Abstract
Interleukin 9 secreting TH9 cells have been proposed as the latest addition to the family of T helper cell subsets. While a growing body of evidence from animal models points to important roles for these cells in allergic inflammation of the lung, autoinflammation of the gastrointestinal tract, and tumor immunity, their role in skin immunity and skin immunopathology remains poorly defined. Interestingly, studies of T helper cells from healthy humans suggest that TH9 cells are predominantly skin-homing and skin-resident and that they are involved in protection against extracellular pathogens. Thus, TH9 cells have entered the stage as potential mediators of cutaneous pathology. However, under which conditions and by which mechanisms these cells contribute to skin immunity and disease still has to be investigated. Here, we review our current understanding of TH9 cells as skin-tropic T helper cells and their involvement in skin pathology. Further, we discuss open questions with regard to the intricate nature of interleukin 9 producing T helper cells.
Introduction
Interleukin 9 secreting TH9 cells are the latest addition to the growing family of T helper cells. Since the description of TH9 cells in 2008, important progress has been made in the understanding of the cellular identity, transcriptional regulation and functional importance of these cells [1]. Besides their well-described functions in immunopathology of the lung and the gut, an increasing body of evidence suggests that these cells play important roles in skin immunity, both in health and disease. Furthermore, the discovery of the superior capacity of TH9 cells to mediate tumor immunity to the skin cancer melanoma has moved these cells into the limelight of cutaneous tumor immunology. In this review, we describe the insights into the functional role of Th9 cells in skin immunity and immunopathology that have been gained since their discovery as putative novel T helper cell subset and we discuss important open questions that will have to be addressed in future studies of cutaneous Th9 biology.
Unanswered questions regarding TH9 cells in skin disorders
As in diseases of other tissues and organs, the role of TH9 cells in skin disorders remains incompletely understood. The available data originates from a limited number of mouse models or correlative studies in humans, making the interpretation of the functional role of cutaneous IL-9 producing TH cells inconclusive [1]. In addition, the existence of a bona fide TH9 cell as a distinct T helper cell subset in skin immunity has formally not been proven. To date, there is no unequivocal data showing stable IL-9 production in T helper cells which are distinct from one of the already defined TH cell subsets. In fact, the “TH9” phenotype appears to be transient in vitro [2], in most disease models [3–5], and in vivo in humans [6]. Similar to its transient expression in T cells, IL-9 is also only transiently expressed after activation by innate lymphoid cells, the putative evolutionary precursors of T cells [7, 8]. In addition, the cytokine co-expression profiles in IL-9 producing TH cells has not been systematically evaluated over time on a single cell level [7, 9], and a transcription factor that serves as master gene regulator of TH9 cells still awaits identification. Therefore, it has proven challenging to unambiguously identify TH9 cells in the skin and differentiate them from other TH cell subsets with the ability to secrete IL-9. These limitations have to be taken into account when reviewing the role of TH9 cells in skin disorders. For ease of readability in this article, however, cells with an IL-9 secreting phenotype will be termed TH9 cells, regardless of the stability of IL-9 production or cytokine co-expression profiles of these cells. Delineating the true existence of a TH9 lineage is currently the subject of intensive study for which models of cutaneous inflammation and immunity will certainly function as important tools [9].
TH9 cells in skin immunity
Th9 cells in skin infection
Studies of tissue-homing and tissue-resident human memory T cells suggest that there is a close link between skin immunity and TH9 cells [2, 10, 11]. Analysis of T cells from human blood and tissues revealed that TH9 cells were predominantly skin-tropic or skin-resident [2, 11]: In vivo primed memory TH cells of healthy donors expressing the major skin-homing receptor cutaneous lymphocyte antigen (CLA) were highly enriched for TH9 cells, whereas gut-homing T cells, identified by their expression of α4β7, contained only very few TH9 cells. CLA on T cells enables them to bind to and roll along endothelium expressing E-selectin. This tethering is crucial for T cells to enter both inflamed and normal skin and thus CLA is regarded as the major skin-homing receptor of T cells together with chemokine receptor 4 (CCR4) [12]. In these skin-tropic TH cells, IL-9 production was transiently expressed after activation and preceded the up-regulation of other inflammatory cytokines. In contrast to these data, T cells from peripheral blood which are polarized in vitro under TH9-inducing conditions have been shown to express the gut-homing integrins α4 and β7 [13]. This discrepancy might be explained by the fact that TH cells which were primed in vivo might differ in terms of their cytokine profile and tissue-homing receptor repertoire from T cells primed in vitro [14]. Future studies are thus needed to define the conditions which regulate homing of TH9 cells into different tissues in health and disease.
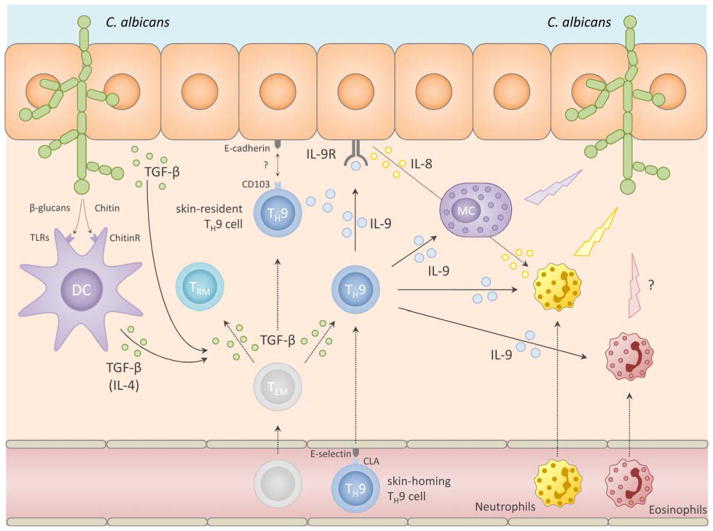
Furthermore, antigen-specificity studies of in vivo differentiated TH cells revealed that many IL-9 producing TH cells are specific for Candida albicans [2]. These C. albicans-specific TH cells showed transient expression of IL-9 without co-production of IL-17, thus showing distinctness from TH17 cells, the TH subset known to be crucial in immunity against C. albicans [14, 15]. Together, these findings indicate that TH9 cells play a critical role in the cutaneous defense against extracellular pathogens in healthy individuals. The mechanism, however, by which TH9 cells participate in the cutaneous immune response to extracellular microbes remains speculative as neither data from human skin infections nor from mouse models are available. Nevertheless, it is possible that, in the context of fungal skin infection, TH9 cells are induced via TGF-β and IL-4 secreted by keratinocytes and antigen-presenting cells (APCs) which were activated via pathogen-recognition receptors. For instance, in monocytes and macrophages. candida-derived β-glucans bind to toll-like receptor (TLR) 2/6 and induce TGF-β secretion whereas chitin induces IL-4 secretion thus providing the key differentiation cytokines for TH9 cells [16]. Thereafter, TH cell derived IL-9 might be important for rapid activation of innate immune cells, such as mast cells, neutrophils and eosinophils, on the one hand, and of keratinocytes on the other hand (Fig. 1: Putative role of TH9 cells in skin immunity):
Fig 1.
putative role of TH9 cells in skin immunity to fungi
First, IL-9 is a key activator and survival factor of mast cells and TH9 cells have been shown to be critical for tissue mast cell accumulation and activation [17, 18]. In mast cells, IL-9 induces the secretion of proinflammatory mediators such as IL-6 and TNF-α, both of which are important for mounting effective anti-fungal immune responses [19–21]. Activated mast cells are specifically able to kill C. albicans [17, 22–24]. In some tumor models, TH9-mediated anti-melanoma immunity is mast cell dependent [10]. Thus, given the abundance of mast cells in human skin, their capacity to produce potent proinflammatory mediators in response to IL-9, and the striking skin-tropism of TH9 cells, it appears likely that mast cells are important effector cells through which TH9 cells exert their function in cutaneous immunity against fungi. Second, also eosinophils may exert effector functions in the TH9-mediated cutaneous immune response to C. albicans, albeit their exact role in anti-fungal immune responses is less well understood. Nevertheless, under distinct circumstances eosinophils have been shown to play a role in anti-fungal skin immunity [25]. Since IL-9 can induce tissue influx, activation, and survival of eosinophils, it appears possible that they too are effectors of TH9-mediated skin immunity [25–28]. Finally, TH9 cells may also attract neutrophils to infected skin via activation of the epithelium. Namely, IL-9 induces the secretion of IL-8 in keratinocytes and thereby the influx of neutrophils which are key effector cells against fungal skin infections [19, 29, 30]. IL-9 is then able to activate these neutrophils and renders them resistant to apoptosis under inflammatory conditions [13]. In summary, rapid and transient secretion of IL-9 by C. albicans-specific skin-resident TH9 cells might function to bridge the adaptive and the innate immune response to common fungal skin infections. The conditions under which this occurs and the precise mechanisms, however, still await elucidation.
TH9 cells as skin resident T cells
In accordance with the predominant skin-homing phenotype of circulating human TH9 cells, IL-9 secreting TH cells were also found in the skin-resident population of healthy humans but not in T cells isolated from healthy gut or lung [2]. These findings were recently confirmed by mass cytometry analysis of TH cells isolated from multiple human tissue [11]. As their circulating skin-homing counter parts, skin-resident TH cells produce IL-9 transiently post activation and this precedes the upregulation of other TH cell subset defining cytokines. IL-9 secreted from skin-tropic TH cells was also found to enhance cytokine production from other T cell subsets, suggesting one function of IL-9 may be the rapid and broad amplification of inflammation [2].
The skin-residency of human TH9 cells under non-inflamed conditions was somewhat unexpected since, in mouse models and some human diseases, TH9 cells have mainly been linked to allergic inflammation of the lung and autoinflammation of the gut [31–35]. It remains to be investigated if under inflammatory conditions in humans, tissue-homing patterns are altered in a way that enables TH9 cells to home to other peripheral organs, which would help explain this discrepancy.
Howsoever, the close association between skin-resident T cells and the TH9 cells might be explained by the role of transforming growth factor-β (TGF-β) in the development of both of these cell types: TGF-β is expressed in the skin epithelium and is central in promoting both the TH9 phenotype and skin-residency of T cells [36–40]: For the development of long-lived skin-resident memory T cells, TGF-β is essential by driving the upregulation of CD103 (also known as αE, which pairs with the β7 integrin chain). CD103 on T cells binds to E-cadherin on keratinocytes and is thought to be crucial for their retention in the skin [36, 40]. In TH9 development, TGF-β together with IL-4 drives the TH9 phenotype in differentiating naïve T cells35,36 and is able to induce IL-9 production in memory TH2 and TH17 cells [38, 39, 41]. Since skin-resident T cells differentiate and accumulate in the skin as a result of cutaneous infections, it is likely that under the TGF-β-rich environment of the skin, pathogen-specific T cells infiltrating the skin in the course of infection can acquire both the skin-resident phenotype as well as the ability to produce IL-9 [42]. It will be interesting to study the tissue-homing and tissue-residency pattern of human TH9 cells under pathological skin conditions to further unravel this close relationship between cutaneous immunity and TH9 cells.
Th9 cells in skin disorders
The majority of published reports and studies on the role of TH9 cells in skin disorders is related to TH2-mediated and allergic inflammation, most notably atopic dermatitis and allergic contact dermatitis. In addition, a growing body of evidence from mouse models points to a superior role of TH9 cells in the mediation of tumor immunity against the skin cancer melanoma (reviewed elsewhere). There are also reports suggesting a role of TH9 cells in other inflammatory and neoplastic disorders of the skin, such as psoriasis and cutaneous T cell lymphoma, respectively.
TH9 cells in atopic dermatitis
Before the first description of TH9 cells in 2008, little was known about the contribution of IL-9 and TH9 cells to atopic dermatitis [38, 39]. At that time, IL-9 was mostly studied in the context of helminth infections and allergic lung inflammation. After the description of TH9 cells however, a number of studies addressed the role of IL-9 in allergic AD. These studies provide evidence for increased expression of IL-9 in atopic dermatitis, both in pediatric patients as well as in adults [43–45]. In these patients, IL-9 levels correlated with clinical severity, IgE levels, and CCL17 levels, indicating an intimate relationship of IL-9 with classical mediators of TH2-induced inflammation [44]. Interestingly, IL-9 expression was shown to be amongst the earliest cytokines to be upregulated in acute canine atopic dermatitis, nicely fitting to the early expression kinetics of IL-9 observed post activation of skin-tropic TH cells [2, 46]. A potential pathogenic role of IL-9 in atopic dermatitis is indicated by the discovery of a significant association between IL-9 and IL-9 receptor gene polymorphisms and atopic dermatitis in the Korean population [47].
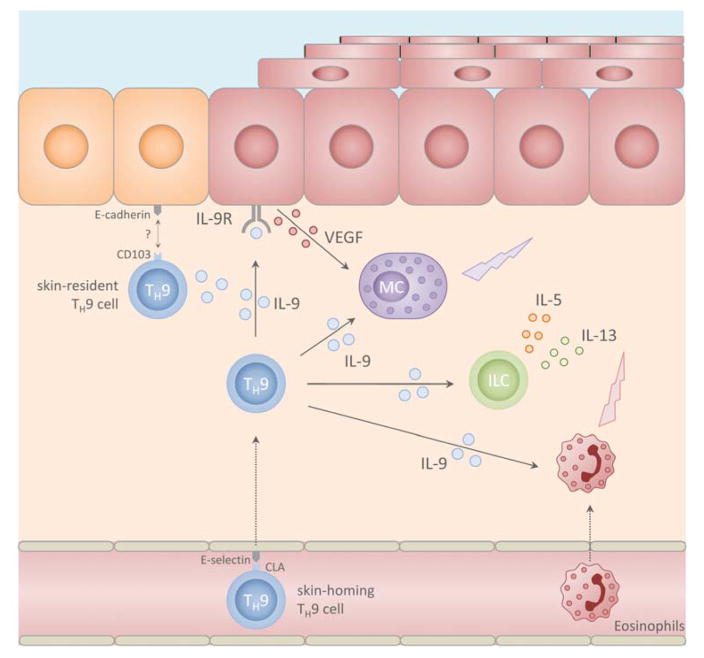
The mechanisms by which IL-9 contributes to AD as well as the cellular sources of IL-9 in AD lesions are incompletely understood. Nevertheless, a few mediators of TH9 driven inflammation in atopic dermatitis have been identified. For instance, Itk, a mediator of T cell receptor signaling and a positive regulator of TH9 differentiation has been shown to be expressed in T cells in lesional skin of AD but not in psoriasis [48, 49]. As for the downstream effects of IL-9 in atopic dermatitis, similar considerations can be made as have been outlined above for their role in skin infection (Fig. 2). IL-9 promotes tissue accumulation, survival and activation of mast cells, eosinophils and innate lymphoid cells, all of which are key cellular contributors to atopic dermatitis pathogenesis [50]. In particular, IL-9 seems to play an important role in early activation of ILCs, in which it enhances the secretion of IL-5 and IL-13, two cytokines which are intimately linked to AD pathogenesis [51, 52]. In keratinocytes, IL-9 induces VEGF expression which has been linked to capillary dilatation, dermal edema and epidermal changes seen in atopic dermatitis [44, 53]. It can be expected that with the advent of biological and targeted therapies in atopic dermatitis, we will soon gain novel insights into the role of TH9 cells in this common skin disease through translational research [50].
Fig 2.
putative role of TH9 cells in allergic skin inflammation
TH9 cells in allergic contact dermatitis & allergen-induced delayed type hypersensitivity
Further evidence for an important role of IL-9 in allergic skin inflammation comes from the analysis of allergic contact dermatitis and allergen-induced delayed type hypersensitivity. IL-9 is upregulated in positive skin patch reactions in patients with allergic contact dermatitis [54, 55]. IL-9 expression seems to be a general pathogenetic event in allergic contact dermatitis, as increased levels of IL-9 were found in reactions to a variety of different contact allergens, including metals, drugs, and polymers [54]. In these patch test reactions, both IL-9 gene expression and that of TH9-associated transcription factors PU.1, ETS-1, IFN regulatory factor 4 (IRF4), and GcN5 were induced, thus suggesting TH9 cells as prominent source of IL-9 in allergic contact dermatitis. In addition to ACD, IL-9 expression is also upregulated in allergen-induced delayed type hypersensitivity (DTH). In DTH reactions to Diphencyprone as well as after intradermal injection of grass pollen in atopic patients, IL-9 was rapidly upregulated within 3 days after challenge but quickly waned again thereafter [6, 56]. Again, these expression kinetics of IL-9 in vivo in humans seem to correspond to those observed in vitro post activation of skin-tropic TH cells. Moreover, levels of IL-9 in the skin correlated with numbers of infiltrating eosinophils indicating a role of IL-9 in their recruitment to the skin [27].
Whether IL-9 is pathogenic in ACD and cutaneous DTH has not been directly addressed. However, there is circumstantial evidence that IL-9 may serve as a proinflammatory mediator in ACD: In contact allergic patients, IL-9 expression in both the skin and in peripheral blood mononuclear cells (PBMCs) seems to correlate with the strength of the allergen stimulus: In vitro stimulation of PBMCs with nickel leads to a dose-dependent production of IL-9 in nickel-allergic patients and elevated levels of IL-9 were found in blister fluids of highly positive patch test reactions but not in fluids of other blistering skin conditions [54, 55]. Moreover, PBMCs of allergic patients but not those of tolerant individuals secrete IL-9 after allergen stimulation [57]. In contrast to these findings, a mouse model of ACD showed decreased ear swelling in IL-9 knockout mice and IL-9 was shown to downregulate interferon-γ in vitro, rather suggesting a regulatory role of IL-9 [54]. How these partially conflicting results can be reconciled will have to be addressed in future investigations.
TH9 cells in psoriasis
Only few reports address the role of IL-9 and TH9 cells in psoriasis. In human psoriasis, IL-9 expressing CD4+ Th cells have been found to be increased in lesional skin [2, 44]. However, in these T cells, cytokine co-expression of IL-9 with other Th cell cytokines was not analyzed. Since IL-17 is one of the major cytokines driving psoriatic inflammation and TH17 cells are able to secrete IL-9 under certain conditions, it remains possible that the IL-9+ cells detected in this study were TH17 cells rather than TH9 cells [41].
Insights into the potential mechanistic role of IL-9 in psoriasis come from a study of K5.hTGF-b1 transgenic mice [53]. In this study, IL-9 induced TH17-dependent psoriasis-like skin inflammation and angiogenesis. Further, it was shown that IL-9 enhances IL-17 production in human psoriasis patients. These findings are particularly intriguing as they place IL-9 upstream of TH17driven inflammation in psoriatic inflammation. Such a scenario awaits further elucidation in additional models of psoriatic inflammation and in translational analysis of psoriasis patients.
TH9 cells in cutaneous T cell lymphoma
IL-9 has long been recognized as an important growth and differentiation factor for transformed lymphoid cells, including malignant T cells [58–61]. In addition, IL-9 is also capable of modulating the inflammatory microenvironment of lymphoid tumors [62]. In a recent study in cutaneous T cell lymphoma (CTCL), IL-9 has now been linked to the pathogenesis of mycosis fungoides (MF), the most common form of CTCL [63]. Lesional skin of MF patients was enriched with cells expressing IL-9 and IL-9 receptor (IL-9R). IL-9 producing cells belonged to both the malignant as well as to the infiltrating benign T cell population. In vitro, IL-9 had an anti-apoptotic effect on malignant T cells, which corresponded to the clinical observation that successful therapy of MF patients was associated with decreased numbers of IL-9 and IL-9R expressing cells in lesional skin. These tumorigenic and regulatory properties of IL-9 in MF pathogenesis were finally substantiated in a mouse model where neutralization of IL-9 inhibited tumor growth and improved the reactive T cell response. This regulatory and tumorigenic role of IL-9 in CTCL stands in contrast to the anti-tumor immunity mediated by TH9 cells in various tumor models [10, 64–66], and shows that IL-9 function is highly context-dependent. Howsoever, the well-established function of IL-9 as growth factor of lymphoid tumors and the skin-tropism of TH9 cells make further investigations of the interrelation of benign and malignant IL-9 secreting T cells in CTCL a promising task.
Involvement of TH9 cells in other skin pathologies
IL-9 and, thereby, potentially TH9 cells have been implicated in few additional pathologies that involve the skin, namely: systemic sclerosis, alopecia areata, and cutaneous lichen planus. In systemic sclerosis, IL-9 serum levels were elevated and found to correlate with better lung function [67]. In alopecia areata, lesional samples showed upregulated IL-9 gene expression [68]. In lichen patients, IL-9 expression was higher in patients with cutaneous LP as compared to oral LP [69]. All of this data is preliminary and thus prevents any conclusions being drawn about the role of TH9 cells in these diseases.
Concluding remarks
The skin-homing and skin-resident properties of human TH9 cells under homeostatic conditions and the many putative target cells of IL-9 residing in the cutaneous compartment make these cells intriguing candidates as key mediators of skin immunity and inflammation [2, 11]. However, much remains to be learned with respect to the cellular identity, the genetic regulation and the functional role of TH9 cells, particularly in humans. The future study of these cells will have to address their peculiarities and intricacies, their phenotypic transience, their genetic regulation, the broader repertoire of their effector molecules and their precise effects on the various targets cells. Based on the superior role of TH9 cells in mediating anti-tumor immunity in mouse models and the current emergence of successful immunotherapy of cancer patients, TH9 cells have entered the limelight for novel T cell-based immunotherapies in cancer. Thus, it is an exciting time to further unravel the true identity of this putative novel skin-resident T helper cell subset.
Abbreviations
- APC
antigen-presenting cell
- C. albicans
Candida albicans
- CCR
Chemokine receptor
- CLA
Cutaneous lymphocyte antigen
- CTCL
cutaneous T cell lymphoma
- DTH
Delayed type hypersensitivity
- IL-9
interleukin 9
- ILC
Innate lymphoid cells
- IRF4
IFN regulatory factor 4
- MF
mycosis fungoides
- PBMC
peripheral blood mononuclear cells
- TH cells
T helper cells
- TLR
toll-like receptor
References
- 1.Kaplan MH, Hufford MM, Olson MR. The development and in vivo function of T helper 9 cells. Nat Rev Immunol. 2015;15(5):295–307. doi: 10.1038/nri3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlapbach C, Gehad A, Yang C, Watanabe R, Guenova E, et al. Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci Transl Med. 2014;6(219):219ra8. doi: 10.1126/scitranslmed.3007828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Licona-Limon P, Henao-Mejia J, Temann AU, Gagliani N, Licona-Limon I, et al. Th9 Cells Drive Host Immunity against Gastrointestinal Worm Infection. Immunity. 2013;39(4):744–57. doi: 10.1016/j.immuni.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan C, Aziz MK, Lovaas JD, Vistica BP, Shi G, et al. Antigen-specific Th9 cells exhibit uniqueness in their kinetics of cytokine production and short retention at the inflammatory site. J Immunol. 2010;185(11):6795–801. doi: 10.4049/jimmunol.1001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richard AC, Tan C, Hawley ET, Gomez-Rodriguez J, Goswami R, et al. The TNF-family ligand TL1A and its receptor DR3 promote T cell-mediated allergic immunopathology by enhancing differentiation and pathogenicity of IL-9-producing T cells. J Immunol. 2015;194(8):3567–82. doi: 10.4049/jimmunol.1401220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ying S, Meng Q, Kay AB, Robinson DS. Elevated expression of interleukin-9 mRNA in the bronchial mucosa of atopic asthmatics and allergen-induced cutaneous late-phase reaction: relationships to eosinophils, mast cells and T lymphocytes. Clin Exp Allergy. 2002;32(6):866–71. doi: 10.1046/j.1365-2222.2002.01376.x. [DOI] [PubMed] [Google Scholar]
- 7.Wilhelm C, Turner JE, Van Snick J, Stockinger B. The many lives of IL-9: a question of survival? Nat Immunol. 2012;13(7):637–41. doi: 10.1038/ni.2303. [DOI] [PubMed] [Google Scholar]
- 8.Vivier E, van de Pavert SA, Cooper MD, Belz GT. The evolution of innate lymphoid cells. Nat Immunol. 2016;17(7):790–4. doi: 10.1038/ni.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitt E, Klein M, Bopp T. Th9 cells, new players in adaptive immunity. Trends Immunol. 2014;35(2):61–8. doi: 10.1016/j.it.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. 2012;18(8):1248–53. doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong MT, Ong DE, Lim FS, Teng KW, McGovern N, et al. A High-Dimensional Atlas of Human T Cell Diversity Reveals Tissue-Specific Trafficking and Cytokine Signatures. Immunity. 2016;45(2):442–56. doi: 10.1016/j.immuni.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176(7):4431–9. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 13.Nalleweg N, Chiriac MT, Podstawa E, Lehmann C, Rau TT, et al. IL-9 and its receptor are predominantly involved in the pathogenesis of UC. Gut. 2015;64(5):743–55. doi: 10.1136/gutjnl-2013-305947. [DOI] [PubMed] [Google Scholar]
- 14.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484(7395):514–8. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 15.Netea MG, Marodi L. Innate immune mechanisms for recognition and uptake of Candida species. Trends Immunol. 2010;31(9):346–53. doi: 10.1016/j.it.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6(1):67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 17.Sehra S, Yao W, Nguyen ET, Glosson-Byers NL, Akhtar N, et al. TH9 cells are required for tissue mast cell accumulation during allergic inflammation. J Allergy Clin Immunol. 2015;136(2):433–40 e1. doi: 10.1016/j.jaci.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CY, Lee JB, Liu B, Ohta S, Wang PY, et al. Induction of Interleukin-9-Producing Mucosal Mast Cells Promotes Susceptibility to IgE-Mediated Experimental Food Allergy. Immunity. 2015;43(4):788–802. doi: 10.1016/j.immuni.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conti HR, Gaffen SL. IL-17-Mediated Immunity to the Opportunistic Fungal Pathogen Candida albicans. J Immunol. 2015;195(3):780–8. doi: 10.4049/jimmunol.1500909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alves de Medeiros AK, Lodewick E, Bogaert DJ, Haerynck F, Van Daele S, et al. Chronic and Invasive Fungal Infections in a Family with CARD9 Deficiency. J Clin Immunol. 2016;36(3):204–9. doi: 10.1007/s10875-016-0255-8. [DOI] [PubMed] [Google Scholar]
- 21.Marehbian J, Arrighi HM, Hass S, Tian H, Sandborn WJ. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104(10):2524–33. doi: 10.1038/ajg.2009.322. [DOI] [PubMed] [Google Scholar]
- 22.Lopes JP, Stylianou M, Nilsson G, Urban CF. Opportunistic pathogen Candida albicans elicits a temporal response in primary human mast cells. Sci Rep. 2015;5:12287. doi: 10.1038/srep12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nieto-Patlan A, Campillo-Navarro M, Rodriguez-Cortes O, Munoz-Cruz S, Wong-Baeza I, et al. Recognition of Candida albicans by Dectin-1 induces mast cell activation. Immunobiology. 2015;220(9):1093–100. doi: 10.1016/j.imbio.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Trevisan E, Vita F, Medic N, Soranzo MR, Zabucchi G, et al. Mast cells kill Candida albicans in the extracellular environment but spare ingested fungi from death. Inflammation. 2014;37(6):2174–89. doi: 10.1007/s10753-014-9951-9. [DOI] [PubMed] [Google Scholar]
- 25.Tsunemi Y, Kadono T, Saeki H, Kikuchi K, Tamaki K, et al. Secondary cutaneous candidiasis with eosinophilia. J Dermatol. 2010;37(2):175–8. doi: 10.1111/j.1346-8138.2009.00782.x. [DOI] [PubMed] [Google Scholar]
- 26.Vultaggio A, Lombardelli L, Giudizi MG, Biagiotti R, Mazzinghi B, et al. T cells specific for Candida albicans antigens and producing type 2 cytokines in lesional mucosa of untreated HIV-infected patients with pseudomembranous oropharyngeal candidiasis. Microbes Infect. 2008;10(2):166–74. doi: 10.1016/j.micinf.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Louahed J, Zhou Y, Maloy WL, Rani PU, Weiss C, et al. Interleukin 9 promotes influx and local maturation of eosinophils. Blood. 2001;97(4):1035–42. doi: 10.1182/blood.v97.4.1035. [DOI] [PubMed] [Google Scholar]
- 28.Gounni AS, Gregory B, Nutku E, Aris F, Latifa K, et al. Interleukin-9 enhances interleukin-5 receptor expression, differentiation, and survival of human eosinophils. Blood. 2000;96(6):2163–71. [PubMed] [Google Scholar]
- 29.Hong CH, Chang KL, Wang HJ, Yu HS, Lee CH. IL-9 induces IL-8 production via STIM1 activation and ERK phosphorylation in epidermal keratinocytes: A plausible mechanism of IL-9R in atopic dermatitis. J Dermatol Sci. 2015;78(3):206–14. doi: 10.1016/j.jdermsci.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Erwig LP, Gow NA. Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol. 2016;14(3):163–76. doi: 10.1038/nrmicro.2015.21. [DOI] [PubMed] [Google Scholar]
- 31.Jabeen R, Goswami R, Awe O, Kulkarni A, Nguyen ET, et al. Th9 cell development requires a BATF-regulated transcriptional network. J Clin Invest. 2013;123(11):4641–53. doi: 10.1172/JCI69489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33(2):192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Yao W, Zhang Y, Jabeen R, Nguyen ET, Wilkes DS, et al. Interleukin-9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity. 2013;38(2):360–72. doi: 10.1016/j.immuni.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niedbala W, Besnard AG, Nascimento DC, Donate PB, Sonego F, et al. Nitric oxide enhances Th9 cell differentiation and airway inflammation. Nat Commun. 2014;5:4575. doi: 10.1038/ncomms5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerlach K, Hwang Y, Nikolaev A, Atreya R, Dornhoff H, et al. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat Immunol. 2014;15(7):676–86. doi: 10.1038/ni.2920. [DOI] [PubMed] [Google Scholar]
- 36.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013;14(12):1294–301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 37.Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, et al. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14(12):1285–93. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9(12):1347–55. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9(12):1341–6. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe R, Gehad A, Yang C, Scott LL, Teague JE, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med. 2015;7(279):279ra39. doi: 10.1126/scitranslmed.3010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beriou G, Bradshaw EM, Lozano E, Costantino CM, Hastings WD, et al. TGF-beta induces IL-9 production from human Th17 cells. J Immunol. 2010;185(1):46–54. doi: 10.4049/jimmunol.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark RA. Resident memory T cells in human health and disease. Sci Transl Med. 2015;7(269):269rv1. doi: 10.1126/scitranslmed.3010641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130(6):1344–54. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma L, Xue HB, Guan XH, Shu CM, Zhang JH, et al. Possible pathogenic role of T helper type 9 cells and interleukin (IL)-9 in atopic dermatitis. Clin Exp Immunol. 2014;175(1):25–31. doi: 10.1111/cei.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ciprandi G, De Amici M, Giunta V, Marseglia A, Marseglia G. Serum interleukin-9 levels are associated with clinical severity in children with atopic dermatitis. Pediatr Dermatol. 2013;30(2):222–5. doi: 10.1111/j.1525-1470.2012.01766.x. [DOI] [PubMed] [Google Scholar]
- 46.Olivry T, Mayhew D, Paps JS, Linder KE, Peredo C, et al. Early Activation of Th2/Th22 Inflammatory and Pruritogenic Pathways in Acute Canine Atopic Dermatitis Skin Lesions. J Invest Dermatol. 2016 doi: 10.1016/j.jid.2016.05.117. [DOI] [PubMed] [Google Scholar]
- 47.Namkung JH, Lee JE, Kim E, Park GT, Yang HS, et al. An association between IL-9 and IL-9 receptor gene polymorphisms and atopic dermatitis in a Korean population. J Dermatol Sci. 2011;62(1):16–21. doi: 10.1016/j.jdermsci.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Gomez-Rodriguez J, Meylan F, Handon R, Hayes ET, Anderson SM, et al. Itk is required for Th9 differentiation via TCR-mediated induction of IL-2 and IRF4. Nat Commun. 2016;7:10857. doi: 10.1038/ncomms10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Bonin A, Rausch A, Mengel A, Hitchcock M, Kruger M, et al. Inhibition of the IL-2-inducible tyrosine kinase (Itk) activity: a new concept for the therapy of inflammatory skin diseases. Exp Dermatol. 2011;20(1):41–7. doi: 10.1111/j.1600-0625.2010.01198.x. [DOI] [PubMed] [Google Scholar]
- 50.Werfel T, Allam JP, Biedermann T, Eyerich K, Gilles S, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol. 2016;138(2):336–49. doi: 10.1016/j.jaci.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12(11):1071–7. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med. 2016 doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 53.Singh TP, Schon MP, Wallbrecht K, Gruber-Wackernagel A, Wang XJ, et al. Involvement of IL-9 in Th17-associated inflammation and angiogenesis of psoriasis. PLoS One. 2013;8(1):e51752. doi: 10.1371/journal.pone.0051752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J, Harberts E, Tammaro A, Girardi N, Filler RB, et al. IL-9 regulates allergen-specific Th1 responses in allergic contact dermatitis. J Invest Dermatol. 2014;134(7):1903–11. doi: 10.1038/jid.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gutin L, Tammaro A, Fishelevich R, Gaspari AA. Elevation of IL-9 in Extreme Patch Test Reactions Suggests It Is an Inflammatory Mediator in Allergic Contact Dermatitis. Dermatitis. 2016;27(1):35–6. doi: 10.1097/DER.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 56.Gulati N, Suarez-Farinas M, Fuentes-Duculan J, Gilleaudeau P, Sullivan-Whalen M, et al. Molecular characterization of human skin response to diphencyprone at peak and resolution phases: therapeutic insights. J Invest Dermatol. 2014;134(10):2531–40. doi: 10.1038/jid.2014.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coulter EM, Jenkinson C, Farrell J, Lavergne SN, Pease C, et al. Measurement of CD4+ and CD8+ T-lymphocyte cytokine secretion and gene expression changes in p-phenylenediamine allergic patients and tolerant individuals. J Invest Dermatol. 2010;130(1):161–74. doi: 10.1038/jid.2009.187. [DOI] [PubMed] [Google Scholar]
- 58.Renauld JC, van der Lugt N, Vink A, van Roon M, Godfraind C, et al. Thymic lymphomas in interleukin 9 transgenic mice. Oncogene. 1994;9(5):1327–32. [PubMed] [Google Scholar]
- 59.Lv X, Feng L, Fang X, Jiang Y, Wang X. Overexpression of IL-9 receptor in diffuse large B-cell lymphoma. Int J Clin Exp Pathol. 2013;6(5):911–6. [PMC free article] [PubMed] [Google Scholar]
- 60.Lv X, Feng L, Ge X, Lu K, Wang X. Interleukin-9 promotes cell survival and drug resistance in diffuse large B-cell lymphoma. J Exp Clin Cancer Res. 2016;35(1):106. doi: 10.1186/s13046-016-0374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J, Wang WD, Geng QR, Wang L, Chen XQ, et al. Serum levels of interleukin-9 correlate with negative prognostic factors in extranodal NK/T-cell lymphoma. PLoS One. 2014;9(4):e94637. doi: 10.1371/journal.pone.0094637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lange K, Uckert W, Blankenstein T, Nadrowitz R, Bittner C, et al. Overexpression of NPM-ALK induces different types of malignant lymphomas in IL-9 transgenic mice. Oncogene. 2003;22(4):517–27. doi: 10.1038/sj.onc.1206076. [DOI] [PubMed] [Google Scholar]
- 63.Vieyra-Garcia PA, Wei T, Naym DG, Fredholm S, Fink-Puches R, et al. STAT3/5-Dependent IL9 Overexpression Contributes to Neoplastic Cell Survival in Mycosis Fungoides. Clin Cancer Res. 2016;22(13):3328–39. doi: 10.1158/1078-0432.CCR-15-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu Y, Hong S, Li H, Park J, Hong B, et al. Th9 cells promote antitumor immune responses in vivo. J Clin Invest. 2012;122(11):4160–71. doi: 10.1172/JCI65459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vegran F, Berger H, Boidot R, Mignot G, Bruchard M, et al. The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat Immunol. 2014;15(8):758–66. doi: 10.1038/ni.2925. [DOI] [PubMed] [Google Scholar]
- 66.Nakatsukasa H, Zhang D, Maruyama T, Chen H, Cui K, et al. The DNA-binding inhibitor Id3 regulates IL-9 production in CD4(+) T cells. Nat Immunol. 2015;16(10):1077–84. doi: 10.1038/ni.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yanaba K, Yoshizaki A, Asano Y, Kadono T, Sato S. Serum interleukin 9 levels are increased in patients with systemic sclerosis: association with lower frequency and severity of pulmonary fibrosis. J Rheumatol. 2011;38(10):2193–7. doi: 10.3899/jrheum.110268. [DOI] [PubMed] [Google Scholar]
- 68.Suarez-Farinas M, Ungar B, Noda S, Shroff A, Mansouri Y, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136(5):1277–87. doi: 10.1016/j.jaci.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 69.Weber B, Schlapbach C, Stuck M, Simon HU, Borradori L, et al. Distinct interferon-gamma and interleukin-9 expression in cutaneous and oral lichen planus. J Eur Acad Dermatol Venereol. 2016 doi: 10.1111/jdv.13989. [DOI] [PubMed] [Google Scholar]