Structured Abstract
Background
Effective decongestion of heart failure patients predicts improved outcomes, but high dose loop diuretics (HDLD) used to achieve diuresis predict adverse outcomes. In the DOSE trial, randomization to a HDLD intensification strategy (HDLD-strategy) improved diuresis but not outcomes. Our objective was to determine if potential beneficial effects of more aggressive decongestion may have been offset by adverse effects of the HDLD used to achieve diuresis.
Methods and Results
A post hoc analysis of the DOSE trial (n=308) was conducted to determine the influence of post-randomization diuretic dose and fluid output on the rate of death, rehospitalization or emergency department visitation associated with the HDLD-strategy. Net fluid output was used as a surrogate for beneficial decongestive effects and cumulative loop diuretic dose for the dose-related adverse effects of the HDLD-strategy.
Randomization to the HDLD-strategy resulted in increased fluid output, even after adjusting for cumulative diuretic dose (p=0.006). Unadjusted, the HDLD-strategy did not improve outcomes (p=0.28). However, following adjustment for cumulative diuretic dose, significant benefit emerged (HR=0.64, 95% CI 0.43–0.95, p=0.028). Adjusting for net fluid balance eliminated the benefit (HR=0.95, 95% CI 0.67–1.4, p=0.79).
Conclusions
A clinically meaningful benefit from a randomized aggressive decongestion strategy became apparent after accounting for the quantity of loop diuretic administered. Adjusting for the diuresis resulting from this strategy eliminated the benefit. These hypothesis-generating observations may suggest a role for aggressive decongestion in improved outcomes.
Keywords: Acute heart failure, outcomes, loop diuretics
Introduction
Acute decompensated heart failure (ADHF) is the most common hospital discharge diagnosis among Medicare beneficiaries and accounts for a substantial percentage of all heart failure related expenditures.1–3 The vast majority of these admissions appears primarily driven by symptoms of congestion rather than low cardiac output.4–7 Importantly, there are substantial theoretical grounds and a modest amount of evidence to suggest that inadequate decongestion directly contributes to heart failure disease progression and poor outcomes.4, 5, 8–11 As a result, loop diuretics have become the cornerstone of pharmacotherapy for ADHF treatment. This is problematic in that loop diuretics directly worsen neurohormonal activation independent of their effects on volume, and in the majority of studies, high doses are associated with significantly worsened outcomes.12–16 However, it remains unclear whether the incremental benefit afforded by aggressive decongestion outweighs any potential direct toxicity of loop diuretic therapy in humans with heart failure.
In observational studies, it is impossible to fully account for the fact that “sicker” patients are usually prescribed more diuretic and “healthier” patients tend to respond better to diuretics at any given dose. The Diuretic Optimization Strategies (DOSE) trial was the most rigorous attempt to date to answer the question of whether more aggressive decongestion can improve outcomes. Importantly, in this trial, patients were randomized to a high vs. low loop diuretic dose intensification strategy but the actual dose of loop diuretic used to implement this strategy was not fixed. The findings with respect to post-discharge outcomes in the DOSE trial were neutral. However, in any trial the observed effect represents the sum of the potential harms and benefits of the intervention. As such, it is possible that a clinically meaningful benefit from the more aggressive decongestion strategy was present, but was nullified by a similar magnitude of harm from high-dose loop diuretics.
The purpose of the current analysis was to determine if important competing effects were present between the potential toxicity of high dose loop diuretics and the benefits of the additional decongestion provided by their use. To accomplish this, we employed the approach of mediation analysis, whereby statistical adjustment for factors which are thought to be in the causal pathway of benefit or harm (i.e., mediators) was undertaken. This analytic approach is well established in the medical and statistical literature and has been applied to cardiovascular randomized trials to better understand how the interventions were either effective or ineffective at improving outcomes.17–22 Since precise measures of diuretic toxicity and decongestion were not available in the DOSE dataset, we adjusted for surrogate measures of these effects as total loop diuretic received (toxicity) and fluid loss (decongestion). Our hypothesis was that there would be important competing effects of high dose loop diuretics with both benefit in the form of additional decongestion and harm from toxicity of the high dose diuretics, and thus we would see significant changes in the outcomes associated with randomization to the high dose strategy after adjustment for the potential mediators.
Methods
The analysis was conducted using data from the NHLBI Heart Failure Network’s DOSE trial. The study design and results of this trial have been previously published.23, 24 Briefly, DOSE was a multicenter, randomized, double blind, placebo controlled trial of diuretic strategies in patients with ADHF. The study used a 2×2 factorial design randomizing patients to a strategy of high- vs. low-dose furosemide treatment and continuous infusion vs. every-12-hour bolus furosemide administration. High-dose treatment consisted of 2.5 times the previous oral diuretic dose and low-dose of 1.0 times the previous oral diuretic dose, administered as IV furosemide on a milligram per milligram basis. Eligibility criteria included an oral loop diuretic dose 80–240 mg of furosemide equivalents for at least 1 month, a systolic blood pressure ≥90 mmHg, and a serum creatinine ≤ 3.0 mg/dl. The randomized treatment was continued for 72 hours with an option for the treating physician to adjust the dose at 48 hours if required while maintaining study treatment concealment.
The 72-hour cumulative dose of diuretic received during the intervention period was calculated as the sum of the total open label intravenous loop diuretics and the amount of study drug, accounting for change in diuretic strategy such as up/down titration or conversion to oral diuretics at 48 hours. Loop diuretic doses were converted to furosemide equivalents with 1 mg bumetanide = 20 mg torsemide = 40 mg furosemide and expressed as IV furosemide equivalents.25, 26 For oral diuretics a bioavailability of 50% was assumed for furosemide, while torsemide and bumetanide were converted 1:1.27 The chronic kidney disease epidemiology collaboration equation was used to calculate estimated glomerular filtration rate (eGFR).28
Statistical analysis
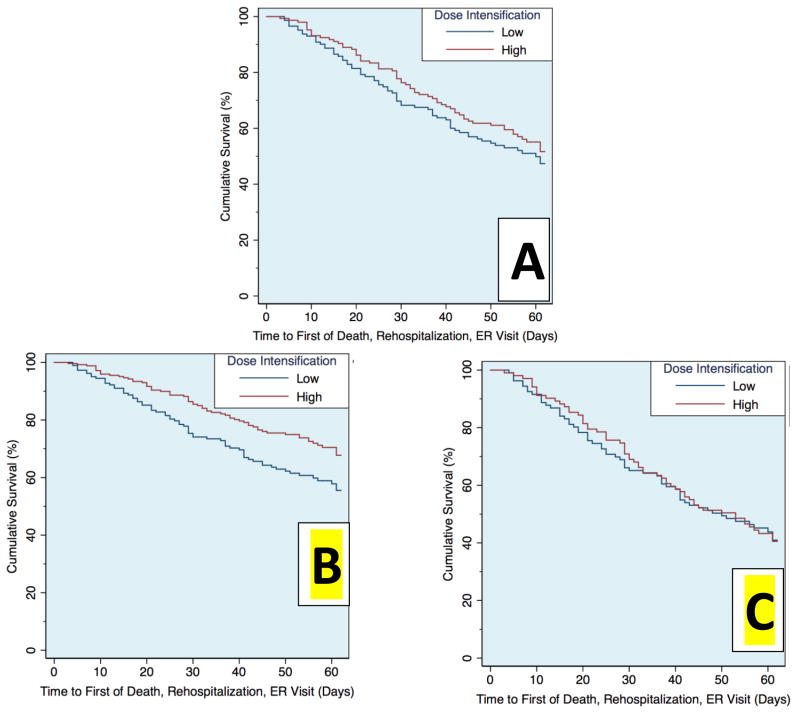
Values reported are mean ± SD, median (quartile 1 – quartile 3) and percentage. Independent Student’s t test or the Wilcoxon Rank Sum test was used to compare continuous variables. The chi-square test was used to evaluate associations between categorical variables. Correlation coefficients reported are Pearson’s in the case of comparisons of two continuous variables and the point biserial coefficient (rpb) when evaluating correlations between randomized strategy and a continuous parameter. Linear regression was used to determine if an independent association between net fluid output and the randomized dose intensification strategy was present after adjusting for the dose of loop diuretic received. Cox proportional hazards modeling was used to evaluate time-to-event associations with the composite endpoint of death, rehospitalization, or emergency room visitation. To maintain consistency with the primary analytic approach of the DOSE trial, survival analyses were adjusted for randomization to continuous infusion vs. bolus administration of loop diuretic therapy. Multivariable Cox regression models were constructed to evaluate the effect of randomization to the high vs. low dose strategy, with adjustment for either net fluid loss or cumulative loop diuretic dose during the 72 hour intervention period. Fluid loss and cumulative loop diuretic dose were evaluated as continuous variables with the exception of the analysis underlying Figure 3. These models were then adjusted for all baseline characteristics with a univariable association with death, rehospitalization or emergency department visitation of p ≤0.2 in addition to any parameter which was significantly associated with randomized strategy after adjustment for either fluid loss or cumulative diuretic dose (Table 1). Of the 21 baseline variables spanning demographics, comorbidities, physical examination findings, medications, and laboratory findings tested for the above criteria, the following parameters were entered into multivariable models: Age, ejection fraction, gout, heart rate, angiotensin converting enzyme inhibitor or receptor blocker use, baseline loop diuretic dose, serum sodium, blood urea nitrogen, eGFR, uric acid, blood urea nitrogen to creatinine ratio, amino-terminal pro B type natriuretic peptide, and hemoglobin. Models were built using backward elimination (likelihood ratio test) where all covariates with a p≤0.2 were retained.29 Given that fluid loss is not an ideal surrogate for decongestion and cumulative diuretic dose is not an ideal surrogate for diuretic-related toxicity, formal quantification of the direct and indirect mediation effects was intentionally not attempted. The relationship between the composite endpoint and randomized dose intensification strategy is visualized in Figure 2. Panel A depicts the Kaplan-Meier estimated survivor functions of the high and low dose intensification groups. Panels B and C depict estimated survival functions from Cox models, stratified on dose intensification strategy, with adjustment for cumulative loop diuretic received or net fluid output as covariates.
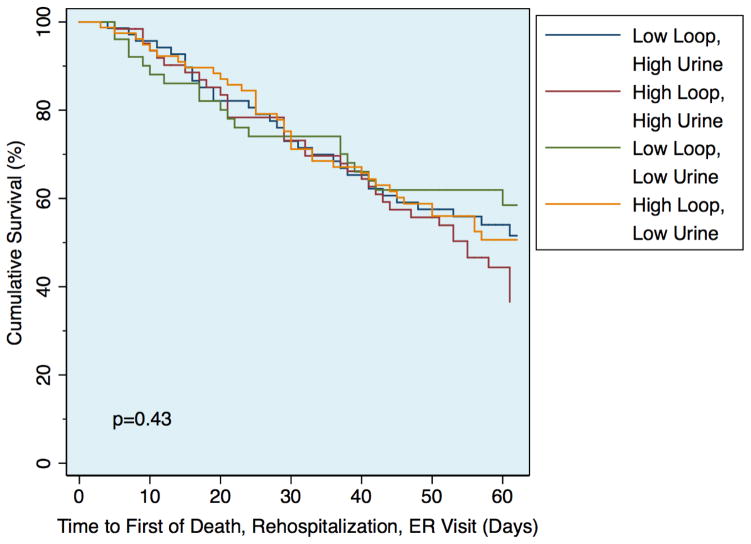
Figure 3.
Kaplan-Meier survival plots of the risk of death, rehospitalization, or emergency department visit between groups defined by cumulative loop diuretic dose and net fluid output above or below the median
Table 1.
Baseline characteristics of the DOSE trial population
| Variable | n=308 |
|---|---|
| Demographics/medical history | |
| Age | 66 ± 14 |
| Male sex | 73.4% (226) |
| White race | 72.1% (222) |
| Ischemic heart disease | 57.1% (176) |
| Diabetes | 51.3% (158) |
| Gout | 22.4% (69) |
| Cardiac function | |
| Ejection fraction (%) | 30 (30–50) |
| Physical examination findings | |
| Systolic Blood Pressure (mmHg) | 119 ± 20 |
| Heart rate (beats per minute) | 78 ± 16 |
| Jugular venous pressure >12 cm water | 58.6% (171) |
| Edema | 78.9% (243) |
| Rales | 58.2% (178) |
| Medications | |
| ACE or ARB | 64.0% (197) |
| Beta blocker | 83.1% (256) |
| Aldosterone antagonist | 27.9% (86) |
| Laboratory parameters | |
| Sodium (mEq/L) | 138 ± 4 |
| Blood urea nitrogen (mg/dL) | 38 ± 23 |
| eGFR (ml/min/1.73m2) | 55 ± 25 |
| Uric Acid | 9.8 ± 2.6 |
| Hemoglobin (g/dl) | 11.7 ± 2.0 |
| NT-proBNP (pg/mL) | 4435 (2466–9882) |
ACE: Angiotensin converting enzyme inhibitor. ARB: Angiotensin receptor blocker. eGFR: Estimated glomerular filtration rate. NT-proBNP: Amino terminal pro B-type natriuretic peptide. Values presented are mean ± standard deviation, median (quartile 1 – quartile 3) and percentage (absolute number).
Figure 2.
Survival plots of the risk of death, rehospitalization, or emergency department visit between the randomized high or low dose intensification strategies prior to adjustment (A), following adjustment for cumulative loop diuretic received (B), and following adjustment for the resulting net fluid output (C)
In addition to our primary survival analyses, we assessed the competing effects of decongestion and diuretic toxicity using the more proximate endpoints of length of stay (via linear regression) and worsening heart failure at 72 hours (via logistic regression). Both models were adjusted for route of administration in order to maintain consistency with the original analytic approach of the DOSE trial.
Statistical analysis was performed with IBM SPSS Statistics version 23 (IBM Corp., Armonk, NY) and Stata version 13.1 (StataCorp, College Station, TX). Statistical significance was defined as 2-tailed p<0.05 for all analyses except for tests for interaction, where p<0.1 was considered significant.
Results
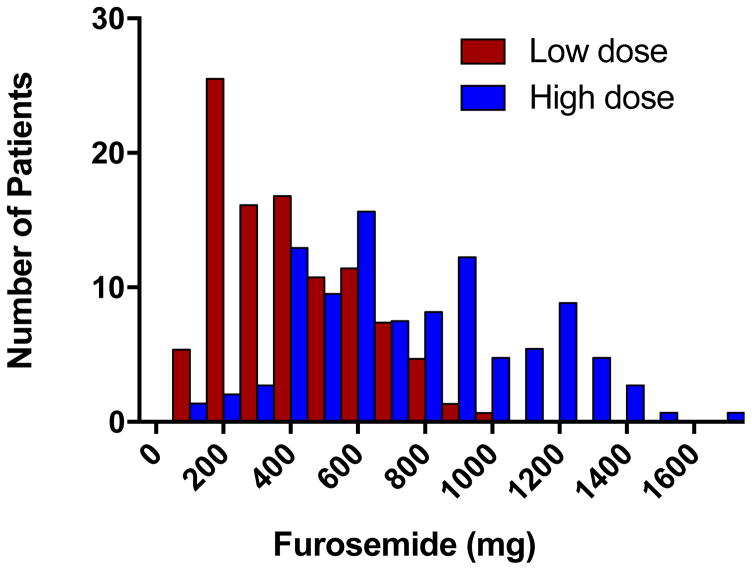
Baseline characteristics of the DOSE trial (n=308) have previously been published.23 In the overall population, the median cumulative dose of IV loop diuretic received over the 72 hour intervention period was 544 mg (335 to 821 mg). Despite the overall higher dose received with the high dose intensification strategy (a median of 773 mg in the high-dose group versus 358 in the low-dose group), there was substantial overlap between groups with respect to the absolute dose of diuretic any individual patient received (Figure 1). The correlation between the randomized dose intensification strategy and the cumulative diuretic received over the 72-hour period was rpb=0.54, p<0.001. The correlation between the randomized intensification strategy and net fluid output was rpb=0.22 (p<0.001), and the correlation between net fluid output and cumulative diuretic received over the 72-hour period was r=0.16 (p=0.009). The randomized intensification strategy appeared to be significantly more important than the dose of diuretic in determining net fluid output, as the intensification strategy remained highly significantly associated with fluid output (beta=0.20, p=0.006) whereas the cumulative diuretic dose was not (beta=0.03, p=0.68) in a multivariable model containing both parameters.
Figure 1.
Frequency plot of the cumulative dose of intravenous loop diuretic received over the 72-hour intervention period demonstrating substantial overlap in absolute dose received between the high and low intensification strategies
Association of outcomes, study arm and individual metrics of decongestion or toxicity
Over a median follow up of 50 days (21 to 61 days), 141 (45.8%) patients experienced the primary composite endpoint of death, rehospitalization, or an emergency room visit. The cumulative amount of intravenous loop diuretic received during the 72-hour intervention period was not associated with the composite endpoint (HR=1.0 per 100 mg furosemide equivalents, 95% CI 0.99–1.1, p=0.14). This lack of association with the composite outcome was similar in both the high- and low-intensification groups (p interaction= 0.51). Similarly, the total fluid output during the first 72 hours had only a small and borderline significant association with outcomes (HR=0.97 per 500 cc, 95% CI 0.94–1.0, p=0.056) which was similar between the high- and low-intensification strategies (p interaction=0.26). As previously reported, randomization to the high-dose strategy was not associated with a statistically significant improvement in death, rehospitalization or emergency room visitation in univariable analysis (HR=0.83, 95% CI 0.60–1.2, p=0.28, Figure 2A; Supplementary Table 1, model 1). This was unchanged after adjusting for baseline characteristics including the baseline loop diuretic dose (p=0.24).
Competing effects between diuretic-mediated decongestion and toxicity
After adjustment for the cumulative diuretic received over the 72-hour intervention period, a significant relationship emerged with improved outcomes in the high-dose intensification strategy group (HR=0.64, 95% CI 0.43–0.95, p=0.028; Figure 2B; Supplementary Table 1, model 2). This relationship remained stable after adjustment for baseline characteristics (HR=0.60, 95% CI 0.38–0.95, p=0.029; Supplementary Table 1, model 3). Further, it did not appear to be confounded by a requirement for higher doses among patients with reduced renal function, as adjustment for eGFR did not eliminate the effect (HR=0.66, p=0.042, 95% CI 0.44–0.99) nor was there any significant interaction between eGFR and cumulative diuretic dose (p interaction=0.67). However, adjusting for net fluid output during the intervention period moved the point estimate closer to unity (HR=0.95, 95% CI 0.67–1.4, p=0.79; Figure 2C; Supplementary Table 1, model 4). After adjustment for multiple metrics of decongestion (change in weight, net fluid loss, dyspnea visual analog scale at 72 hours, global symptom visual analog scale at 72 hours, congestion status, worsening heart failure, early transition to oral diuretics, length of stay), the point estimate further diverged from that consistent with benefit (HR=1.1, 95% CI 0.7–1.5, p=0.76; Supplementary Table 1, model 5). Adjusting for the cumulative dose of loop diuretic in the above multivariable model did not “rescue” the association (HR=0.98, 95% CI 0.61–1.6, p=0.94; Supplementary Table 1, model 6) suggesting that the benefit of the high intensification strategy was mediated via decongestion. Moreover, these findings did not appear to be the product of identifying “super-responders” or diuretic resistant patients since there was no interaction between the cumulative dose of loop diuretic received and the resultant fluid output on the rate of adverse outcomes (p interaction=0.70) and outcomes were similar between the four groups characterized by high vs. low fluid loss and high vs. low cumulative loop diuretic dose (Figure 3).
Proximate outcomes: length of stay and worsening heart failure
To address potential confounding introduced during the 60-day interval between randomization and assessment of our primary endpoint, we performed a secondary analysis on the more proximate outcomes of length of stay and worsening heart failure. In a linear model assessing the influence of high- versus low-intensity dosing and route of administration on length of stay, no significant effect was attributable to the high-versus low-dose arm (beta=−0.01, p=0.80). However, upon adjustment for the cumulative dose of diuretic, a significant effect emerged (beta=−0.15, p=0.03). Similar to the primary analysis, after adjustment for fluid output, the effect of dose intensification on length of stay was minimal (beta=−0.01, p=0.86). In a logistic model with the outcome of worsening heart failure at 72 hours, there was a borderline significant protective effect attributable to randomization to the high dose strategy (OR=0.55, p=0.049, 95% CI 0.30–0.998). However, upon adjustment for total diuretic dose, this effect was significantly strengthened (OR=0.25, p=0.001, 95% CI 0.11–0.56). Again, after adjustment for net fluid output, the effect was weakened (OR=0.60, p=0.11, 95% CI 0.32–1.13). Thus, similar to what was observed with hard clinical outcomes (death, rehospitalization, or ER visit), there appeared to be competing effects between dose-mediated toxicity and the beneficial effects of decongestion on short-term outcomes such as in-hospital worsening heart failure and length of stay.
Discussion
The principal finding from this exploratory analysis is that the outcomes associated with randomization to a more aggressive diuretic strategy appear to be mediated by the balance between the benefit from more aggressive decongestion and the risk associated with higher doses of loop diuretics. Notably, after accounting for the total amount of loop diuretic received during the intervention period, a substantial and statistically significant benefit of randomization to a more aggressive decongestion strategy emerged. To the contrary, adjusting for the degree of decongestion achieved during the intervention period removed any trend toward a beneficial effect of the high intensification strategy. These hypothesis-generating observations provide additional support that aggressive decongestion may be an important therapeutic target in patients with ADHF.
It is well described in animals and in humans that loop diuretics have direct and potentially harmful effects.15, 30–37 Much of this may be secondary to interaction with the sodium potassium 2-chloride cotransporter (NKCC), the primary pharmacologic target of the loop diuretics responsible for their diuretic effect. However, this cotransporter also functions to deliver chloride to the cells of the macula densa, allowing the kidney to sense tubular delivery of sodium chloride.38 When this channel is blocked, the kidney perceives reduced salt delivery, and increases renin secretion and sympathetic nervous system activity accordingly.38 These effects have been demonstrated to have clinically measurable effects on hemodynamics in humans with decompensated heart failure, resulting in a decrease in stroke volume, increase in wedge pressure, and increase in systemic vascular resistance, all of which occur prior to meaningful natriuresis.15 Notably, in an animal model of experimentally induced heart failure, randomization to furosemide significantly accelerates the development of left ventricular systolic dysfunction.37 These mechanisms have been entertained as a possible causal explanation for the strong, dose-dependent association between loop diuretic dose and outcomes commonly observed in observational populations. However, higher doses of loop diuretics also likely lead to improved decongestion, which likely provides benefit that could offset the above toxic effects.
The randomized intervention in the DOSE trial provides a unique opportunity to investigate the competing risks and benefits from high dose loop diuretics. First, the confounding by indication that limits observational studies of diuretic use was eliminated by randomization. Additionally, disassociation of the intensity of decongestion (i.e., additional fluid loss) from the absolute dose of diuretic (i.e., the dose related adverse effects) was possible since the randomized high- vs. low-dose intervention was relative (to the baseline diuretic dose) rather than absolute, as shown by the overlap in absolute loop diuretic doses received between randomized groups. Further, randomization to the high dose intensification strategy was a much more important determinant of net fluid output than was the absolute loop diuretic dose administered. It should be emphasized that future randomized trials will be required to truly prove causality with respect to the importance of aggressive decongestion vs. dose-related adverse effects. However, the current results provide optimism that additional optimization of the risk/benefit ratio of our decongestive therapies will improve outcomes.
In addition to accounting for possible adverse effects of high doses of loop diuretics, this analysis also helps to account for the fact that some patients in the high-dose group did not receive low doses of diuretics compared to the overall study population, and some in the low-dose group received high doses compared to the study population. In the DOSE trial, the use of off-protocol loop diuretics or early discontinuation of study therapy prior to 72 hours was not uncommon. Furthermore, per protocol, dose adjustment was permitted at 48 hours and the rate of dose adjustment was not the same between randomized groups. Perhaps more importantly, the diuretic dose used to carry out the high vs. low dose strategy intervention was calculated from the home diuretic dose. Given that outpatient diuretic doses are largely empirically determined, in many cases the patient may have received a multiplier of the “wrong” outpatient dose as the study intervention. Of course, many of these factors were not avoidable in a real world clinical trial; however, they would lead to dilution of the actual intensity of diuretic therapy in each group, biasing the results toward the null. Adjusting for the cumulative amount of diuretic actually prescribed reduces these influences and, in some ways, provides an “on treatment” analysis focusing on the strategy of more versus less aggressive decongestion. It is not possible to determine to what degree the positive findings from the current analysis were driven by correcting for dose related loop diuretic toxicity or from correcting for dilution/crossover between randomized groups. However, in either case, the current results suggest that additional strategies that go beyond a non-selective relative increase in the dose of loop diuretic should be investigated.
Limitations
Given the post hoc-post randomization nature of this study, the limitations of retrospective analyses apply, residual confounding cannot be excluded, and causality is impossible to determine. First, it should be stressed that while the DOSE trial is the highest-quality trial of loop diuretic dosing ever conducted, there remain multiple limitations: 1) it was a small trial, not powered for outcomes, 2) the actual dose of diuretics was individualized based on pre-admission diuretic dose, 3) significant on- and off- protocol crossover between groups occurred, 4) enrolled participants were not selected to be in need of an aggressive diuretic dosing strategy, and 5) the number of mortality events was low and follow-up short. Furthermore, the DOSE trial did not reliably collect data on fluid output or other decongestion variables beyond the 72-hour intervention period, in addition to the fact that the randomized intervention only applied to the first 72 hours. As a result, the findings of this analysis may only apply to the early decongestive period. Additionally, the available surrogates used to determine decongestion in this analysis were crude and hemoconcentration or right heart catheterization parameters were unavailable. Although significant change in the parameter estimate and statistical significance between the randomized intervention and outcomes emerged with adjustment for either fluid loss or amount of diuretic received, because of the fact that the parameters used for adjustment were determined post-randomization, confounding may still be responsible for the effects. It is possible that the risks of randomization to a high dose loop diuretic strategy would be different after better accounting for the true degree of decongestion. Additionally, recent data have introduced equipoise regarding the degree of neurohormonal activation attributable to loop diuretic therapy in the modern era of neurohormonal blockade.39 As a result of the above limitations, these data should be regarded as hypothesis generating only and serve primarily to motivate additional research.
Conclusion
After accounting for the actual dose of loop diuretic administered, a benefit from a randomized strategy for more aggressive diuresis became apparent. However, adjusting for the diuresis resulting from this strategy completely eliminated this benefit. These hypothesis-generating observations suggest that a more aggressive approach toward diuresis may directly improve long term outcomes, should steps be taken to limit the dose-related adverse effects of loop diuretics. Given the current epidemic of ADHF hospitalizations and the poor outcomes in these patients, these findings indicate that additional prospective research on diuretic and decongestive strategies is critically needed.
Supplementary Material
Acknowledgments
Funding sources: This work was supported by NIH Grants, K23HL114868, L30HL115790 (JT), and K23DK097201 (FPW): The funding source had no role in study design, data collection, analysis or interpretation.
This manuscript was prepared using DOSE research materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the DOSE study investigators or the NHLBI.
Footnotes
Conflict of Interest Disclosures: The authors report no relationships that could be construed as a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giamouzis G, Kalogeropoulos A, Georgiopoulou V, Laskar S, Smith AL, Dunbar S, Triposkiadis F, Butler J. Hospitalization epidemic in patients with heart failure: risk factors, risk prediction, knowledge gaps, and future directions. Journal of Cardiac Failure. 2011;17(1):54–75. doi: 10.1016/j.cardfail.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Fonarow GC, Corday E. Overview of acutely decompensated congestive heart failure (ADHF): a report from the ADHERE registry. Heart Fail Rev. 2004;9(3):179–85. doi: 10.1007/s10741-005-6127-6. [DOI] [PubMed] [Google Scholar]
- 3.Bradley SM, Levy WC, Veenstra DL. Cost-consequences of ultrafiltration for acute heart failure: a decision model analysis. Circulation Cardiovascular quality and outcomes. 2009;2(6):566–73. doi: 10.1161/CIRCOUTCOMES.109.853556. [DOI] [PubMed] [Google Scholar]
- 4.Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. The American Journal of Medicine. 2006;119(12 Suppl 1):S3–S10. doi: 10.1016/j.amjmed.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail. 2010;12(5):423–33. doi: 10.1093/eurjhf/hfq045. [DOI] [PubMed] [Google Scholar]
- 6.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53(7):557–573. doi: 10.1016/j.jacc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for Heart Failure: Problems and Perspectives. J Am Coll Cardiol. 2012 doi: 10.1016/j.jacc.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 8.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122(3):265–72. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Testani JM, Brisco MA, Chen J, McCauley BD, Parikh CR, Tang WH. Timing of hemoconcentration during treatment of acute decompensated heart failure and subsequent survival: importance of sustained decongestion. J Am Coll Cardiol. 2013;62(6):516–24. doi: 10.1016/j.jacc.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Meer P, Postmus D, Ponikowski P, Cleland JG, O’Connor CM, Cotter G, Metra M, Davison BA, Givertz MM, Mansoor GA, Teerlink JR, Massie BM, Hillege HL, Voors AA. The predictive value of short term changes in hemoglobin concentration in patients presenting with acute decompensated heart failure. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2012.12.050. [DOI] [PubMed] [Google Scholar]
- 11.Greene SJ, Gheorghiade M, Vaduganathan M, Ambrosy AP, Mentz RJ, Subacius H, Maggioni AP, Nodari S, Konstam MA, Butler J, Filippatos G. Haemoconcentration, renal function, and post-discharge outcomes among patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. European journal of heart failure. 2013;15(12):1401–11. doi: 10.1093/eurjhf/hft110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neuberg GW, Miller AB, O’Connor CM, Belkin RN, Carson PE, Cropp AB, Frid DJ, Nye RG, Pressler ML, Wertheimer JH, Packer M. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144(1):31–8. doi: 10.1067/mhj.2002.123144. [DOI] [PubMed] [Google Scholar]
- 13.Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. The American Journal of Cardiology. 2006;97(12):1759–1764. doi: 10.1016/j.amjcard.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 14.Felker GM, Mentz RJ. Diuretics and ultrafiltration in acute decompensated heart failure. J Am Coll Cardiol. 2012;59(24):2145–53. doi: 10.1016/j.jacc.2011.10.910. [DOI] [PubMed] [Google Scholar]
- 15.Francis GS, Siegel RM, Goldsmith SR, Olivari MT, Levine TB, Cohn JN. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohumoral axis. Ann Intern Med. 1985;103(1):1–6. doi: 10.7326/0003-4819-103-1-1. [DOI] [PubMed] [Google Scholar]
- 16.Testani JM, Cappola TP, Brensinger CM, Shannon RP, Kimmel SE. Interaction between loop diuretic-associated mortality and blood urea nitrogen concentration in chronic heart failure. J Am Coll Cardiol. 2011;58(4):375–82. doi: 10.1016/j.jacc.2011.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mascha EJ, Dalton JE, Kurz A, Saager L. Statistical grand rounds: understanding the mechanism: mediation analysis in randomized and nonrandomized studies. Anesth Analg. 2013;117(4):980–94. doi: 10.1213/ANE.0b013e3182a44cb9. [DOI] [PubMed] [Google Scholar]
- 18.Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res. 2010;19(3):237–70. doi: 10.1177/0962280209105014. [DOI] [PubMed] [Google Scholar]
- 19.Richiardi L, Bellocco R, Zugna D. Mediation analysis in epidemiology: methods, interpretation and bias. Int J Epidemiol. 2013;42(5):1511–9. doi: 10.1093/ije/dyt127. [DOI] [PubMed] [Google Scholar]
- 20.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 21.Bohm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376(9744):886–94. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 22.Vergeer M, Bots ML, van Leuven SI, Basart DC, Sijbrands EJ, Evans GW, Grobbee DE, Visseren FL, Stalenhoef AF, Stroes ES, Kastelein JJ. Cholesteryl ester transfer protein inhibitor torcetrapib and off-target toxicity: a pooled analysis of the rating atherosclerotic disease change by imaging with a new CETP inhibitor (RADIANCE) trials. Circulation. 2008;118(24):2515–22. doi: 10.1161/CIRCULATIONAHA.108.772665. [DOI] [PubMed] [Google Scholar]
- 23.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O’Connor CM. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364(9):797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felker GM, O’Connor CM, Braunwald E. Loop diuretics in acute decompensated heart failure: necessary? Evil? A necessary evil? Circulation Heart failure. 2009;2(1):56–62. doi: 10.1161/CIRCHEARTFAILURE.108.821785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brater DC, Day B, Burdette A, Anderson S. Bumetanide and furosemide in heart failure. Kidney Int. 1984;26(2):183–9. doi: 10.1038/ki.1984.153. [DOI] [PubMed] [Google Scholar]
- 26.Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin Pharmacol Ther. 1995;57(6):601–9. doi: 10.1016/0009-9236(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 27.Brenner BM, Rector FC. Brenner & Rector’s the kidney. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Annals of Internal Medicine. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 30.Tuck ML, Sambhi MP, Kramer SB, Eggena P, Barrett J. Enhanced renin levels after discontinuation of furosemide: additional effects of loop diuretics on renin release. Clin Exp Hypertens A. 1982;4(8):1359–75. doi: 10.3109/10641968209060795. [DOI] [PubMed] [Google Scholar]
- 31.Kaissling B, Bachmann S, Kriz W. Structural adaptation of the distal convoluted tubule to prolonged furosemide treatment. Am J Physiol. 1985;248(3 Pt 2):F374–81. doi: 10.1152/ajprenal.1985.248.3.F374. [DOI] [PubMed] [Google Scholar]
- 32.Mulder H, Schopman W, Jr, van der Lely AJ, Schopman W., Sr Acute changes in plasma renin activity, plasma aldosterone concentration and plasma electrolyte concentrations following furosemide administration in patients with congestive heart failure--interrelationships and diuretic response. Horm Metab Res. 1987;19(2):80–3. doi: 10.1055/s-2007-1011744. [DOI] [PubMed] [Google Scholar]
- 33.Ellison DH, Velazquez H, Wright FS. Adaptation of the distal convoluted tubule of the rat. Structural and functional effects of dietary salt intake and chronic diuretic infusion. J Clin Invest. 1989;83(1):113–26. doi: 10.1172/JCI113847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gottlieb SS, Skettino SL, Wolff A, Beckman E, Fisher ML, Freudenberger R, Gladwell T, Marshall J, Cines M, Bennett D, Liittschwager EB. Effects of BG9719 (CVT-124), an A1-adenosine receptor antagonist, and furosemide on glomerular filtration rate and natriuresis in patients with congestive heart failure. J Am Coll Cardiol. 2000;35(1):56–59. doi: 10.1016/s0735-1097(99)00532-x. [DOI] [PubMed] [Google Scholar]
- 35.Abdallah JG, Schrier RW, Edelstein C, Jennings SD, Wyse B, Ellison DH. Loop diuretic infusion increases thiazide-sensitive Na(+)/Cl(-)-cotransporter abundance: role of aldosterone. J Am Soc Nephrol. 2001;12(7):1335–41. doi: 10.1681/ASN.V1271335. [DOI] [PubMed] [Google Scholar]
- 36.Chen HH, Redfield MM, Nordstrom LJ, Cataliotti A, Burnett JC., Jr Angiotensin II AT1 receptor antagonism prevents detrimental renal actions of acute diuretic therapy in human heart failure. American journal of physiology Renal physiology. 2003;284(5):F1115–9. doi: 10.1152/ajprenal.00337.2002. [DOI] [PubMed] [Google Scholar]
- 37.McCurley JM, Hanlon SU, Wei SK, Wedam EF, Michalski M, Haigney MC. Furosemide and the progression of left ventricular dysfunction in experimental heart failure. J Am Coll Cardiol. 2004;44(6):1301–1307. doi: 10.1016/j.jacc.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 38.Castrop H, Schiessl IM. Physiology and pathophysiology of the renal Na-K-2Cl cotransporter (NKCC2) American journal of physiology Renal physiology. 2014;307(9):F991–F1002. doi: 10.1152/ajprenal.00432.2014. [DOI] [PubMed] [Google Scholar]
- 39.Mentz RJ, Stevens SR, DeVore AD, Lala A, Vader JM, AbouEzzeddine OF, Khazanie P, Redfield MM, Stevenson LW, O’Connor CM, Goldsmith SR, Bart BA, Anstrom KJ, Hernandez AF, Braunwald E, Felker GM. Decongestion strategies and renin-angiotensin-aldosterone system activation in acute heart failure. JACC Heart failure. 2015;3(2):97–107. doi: 10.1016/j.jchf.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.