Abstract
CYP11B1 and CYP11B2 play pivotal roles in adrenocorticosteroids synthesis. We performed semi-quantitative immunohistochemical analysis of these proteins in adrenals from patients with primary aldosteronism using novel monoclonal antibodies. Clusters of cortical cells positive for CYP11B2 were detected in the zona glomerulosa (ZG) of normal adrenal gland (NA), idiopathic hyperaldosteronism (IHA) and the adjacent adrenal of aldosterone-producing adenoma (APA). In APA, heterogenous immunolocalization of CYP11B2 and diffuse immunoreactivity of CYP11B1 were detected in tumor cells, respectively. The relative immunoreactivity of CYP11B2 in the ZG of adjacent adrenal of APA was significantly lower than that of NA, IHA and APA tumor cells, suggestive of suppressed aldosterone biosynthesis in these cells. These findings did indicate the regulatory mechanisms of aldosterone biosynthesis were different between normal/hyperplastic and neoplastic aldosterone-producing cells in human adrenals. CYP11B2 immunoreactivity in the ZG could also serve as a potential immunohistochemical marker differentiating morphologically hyperplastic ZG of IHA and APA adjacent adrenal.
Keywords: CYP11B1, CYP11B2, Adrenal cortex, APA, IHA, Monoclonal antibody
1. Introduction
Increasing numbers of patients with primary aldosteronism (PA) have been recently identified using advanced diagnostic techniques, and 6–10% of all hypertensive patients are currently considered to harbor PA (Funder et al., 2008). PA patients have an increased risk of developing cardiovascular events compared to matched patients with essential hypertension (Milliez et al., 2005). There are two main causes of PA: aldosterone-producing adenomas (APA) in 30–60% of patients and idiopathic hyperaldosteronism (IHA) in 30–70% of patients (Young, 2007). It has become pivotal to examine the regulation of aldosterone mechanism in these adrenal tissues because of different clinical approaches in these two conditions (Young, 2007).
The final steps in glucocorticoids and mineralocorticoids biosynthesis are catalyzed by two closely related mitochondrial enzymes: aldosterone synthase (CYP11B2) and 11β-hydroxylase (CYP11B1) (Domalik et al., 1991; Fardella et al., 1996; Pascoe et al., 1992). In the normal adrenal gland (NA), CYP11B1 is the classical 11β-hydroxylase which converts 11-deoxycortisol to cortisol and deoxycorticosterone to corticosterone and is expressed only in the zona fasciculata (ZF) and zona reticularis (ZR) (Nishimoto et al., 2010). CYP11B2 is present only in the zona glomerulosa (ZG), where its 11β-hydroxylase, 18-hydroxylase and 18-methyl oxidase activities are required to convert deoxycorticosterone to aldosterone (Ogishima et al., 1991; Curnow et al., 1991; Kawamoto et al., 1992). Although CYP11B2 expression appears to be high in IHA and APA (Takeda et al., 1999; Miyamori et al., 2000; Fallo et al., 2002; Bassett et al., 2005), only descriptive low resolution studies using polyclonal antibodies have been published (Nishimoto et al., 2010; Nanba et al., 2013).
We recently developed monoclonal antibodies against these proteins and reported their expression in human normal adrenal gland (Gomez-Sanchez et al., 2014). In this study, we used these novel rat anti-human CYP11B1 and mouse anti-human CYP11B2 monoclonal antibodies to quantify the expression of these steroidogenic enzymes in adrenals associated with PA.
2. Materials and methods
2.1. Human adrenals
Fifty cases of adrenocortical surgical pathology materials (10 NA, 9 IHA and 31 APA) were retrieved from surgical pathology files of Tohoku University Hospital (Sendai, Japan). The specimens had been fixed in 10% formalin and embedded in paraffin. The research protocol was approved by the ethics committee at Tohoku University Graduate School of Medicine (Sendai, Japan) (No. 2011-544).
2.2. Antibodies
Mouse monoclonal antibody for CYP11B2 and rat monoclonal antibody for CYP11B1 were recently developed in the laboratory of Dr. Gomez Sanchez (University of Mississippi Medical Center, Jackson, MS, USA) (Gomez-Sanchez et al., 2014). Rabbit polyclonal antibody against 3β-hydroxysteroid dehydrogenase/Δ5−Δ4 isomerase (HSD3B) was kindly provided by Dr. Mason (University of Edinburgh, Edinburgh, UK) (Suzuki et al., 2000). Rabbit polyclonal antibody against 17α-hydroxylase (CYP17) was previously described (Sakuma et al., 2013).
2.3. Immunofluorescence analysis
The slides were thoroughly de-paraffinized and placed in Trilogy™ solution (Cell Marque Corporation, Rocklin, CA, USA) in a steamer for 45 min. Nonspecific staining was blocked with Tris 0.1 M pH 7.4 with 5% goat serum and 0.5% SDS for 1 h. The slides were then incubated with mixture of mouse monoclonal anti-hCYP11B2-41-17B (1:100), rat monoclonal anti-hCYP11B1-80-2-2 (1:10) and rabbit polyclonal anti CYP17 antibody (1:600) overnight in Tris 0.1 M, 5% goat serum and 0.1% tween-20. Samples were thin incubated with second antibody: goat anti-mouse IgG-Alexa 488, goat anti-rat IgG Alexa 594 and goat anti-rabbit IgG Alexa 647 (Jackson Immunoresearch Inc. Allentown, PA, USA) at 1:500 each for an hour using the same buffer as above. Coverslips were mounted using Vector Laboratories Vectashield mounting media with DAPI (Vector Labs, Burlingame, CA, USA).
2.4. Immunohistochemical staining analysis
For CYP11B1 and CYP11B2, immunostaining was performed using the ImmPRESS REAGENT (VECTOR, Burlingame, CA, USA). Antigen retrieval was performed by heating the glue-coated slides in an autoclave for 5 min in EDTA buffer (pH 9.0). Blocking was performed for 1 h using Blocking buffer (normal horse serum 2.5% with SDS 0.5% for CYP11B2 and normal goat serum 2.5% with SDS 0.5% for CYP11B1) at room temperature. For HSD3B, immunohistochemical analysis was performed employing the streptavidin–biotin amplification method using a Histofine Kit (Nichirei, Tokyo, Japan) without antigen retrieval. The dilutions of the primary antibodies used in this study were summarized as follows: 1:750 (CYP11B2), 1:200 (CYP11B1) and 1:2500 (HSD3B), respectively. The antigen–antibody complex was visualized with 3,3′-diaminobenzidine solution [1 mM 3,3′-diaminobenzidine, 50 mM Tris–HCl buffer (pH 7.6), and 0.006% H2O2] and counterstained with hematoxylin.
2.5. Immunohistochemical evaluation and statistical analysis
Five hundred parenchymal cells were evaluated in each corresponding region and the ratio of positive cells was subsequently obtained in relevant areas of the specimens, i.e., the ZG, ZF and ZR of NA, the ZG of IHA, the ZG of adjacent adrenal gland of APA and tumor lesion of APA.
Immunoreactivity was semi-quantitatively assessed using McCarty’s H-scoring system, in which the percentage of stained cells is multiplied by a number, 0–3, reflecting the intensity of their immunopositivities (McCarty et al., 1985). The relative immunointensity of specific immunoreactivity was characterized as not present (0), weak but detectable above control (1+), distinct (2+), and very strong (3+) (Budwit-Novotny et al., 1986).
Statistical analysis was performed using R software version 3.0.2. and data from the quantification of CYP11B2 and HSD3B protein levels were evaluated in groups of 4 (NA, IHA, APA-adjacent and APA-tumor) using Mann–Whitney multiple comparison tests with significance level set to α = 0.05; Bonferroni inequality was used to correct multiple comparison, with 0.05/6 = 0.0083, which determined P < 0.0083 as the statistically significant value, as previously described by our group (Felizola et al., 2014a,b).
3. Results
3.1. Localization of CYP11B2 and CYP11B1 in immunofluorescence analysis
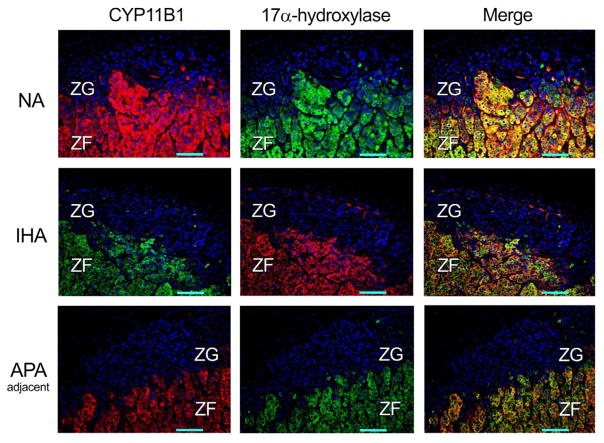
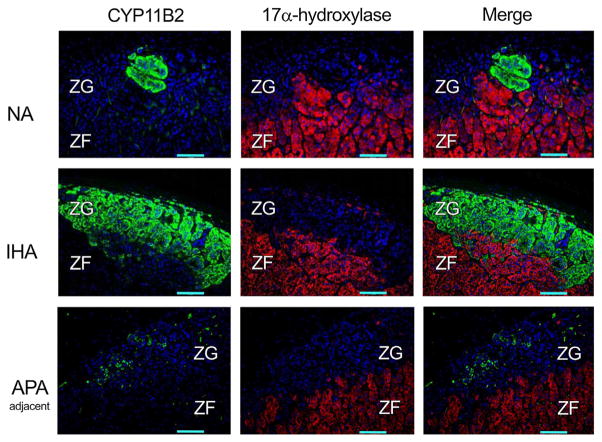
In NA, IHA and adjacent adrenal of APA cases, CYP11B1 immunoreactivity was diffusely detected in the ZF and ZR (data not shown) but not in the ZG, compatible with our recent report (Gomez-Sanchez et al., 2014) (Fig. 1). The clusters of cortical cells positive for CYP11B2 were sporadically detected in the ZG of NA, IHA and adjacent adrenal of APA, where CYP17 and CYP11B1 were absent (Gomez-Sanchez et al., 2014) (Fig. 2). CYP11B2 immunoreactivity was relatively abundant in the ZG of IHA, while barely detectable in the histologically hyperplastic ZG of the adjacent adrenal of APA (Fig. 2).
Fig. 1.
Immunofluorescence findings of CYP11B1 and 17α-hydroxilase (CYP17) in non-pathological adrenals (NA), idiopathic hyperaldosteronism (IHA), and the adrenal cortex adjacent to aldosterone-producing adrenocortical adenoma (APA-adjacent). CYP11B1 and CYP17 immunofluorescence were diffusely detected in the zona fasciculata (ZF), but was virtually absent in the zona glomerulosa (ZG). Images on the right represent the merged photographs of CYP11B1 or CYP11B2 and CYP17. The cases illustrated in this figure are: NA 05, IHA 01, APA 08 (adjacent) as listed in Table 1.
Fig. 2.
Immunofluorescence findings of aldosterone synthase (CYP11B2), and 17α-hydroxilase (CYP17) in non-pathological adrenals (NA), idiopathic hyperaldosteronism (IHA), and the adrenal cortex adjacent to aldosterone-producing adrenocortical adenoma (APA-adjacent). CYP11B2 was detected in clusters of the zona glomerulosa (ZG) cells of NA and IHA, but was scantly detected or absent in the ZG of APA-adjacent adrenal cortex. CYP17 immunofluorescence was diffusely detected in the zona fasciculata (ZF), but virtually absent in the ZG. Images on the right represent the merged photographs of CYP11B2 and CYP17. The tissue samples illustrated in this figure are: NA 05, IHA 01, APA 08 (adjacent) as listed in Table 1.
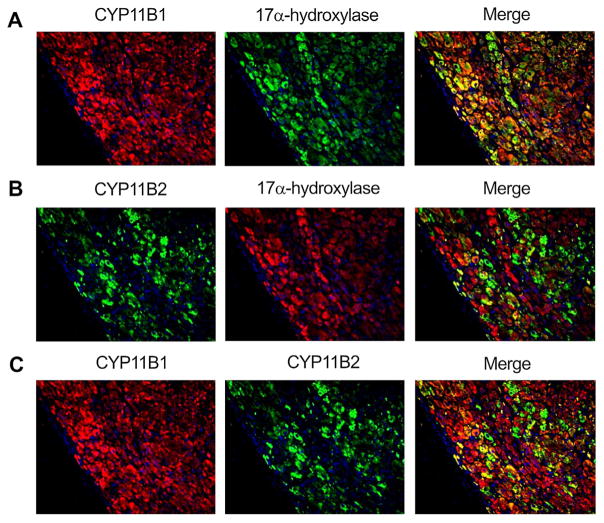
In APA, CYP11B1 immunoreactivity was diffusely detected, while CYP11B2 immunoreactivity was heterogeneously present (Fig. 3A and B). In these areas, CYP11B1 and CYP17 were colocalized in the great majority of the tumor cells of APA, while CYP11B2 immunoreactivity focally present in the tumor cells double positive for CYP11B1 and CYP17 (Fig. 3A–C).
Fig. 3.
Immunofluorescence of CYP11B2, CYP11B1 and CYP17 in the aldosterone-producing adenoma (APA): [A] CYP11B1 and CYP17 were diffusely detected in the tumoral cells of APA. The merged photograph of CYP11B1 and CYP17 is presented on the right. [B] CYP11B2 was detected in patches of tumoral cells in a diffuse manner along-with non-fluorescent CYP11B2 negative cells in-between. CYP17 showed the same pattern described in [A]. The merged photograph of CYP11B2 and CYP17 is presented on the right. [C] The merged photograph of CYP11B1 and CYP11B2 is represented on the right. The tissue sample illustrated in this figure is: APA 08 (tumor), as listed in Table 1.
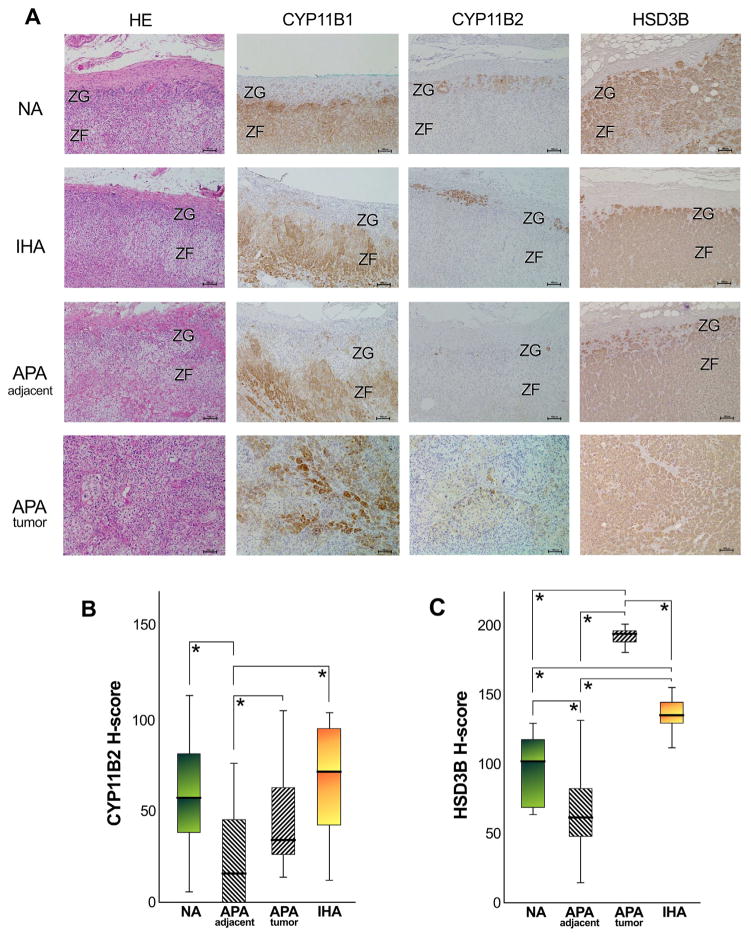
3.2. Immunohistochemical evaluation of adrenocortical tissues using H-scoring system
The representative immunohistochemical finding of NA, IHA, adjacent adrenal gland of APA and APA tumorous lesion were illustrated in Fig. 4A. Results were consistent with those in the immunofluorescence analysis. Semi-quantitative results using H-scoring system were summarized in Fig. 4B, C and Table 1. The range of relative levels for CYP11B2 and HSD3B immunoreactivity was identified in the ZG of NA, IHA and adjacent adrenal of APA, respectively (Table 1). In tumor cells of APA cases, a wide range of relative levels for CYP11B2 immunoreactivity was also detected, and H-score tended to be lower compared to that of CYP11B1 (Table 1). H-score of CYP11B2 in the hyperplastic ZG of adjacent adrenal of APA (H-score 24.5 ± 4.9 SE) was significantly lower than that of NA (H-score 58.6 ± 9.5 SE), IHA (H-score 68.4 ± 10.4 SE) and tumor cells of APA (H-score 44.0 ± 5.1 SE), respectively (Fig. 4B). The status of HSD3B immunoreactivity was 97.8 ± 8.0 (SE) in the ZG of NA, 136.3 ± 4.1(SE) in the hyperplastic ZG of IHA, 67.8 ± 4.7 (SE) in that of adjacent adrenal of APA and 190.3 ± 1.8 (SE) in the tumor lesion of APA, respectively (Fig. 4C). There was a significant difference of HSD3B immunoreactivity between these two groups (Fig. 4C). There were no significant correlations between the patterns of the immunoreactivity of CYP11B1, CYP11B2 and HSD3B versus sex, age or tumor size, plasma aldosterone levels or plasma renin activity (PRA) in the patients with APA examined in this study (data not shown).
Fig. 4.
Immunohistochemistry of CYP11B1, CYP11B2 and HSD3B in non-pathological adrenals (NA), in idiopathic hyperaldosteronism (IHA), in the adrenal cortex adjacent to aldosterone-producing adrenocortical adenoma (APA-adjacent), and in the tumor lesion of APA (APA-tumor). [A] CYP11B1 was diffusely detected in the zona fasciculata (ZF) of NA, IHA, and APA-adjacent adrenal cortex, but was virtually absent in the zona glomerulosa (ZG); in addition, it was detected in patches of tumor cells of APA in a diffuse manner. CYP11B2 was detected in patches of ZG cells of NA and IHA, but was scantly detected or absent in the ZG of APA-adjacent adrenal cortex; the immunostaining of CYP11B2 was detected in islets or patches of cells surrounded by large areas of non-immunostained negative cells. Marked HSD3B immunoreactivity was detected in the ZG of NA and IHA, with a diffuse HSD3B immunostaining detected in the ZF of NA, IHA; however, its immunoreactivity was weaker in the APA-adjacent ZG. Marked HSD3B immunoreactivity was also detected in the APA-tumor cases. [B] The quantitative analysis of CYP11B2 immunoreactivity using H-score demonstrated that its levels were significantly lower in APA-adjacent adrenocortical samples, compared to NA (*P = 0.0020), to IHA (*P = 0.0008) and APA-tumor samples (*P = 0.0011). [C] The quantitative analysis of HSD3B immunoreactivity demonstrated that its levels were significantly lower in APA-adjacent adrenocortical samples, compared to NA (*P = 0.0034), to IHA (*P < 0.0001) and APA-tumor samples (*P < 0.0001); in addition, the HSD3B immunoreactivity was significant lower in NA compared to APA (*P < 0.0001) and IHA (*P = 0.0017), and significantly higher in APA compared to IHA (*P < 0.0001). The tissue samples illustrated in this figure are: NA 04, IHA 04, APA 16 (adjacent) and APA 07 (tumor) as listed in Table 1.
Table 1.
H-score Evaluation of CYP11B1 and CYP11B2 in NA, IHA and APA.
| Case No. | Age | Sex | Clinical diagnosis | Tumor
|
Adrenal cortex
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (mm) | CYP11B1 | CYP11B2 | HSD3B | ZG*
|
ZF
|
ZR
|
||||||||
| CYP11B1 | CYP11B2 | HSD3B | CYP11B1 | CYP11B2 | CYP11B1 | CYP11B2 | ||||||||
| NA 01 | 74 | m | PC | 0.0 | 50.6 | 121.4 | 178.9 | 0.0 | 153.9 | 0.0 | ||||
| NA 02 | 76 | m | RCC | 0.0 | 38.3 | 115.9 | 185.6 | 0.0 | 154.7 | 0.0 | ||||
| NA 03 | 60 | m | RCC | 0.0 | 5.0 | 65.2 | 181.8 | 0.0 | 167.9 | 0.0 | ||||
| NA 04 | 82 | m | RCC | 0.0 | 64.4 | 70.5 | 185.8 | 0.0 | 135.7 | 0.0 | ||||
| NA 05 | 52 | f | RCC | 0.0 | 47.1 | 87.2 | 181.0 | 0.0 | 199.9 | 0.0 | ||||
| NA 06 | 61 | m | PC | 0.0 | 113.9 | 89.3 | 169.4 | 0.0 | 161.0 | 0.0 | ||||
| NA 07 | 71 | f | RCC | 0.0 | 65.5 | 117.3 | 187.1 | 0.0 | 207.7 | 0.0 | ||||
| NA 08 | 71 | m | RCC | 0.0 | 83.2 | 129.9 | 204.3 | 0.0 | 160.6 | 0.0 | ||||
| NA 09 | 75 | m | PC | 0.0 | 37.2 | 116.8 | 189.6 | 0.0 | 192.5 | 0.0 | ||||
| NA 10 | 39 | m | RCC | 0.0 | 80.9 | 64.4 | 186.1 | 0.0 | 167.2 | 0.0 | ||||
| IHA 01 | 30 | m | PA | 0.0 | 51.2 | 128.1 | ||||||||
| IHA 02 | 40 | m | PA | 0.0 | 34.2 | 112.0 | ||||||||
| IHA 03 | 43 | f | PA | 0.0 | 62.7 | 145.1 | ||||||||
| IHA 04 | 32 | f | PA | 0.0 | 97.0 | 141.9 | ||||||||
| IHA 05 | 36 | f | PA | 0.0 | 12.3 | 135.2 | ||||||||
| IHA 06 | 25 | m | PA | 0.0 | 72.5 | 144.0 | ||||||||
| IHA 07 | 44 | m | PA | 0.0 | 84.5 | 155.5 | ||||||||
| IHA 08 | 43 | f | PA | 0.0 | 104.9 | 131.0 | ||||||||
| IHA 09 | 44 | f | PA | 0.0 | 96.0 | 134.0 | ||||||||
| APA 01 | 46 | m | PA | 19.0 | 104.0 | 18.4 | 180.4 | 0.0 | 1.0 | 14.1 | ||||
| APA 02 | 51 | f | PA | 15.0 | 140.0 | 18.9 | 193.6 | 0.0 | 1.1 | 42.6 | ||||
| APA 03 | 62 | m | PA | 12.0 | 47.9 | 32.8 | 190.2 | 0.0 | 0.0 | 121.2 | ||||
| APA 04 | 43 | f | PA | 18.0 | 182.7 | 32.2 | 190.1 | 0.0 | 15.5 | 73.2 | ||||
| APA 05 | 53 | f | PA | 17.0 | 148.2 | 13.5 | 194.3 | 0.0 | 4.9 | 47.6 | ||||
| APA 06 | 51 | f | PA | 20.0 | 0.0 | 43.0 | 200.4 | 0.0 | 45.5 | 115.3 | ||||
| APA 07 | 54 | f | PA | 13.0 | 61.2 | 34.3 | 191.9 | 0.0 | 34.1 | 60.7 | ||||
| APA 08 | 28 | f | PA | 18.0 | 141.4 | 32.0 | 180.1 | 0.0 | 20.5 | 47.3 | ||||
| APA 09 | 52 | f | PA | 37.0 | 188.8 | 32.2 | 164.7 | 0.0 | 60.8 | 76.6 | ||||
| APA 10 | 35 | m | PA | 16.0 | 81.2 | 52.1 | 195.7 | 0.0 | 7.6 | 92.1 | ||||
| APA 11 | 38 | m | PA | 16.0 | 167.0 | 82.4 | 196.3 | 0.0 | 0.0 | 63.3 | ||||
| APA 12 | 37 | m | PA | 12.0 | 1.8 | 26.1 | 192.1 | 0.0 | 8.1 | 73.4 | ||||
| APA 13 | 46 | m | PA | 27.0 | 198.4 | 21.7 | 154.3 | 0.0 | 0.0 | 58.2 | ||||
| APA 14 | 51 | m | PA | 19.0 | 100.3 | 36.7 | 193.5 | 0.0 | 0.0 | 61.2 | ||||
| APA 15 | 50 | m | PA | 16.0 | 78.8 | 106.0 | 193.7 | 0.0 | 5.0 | 27.8 | ||||
| APA 16 | 53 | m | PA | 6.0 | 134.2 | 135.5 | 195.5 | 0.0 | 37.5 | 83.8 | ||||
| APA 17 | 38 | f | PA | 27.0 | 56.2 | 32.1 | 196.2 | 0.0 | 16.0 | 57.5 | ||||
| APA 18 | 36 | f | PA | 17.0 | 122.6 | 71.5 | 197.2 | 0.0 | 76.9 | 62.2 | ||||
| APA 19 | 51 | m | PA | 18.0 | 187.1 | 65.1 | 193.2 | 0.0 | 0.0 | 41.5 | ||||
| APA 20 | 57 | f | PA | 15.0 | 181.1 | 63.3 | 195.9 | 0.0 | 30.7 | 58.6 | ||||
| APA 21 | 44 | m | PA | 28.0 | 174.0 | 56.9 | 184.8 | 0.0 | 0.0 | 40.8 | ||||
| APA 22 | 53 | m | PA | 18.0 | 36.8 | 26.8 | 191.7 | 0.0 | 61.3 | 131.3 | ||||
| APA 23 | 48 | f | PA | 17.0 | 45.4 | 24.9 | 185.8 | 0.0 | 58.6 | 82.0 | ||||
| APA 24 | 51 | m | PA | 17.0 | 53.1 | 23.1 | 195.2 | 0.0 | 0.8 | 100.6 | ||||
| APA 25 | 51 | m | PA | 31.0 | 86.2 | 42.2 | 187.2 | 0.0 | 0.0 | 68.5 | ||||
| APA 26 | 62 | m | PA | 8.0 | 20.6 | 24.1 | 198.3 | 0.0 | 0.0 | 54.7 | ||||
| APA 27 | 59 | m | PA | 10.0 | 66.7 | 52.5 | 192.9 | 0.0 | 54.8 | 49.4 | ||||
| APA 28 | 46 | f | PA | 14.0 | 23.1 | 53.7 | 196.3 | 0.0 | 59.4 | 91.3 | ||||
| APA 29 | 56 | f | PA | 5.0 | 159.4 | 93.7 | 191.3 | 0.0 | 31.2 | 36.4 | ||||
| APA 30 | 59 | m | PA | 17.0 | 171.6 | 33.9 | 185.0 | 0.0 | 74.6 | 74.1 | ||||
| APA 31 | 67 | f | PA | 17.0 | 0.0 | 104.7 | 194.2 | 0.0 | 31.0 | 40.1 | ||||
NA, normal adrenal gland; IHA, idiopathic hyperaldosteronism; APA, aldosterone-producing adenoma; ZG, zona gromerulosa; ZF, zona fasciculata; ZR, zona reticularis; m, male; f, female; PA, primary aldosteronism; PC, pancreas carcinoma; RCC, renal cell carcinoma.
ZG of APA was the adjacent adrenal tissue of APA.
4. Discussion
The results of our present study clearly demonstrate the presence of cortical cells immunohistochemically positive for CYP11B2 in the ZG of NA, IHA and adjacent adrenal of APA as well as tumor lesion of APA. IHA, one of the important etiologies of PA, is morphologically characterized by the hyperplasia of the ZG with or without concomitant cortical nodules. The expression of HSD3B in the ZG of IHA has been reported to be markedly elevated compared to the ZG of NA, compatible with the results of our present study (Sasano, 1994). However, results of our immunohistochemical analysis demonstrated that the relative levels of CYP11B2 immunoreactivity were not significantly different between the ZG of NA and IHA. These results also did indicated that elevated levels of CYP11B2 in the ZG of IHA may not necessarily represent the presence of aldosterone overproduction in IHA cases. Therefore, the overexpression of the upstream enzymes in the steroid synthesis cascade, such as HSD3B, may be reasonably suggested to play more important roles in the pathophysiology of IHA, but it awaits further investigations for clarification.
CYP11B2 immunoreactive cells have been reported in the ZG of adjacent adrenal of APA in immunohistochemical studies using polyclonal antibodies (Nishimoto et al., 2010; Nanba et al., 2013). Results of our present study demonstrated the relative levels of CYP11B2 immunoreactivity in the ZG of adjacent adrenal of APA were significantly lower than that of NA and IHA. As demonstrated in our results, HSD3B immunopositivity is weak in the ZG of attached adrenals of APA. Despite the histopathological hyperplasia observed in these tissues (Sasano, 1994). Wang et al. previously demonstrated the levels of CYP11B2 mRNA in the adjacent adrenal of APA was markedly lower than that in APA tumor using microarray analysis (Wang et al., 2011). We have also shown the relative CYP11B2 immunoreactivity in the ZG of adjacent adrenal of APA was significantly lower than that in tumor lesion of APA. These results also suggest that aldosterone production may be suppressed via decreased expression levels of steroidogenic enzymes including HSD3B and CYP11B2 in the ZG of adjacent adrenal in APA cases.
The levels of CYP11B2 mRNA in the whole tumor specimens have been reported to be consistently higher in APA than that in NA (Fallo et al., 2002; Bassett et al., 2005). In addition, results of CYP11B2 score adjusted for tumor volume was positively correlated with aldosterone synthesis (Nanba et al., 2013). In our study, CYP11B2 immunoreactivity was weakly and heterogeneously detected in most tumor lesions of APA, but the percentage and intensity of CYP11B2 immunoreactivity in APA obtained with the H-score was not necessarily significantly higher compared to those of the ZG in NA. Therefore, it is reasonable to suggest that the relative immunointensity and percentage of CYP11B2 immunoreactivity do not reflect overproduction of aldosterone in APA.
Results of our present study demonstrated that CYP11B1 was diffusely detected in the tumorous lesion of APA, compared to CYP11B2. Both CYP11B1 and CYP11B2 are well known to convert deoxycorticosterone to corticosterone and corticosterone to 18OH-corticosterone, respectively, but only CYP11B2 can convert 18OH-corticosterone to aldosterone (Curnow et al., 1991). Therefore, both CYP11B1 and CYP11B2 could play roles in the tumor of APA, while only CYP11B2 is the corresponding enzyme for synthesis of corticosterone, 18OH-corticosterone and aldosterone. The expression of CYP17 has been postulated to be low in the tumor of APA (Sasano, 1994). However, our results clearly demonstrated the focal but distinctive colocalization of CYP11B1/CYP17 or CYP11B2/CYP17 in the tumor lesion of APA. The colocalized CYP11B1 and CYP17 immunoreactivity had been previously reported in the same cells of APA (Nishimoto et al., 2010), but not CYP11B2 and CYP17. In addition, APA is generally postulated to be composed of heterogeneous or a mixture of the cortical cells from different adrenal zones, which could explain the expression patterns previously reported (Azizan et al., 2012; Neville and O’Hare, 1985). However, further studies are required to clarify the functional significance of the co-expression of CYP11B1/CYP11B2 and CYP11B2/CYP17 in the tumor lesion of APA.
5. Conclusion
The results of our present study demonstrate the status of CYP11B2 and CYP11B1 in various types of human adrenocortical tissues from the patients with PA, including APA and IHA. The newly developed CYP11B2 and CYP11B1 antibodies we used could provide new insights into the analysis of endocrine-pathological evaluation of various adrenals associated with primary aldosteronism.
Acknowledgments
The authors have no conflicts of interest to disclose.
Abbreviations
- CYP11B1
Cytochrome P450, family 11, subfamily B, polypeptide 1
- CYP11B2
Cytochrome P450, family 11, subfamily B, polypeptide 2
- CYP17
17α-hydroxylase
- NA
normal adrenal gland
- IHA
idiopathic hyperaldosteronism
- APA
aldosterone-producing adenoma
- ZG
zona glomerulosa
- ZF
zona fasciculate
- ZR
zona reticularis
- HSD3B
3 beta hydroxysteroid dehydrogenase
- PA
primary aldosteronism.
References
- Azizan EA, Lam BY, Newhouse SJ, Zhou J, Kuc RE, Clarke J, Happerfield L, Marker A, Hoffman GJ, Brown MJ. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J Clin Endocrinol Metab. 2012;97:E819–E829. doi: 10.1210/jc.2011-2965. [DOI] [PubMed] [Google Scholar]
- Bassett MH, Mayhew B, Rehman K, White PC, Mantero F, Arnaldi G, Stewart PM, Bujalska I, Rainey WE. Expression profiles for steroidogenic enzymes in adrenocortical disease. J Clin Endocrinol Metab. 2005;90:5446–5455. doi: 10.1210/jc.2005-0836. [DOI] [PubMed] [Google Scholar]
- Budwit-Novotny DA, McCarty KS, Cox EB, Soper JT, Mutch DG, Creasman WT, Flowers JL, McCarty KS., Jr Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46:5419–5425. [PubMed] [Google Scholar]
- Curnow KM, Tusie-Lunaf MT, Pascoe L, Natarajan R, Gu JL, Nadler JL, White PC. The product of the CYP11B2 gene is required for aldosterone biosynthesis in the human adrenal cortex. Mol Endocr. 1991;5:1513–1522. doi: 10.1210/mend-5-10-1513. [DOI] [PubMed] [Google Scholar]
- Domalik LJ, Chaplin DD, Kirkman MS, Wu RC, Liu W, Howard TA, Seldin MF, Parker KL. Different isozymes of mouse 11β-hydroxylase produce mineralocorticoids and glucocorticoids. Mol Endocrinol. 1991;5:1853–1861. doi: 10.1210/mend-5-12-1853. [DOI] [PubMed] [Google Scholar]
- Fallo F, Pezzi V, Barzon L, Mulatero P, Veglio F, Sonino N, Mathis JM. Quantitative assessment of CYP11B1 and CYP11B2 expression in aldosterone-producing adenomas. Eur J Endocrinol. 2002;147:795–802. doi: 10.1530/eje.0.1470795. [DOI] [PubMed] [Google Scholar]
- Fardella CE, Hum DW, Rodriguez H, Zhang G, Barry FL, Ilicki A, Bloch CA, Miller WL. Gene conversion in the CYP11B2 gene encoding P450c11AS is associated with, but does not cause, the syndrome of corticosterone methyloxidase II deficiency. J Clin Endocrinol Metab. 1996;81:321–326. doi: 10.1210/jcem.81.1.8550772. [DOI] [PubMed] [Google Scholar]
- Felizola SJA, Nakamura Y, Satoh F, Morimoto R, Kikuchi K, Nakamura T, Hozawa A, Wang L, Onodera Y, Ise K, McNamara KM, Midorikawa S, Suzuki S, Sasano H. Glutamate receptors and the regulation of steroidogenesis in the human adrenal gland: the metabotropic pathway. Mol Cell Endocrinol. 2014a;382:170–177. doi: 10.1016/j.mce.2013.09.025. [DOI] [PubMed] [Google Scholar]
- Felizola SJA, Nakamura Y, Ono Y, Kitamura K, Kikuchi K, Onodera Y, Ise K, Takase K, Sugawara A, Hattangady N, Rainey WE, Satoh F, Sasano H. PCP4: a regulator of aldosterone synthesis in human adrenocortical tissues. J Mol Endocrinol. 2014b;52:159–167. doi: 10.1530/JME-13-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF, Jr, Montori VM. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey WE, Satoh F, Maekawa T, Nakamura Y, Sasano H, Gomez-Sanchez EP. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383:111–117. doi: 10.1016/j.mce.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T, Mitsuuchi Y, Toda K, Yokoyama Y, Miyahara K, Miura S, Ohnishi T, Ichikawa Y, Nakao K, Imura H. Role of steroid 11, 6-hydroxylase and steroid 18-hydroxylase in the biosynthesis of glucocorticoids and mineralocorticoids in humans. Proc Natl Acad Sci USA. 1992;89:1458–1462. doi: 10.1073/pnas.89.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty KS, Jr, Miller LS, Cox EB, Konrath J, McCarty KS., Sr Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109:716–721. [PubMed] [Google Scholar]
- Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Miyamori I, Inaba S, Hatakeyama H, Taniguchi N, Takeda Y. Idiopathic hyperaldosteronism: analysis of aldosterone synthase gene. Biomed Pharmacother. 2000;54(Suppl 1):77–79. doi: 10.1016/s0753-3322(00)80017-7. [DOI] [PubMed] [Google Scholar]
- Nanba K, Tsuiki M, Sawai K, Mukai K, Nishimoto K, Usui T, Tagami T, Okuno H, Yamamoto T, Shimatsu A, Katabami T, Okumura A, Kawa G, Tanabe A, Naruse M. Histopathological diagnosis of primary aldosteronism using CYP11B2 immunohistochemistry. J Clin Endocrinol Metab. 2013;98:1567–1574. doi: 10.1210/jc.2012-3726. [DOI] [PubMed] [Google Scholar]
- Neville AM, O’Hare MJ. Histopathology of the human adrenal cortex. Clin Endocrinol Metab. 1985;14:791–820. doi: 10.1016/s0300-595x(85)80078-5. [DOI] [PubMed] [Google Scholar]
- Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, Ogishima T, Suematsu M, Mukai K. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95:2296–2305. doi: 10.1210/jc.2009-2010. [DOI] [PubMed] [Google Scholar]
- Ogishima T, Shibata H, Shimada H, Mitani F, Suzuki H, Saruta T, Ishimura Y. Aldosterone synthase cytochrome P-450 expressed in the adrenals of patients with primary aldosteronism. J Biol Chem. 1991;266:10731–10734. [PubMed] [Google Scholar]
- Pascoe L, Curnow KM, Slutsker L, Connell JM, Speiser PW, New MI, White PC. Glucocorticoid-suppressible hyperaldosteronism results from hybrid genes created by unequal crossovers between CYP11B1 and CYP11B2. Proc Natl Acad Sci USA. 1992;89:8327–8331. doi: 10.1073/pnas.89.17.8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma I, Suematsu S, Matsuzawa Y, Saito J, Omura M, Maekawa T, Nakamura Y, Sasano H, Nishikawa T. Characterization of steroidogenic enzyme expression in aldosterone-producing adenoma: a comparison with various human adrenal tumors. Endocr J. 2013;60:329–336. doi: 10.1507/endocrj.ej12-0270. [DOI] [PubMed] [Google Scholar]
- Sasano H. Localization of steroidogenic enzymes in adrenal cortex and its disorders. Endocr J. 1994;41:471–482. doi: 10.1507/endocrj.41.471. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 2000;53:739–747. doi: 10.1046/j.1365-2265.2000.01144.x. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Furukawa K, Inaba S, Miyamori I, Mabuchi H. Genetic analysis of aldosterone synthase in patients with idiopathic hyperaldosteronism. J Clin Endocrinol Metab. 1999;84:1633–1637. doi: 10.1210/jcem.84.5.5671. [DOI] [PubMed] [Google Scholar]
- Wang T, Satoh F, Morimoto R, Nakamura Y, Sasano H, Auchus RJ, Edwards MA, Rainey WE. Gene expression profiles in aldosterone-producing adenomas and adjacent adrenal glands. Eur J Endocrinol. 2011;164:613–619. doi: 10.1530/EJE-10-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol. 2007;66:607–618. doi: 10.1111/j.1365-2265.2007.02775.x. [DOI] [PubMed] [Google Scholar]