang L. A Biomimetic Nanosponge That Absorbs Pore-Forming Toxins. Nat Nanotechnol. 2013;8:336–340. doi: 10.1038/nnano.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu ZG, Li TL, Gao W, Xu TL, Jurado-Sanchez B, Li JX, Gao WW, He Q, Zhang LF, Wang J. Cell-Membrane-Coated Synthetic Nanomotors for Effective Biodetoxification. Adv Funct Mater. 2015;25:3881–3887. [Google Scholar]
- 72.Wu ZG, Li JX, de Avila BEF, Li TL, Gao WW, He Q, Zhang LF, Wang J. Water-Powered Cell-Mimicking Janus Micromotor. Adv Funct Mater. 2015;25:7497–7501. [Google Scholar]
- 73.Wang F, Gao WW, Thamphiwatana S, Luk BT, Angsantikul P, Zhang QZ, Hu CMJ, Fang RH, Copp JA, Pornpattananangkul D, et al. Hydrogel Retaining Toxin-Absorbing Nanosponges for Local Treatment of Methicillin-Resistant Staphylococcus Aureus Infection. Adv Mater. 2015;27:3437–3443. doi: 10.1002/adma.201501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li LL, Xu JH, Qi GB, Zhao XZ, Yu FQ, Wang H. Core-Shell Supramolecular Gelatin Nanoparticles for Adaptive and “On-Demand” Antibiotic Delivery. ACS Nano. 2014;8:4975–4983. doi: 10.1021/nn501040h. [DOI] [PubMed] [Google Scholar]
- 75.Eddleston M, Buckley NA, Eyer P, Dawson AH. Management of Acute Organophosphorus Pesticide Poisoning. Lancet. 2008;371:597–607. doi: 10.1016/S0140-6736(07)61202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eggert M, Zettl UK, Neeck G. Autoantibodies in Autoimmune Diseases. Curr Pharm Des. 2010;16:1634–1643. doi: 10.2174/138161210791164144. [DOI] [PubMed] [Google Scholar]
- 77.Tabas I, Glass CK. Anti-Inflammatory Therapy in Chronic Disease: Challenges and Opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valent P, Lechner K. Diagnosis and Treatment of Autoimmune Haemolytic Anaemias in Adults: A Clinical Review. Wien Klin Wochenschr. 2008;120:136–151. doi: 10.1007/s00508-008-0945-1. [DOI] [PubMed] [Google Scholar]
- 79.Copp JA, Fang RH, Luk BT, Hu CM, Gao W, Zhang K, Zhang L. Clearance of Pathological Antibodies Using Biomimetic Nanoparticles. Proc Natl Acad Sci USA. 2014;111:13481–13486. doi: 10.1073/pnas.1412420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu CM, Fang RH, Luk BT, Zhang L. Nanoparticle-Detained Toxins for Safe and Effective Vaccination. Nat Nanotechnol. 2013;8:933–938. doi: 10.1038/nnano.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang F, Fang RH, Luk BT, Hu CJ, Thamphiwatana S, Dehaini D, Angsantikul P, Kroll AV, Pang Z, Gao W, et al. Nanoparticle-Based Antivirulence Vaccine for the Management of Methicillin-Resistant Staphylococcus Aureus Skin Infection. Adv Funct Mater. 2016;26:1628–1635. doi: 10.1002/adfm.201505231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao W, Fang RH, Thamphiwatana S, Luk BT, Li J, Angsantikul P, Zhang Q, Hu CM, Zhang L. Modulating Antibacterial Immunity Via Bacterial Membrane-Coated Nanoparticles. Nano Lett. 2015;15:1403–1409. doi: 10.1021/nl504798g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY. Therapeutic Cancer Vaccines: Past, Present, and Future. Adv Cancer Res. 2013;119:421–475. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fang RH, Kroll AV, Zhang L. Nanoparticle-Based Manipulation of Antigen-Presenting Cells for Cancer Immunotherapy. Small. 2015;11:5483–5496. doi: 10.1002/smll.201501284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lokhov PG, Balashova EE. Cellular Cancer Vaccines: An Update on the Development of Vaccines Generated from Cell Surface Antigens. J Cancer. 2010;1:230–241. doi: 10.7150/jca.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ. Vaccines for Established Cancer: Overcoming the Challenges Posed by Immune Evasion. Nat Rev Cancer. 2016;16:219–233. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- 87.Delcayre A, Le Pecq JB. Exosomes as Novel Therapeutic Nanodevices. Curr Opin Mol Ther. 2006;8:31–38. [PubMed] [Google Scholar]
- 88.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fang RH, Hu CM, Chen KN, Luk BT, Carpenter CW, Gao W, Li S, Zhang DE, Lu W, Zhang L. Lipid-Insertion Enables Targeting Functionalization of Erythrocyte Membrane-Cloaked Nanoparticles. Nanoscale. 2013;5:8884–8888. doi: 10.1039/c3nr03064d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou H, Fan Z, Lemons PK, Cheng H. A Facile Approach to Functionalize Cell Membrane-Coated Nanoparticles. Theranostics. 2016;6:1012–1022. doi: 10.7150/thno.15095. [DOI] [PMC free article] [PubMed] [Google Scholar]


