Abstract
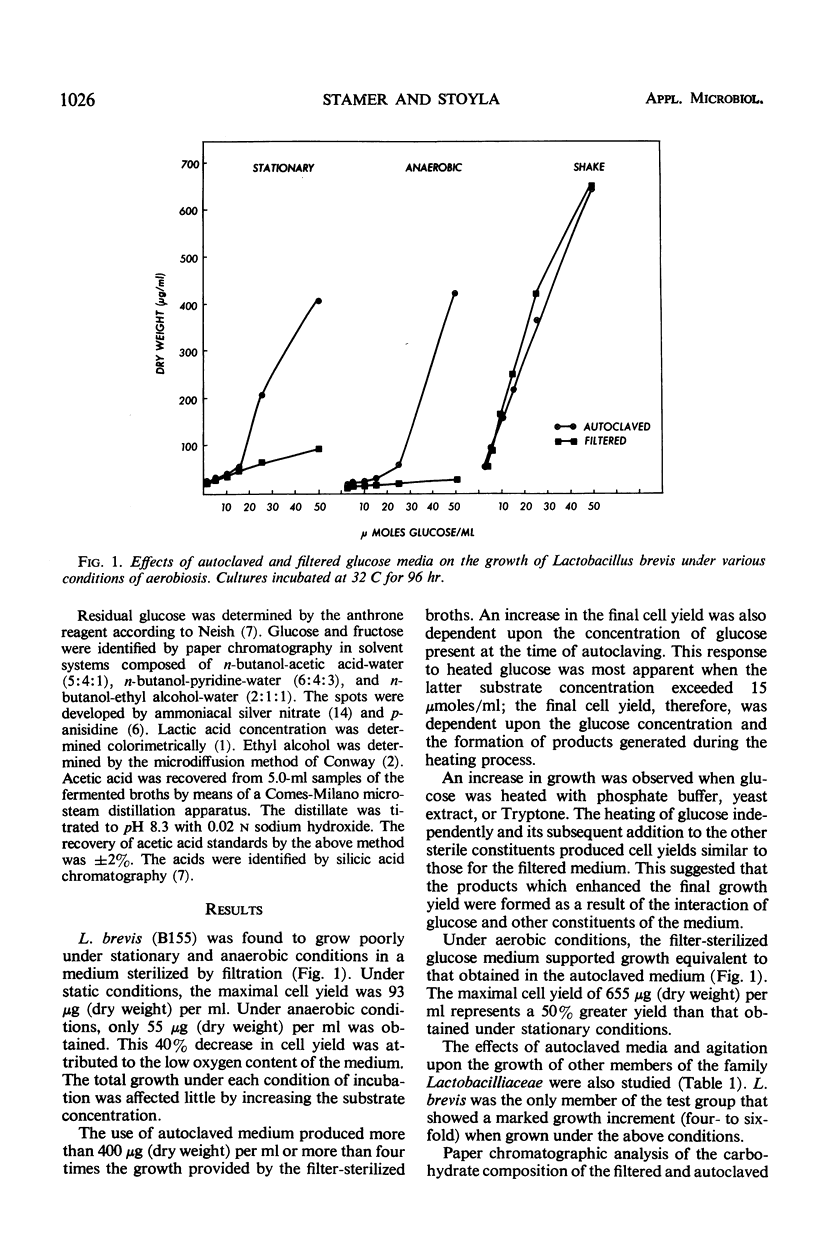
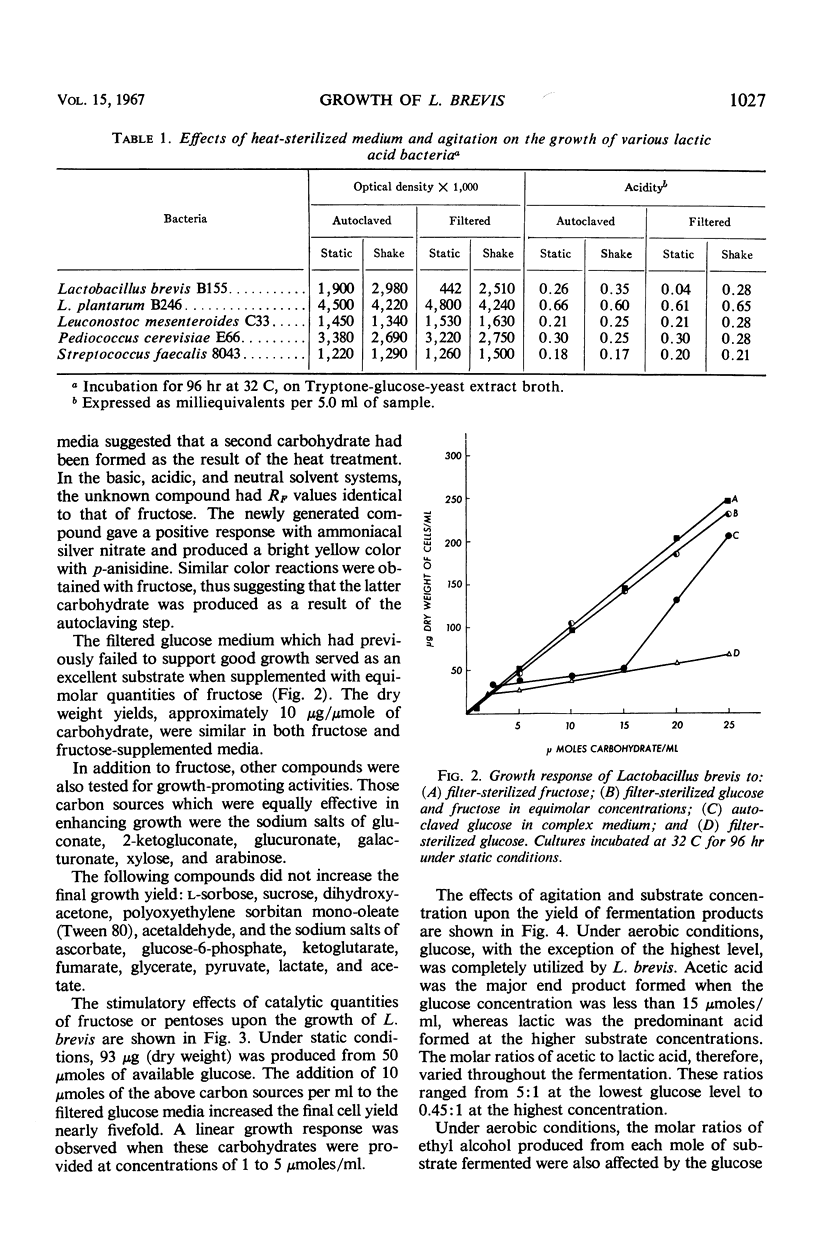
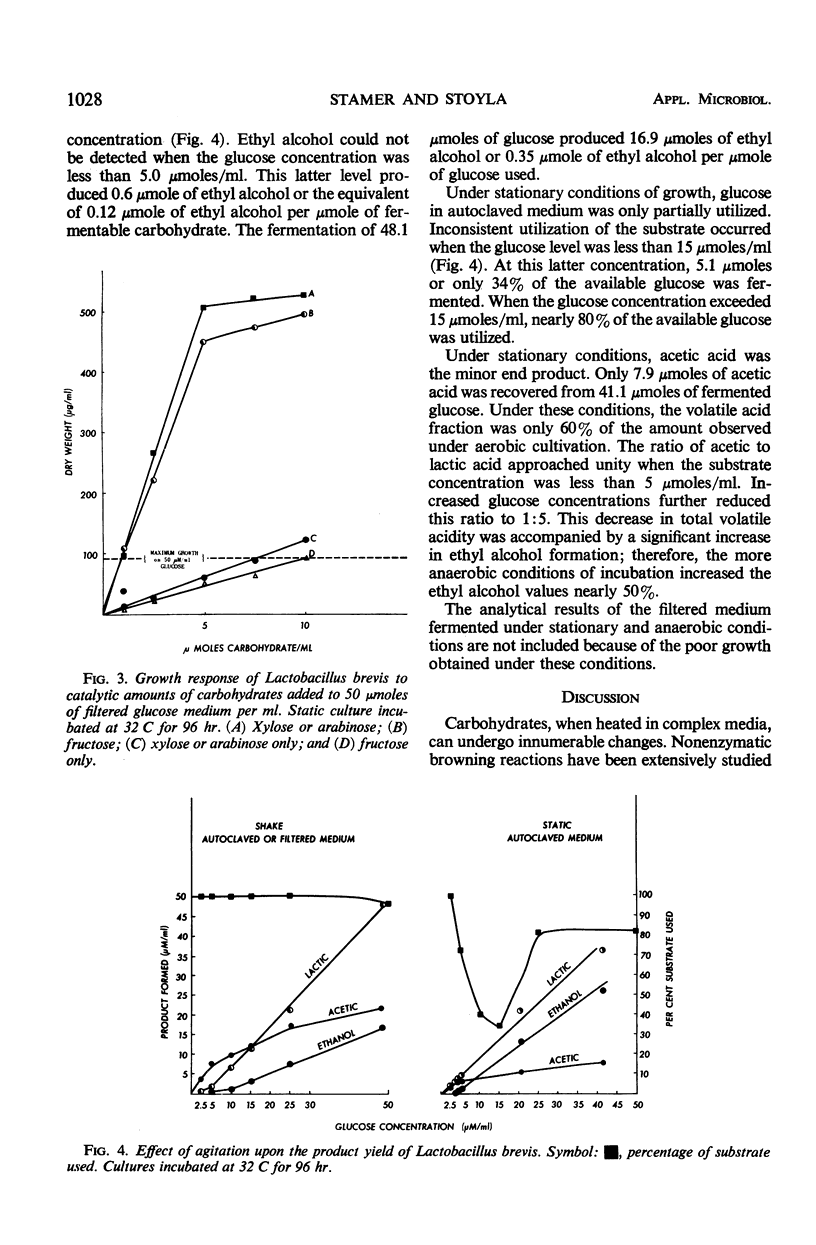
Under stationary and anaerobic conditions, greater cell yields of Lactobacillus brevis were obtained from autoclaved than from filter-sterilized glucose media. Fructose, tentatively identified as a product generated by the heating process, served as an excellent catalyst for inducing growth. The addition of micromolar quantities of pentoses or potential pentose precursors to the filter-sterilized medium was equally effective in stimulating growth. These organic catalysts were not essential for growth under aerobic conditions. Upon agitation, similar cell yields were obtained from the autoclaved and filter-sterilized media. The micromolar quantities of lactic acid produced per micromole of carbohydrate fermented appeared to be similar under aerobic and static conditions of incubation. The final concentration of acetic acid increased as the result of agitation. This increase in volatile acidity was accompanied by a significant decrease in ethyl alcohol production. The cell yield was increased nearly 50% under aerobic conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ELTZ R. W., VANDEMARK P. J. Fructose dissimilation of Lactobacillus brevis. J Bacteriol. 1960 Jun;79:763–776. doi: 10.1128/jb.79.6.763-776.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANKFORD C. E., RAMSEY H. H. Stimulation of growth initiation by heat degradation products of glucose. J Bacteriol. 1956 Oct;72(4):511–518. doi: 10.1128/jb.72.4.511-518.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDS E. L. Non-enzymic browning; the reaction between D-glucose and glycine in the dry state. Biochem J. 1956 Dec;64(4):639–644. doi: 10.1042/bj0640639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS D., KING T. E., CHELDELIN V. H. Growth stimulation of Lactobacillus gayoni by N-D-glucosylglycine. Proc Soc Exp Biol Med. 1953 Jan;82(1):140–144. doi: 10.3181/00379727-82-20046. [DOI] [PubMed] [Google Scholar]
- SNELL N., LEWIS J. C. Nutritional studies with Lactobacillus fermenti. J Bacteriol. 1953 Jun;65(6):671–677. doi: 10.1128/jb.65.6.671-677.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARR M. P., DE LEY J., KILGORE W. W. Catabolism of hexuronic acids by Erwinia and Aerobacter. Science. 1957 May 10;125(3254):929–929. doi: 10.1126/science.125.3254.929. [DOI] [PubMed] [Google Scholar]
- Smiley K. L., Niven C. F., Sherman J. M. The Nutrition of Streptococcus salivarius. J Bacteriol. 1943 May;45(5):445–454. doi: 10.1128/jb.45.5.445-454.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- WHITTENBURY R. THE USE OF SOFT AGAR IN THE STUDY OF CONDITIONS AFFECTING THE UTILIZATION OF FERMENTABLE SUBSTRATES BY LACTIC ACID BACTERIA. J Gen Microbiol. 1963 Sep;32:375–384. doi: 10.1099/00221287-32-3-375. [DOI] [PubMed] [Google Scholar]


