Abstract
Aims
Hyponatraemia is strongly associated with adverse outcomes in heart failure. However, accumulating evidence suggests that chloride may play an important role in renal salt sensing and regulation of neurohormonal and sodium-conserving pathways. Our objective was to determine the prognostic importance of hypochloraemia in patients with heart failure.
Methods and results
Patients in the BEST trial with baseline serum chloride values were evaluated (n = 2699). Hypochloraemia was defined as a serum chloride ≤96 mmol/L and hyponatraemia as serum sodium ≤135 mmol/L. Hypochloraemia was present in 13.0% and hyponatraemia in 13.7% of the population. Chloride and sodium were only modestly correlated (r = 0.53), resulting in only 48.7% of hypochloraemic patients having concurrent hyponatraemia. Both hyponatraemia and hypochloraemia identified a population with greater disease severity; however, renal function tended to be worse and loop diuretic doses higher with hypochloraemia. In univariate analysis, lower serum sodium or serum chloride as continuous parameters were each strongly associated with mortality (P < 0.001). However, when both parameters were included in the same model, serum chloride remained strongly associated with mortality [hazard ratio (HR) 1.3 per standard deviation decrease, 95% confidence interval (CI) 1.18–1.42, P < 0.001], whereas sodium was not (HR 0.97 per standard deviation decrease, 95% CI 0.89–1.06, P = 0.52).
Conclusion
Serum chloride is strongly and independently associated with worsened survival in patients with chronic heart failure and accounted for the majority of the risk otherwise attributable to hyponatraemia. Given the critical role of chloride in a number of regulatory pathways central to heart failure pathophysiology, additional research is warranted in this area.
Keywords: Hypochloraemia, Serum chloride, Hyponatraemia, Serum sodium, Heart failure, Mortality
Introduction
Hyponatraemia is a common electrolyte disorder in patients with heart failure.1 A substantial body of literature now exists indicating that even mild hyponatraemia is a robust predictor of adverse events including worsened survival, rehospitalization, prolonged length of stay, and increased hospital resource utilization and cost.1–3 This consistent signal for adverse outcomes, which is independent of traditional disease severity indicators, has motivated the hypothesis that correction of hyponatraemia may improve outcomes.4–9 Unfortunately, recent trials of vasopressin antagonists have yielded disappointing effects on short- and long-term outcomes despite significant improvement in serum sodium levels.10,11
Reductions in serum sodium concentrations, by definition, cannot occur in isolation. In order to preserve electroneutrality, an anion such as chloride or bicarbonate must be reduced in parallel with the sodium. Serum chloride is traditionally viewed as a passive anion linked to sodium concentration; however, accumulating evidence would argue to the contrary.12 It has been recognized for decades that chloride is the predominant ion responsible for renal salt-sensing mechanisms, with a limited direct role for sodium.13–16 Furthermore, a family of serine-threonine kinases [With-No-Lysine (K), WNK] has recently been demonstrated to play a key role in the regulation of sodium chloride homeostasis, the actions of the renin–angiotensin–aldosterone system, and the transporters upon which loop and thiazide diuretics work.17 Notably, chloride appears to bind directly to the catalytic site of these kinases, regulating their ability to phosphorylate important sodium-regulatory pathways.18,19 Analogous direct regulatory functions of sodium have not been described.
Given the direct role of chloride in regulating neurohormonal activation and sodium homeostasis, two critical pathways in heart failure, we hypothesized that serum chloride may be an important prognostic factor and may explain much of the risk otherwise attributable to serum sodium. Our goal in the current analysis was to describe the clinical characteristics associated with low serum chloride and determine the independent prognostic importance of serum sodium and serum chloride in patients with chronic heart failure.
Methods
The Beta-Blocker Evaluation of Survival Trial (BEST) was a National Heart, Lung, and Blood Institute- (NHLBI) supported randomized placebo-controlled trial investigating the impact of bucindolol, a non-selective beta-blocker, on all-cause mortality in chronic heart failure patients. The design and primary results have been previously published.20 Briefly, 2708 patients with NYHA functional class III or IV heart failure, an LVEF of ≤35%, and use of an ACE inhibitor for ≥1 month (unless contraindicated) were randomized to bucin-dolol or placebo. Exclusion criteria included reversible heart failure, uncorrected primary valvular disease, decompensated heart failure, life expectancy of <3 years, a serum creatinine level of ≥3.0 mg/dL, or the use of a beta-blocker within 30 days of baseline.
Hyponatraemia was defined as a serum sodium concentration ≤135 mmol/L and hypochloraemia as a serum chloride concentration ≤96 mmol/L, consistent with previously published definitions.21,22 Baseline laboratory studies, including serum chloride and sodium levels, were obtained at the screening visit, which could occur in either outpatients or hospitalized patients. The primary results were similar regardless of the location of the screening visit (P for interaction = 0.42) and thus the cohort was analysed as a whole. Loop diuretic doses were converted to furosemide equivalents, with 1 mg of bumetanide = 20 mg of torsemide = 40 mg of furosemide.23 The chronic kidney disease epidemiology collaboration equation was used to calculate estimated glomerular filtration rate (eGFR). This research was determined to qualify as exempt by the Yale institutional review board.
Statistical analysis
Values reported are mean ± standard deviation, median (quartile 1–quartile 3), and percentile. Independent Student’s t-test or the Wilcoxon Rank Sum test was used to compare continuous variables. The χ2 test was used to evaluate associations between categorical variables. Correlation coefficients reported are Spearman’s rho for continuous variables and, in the case of two dichotomous variables, correlations are reported are phi. Linear regression was used to determine if an independent association between either log-transformed loop diuretic dose or log-transformed norepinephrine and serum chloride was present after controlling for serum sodium and/or bicarbonate concentrations. Cox proportional hazards modelling was used to evaluate time to event associations between serum electrolyte levels (both categorical and continuous) and all-cause mortality. Candidate covariates entered in the model were baseline characteristics with univariate all-cause mortality associations P ≤0.2. Covariates that had a P > 0.2 but a theoretical basis for potential confounding were forced into the model. Models were built using backward elimination (likelihood ratio test) where all covariates with a P < 0.2 were retained. Kaplan–Meier curves for death from any cause were plotted for the four combinations of groups between hypochloraemia and hyponatraemia. The x-axis was terminated when the remaining number at risk was <10%. The primary mortality findings were validated in patients with data available on serum chloride surviving to the 3-month (n = 2481) and 12-month (n = 1936) study visits. To visually assess non-linear relationships between serum sodium and chloride and mortality risk, univariate Cox regression using restricted cubic spline-smoothed baseline sodium and chloride was performed. Plots of mortality risk over sodium and chloride were then created. The added prognostic value of serum chloride and sodium with respect to mortality compared with a multivariable model was assessed using the gain in receiver operating characteristic (ROC) area under the curve (AUC), category-free net reclassification improvement (NRI), and integrated discrimination index (IDI). AUC comparison was performed using nested likelihood ratio tests The Hosmer–Lemeshow test was used to assess calibration for each model. Statistical analysis was performed with IBM SPSS Statistics version 21 (IBM Corp., Armonk, NY, USA) and Stata version 13 (StataCorp, College Station, TX, USA), and statistical significance was defined as two-tailed P < 0.05 excluding interactions, where a P < 0.10 was considered significant.
Results
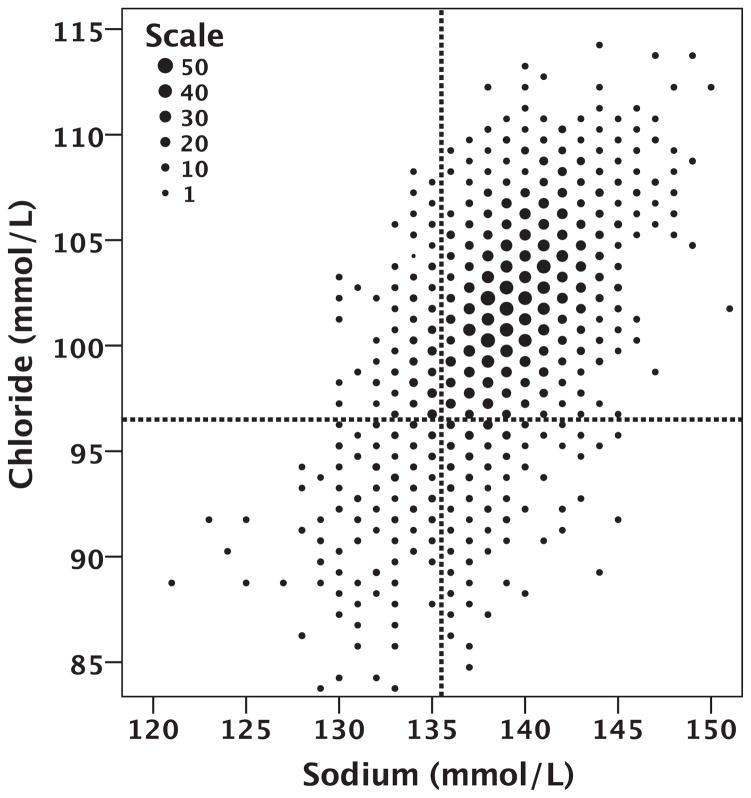
Of the 2708 patients enrolled in the BEST trial, 2699 (99.7%) had data on baseline serum sodium and serum chloride levels available for analysis. The mean serum sodium was 139.0 ± 3.4 mmol/L and the mean serum chloride level was 101.3 ± 4.5 mmol/L. The distribution of values in the population can be seen in Figure 1 and in the Supplementary material online, Figure S1. Hyponatraemia (serum sodium ≤135 mmol/L) was present in 13.7% of the population, and hypochloraemia (serum chloride ≤96 mmol/L) was present in a similar 13.0% of the population. The correlation between serum sodium and chloride was surprisingly modest (r = 0.53, P < 0.001; Figure 1; Table 2). Due to this limited overall correlation, amongst patients with hyponatraemia, only 46.3% also had hypochloraemia and, amongst those with hypochloraemia, only 48.7% had hyponatraemia (Figure 1).
Figure 1.
Scatterplot of baseline serum chloride and serum sodium. The size of the dot indicates the number of patients at each integer value of sodium and chloride with that value as per the ‘Scale’ legend.
Table 2.
Correlation of serum chloride and sodium with parameters of renal function
| Sodium
|
Chloride
|
|||
|---|---|---|---|---|
| r | P-value | r | P-value | |
| Sodium | – | – | 0.53 | <0.001* |
| Chloride | 0.53 | <0.001* | – | – |
| Bicarbonate | 0.05 | 0.02* | −0.35 | <0.001* |
| eGFR | 0.01 | 0.67 | 0.08 | <0.001* |
| BUN | −0.13 | <0.001* | −0.19 | <0.001* |
| Creatinine | −0.01 | 0.755 | −0.06 | 0.004* |
| BUN/creatinine ratio | −0.18 | <0.001* | −0.21 | <0.001* |
BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate.
Significant P-value.
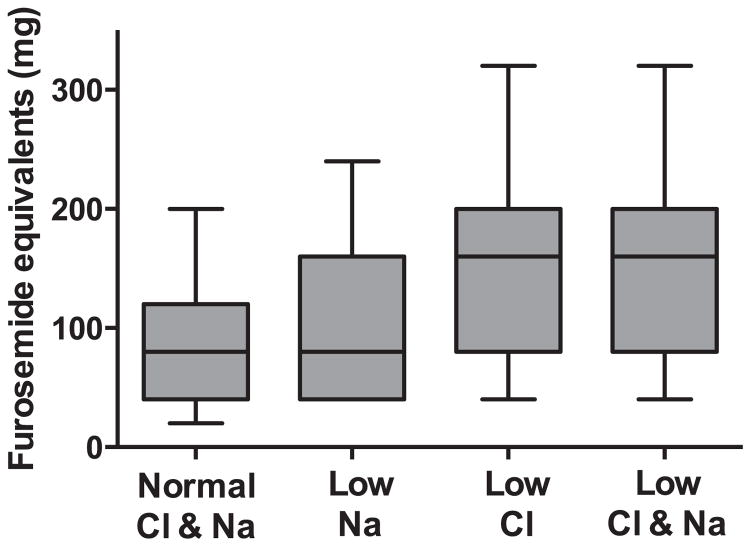
Similar to previous reports, in patients with hyponatraemia the majority of baseline characteristics were consistent with a greater burden of disease severity (Table 1). This overall pattern was also observed in patients with hypochloraemia (Table 1). Notable differences in the pattern of baseline characteristics were a more pronounced relationship between serum chloride and metrics of renal function and serum bicarbonate than that observed with sodium (Tables 1 and 2). The association with baseline loop diuretic dose tended to be stronger for serum chloride (r = −0.34, P < 0.001; Figure 2) than for serum sodium (r = −0.14, P < 0.001; Figure 2) or serum bicarbonate (r = 0.17, P < 0.001). In a linear regression model including all three parameters, only serum chloride remained significantly associated with the dose of loop diuretic (P < 0.001). The correlation between plasma norepinephrine and chloride was very weak but statistically significant (r = −0.08, P < 0.001). A similar significant correlation was not observed with serum sodium (r = −0.04, P = 0.07). When both parameters were included in a regression equation, log-transformed norepinephrine remained associated with chloride (P = 0.004) but not with sodium (P = 0.94).
Table 1.
Baseline characteristics of the study population grouped by hypochloraemia and hyponatraemia
| Characteristics | Hypochloraemia (≤96 mmol/L)
|
Hyponatraemia (≤135 mmol/L)
|
||||
|---|---|---|---|---|---|---|
| No (n =2348) | Yes (n =351) | P-value | No (n =2330) | Yes (n =369) | P-value | |
| Demographics | ||||||
| Age | 60.34 ± 12.4 | 59.6 ± 12.2 | 0.268 | 60.6 ± 12.4 | 58.3 ± 12.2 | 0.001* |
| White race | 69.6% | 72.1% | 0.343 | 69.7% | 71.0% | 0.624 |
| Male | 78.0% | 78.6% | 0.797 | 77.9% | 78.1% | 0.607 |
| Past medical history | ||||||
| Obstructive coronary artery disease | 47.9% | 53.6% | 0.047* | 48.8% | 47.4% | 0.624 |
| Hypertension | 40.8% | 43.9% | 0.268 | 39.8% | 49.6% | <0.001* |
| Diabetes | 67.0% | 46.7% | <0.001* | 66.0% | 54.2% | <0.001* |
| Physical examination | ||||||
| Heart rate | 81.6 ± 13.2 | 86.0 ± 14.0 | <0.001* | 81.7 ± 13.2 | 85.4 ± 14.5 | <0.001* |
| Systolic blood pressure (mmHg) | 119.5 ± 19.4 | 111.4 ± 17.7 | <0.001* | 119.4 ± 19.4 | 112.4 ± 18.4 | <0.001* |
| Medications (baseline) | ||||||
| Digoxin | 91.5% | 96.3% | 0.002* | 91.6% | 95.7% | 0.007* |
| Vasodilators | 43.6% | 44.7% | 0.694 | 43.4% | 45.8% | 0.395 |
| ACE inhibitor | 91.7% | 92.0% | 0.856 | 91.7% | 92.1% | 0.783 |
| Bucindolol | 49.9% | 50.1% | 0.937 | 49.2% | 54.5% | 0.061 |
| Median furosemide equivalents (mg) | 80 (40–120) | 160 (80–200) | <0.001* | 80 (40–120) | 80 (40–160) | <0.001* |
| Mean furosemide equivalents (mg) | 86 ± 78 | 157 ± 118 | 90 ± 85 | 127 ± 100 | ||
| Laboratory value | ||||||
| Haemoglobin (g/dL) | 13.9 ± 1.6 | 14.0 ± 1.8 | 0.365 | 14.0 ± 1.7 | 13.9 ± 1.7 | 0.628 |
| Serum sodium (mmol/L) | 139.5 ± 2.9 | 135.2 ± 3.7 | <0.001* | 139.9 ± 2.5 | 133.2 ± 2.1 | <0.001* |
| Serum chloride (mmol/L) | 102.5 ± 3.3 | 93.2 ± 2.8 | <0.001* | 102.1 ± 4.0 | 96.5 ± 4.6 | <0.001* |
| Serum bicarbonate (mmol/L) | 27.0 ± 3.2 | 29.2 ± 3.9 | <0.001* | 27.3 ± 3.4 | 27.0 ± 3.5 | 0.120 |
| Glomerular filtration rate (mL/min/1.73 m2) | 66.2 ± 22.7 | 60.0 ± 25.3 | <0.001* | 63.8 ± 23.0 | 63.1 ± 23.9 | 0.042* |
| Glomerular filtration rate <60 mL/min/1.73 m2 | 38.8% | 55.0% | <0.001* | 40.1% | 46.1% | 0.030* |
| Blood urea nitrogen (mg/dL) | 23.3 ± 14.0 | 34.9 ± 20.1 | <0.001* | 23.8 ± 14.3 | 31.3 ± 14.3 | <0.001* |
| Creatinine (mg/dL) | 1.2 ± 0.4 | 1.4 ± 0.5 | <0.001* | 1.2 ± 0.4 | 1.3 ± 0.4 | 0.003* |
| Blood urea nitrogen to creatinine ratio | 18.7 ± 7.5 | 24.9 ± 10.2 | <0.001* | 18.9 ± 7.9 | 23.0 ± 9.2 | <0.001* |
| Functional status/ejection fraction | ||||||
| Left ventricular ejection fraction (%) | 23.3 ± 7.3 | 21.2 ± 7.0 | <0.001* | 23.3 ± 7.3 | 21.3 ± 6.9 | <0.001* |
| New York Heart Association class | 3.1 ± 0.3 | 3.2 ± 0.4 | 0.001* | 3.1 ± 0.3 | 3.1 ± 0.3 | 0.063 |
Significant P-value.
Figure 2.
Box plot of baseline loop diuretic dose based on groups defined by the presence or absence of hyponatraemia or hypochloraemia. Boxes represent the 25th to 75th percentile, the line represents the median, and whiskers the 10th and 90th percentile. P < 0.001 for all comparisons apart from the comparison between patients with isolated hypochloraemia and those with hypochloraemia and hyponatraemia (rightward two boxes) where P = 0.72.
Associations with mortality
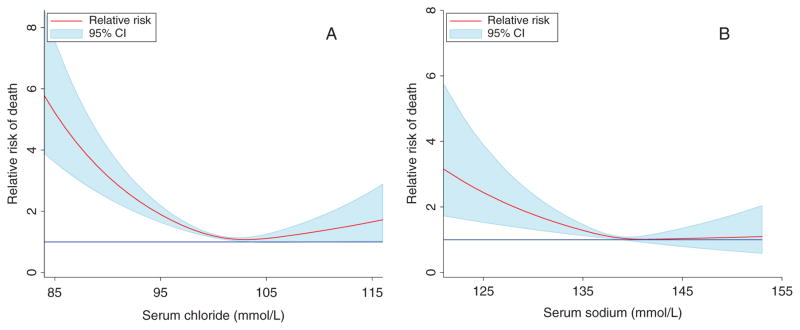
Over a median follow-up of 737 days (400–1049), 857 patients (31.8%) died. Both serum sodium and serum chloride were strongly related to mortality in univariate models, whereas the serum bicarbonate level was unrelated to mortality (Table 3). However, in a multivariable model containing both serum sodium and chloride, serum chloride remained highly significantly associated with survival, whereas the association between serum sodium and mortality was completely eliminated (Table 3). This relationship held following adjustment for baseline characteristics including medication use, CAD, diabetes, hypertension, age, gender, EF, systolic blood pressure, NYHA class, loop diuretic dose, haemoglobin level, eGFR, blood urea nitrogen, and the blood urea nitrogen to creatinine ratio (Table 3). The prognostic importance of serum chloride was not different in subgroups defined by an eGFR <60 mL/min/1.73 m2, an EF below the median value (23%), randomization to bucindolol, ACE inhibitor use, or a loop diuretic dose >160 mg/day (P for interaction ≥0.13 for all). Adjustment for serum potassium did not diminish the prognostic importance of serum chloride (adjusted for sodium and potassium HR 1.33, 95% CI 1.22–1.45, P < 0.0001). The association of both serum chloride and sodium with mortality was J-shaped (Figure 4). The prognostic value of serum chloride and sodium on parameters such as the improvement in ROC AUC, category-free NRI, and IDI can be found in the Supplementary material online, Table S1.
Table 3.
Adjusted and unadjusted associations with mortality at baseline, 3 months, and 12 months
| Baseline
|
3 months
|
12 months
|
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Univariate | ||||||
| Sodium | 1.13 (1.05–1.20) | 0.001* | 1.09 (1.01–1.17) | 0.022* | 1.15 (1.05–1.27) | 0.003* |
| Chloride | 1.27 (1.18–1.35) | <0.001* | 1.25 (1.16–1.34) | <0.001* | 1.34 (1.22–1.46) | <0.001* |
| Bicarbonate | 1.05 (0.98–1.13) | 0.152 | 1.01 (0.94–1.09) | 0.729 | 1.03 (0.93–1.13) | 0.611 |
| Multivariable | ||||||
| Sodium | 0.97 (0.89–1.06) | 0.521 | 0.89 (0.81–0.98) | 0.015* | 0.90 (0.80–1.02) | 0.091 |
| Chloride | 1.30 (1.18–1.42) | <0.001* | 1.38 (1.25–1.52) | <0.001* | 1.49 (1.31–1.69) | <0.001* |
| Bicarbonate | 1.03 (0.96–1.11) | 0.430 | 1.11 (1.02–1.20) | 0.022* | 1.13 (1.01–1.25) | 0.031* |
| Multivariable with adjustment for baseline characteristics | ||||||
| Sodium | 0.93 (0.86–1.02) | 0.147 | 0.91 (0.83–1.01) | 0.063 | 0.95 (0.84–1.07) | 0.392 |
| Chloride | 1.19 (1.08–1.31) | <0.001* | 1.24 (1.12–1.38) | <0.001* | 1.34 (1.17–1.53) | <0.001* |
| Bicarbonate | 1.02 (0.95–1.11) | 0.547 | 1.09 (1.00–1.19) | 0.063 | 1.08 (0.97–1.21) | 0.148 |
Baseline characteristics adjusted for include: medications (bucindolol, digoxin, loop diuretic dose, ACE inhibitor, and vasodilators), coronary artery disease, diabetes, hypertension, age, gender, ejection fraction, systolic blood pressure, New York Heart Association class, haemoglobin level, estimated glomerular filtration rate, blood urea nitrogen, and the blood urea nitrogen to creatinine ratio.
CI, confidence interval; HR, hazard ratio.
The HR is expressed per standard deviation decrease for chloride and sodium and per standard deviation increase for bicarbonate.
Figure 4.
Relationship between serum chloride (A) and serum sodium (B) and mortality risk. Risk was calculated relative to the cohort mean value (101.3 mmol/L for serum chloride and 138.9 mmol/L for serum sodium.) The blue reference line indicates a relative risk of 1. CI, confidence interval.
Similar observations with respect to chloride and sodium were evident at both 3 and 12 months (Table 3). Despite the fact that serum electrolyte levels were stable over time on a population level (Supplementary material online, Figure S2), there was significant prognostic value with serial values of serum chloride (Supplementary material online, Figure S3). Notably, the presence of hypochloraemia at both baseline and 3 or 12 months was consistently associated with worse prognosis than for those patients who never had hypochloraemia or had transient hypochloraemia (Supplementary material online, Figure S3).
Associations with categorical variables
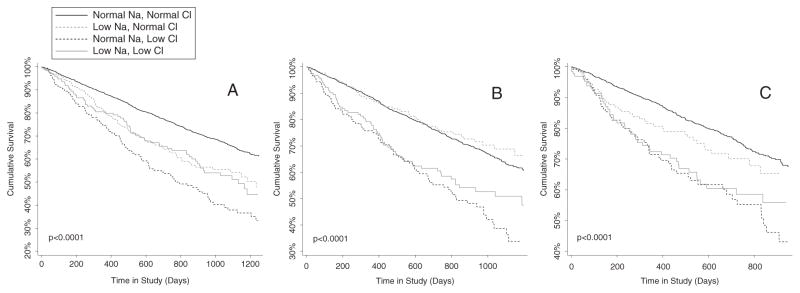
Similar observations were noted when evaluating the dichotomous variables, where hyponatraemia (HR 1.5, 95% CI 1.2–1.8, P < 0.001) and hypochloraemia (HR 1.9, 95% CI 1.6–2.3, P < 0.001) were strongly associated with mortality in univariate models. However, despite a limited correlation between these parameters (r = 0.39, P < 0.001), when the two were evaluated in the same model hypochloraemia remained significantly associated with survival (HR 1.8, 95% CI 1.5–2.2, P < 0.001) whereas hyponatraemia was not (HR 1.1, 95% CI 0.93–1.4, P = 0.23). These findings were consistently identified at baseline, 3, and 12 months where hypochloraemia was consistently associated with a higher risk of death (Figure 3A–C).
Figure 3.
Survival plots of the groups defined by hypochloraemia and hyponatraemia at baseline (A), 3 months (B), and 12 months (C). Low Na: hyponatraemia defined as a serum sodium ≤135 mmol/L. Low Cl: hypochloraemia defined as serum chloride ≤96 mmol/L. P < 0.001 for overall differences between groups. P ≤0.017 for the difference between isolated hypochloraemia and isolated hyponatraemia at all time points.
Interaction with serum bicarbonate level
As discussed above, serum bicarbonate did not have a univariate or multivariable association with mortality (Table 3). Similarly, there were no differences in survival between tertiles of baseline serum bicarbonate (P = 0.23). However, a highly significant interaction was present between serum chloride and serum bicarbonate (adjusted P for interaction <0.0001). Notably, serum chloride demonstrated a much stronger association with mortality in patients with higher serum bicarbonate levels (Table 4). Similar trends were noted for serum sodium, with greater risk found in patients with higher serum bicarbonate levels (Table 4) but the interaction with sodium did not reach statistical significance (P for interaction = 0.23). A borderline significant interaction was present (P = 0.08) between the risk associated with hypochloraemia and a loop diuretic dose above or below the median (80 mg of furosemide equivalents). However, this interaction did not persist following multivariable adjustment (P = 0.38).
Table 4.
Risk of death associated with serum chloride and sodium levels across tertiles of serum bicarbonate
| Serum bicarbonate tertile
|
Serum chloride
|
Serum sodium | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| <26 mEq/L | 1.06 (0.93–1.21) | 0.386 | 1.07 (0.95–1.21) | 0.271 |
| 26–28 mEq/L | 1.21 (1.06–1.38) | 0.006* | 1.10 (0.98–1.24) | 0.119 |
| ≥29 mEq/L | 1.52 (1.37–1.70) | <0.001* | 1.21 (1.08–1.35) | 0.001* |
CI, confidence interval; HR, hazard ratio.
The HR is expressed per standard deviation decrease.
P < 0.0001 for chloride by tertiles of bicarbonate interaction and P = 0.14 for sodium by tertiles of bicarbonate interaction.
Significant P-value.
Discussion
The principal finding in this analysis is that a low serum chloride is strongly and independently associated with reduced survival in patients with chronic heart failure. Although serum sodium and chloride are often assumed to be very tightly correlated, we found that the serum sodium level could explain <30% of the variability in the serum chloride concentration. Notably, many patients had either hyponatraemia or hypochloraemia in the absence of the other electrolyte abnormality. Serum chloride had a stronger relationship with metrics of renal function and loop diuretic dose than did serum sodium. Importantly, the entirety of the survival disadvantage associated with low serum sodium was explainable by the serum chloride concentration, and hypochloraemia in the absence of hyponatraemia was a particularly ominous prognostic indicator. In the light of the growing literature demonstrating the regulatory importance of chloride, these results raise the question of whether chloride rather than sodium is the dominant cardio-renal electrolyte marker, and possibly target, in patients with heart failure.
The association between serum sodium and adverse outcomes has been the subject of a large number of studies; however, this is one of the first studies we are aware of in patients with heart failure that has evaluated the importance of the effects of serum chloride on outcomes.1,24,25 This lack of attention may have been driven by the fact that traditional wisdom has maintained that chloride is primarily the counter ion for sodium and of secondary importance.12 Searching the term ‘hyponatremia’ in Medline results in >11 000 citations, whereas searching ‘hypochloremia’ results in only 290 citations. Potentially as a result, the majority of heart failure studies that we have access to or are aware of have not collected serum chloride levels. These factors may explain why the current observations have not previously been identified, given that the driving force for hypothesis-driven research was absent and data availability was lacking.
The recent characterization of the WNK kinases as chloride-sensing kinases provides a possible mechanism by which hypochloraemia could directly participate in the pathophysiology of heart failure. Since their initial description, WNK kinases, and in particular WNK1 and WNK4, have emerged as key regulators of blood pressure and electrolyte homeostasis.26–29 Mutations in WNK1 and WNK4 have been demonstrated to cause hypertension, hyperchloraemic metabolic acidosis, and hyperkalaemia through increased renal sodium chloride reabsorption.27,30,31 In recent years, it has become clear that one of the key functions of WNKs is related to sensing and regulating chloride.18,32,33 Multiple studies have now shown that the WNK kinase network is critically involved in the activation and regulation of the renin–angiotensin–aldosterone system and the associated downstream sodium transport pathways.34–37 Notably, WNK3 was found to regulate the activation of the sodium–potassium–2-chloride co-transporter (NKCC2), the target of loop diuretics, in response to changes in intracellular chloride.33 Additionally, the WNK4-mediated regulation of the Na–Cl co-transporter (NCC), the target of thiazide diuretics, is also a chloride-dependent process.32 Of note, in the current analysis, serum chloride had a much stronger association than serum sodium with loop diuretic dose. In addition to the WNK system, several chloride channels with important regulatory functions have recently been identified or further characterized.12,38 For example, Cappola and colleagues employed a large-scale candidate gene approach to identify genetic variants associated with risk of advanced heart failure. Of the two candidate genes identified through this investigation, one of them appears to code for a renal chloride channel.39,40 Given the importance of chloride in renal signalling related to neurohormonal activation, sodium homeostasis, and regulation of the site of action of commonly used diuretics, it is biologically plausible that states of chloride depletion could directly contribute to the genesis of adverse events.
In light of this critical role of chloride in regulating physiology central to heart failure, and the strong association between low serum chloride and mortality, a viable hypothesis would be that strategies targeted specifically at normalizing chloride levels could potentially improve outcomes. In the current analysis, the non-electrolyte parameter most strongly associated with chloride levels was the loop diuretic dose, and it is plausible that diuretic use was the driver for much of the chloride depletion in this cohort. Notably, loop diuretics are known to increase chloride excretion by as much as 20-fold, with a 10–20% excess of chloride loss in relation to the amount of sodium and potassium excreted.41 The finding that hypochloraemia was associated with the greatest risk in patients with higher levels of serum bicarbonate and in those receiving high doses of loop diuretics supports the hypothesis that total body chloride depletion via diuretics may be more important than dilutional hypochloraemia. Although extensive research in the area of tubular chloride handling in response to non-loop diuretics has not been conducted, it has been reported that co-administration of acetazolamide with loop diuretics can reduce chloride losses by ~5%.42 Furthermore, a theoretical improvement in the urinary loss of chloride could be accomplished with adjuvant thiazide diuretics which do not directly cause excess chloride loss.43 Additionally, the use of vasopressin receptor antagonists would be expected to improve sodium and chloride in a 1:1 molar ratio in the setting of dilutional states.43 However, the effectiveness of these approaches in improving serum chloride, and more importantly hypochloraemia-associated outcomes, remains unknown.
Supplementation of additional chloride may be an alternative approach to normalizing chloride stores and possibly improving outcomes. Some possible support for this hypothesis is provided by the counterintuitive findings that administration of additional sodium chloride, either by diet or intravenously, paradoxically leads to either no change or an actual improvement in diuresis, renal function, rehospitalization, and death in several small studies.44–46 Given that the primary goal in the majority of decompensated heart failure hospitalizations is removal of sodium and fluid from the patient, the direct effect of additional sodium administration is intuitively counterproductive. As a result, administration of sodium chloride must be providing benefit via some alternative mechanism to offset the negative stoichiometric effect of additional sodium.47 In light of the findings from the current analysis and the key role of chloride in regulating renal sodium homeostasis and neuro-hormonal activation, it is plausible to hypothesize that the beneficial effects of sodium chloride administration are derived from the supplemental chloride. Given that administration of sodium chloride-based intravenous fluids outside of the confines of a randomized clinical trial has been linked with adverse outcomes, investigation of the effects of administration of sodium-free chloride salts (e.g. choline chloride or lysine chloride) is warranted.47
Limitations
Due to the post-hoc nature of this study, the limitations of retrospective analyses apply and uncontrolled confounding cannot be excluded. The BEST trial completed enrolment in 1998. Although the majority of participants were on ACE inhibitors, other medical therapy was inconsistent with contemporary standards. As a result, it is unclear how the current findings will apply to more contemporary populations. Although inclusion of patients with a serum creatinine up to 3.0 mg/dL was permitted in the trial, the median creatinine value was 1.2 mg/dL, with ~80% of patients having a creatinine ≤1.5 mg/dL. As a result, these findings probably do not apply to a population with more advanced renal insufficiency. The BEST trial required an LVEF ≤35% and an NYHA class of III or IV for enrolment and thus these results may not be applicable to populations with less severe heart failure symptoms or higher EFs. Although the BEST trial collected the dose of loop diuretic, no additional information was available quantifying diuretic responsiveness. As a result, likely important interactions between the genesis of hypochloraemia and diuretic response are not testable with this data set. Furthermore, similar to many clinical trial populations, the average age in BEST was younger than observed in routine clinical care. Additionally, data on BNP were not available in the BEST cohort, preventing adjustment for this prognostically important parameter. However, previously published work in two cohorts has found that the prognostic effect of serum chloride persists after adjustment for BNP and NT-proBNP.24 Finally, treating physicians were not blinded to the serum chloride or sodium concentrations and may have modified treatment differentially based on these values. As a result of the above limitations, replication of these findings in additional settings will be important.
Conclusion
Serum chloride is strongly and independently associated with worsened survival in patients with chronic heart failure and accounted for the majority of the risk otherwise attributable to hyponatraemia. Given the critical role of chloride in a number of regulatory pathways central to heart failure, it is possible that serum chloride may represent a therapeutic target rather than simply a marker of disease severity. Additional research is needed to validate these findings and to explore whether therapeutic manipulation of chloride can improve outcomes.
Supplementary Material
Acknowledgments
This manuscript was prepared using BEST research materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the BEST study investigators or the NHLBI.
Funding
This work was supported in part by NIH Grants 1K23HL114868, L30HL115790 (to J.T.), K23DK097201 (to F.P.W.), and K24DK090203 (to C.P.). The funding source had no role in study design, data collection, analysis, or interpretation.
Footnotes
Conflict of interest: none declared.
References
- 1.Filippatos TD, Elisaf MS. Hyponatremia in patients with heart failure. World J Cardiol. 2013;5:317–328. doi: 10.4330/wjc.v5.i9.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauptman PJ. Clinical challenge of hyponatremia in heart failure. J Hosp Med. 2012;7(Suppl 4):S6–S10. doi: 10.1002/jhm.1913. [DOI] [PubMed] [Google Scholar]
- 3.Rusinaru D, Tribouilloy C, Berry C, Richards AM, Whalley GA, Earle N, Poppe KK, Guazzi M, Macin SM, Komajda M, Doughty RN. Relationship of serum sodium concentration to mortality in a wide spectrum of heart failure patients with preserved and with reduced ejection fraction: an individual patient data meta-analysis: Meta-Analysis Global Group in Chronic heart failure (MAGGIC) Eur J Heart Fail. 2012;14:1139–1146. doi: 10.1093/eurjhf/hfs099. [DOI] [PubMed] [Google Scholar]
- 4.Goldsmith SR. Current treatments and novel pharmacologic treatments for hyponatremia in congestive heart failure. Am J Cardiol. 2005;95:14B–23B. doi: 10.1016/j.amjcard.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 5.De Luca L, Klein L, Udelson JE, Orlandi C, Sardella G, Fedele F, Gheorghiade M. Hyponatremia in patients with heart failure. Am J Cardiol. 2005;96:19 L–23 L. doi: 10.1016/j.amjcard.2005.09.066. [DOI] [PubMed] [Google Scholar]
- 6.Adrogue HJ. Consequences of inadequate management of hyponatremia. Am J Nephrol. 2005;25:240–249. doi: 10.1159/000086019. [DOI] [PubMed] [Google Scholar]
- 7.Brandimarte F, Fedele F, De Luca L, Fonarow GC, Gheorghiade M. Hyponatremia in acute heart failure syndromes: a potential therapeutic target. Curr Heart Fail Rep. 2007;4:207–213. doi: 10.1007/s11897-007-0014-5. [DOI] [PubMed] [Google Scholar]
- 8.Hoorn EJ, Zietse R. Hyponatremia and mortality: moving beyond associations. Am J Kidney Dis. 2013;62:139–149. doi: 10.1053/j.ajkd.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Schrier RW, Sharma S, Shchekochikhin D. Hyponatraemia: more than just a marker of disease severity? Nat Rev Nephrol. 2013;9:37–50. doi: 10.1038/nrneph.2012.246. [DOI] [PubMed] [Google Scholar]
- 10.Gheorghiade M, Konstam MA, Burnett JC, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 11.Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 12.Berend K, van Hulsteijn LH, Gans RO. Chloride: the queen of electrolytes? Eur J Intern Med. 2012;23:203–211. doi: 10.1016/j.ejim.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Wesson DE. Glomerular filtration effects of acute volume expansion: importance of chloride. Kidney Int. 1987;32:238–245. doi: 10.1038/ki.1987.198. [DOI] [PubMed] [Google Scholar]
- 14.Briggs JP, Schnermann JB. Whys and wherefores of juxtaglomerular apparatus function. Kidney Int. 1996;49:1724–1726. doi: 10.1038/ki.1996.255. [DOI] [PubMed] [Google Scholar]
- 15.Briggs J. The macula densa sensing mechanism for tubuloglomerular feedback. Fed Proc. 1981;40:99–103. [PubMed] [Google Scholar]
- 16.Kotchen TA, Welch WJ, Lorenz JN, Ott CE. Renal tubular chloride and renin release. J Lab Clin Med. 1987;110:533–540. [PubMed] [Google Scholar]
- 17.Subramanya AR, Yang CL, McCormick JA, Ellison DH. WNK kinases regulate sodium chloride and potassium transport by the aldosterone-sensitive distal nephron. Kidney Int. 2006;70:630–634. doi: 10.1038/sj.ki.5001634. [DOI] [PubMed] [Google Scholar]
- 18.Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal. 2014;7:ra41. doi: 10.1126/scisignal.2005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponce-Coria J, San-Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, de Los Heros P, Juarez P, Munoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci USA. 2008;105:8458–8463. doi: 10.1073/pnas.0802966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 21.Yunos NM, Bellomo R, Story D, Kellum J. Bench-to-bedside review: chloride in critical illness. Crit Care. 2010;14:226. doi: 10.1186/cc9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adrogue HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581–1589. doi: 10.1056/NEJM200005253422107. [DOI] [PubMed] [Google Scholar]
- 23.Testani JM, Cappola TP, Brensinger CM, Shannon RP, Kimmel SE. Interaction between loop diuretic-associated mortality and blood urea nitrogen concentration in chronic heart failure. J Am Coll Cardiol. 2011;58:375–382. doi: 10.1016/j.jacc.2011.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grodin JL, Simon J, Hachamovitch R, Wu Y, Jackson G, Halkar M, Starling RC, Testani JM, Tang WH. Prognostic role of serum chloride levels in acute decompensated heart failure. J Am Coll Cardiol. 2015;66:659–666. doi: 10.1016/j.jacc.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor CM, Ahmad T. The role of sodium and chloride in heart failure: does it take two to Tango? J Am Coll Cardiol. 2015;66:667–669. doi: 10.1016/j.jacc.2015.05.070. [DOI] [PubMed] [Google Scholar]
- 26.Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na–Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci USA. 2003;100:680–684. doi: 10.1073/pnas.242735399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 28.McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev. 2011;91:177–219. doi: 10.1152/physrev.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arroyo JP, Gamba G. Advances in WNK signaling of salt and potassium metabolism: clinical implications. Am J Nephrol. 2012;35:379–386. doi: 10.1159/000337479. [DOI] [PubMed] [Google Scholar]
- 30.Kahle KT, Wilson FH, Leng Q, Lalioti MD, O’Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet. 2003;35:372–376. doi: 10.1038/ng1271. [DOI] [PubMed] [Google Scholar]
- 31.Vidal-Petiot E, Elvira-Matelot E, Mutig K, Soukaseum C, Baudrie V, Wu S, Cheval L, Huc E, Cambillau M, Bachmann S, Doucet A, Jeunemaitre X, Hadchouel J. WNK1-related familial hyperkalemic hypertension results from an increased expression of L-WNK1 specifically in the distal nephron. Proc Natl Acad Sci USA. 2013;110:14366–14371. doi: 10.1073/pnas.1304230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bazua-Valenti S, Chavez-Canales M, Rojas-Vega L, Gonzalez-Rodriguez X, Vazquez N, Rodriguez-Gama A, Argaiz ER, Melo Z, Plata C, Ellison DH, Garcia-Valdes J, Hadchouel J, Gamba G. The effect of WNK4 on the Na + –Cl–cotransporter is modulated by intracellular chloride. J Am Soc Nephrol. 2015;26:1781–1786. doi: 10.1681/ASN.2014050470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponce-Coria J, San Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, De Los Heros P, Juarez P, Munoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci USA. 2008;105:8458–8463. doi: 10.1073/pnas.0802966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ring AM, Cheng SX, Leng Q, Kahle KT, Rinehart J, Lalioti MD, Volkman HM, Wilson FH, Hebert SC, Lifton RP. WNK4 regulates activity of the epithelial Na + channel in vitro and in vivo. Proc Natl Acad Sci USA. 2007;104:4020–4024. doi: 10.1073/pnas.0611727104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.San Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, Hebert SC, Alessi DR, Lifton RP, Gamba G. Angiotensin II signaling increases activity of the renal Na–Cl cotransporter through a WNK4–SPAK-dependent pathway. Proc Natl Acad Sci USA. 2009;106:4384–4389. doi: 10.1073/pnas.0813238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naray-Fejes-Toth A, Snyder PM, Fejes-Toth G. The kidney-specific WNK1 isoform is induced by aldosterone and stimulates epithelial sodium channel-mediated Na + transport. Proc Natl Acad Sci USA. 2004;101:17434–17439. doi: 10.1073/pnas.0408146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arroyo JP, Lagnaz D, Ronzaud C, Vazquez N, Ko BS, Moddes L, Ruffieux-Daidie D, Hausel P, Koesters R, Yang B, Stokes JB, Hoover RS, Gamba G, Staub O. Nedd4-2 modulates renal Na + –Cl– cotransporter via the aldosterone–SGK1–Nedd4-2 pathway. J Am Soc Nephrol. 2011;22:1707–1719. doi: 10.1681/ASN.2011020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luke RG, Galla JH. It is chloride depletion alkalosis, not contraction alkalosis. J Am Soc Nephrol. 2012;23:204–207. doi: 10.1681/ASN.2011070720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cappola TP, Matkovich SJ, Wang W, van Booven D, Li M, Wang X, Qu L, Sweitzer NK, Fang JC, Reilly MP, Hakonarson H, Nerbonne JM, Dorn GW., 2nd Loss-of-function DNA sequence variant in the CLCNKA chloride channel implicates the cardio-renal axis in interindividual heart failure risk variation. Proc Natl Acad Sci USA. 2011;108:2456–2461. doi: 10.1073/pnas.1017494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cappola TP, Li M, He J, Ky B, Gilmore J, Qu L, Keating B, Reilly M, Kim CE, Glessner J, Frackelton E, Hakonarson H, Syed F, Hindes A, Matkovich SJ, Cresci S, Dorn GW., 2nd Common variants in HSPB7 and FRMD4B associated with advanced heart failure. Circ Cardiovasc Genet. 2010;3:147–154. doi: 10.1161/CIRCGENETICS.109.898395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward A, Heel RC. Bumetanide. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use. Drugs. 1984;28:426–464. doi: 10.2165/00003495-198428050-00003. [DOI] [PubMed] [Google Scholar]
- 42.Chou SY, Porush JG, Slater PA, Flombaum CD, Shafi T, Fein PA. Effects of acetazolamide on proximal tubule C1, Na, and HCO3 transport in normal and acidotic dogs during distal blockade. J Clin Invest. 1977;60:162–170. doi: 10.1172/JCI108752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brenner BM, Rector FC. Brenner & Rector’s the kidney. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 44.Gandhi S, Mosleh W, Myers RB. Hypertonic saline with furosemide for the treatment of acute congestive heart failure: a systematic review and meta-analysis. Int J Cardiol. 2014;173:139–145. doi: 10.1016/j.ijcard.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 45.De Vecchis R, Esposito C, Ariano C, Cantatrione S. Hypertonic saline plus i.v. furosemide improve renal safety profile and clinical outcomes in acute decompensated heart failure: a meta-analysis of the literature. Herz. 2015;40:423–435. doi: 10.1007/s00059-013-4041-6. [DOI] [PubMed] [Google Scholar]
- 46.Aliti GB, Rabelo ER, Clausell N, Rohde LE, Biolo A, Beck-da-Silva L. Aggressive fluid and sodium restriction in acute decompensated heart failure: a randomized clinical trial. JAMA Intern Med. 2013;173:1058–1064. doi: 10.1001/jamainternmed.2013.552. [DOI] [PubMed] [Google Scholar]
- 47.Bikdeli B, Strait KM, Dharmarajan K, Li SX, Mody P, Partovian C, Coca SG, Kim N, Horwitz LI, Testani JM, Krumholz HM. Intravenous fluids in acute decompensated heart failure. JACC Heart Fail. 2015;3:127–133. doi: 10.1016/j.jchf.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.