Abstract
Key points
In a rat model of ageing that is free of atherosclerosis or hypertension, E/A, a diagnostic measure of diastolic filling, decreases, and isovolumic relaxation time increases, indicating that both active and passive ventricular relaxation are impaired with advancing age.
Resting coronary blood flow and coronary functional hyperaemia are reduced with age, and endothelium‐dependent vasodilatation declines with age in coronary resistance arterioles.
Exercise training reverses age‐induced declines in diastolic and coronary microvascular function.
Thus, microvascular dysfunction and inadequate coronary perfusion are likely mechanisms of diastolic dysfunction in aged rats.
Exercise training, initiated at an advanced age, reverses age‐related diastolic and microvascular dysfunction; these data suggest that late‐life exercise training can be implemented to improve coronary perfusion and diastolic function in the elderly.
Abstract
The risk for diastolic dysfunction increases with advancing age. Regular exercise training ameliorates age‐related diastolic dysfunction; however, the underlying mechanisms have not been identified. We investigated whether (1) microvascular dysfunction contributes to the development of age‐related diastolic dysfunction, and (2) initiation of late‐life exercise training reverses age‐related diastolic and microvascular dysfunction. Young and old rats underwent 10 weeks of exercise training or remained as sedentary, cage‐controls. Isovolumic relaxation time (IVRT), early diastolic filling (E/A), myocardial performance index (MPI) and aortic stiffness (pulse wave velocity; PWV) were evaluated before and after exercise training or cage confinement. Coronary blood flow and vasodilatory responses of coronary arterioles were evaluated in all groups at the end of training. In aged sedentary rats, compared to young sedentary rats, a 42% increase in IVRT, a 64% decrease in E/A, and increased aortic stiffness (PWV: 6.36 ± 0.47 vs.4.89 ± 0.41, OSED vs. YSED, P < 0.05) was accompanied by impaired coronary blood flow at rest and during exercise. Endothelium‐dependent vasodilatation was impaired in coronary arterioles from aged rats (maximal relaxation to bradykinin: 56.4 ± 5.1% vs. 75.3 ± 5.2%, OSED vs. YSED, P < 0.05). After exercise training, IVRT, a measure of active ventricular relaxation, did not differ between old and young rats. In old rats, exercise training reversed the reduction in E/A, reduced aortic stiffness, and eliminated impairment of coronary blood flow responses and endothelium‐dependent vasodilatation. Thus, age‐related diastolic and microvascular dysfunction are reversed by late‐life exercise training. The restorative effect of exercise training on coronary microvascular function may result from improved endothelial function.
Keywords: aortic stiffness, coronary arterioles, E/A, endothelium, rat
Key points
In a rat model of ageing that is free of atherosclerosis or hypertension, E/A, a diagnostic measure of diastolic filling, decreases, and isovolumic relaxation time increases, indicating that both active and passive ventricular relaxation are impaired with advancing age.
Resting coronary blood flow and coronary functional hyperaemia are reduced with age, and endothelium‐dependent vasodilatation declines with age in coronary resistance arterioles.
Exercise training reverses age‐induced declines in diastolic and coronary microvascular function.
Thus, microvascular dysfunction and inadequate coronary perfusion are likely mechanisms of diastolic dysfunction in aged rats.
Exercise training, initiated at an advanced age, reverses age‐related diastolic and microvascular dysfunction; these data suggest that late‐life exercise training can be implemented to improve coronary perfusion and diastolic function in the elderly.
Abbreviations
- Dea‐NONOate
diethylamineNONOate
- E/A
ratio of early diastolic velocity to peak velocity with atrial contraction
- HFpEF
heart failure with preserved ejection fraction
- IVCT
isovolumic contraction time
- IVRT
isovolumic relaxation time
- MPI
myocardial performance index
- PWV
pulse wave velocity
- OSED
old sedentary
- OET
old exercise‐trained
- SOD
superoxide dismutase
- YET
young exercise‐trained
- YSED
young sedentary
Introduction
The contribution of microvascular dysfunction has been increasingly recognized in the development of heart failure with preserved ejection fraction (HFpEF) in hypertensive and diabetic patients (Mohammed et al. 2015; Franssen et al. 2016; Teo et al. 2016; van Heerebeek & Paulus, 2016). Similarly, reduced coronary blood flow has been related to left ventricular diastolic dysfunction in hypertrophic obstructive cardiomyopathy (Kyriakidis et al. 1998), and in patients with type 2 diabetes, coronary flow reserve is independently associated with left ventricular filling pressure (Kawata et al. 2015). Since myocardial relaxation is an energetically demanding process that is sensitive to ischaemia (Varma et al. 2000; Phan et al. 2009b), regulation of coronary blood flow and delivery of oxygen and substrates, mediated by the coronary resistance vasculature, is critical for normal diastolic function (Sievers et al. 1983; Aroesty et al. 1985; Labovitz et al. 1987; Miyazaki et al. 1990). Reduced myocardial flow reserve has been reported in HFpEF patients without history of obstructive coronary artery disease (Srivaratharajah et al. 2016); however, the role of dysfunction of the coronary resistance vasculature in development of age‐related diastolic dysfunction and related HFpEF (Upadhya et al. 2015; van Heerebeek & Paulus, 2016) has not been explored.
Exercise training has been demonstrated to improve diastolic function in patients with HFpEF (Chan et al. 2016); however, much remains unknown about the mechanisms by which exercise training improves diastolic function. Ageing impairs vasodilatory function of coronary resistance arteries and arterioles (Csiszar et al. 2002; Shipley & Muller‐Delp, 2005; Kang et al. 2009, 2011; Leblanc et al. 2013), but whether exercise training can restore coronary microvascular function in the aged heart remains unknown. In conscious humans, coronary microvascular flow reserve, an indicator of microvascular dysfunction, can be assessed by imaging at rest and during pharmacologically‐induced stress (Abdelmoneim et al. 2011; Larghat et al. 2014; Ong & Sechtem, 2016; Srivaratharajah et al. 2016); however, the reactivity of the coronary resistance vasculature cannot be assessed directly in humans. In contrast, accurate imaging of coronary blood flow in animal models can only occur under anaesthesia which alters coronary vascular resistance (Hartley et al. 2007), and cannot provide information about coronary vasodilatation during the physiological stress of exercise. In this study, we hypothesized that microvascular endothelial dysfunction and dysregulation of coronary blood flow distribution contributes to age‐related diastolic dysfunction. We also hypothesized that both diastolic and microvascular endothelial dysfunction can be reversed by late‐life exercise training. Therefore, we employed an animal model of ageing, the Fischer 344 rat, in order to study regional coronary blood flow and reactivity of the coronary resistance vasculature in the absence of atherosclerosis and hypertension. We assessed coronary microvascular perfusion at rest and during submaximal treadmill exercise through the infusion of radioactive microspheres into conscious rats. To specifically assess endothelium‐dependent function of the coronary resistance vasculature, the vasodilatory responses of isolated coronary arterioles to endothelium‐dependent and endothelium‐independent agents were assessed in the absence of neural and humoral influences.
Methods
Animals
Young (3–4 months) and old (20–21 months) male Fisher 344 rats were obtained from the National Institute of Aging and assigned to young sedentary (YSED, n = 22), young exercise‐trained (YET, n = 26), old sedentary (OSED, n = 28) or old exercise‐trained (OET, n = 34) groups, respectively. All animal procedures in this study were approved by the Institutional Animal Care and Use Committees at University of Florida and Florida State University, and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Eighth edition, 2011).
Exercise training
Exercise‐trained rats underwent 10–12 weeks of treadmill exercise training. Exercise trained rats performed treadmill exercise at 15 m · min−1 (15 deg incline), 5 days · week−1, for 10–12 weeks. The duration of exercise was increased incrementally in the first 4 weeks until a 60 min duration was reached. The rats continued to exercise 5 days week−1 for 60 min · day−1 for the remainder of the 10–12 week training period (Hanna et al. 2014). Only rats which completed the entire 10‐week training protocol were studied. All young rats completed the protocol, and approximately 80% of aged rats completed the protocol. Cardiac function, coronary blood flow, and vascular reactivity were determined at least 24 h after the last exercise bout in exercise trained rats (Spier et al. 2004). To determine the efficacy of the training protocol, the soleus muscle was stored at −80°C for determination of citrate synthase activity, a mitochondrial enzyme and marker of muscle oxidative potential, according to the method of Srere (Srere, 1969).
Doppler ultrasound
Cardiac function was determined using non‐invasive Doppler ultrasound before and during the tenth week of exercise training (Indus Instruments, Webster, TX, USA) as developed by Reddy and colleagues (Reddy et al. 2005a,b, 2009). Briefly, the rat was anaesthetized with 1.5% isoflurane‐balance O2, and taped supine to electrocardiogram electrodes on a procedure board that was designed for the size of rats. A 20‐MHz Doppler probe was placed at the inferolateral region for the assessment of blood flow velocities from mitral inflow and aortic outflow. A 20‐MHz Doppler probe was also placed at the aortic arch and the abdominal aorta for the assessment of pulse wave velocity. Electrocardiogram was recorded simultaneously to ensure appropriate timing of obtainment of blood flow signals and to determine heart rate. In order to evaluate cardiovascular function, the following indexes were obtained: ratio of early diastolic velocity to peak velocity with atrial contraction (E/A), isovolumic relaxation time (IVRT), myocardial performance index (MPI), and pulse wave velocity (PWV). IVRT is an interval in the cardiac cycle, from the closure of the aortic valve to onset of filling by opening of the mitral valve. E/A and IVRT were used for diastolic function evaluation. To calculate MPI, isovolumic contraction time (IVCT) was measured. IVCT is an interval in the cardiac cycle, from the closure of the mitral valve to the onset of the left ventricular outflow by opening of the aortic valve. MPI was defined as the sum of the IVCT and the IVRT divided by the left ventricular ejection time. MPI is a measure of general cardiac function, including systolic and diastolic function. PWV was determined by dividing the distance of measurement points by the time taken to travel this distance as measured from R‐peaks of the electrocardiogram. PWV was obtained to evaluate aortic stiffness.
Coronary blood flow
Prior to blood flow evaluations, sedentary rats were familiarized with treadmill running. During three familiarization bouts, rats exercised on the treadmill 10 min · day−1 at 15 m · min−1 at a 0 deg incline. A minimum of 48 h was maintained between bouts of treadmill exercise and experimental procedures. Rats were anaesthetized with 2.5% isoflurane–balance O2, and a catheter (PE‐50 connected to PE‐10) filled with heparinized saline solution was advanced into the left ventricle via the right carotid artery in order to infuse radiolabelled microspheres for cardiac blood flow measurements. The carotid catheter was externalized at the base of the neck and secured to the skin between the shoulder blades with 4‐0 silk suture. A second catheter was implanted in the caudal tail artery and externalized at the tail. This catheter was used to monitor mean arterial pressure and to obtain a reference blood sample, which serves as an artificial organ for calculating tissue flows. After closure of the incisions, the animals were given ≥4 h to recover, as previous studies demonstrated that circulatory dynamics, regional blood flow, arterial blood gases, and acid–base status are stable in the awake rat 1–6 h after gas anaesthesia (Flaim et al. 1984).
After the recovery period, the rat was placed on the treadmill, and exercise was initiated (15 m · min−1 at a 0 deg incline). After 3 min of total exercise time, blood withdrawal from the caudal artery at 0.25 ml · min−1 was begun. Radiolabelled microspheres (57Co and 85Sr; 15 μm diameter; ∼2.5 × 105 in number) were infused into the ventricle through the right carotid artery catheter. Blood withdrawal from the caudal artery continued for 45 s after microsphere infusion. After a 30 min recovery period, a second microsphere infusion was performed in conscious, standing rats. This strategy was utilized to minimize the pre‐exercise anticipatory response and facilitates an accurate ‘resting’ measurement. After the second microsphere infusion, animals were killed with pentobarbital sodium (>100 mg · kg−1 i.p.). The heart was dissected into right ventricular free wall, ventricular septum, papillary muscle, endocardium and epicardium of left ventricular free wall, and these tissue sections were weighed. The radioactivity level of each discrete tissue section was determined by a gamma scintillation counter (Cobra II Auto Gamma Counter, Packard, Downers Grove, IL, USA). Blood flow to each tissue section was calculated by reference sample method and expressed in millilitres per minute per 100 g of tissue. Blood flows in the tissues were calculated by the following formula:
The blood withdrawals from tail artery were utilized as reference samples. Soleus and gastrocnemius muscles were dissected as positive control tissues and blood flows calculated in these muscles were compared to previously published values (Delp et al. 1998). Kidney blood flows were used as an indicator of adequate mixing of microspheres; blood flow values were only considered valid if left and right kidney flows were within 15% of each other, as previously described (Delp et al. 1998).
Evaluation of vasodilatory responses
Rats were weighed and anaesthetized with 2.5% isoflurane–O2 balance and killed by excision of the heart, which was immediately placed in cold (4°C) filtered physiological saline solution. Coronary resistance arterioles (<150 μm; Chilian et al. 1986) were isolated from the left anterior descending coronary artery distribution. Arterioles were then cannulated on pipettes and pressurized to 60 cmH2O (40–50 mmHg) in a Lucite chamber that contained warm (37°C) filtered physiological saline solution as previously described (Kang et al. 2009, 2011; LeBlanc et al. 2009). Once at least 20% steady tone was achieved, vasodilator responses to the cumulative addition of the endothelium‐dependent vasodilator bradykinin (10–13–10–7 m), 3 min exposure at each consecutive dose) were determined. To evaluate vascular smooth muscle responsiveness to exogenous NO, a concentration–response relationship for diethylamineNONOate (Dea‐NONOate, 10–9–10–3 m) was determined.
Immunohistochemical analysis of arteriolar proteins
To determine whether ageing and exercise training alter protein levels of superoxide dismutase (SOD) and endothelial nitric oxide synthase (eNOS), coronary arterioles were cannulated, pressurized to 60 cmH2O, warmed to 37°C, incubated in Ca2+‐free PSS and 100 um sodium nitroprusside for 1 h, and then fixed in 50% Bouin's solution. Fixed arterioles were frozen in O.C.T. compound and stored at −80°C until use. 5‐micron frozen cross‐sections of arterioles were cut on a cryostat. Thawed sections were washed with phosphate buffered saline (PBS) before adding a blocking solution of 0.3% Triton‐X and 10% normal donkey serum at room temperature for 1 h. Arterioles were then incubated with primary antibodies against either SOD (Anti‐Superoxide Dismutase 1 antibody, 1:50, Abcam, no. ab13498) or eNOS (Anti‐eNOS antibody, 1:200, Sigma, no. ab‐1177) at 4°C overnight. After PBS washes, species‐specific anti‐IgG (FITC, Abcam, no. ab6785) was added at a dilution of 1:100 for 1 h at room temperature. After washing, DAPI (Fisher Scientific, no. P36962) was added, and images were obtained using a fluorescence microscope (BX43, Olympus, Tokyo, Japan). To exclude adventitial staining, a region of interest was established manually using ImageJ Software after isolating the images for green fluorescence only. For each section, the average pixel intensity in the region of interest was obtained (Image J). Background subtraction was performed by subtracting the average pixel intensity obtained in an adjacent section from the same vessel that had been treated in the same manner as the stained sections, but in the absence of both primary and secondary antibodies. The average of the values obtained from analysis of 2–5 cross‐sections per vessel was calculated and used for statistical analysis.
Statistics
Vasodilatory responses were expressed according to the following formula:
where D S is the arteriolar diameter measured after addition of each dose of the drug being tested, D B is the diameter recorded immediately prior to initiation of the concentration–diameter curves, and D M is the maximal diameter for the arteriole. Vessel responses to bradykinin and Dea‐NONOate were evaluated using three‐way ANOVA (age, exercise training, and dose/time) with repeated measurement (dose/time). Group differences in animal characteristics, vessel characteristics and coronary blood flow were assessed by two‐way ANOVA (age and exercise training). Doppler measurements made pre‐ and post‐exercise training were compared by two‐way ANOVA (age and exercise training) with one repeated factor (exercise training). Post hoc analyses were performed using Bonferroni's test for pairwise comparisons if either significant main effects or significant interaction was found. Statistical significance was defined as P ≤ 0.05. All data are presented as mean ± SEM.
Results
Body weight increased with age (Table 1). Exercise training reduced body weight in old, but not in young rats. Heart weight increased with age (Table 1). Heart weight‐to‐body weight ratio increased with exercise training in old rats, but not in young rats. Exercise training increased soleus muscle citrate synthase activity by 20.9% in the young rats, and 30.3% in the old rats, confirming the efficacy of the exercise training regimen (Table 1), as previously demonstrated (Hanna et al. 2014).
Table 1.
Animal and coronary vessel characteristics
| Sedentary | Exercise training | ||
|---|---|---|---|
| Body weight (g) | Young | 374 ± 10 (22) | 358 ± 4 (26) |
| Old | 456 ± 7* (28) | 428 ± 5*† (34) | |
| Heart weight (mg) | Young | 934 ± 17 (15) | 934 ± 17 (25) |
| Old | 1164 ± 34* (28) | 1286 ± 46* (33) | |
| Left ventricular weight (mg) | Young | 746 ± 10 (10) | 753 ± 16 (10) |
| Old | 780 ± 28 (8) | 836 ± 25*† (14) | |
| HW/BW (mg · g−1) | Young | 2.32 ± 0.05 (15) | 2.62 ± 0.07 (25) |
| Old | 2.58 ± 0.07 (27) | 3.00 ± 0.10*† (33) | |
| LV /BW (mg · g−1) | Young | 1.83 ± 0.04 (10) | 2.08 ± 0.05 (10) |
| Old | 1.93 ± 0.07 (27) | 2.01 ± 0.21 (33) | |
| Soleus muscle citrate synthase activity (μmol · min−1 · g−1) | Young | 19.1 ± 0.5 (10) | 23.1 ± 1.1† (9) |
| Old | 14.2 ± 0.7* (8) | 18.5 ± 0.9† (9) | |
| Maximal diameter (μm) | Young | 145 ± 8 (9) | 143 ± 9 (11) |
| Old | 143 ± 7 (9) | 142 ± 9 (13) | |
| Spontaneous tone (%) | Young | 23.1 ± 1.6 (9) | 26.6 ± 2.8 (11) |
| Old | 21.4 ± 0.8 (9) | 29.2 ± 1.9† (13) |
Values are mean ± SEM; n (in parentheses), number of rats in each group; HW/BW, heart weight‐to‐body weight ratio; LV/BW, left ventricle weight‐to‐body weight ratio. *Effect of age (P ≤ 0.05). †Effect of exercise training (P ≤ 0.05).
Mean arterial pressure and heart rate
Neither age nor exercise training altered mean arterial pressure at rest (Table 2). Mean arterial pressure increased during exercise compared to rest in both young and old rats (Table 2). Exercise training did not alter mean arterial pressure during exercise in either young or old rats (Table 2). Heart rate increased during exercise in both young and old rats; however, resting and exercising heart rates were not changed by either age or exercise training. Similarly, heart rates measured at rest, but under isoflurane anaesthesia, during Doppler analysis, were similar in all four groups of rats (data not shown).
Table 2.
Blood pressure and heart rate
| Sedentary | Exercise training | ||
|---|---|---|---|
| Resting MAP (mmHg) | Young | 142.7 ± 2.2 (8) | 142.6 ± 2.6 (21) |
| Old | 138.2 ± 7.6 (19) | 131.5 ± 3.1 (14) | |
| Exercising MAP (mmHg) | Young | 159.5 ± 0.2‡ (8) | 151.2 ± 3.3‡ (21) |
| Old | 158.2 ± 5.8‡ (19) | 149.9 ± 5.5‡ (14) | |
| Resting heart rate (beats · min−1) | Young | 375 ± 6 (8) | 383 ± 6 (9) |
| Old | 362 ± 18 (5) | 331 ± 26 (10) | |
| Exercising heart rate (beats · min−1) | Young | 440 ± 15‡ (7) | 416 ± 13‡ (9) |
| Old | 427 ± 18‡ (5) | 422 ± 24‡ (8) |
Values are mean ± SEM; n (in parentheses), number of rats in each group; MAP, mean arterial pressure. ‡P ≤ 0.05 vs. rest.
Cardiac function
E/A decreased with age (YSED: 1.75 ± 0.09 vs. OSED: 0.64 ± 0.05), and increased with exercise training in old (OET: 1.17 ± 0.11 vs. OSED: 0.64 ± 0.05), but not young rats (YET: 1.76 ± 0.10 vs. YSED: 1.75 ± 0.09) (Fig. 1 A). In old sedentary rats, E/A remained unchanged over the 10‐week period of cage confinement (Fig. 1 B). Ten weeks of exercise training increased E/A in old rats (Fig. 1 C), partially reversing the age‐related decrease in E/A (Fig. 1 A). IVRT was prolonged in OSED vs. YSED rats (42.00 ± 0.84 ms vs. 29.56 ± 1.63 ms; Fig. 2 A). Exercise training reduced IVRT in old (OET: 31.84 ± 0.83 ms), but not young rats (YET: 28.98 ± 1.20 ms) so that IVRT was not different between OET, YSED, and YET rats (Fig. 2 A). In OSED rats, IVRT was elevated at 21 months (compared to young rats; data not shown) and continued to increase during the 10 weeks of cage confinement (from 21 to 24 months, Fig. 2 B). Ten weeks of exercise training prevented this increase in IVRT in old rats and resulted in an overall reduction of IVRT (Fig. 2 C). MPI increased with age (Fig. 3 A). Exercise training reduced MPI in old, but not in young rats (Fig. 3 A). During 10 weeks of cage confinement MPI did not change in old rats (Fig. 3 B). In contrast, 10 weeks of exercise training decreased MPI in old rats (Fig. 3 C), indicating that exercise training improved overall cardiac (systolic and diastolic) performance.
Figure 1. Effects of age and exercise training on E/A.
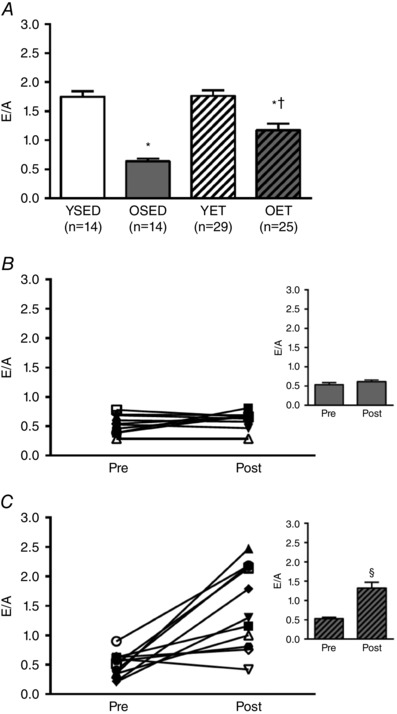
A, ratio of early diastolic peak velocity to peak velocity during left atrial contraction (E/A) in young sedentary (YSED), old sedentary (OSED), young exercise trained (YET), and old exercise trained (OET) rats. B and C, E/A before (pre) and after (post) 10 weeks of cage confinement (B, n = 10) or exercise training (C, n = 18) in old rats. Values are means ± SEM; n, no. of rats. *Effect of age (P ≤ 0.05). †Effect of exercise training (P ≤ 0.05). §Significant difference post vs. pre (P ≤ 0.05).
Figure 2. Effects of age and exercise training on isovolumic relaxation time.
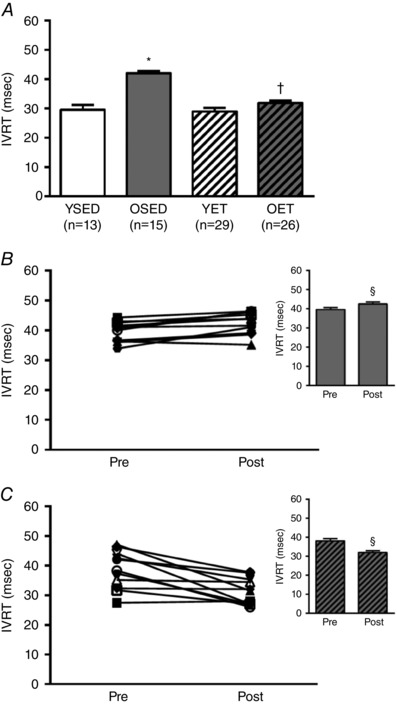
A, isovolumic relaxation time (IVRT) in young sedentary (YSED), old sedentary (OSED), young exercise trained (YET), and old exercise trained (OET) rats. B and C, IVRT before (pre) and after (post) 10 weeks of cage confinement (B, n = 11) or exercise training (C, n = 20) in old rats. *Effect of age (P ≤ 0.05). †Effect of exercise training (P ≤ 0.05). §Significant difference post vs. pre (P ≤ 0.05).
Figure 3. Effects of age and exercise training on myocardial performance index.
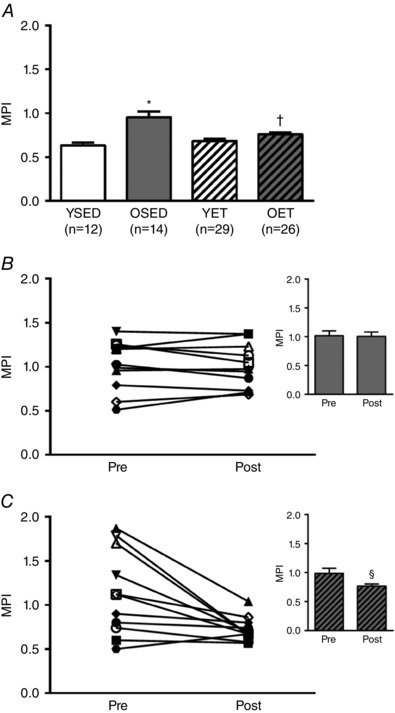
A, myocardial performance index (MPI) in young sedentary (YSED), old sedentary (OSED), young exercise trained (YET), and old exercise trained (OET) rats. B and C, values for MPI before (pre) and after (post) 10 weeks of cage confinement (B, n = 11) or exercise training (C, n = 18) in old rats. Values are means ± SEM; n, no. of rats. *Effect of age (P ≤ 0.05). †Effect of exercise training (P ≤ 0.05). §Significant difference post vs. pre (P ≤ 0.05).
Aortic stiffness
PWV increased with age (Fig. 4 A). Exercise training reversed the age‐related increase in PWV in old rats, but did not alter PWV in young rats (Fig. 4 A). In old rats, PWV did not change during 10 weeks of cage confinement (Fig. 4 B), whereas PWV decreased significantly during 10 weeks of exercise training (Fig. 4 C).
Figure 4. Effects of age and exercise training on aortic stiffness.
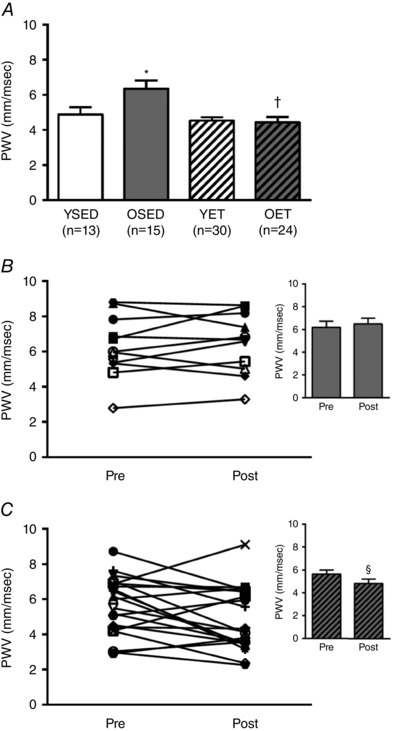
A, pulse wave velocity (PWV) in young sedentary (YSED), old sedentary (OSED), young exercise trained (YET), and old exercise trained (OET) rats. B and C, values for PWV before (pre) and after (post) 10 weeks of cage confinement (B, n = 11) or exercise training (C, n = 21) in old rats. *Effect of age (P ≤ 0.05). †Effect of exercise training (P ≤ 0.05). §Significant difference post vs. pre (P ≤ 0.05).
Coronary blood flow at rest and during exercise
Right ventricle
Resting blood flow to the right ventricular free wall decreased with age (Fig. 5 A). During exercise, blood flow to the right ventricular free wall increased in all groups but old sedentary rats (mean blood flow values in Fig. 5 B vs. Fig. 5 A). In old rats, resting blood flow to the right ventricular free wall was not altered by exercise training, whereas in young rats, resting blood flow to the right ventricular free wall was decreased with exercise training (Fig. 5 A). During exercise, blood flow to the right ventricular free wall was decreased by age, but restored by exercise training in old rats (Fig. 5 B). In young rats, exercise training did not alter blood flow to the right ventricular free wall during exercise (Fig. 5 B).
Figure 5. Right ventricular blood flow.
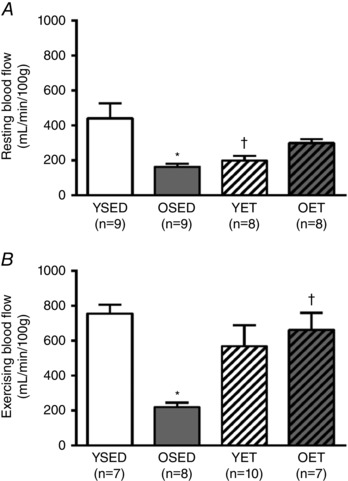
A and B, blood flow to the right ventricular free wall at rest (A) and during treadmill exercise (B) in young sedentary (YSED), old sedentary (OSED), young exercise trained (YET), and old exercise trained (OET) rats. Values are means ± SEM; n, no. of rats. *Effect of age (P ≤ 0.05). †Effect of exercise training (P ≤ 0.05).
Left ventricle
In the left ventricular free wall, resting blood flows to both the endocardium (OSED: 249 ± 42 ml · min−1 · 100 g−1 vs. YSED: 390 ± 54 ml · min−1 · 100 g−1; Fig. 6 A) and the epicardium (OSED: 166 ± 19 ml · min−1 · 100 g−1 vs. YSED: 417 ± 72 ml · min−1 · 100 g−1; Fig. 6 C) were decreased by age, but restored by exercise training in old rats. Exercise training did not alter either resting endocardial or epicardial flows in young rats (Fig. 6 A and C). During exercise, blood flow increased above resting in both the endocardium and epicardium in all groups of rats (values in Fig. 6 B vs. A and values in Fig. 6 D vs. C). Ageing decreased blood flow during exercise in both the endocardium (OSED: 395 ± 70 ml · min−1 · 100 g−1 vs. YSED: 726 ± 85 ml · min−1 · 100 g−1; Fig. 6 B) and the epicardium (OSED: 316 ± 50 ml · min−1 · 100 g−1 vs. YSED: 798 ± 85 ml · min−1 · 100 g−1 Fig. 6 D). Blood flow to both the endocardium (OET: 724 ± 65 ml · min−1 · 100 g−1 vs. YET: 634 ± 72 ml · min−1 · 100 g−1; Fig. 6 B, P = 0.370) and epicardium (OET: 690 ± 62 ml · min−1 · 100 g−1 vs. YET: 715 ± 67 ml · min−1 · 100 g−1 Fig. 6 D, P = 0.788) during exercise were increased by training in old, but not young rats; thus, in exercise trained rats, age‐related differences in exercising blood flows were eliminated.
Figure 6. Endocardial and epicardial blood flow in the left ventricle.
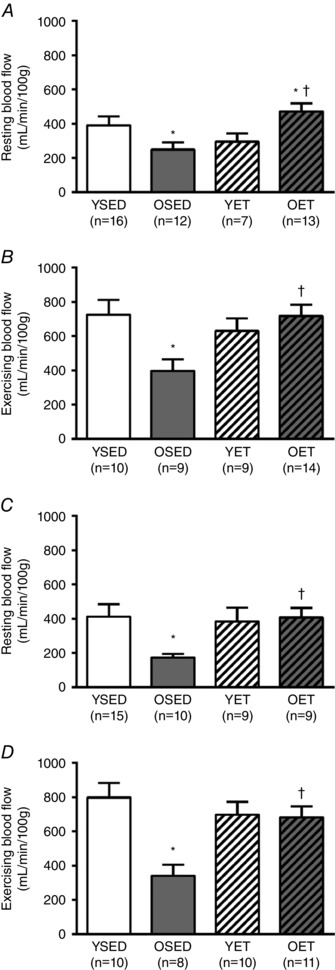
Blood flow to the endocardium (A and B) and epicardium (C and D) of the left ventricular free wall at rest (A and C) and during treadmill exercise (B and D) in young sedentary (YSED), old sedentary (OSED), young exercise trained (YET), and old exercise trained (OET) rats. Values are means ± SEM; n, no. of rats. *Effect of age (P ≤ 0.05). †Effect of exercise training (P ≤ 0.05).
Septum and papillary muscle
Ageing did not change resting blood flow in the septum or papillary muscle (Fig. 7 A and C). Exercise training increased resting blood flow to both the septum (Fig. 7 A) and papillary muscle (Fig. 7 C) in old rats, and to papillary muscle (Fig. 7 C) in young rats. Compared to resting levels, blood flow to both the septum and papillary muscle increased during exercise in all groups of rats. Ageing reduced the overall magnitude of septal blood flow during exercise (Fig. 7 B), but did not change overall blood flow to papillary muscle during exercise (Fig. 7 D). In old rats, exercise training increased septal blood flow during exercise but did not change blood flow to papillary muscle during exercise. In young rats, exercise training did not alter blood flow during exercise to either the septum or papillary muscle (Fig. 7 B and D).
Figure 7. Blood flow to septum and papillary muscle.
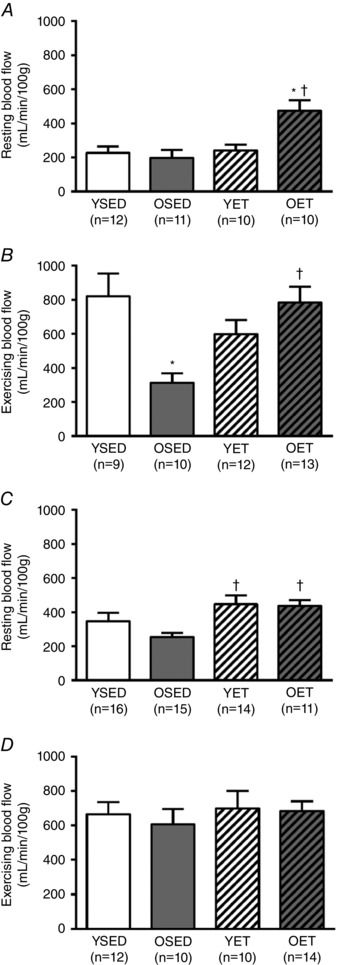
Blood flow to the ventricular septum (A and B) and papillary muscle (C and D) at rest (A and C) and during treadmill exercise (B and D) in young sedentary (YSED), old sedentary (OSED), young exercise trained (YET), and old exercise trained (OET) rats. Values are means ± SEM; n, no. of rats. *Effect of age (P ≤ 0.05). †Effect of exercise training (P ≤ 0.05).
Vasodilatory responses to bradykinin
Aging decreased overall responsiveness and sensitvity of coronary arterioles to the endothelium‐dependent vasodilator, bradykinin (Fig. 8 A). Exercise training increased overall vasodilatation and sensitivity to bradykinin in coronary arterioles from old rats (Fig. 8 D), but did not alter responses to bradykinin in arterioles from young rats (Fig. 8 C). Vasodilatory responsiveness to bradykinin was not different between arterioles from young and old rats after exercise training (Fig. 8 B).
Figure 8. Effects of age and exercise training on endothelium‐dependent vasodilation.
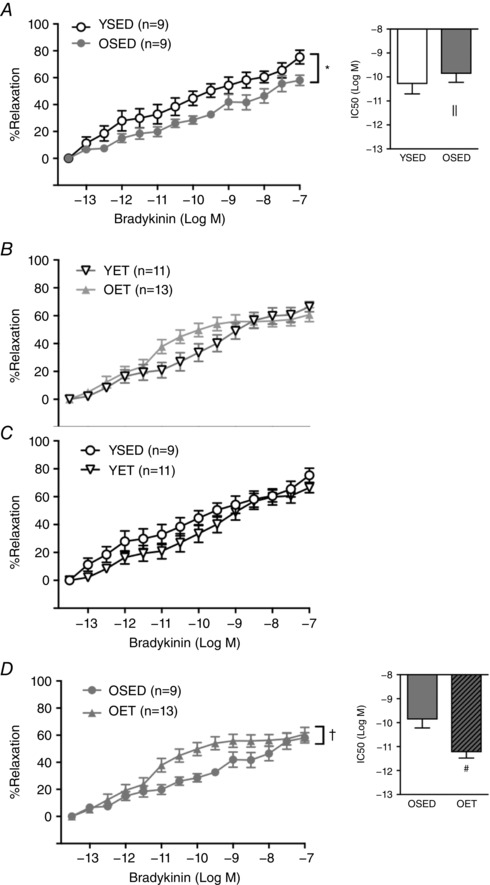
A and B, effect of age on vasodilatation to bradykinin in coronary arterioles from sedentary (young sedentary, YSED; old sedentary, OSED) rats (A), and in coronary arterioles from exercise trained (young exercise trained, YET; old exercise trained, OET) rats (B). C and D, effects of exercise training on vasodilatation in respose to bradykinin in coronary arterioles from young (C) and old (D) rats. Values are means ± SEM; n, no. of rats. *Effect of age (P ≤ 0.05), †Effect of exercise training (P ≤ 0.05) on overall relaxation. ||Effect of age (P ≤ 0.05), #Effect of exercise training (P ≤ 0.05) on EC50.
Vasodilatory responses to DEA‐NONOate
Endothelium‐independent vasodilatation was assessed with the NO donor, DEA‐NONOate. Ageing did not alter maximal vasodilatation or sensitivity to DEA‐NONOate, confirming earlier reports in coronary arterioles from aged rats (LeBlanc et al. 2008). Exercise training did not alter responsiveness to DEA‐NONOate in arterioles from either old or young rats (maximal relaxation: OSED: 85.3 ± 2.9%, YSED: 90.1 ± 3.4%, OET: 84.9 ± 5.1%, YET: 87.7 ± 1.4%).
SOD and eNOS protein levels
The level of the superoxide scavenging protein, superoxide dismutase (SOD) was 50% lower in coronary arterioles from old rats (Fig. 9). In arterioles from old rats, exercise training tended to increase SOD protein levels (62% increase in average pixel intensity, P = 0.17, OET vs. OSED) so that after exercise training there was no difference between SOD protein levels in arterioles from old and young rats (P = 0.36, OET vs. YET). The level of eNOS protein in coronary arterioles was not altered by age or exercise training (data not shown).
Figure 9. Effects of age and exercise training on SOD protein levels in coronary arterioles.
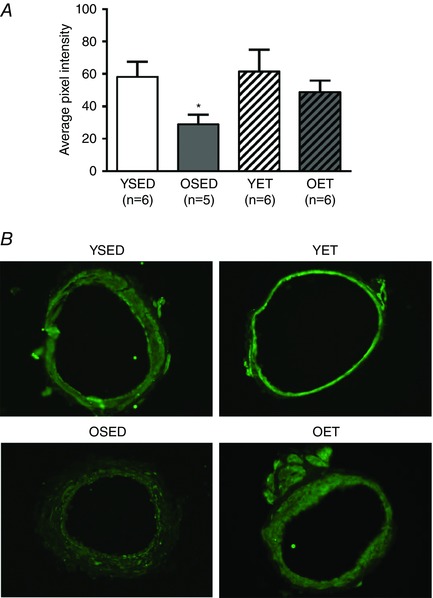
Summary (A) and representative images (B) of immunohistochemical staining for superoxide dismutase‐1 (SOD‐1) protein in coronary arterioles from young sedentary (YSED), old sedentary (OSED), young exercise trained (YET), and old exercise trained (OET) rats. *Effect of age (P ≤ 0.05).
Discussion
Two important findings emerge from this study. The first finding of importance is that both diastolic and microvascular function decline in the aged heart. The second significant finding is that exercise training, initiated at an advanced age, reverses age‐related diastolic and coronary microvascular dysfunction. Because the heart is predominantly aerobic, and since active cardiac relaxation is a process that requires ATP (Phan et al. 2009a,b), there is a necessary link between microvascular delivery of oxygen and substrate to the myocardium and maintenance of appropriate relaxation during diastole. Diastole begins with active isovolumic relaxation, caused by a decrease in cytoplasmic Ca2+ and sarcomeric relaxation after aortic valve closure (Groban, 2005; Borlaug & Kass, 2006). Once intraventricular pressure falls below left atrial pressure, the mitral valve opens, and passive relaxation properties influence the rate of rapid filling. In this study, we document an increase in IVRT and a decrease in E/A with age, indicating that both active and passive ventricular relaxation are impaired with advancing age. Age‐induced impairment of active relaxation (prolongation of IVRT) has been linked to reduced cardiac energetic reserve (Phan et al. 2009a; Kang et al. 2015) and the reduction of cardiac reserve of high energy phosphates is likely to be linked to impaired delivery of oxygen and substrate during periods of increased stress, e.g. exercise. The decrease in passive relaxation indicated by a reduction in E/A is most likely to be due to increasing fibrosis and stiffening of the ventricular wall (Benjamin et al. 1992). Both mitochondrial dysfunction of cardiac myocytes and cardiac fibrosis have been linked to oxidative stress and endothelial dysfunction (Nickel et al. 2014; Münzel et al. 2015; Franssen et al. 2016). Our data suggest that the microvascular dysfunction and, more specifically, impairment of endothelium‐dependent vasodilatation in coronary resistance arterioles, are underlying mechanism(s) that contribute to impairment of diastolic function. Importantly, our data indicate that late‐life exercise training improves endothelial function of coronary resistance arterioles, restores coronary microvascular perfusion at rest and during exercise, and reverses impairment of active ventricular relaxation and early diastolic filling.
Our data indicate that exercise training reverses the age‐associated decrease in the E/A, which is consistent with results from a study performed by Prasad and colleagues (Prasad et al. 2007). These authors reported that E/A was lower in healthy sedentary seniors compared with young healthy subjects (Prasad et al. 2007). They also showed that lifelong exercise‐trained seniors achieved an E/A of 1.0, with or without increasing cardiac filling by saline infusion, but sedentary seniors did not (Prasad et al. 2007). Although these data indicate that exercise training prevents age‐related ventricular stiffening, these results do not indicate the mechanism(s) by which exercise training affords this protection. Our data indicate that at least one mechanism that contributes to age‐induced diastolic dysfunction is microvascular dysfunction and inadequate blood flow responses to acute exercise (inadequate functional hyperaemic response). Our data also indicate that exercise training initiated late in life can reverse microvascular dysfunction, improving blood flow responses to acute exercise, potentially contributing to reversal of diastolic dysfunction. Mechanistically, this reversal may be associated with reduced oxidant stress and reversal of fibrosis, but further studies would be needed to investigate these possibilities.
Brenner et al. (2001) investigated the effects of age and exercise training on diastolic function in F344/BN rats and showed that the amplitude of the E wave declined by 24 months; however, the E/A was not significantly reduced in 24‐month old rats compared to adult (6‐month‐old) rats. In Brenner's study, 24‐month‐old F344/BN rats had an E/A of 1.4 ± 0.1, whereas 23‐ to 25‐month‐old F344 rats in our study demonstrated an E/A of 0.6 ± 0.01. The difference between our results and those of Brenner et al. is likely to be attributable to differences in relative age. The lifespan of F344/BN rats is about 40 months, whereas the lifespan of F344 rats is about 26 months (NIA colony database). In the present study, IVRT, a load‐independent measure of active left ventricular relaxation, was prolonged in OSED rats compared with YSED rats. Furthermore, during 10 weeks of longitudinal observation, IVRT continued to increase in OSED rats, and this indication of continuous decline in active ventricular relaxation was prevented by 10 weeks of exercise training. Results from Brenner et al. (2001) indicate that exercise training initiated at middle age helped prevent the decline of diastolic function. Our study indicates that 10 weeks of exercise training, undertaken at an advanced age, reverses age‐related impairment of passive left ventricular dilatation, i.e., the age‐induced decrease in E/A was reversed by exercise training (Fig. 1 A and C). Exercise training also prevented further age‐related lengthening of IVRT (Fig. 2 B and C), indicating improved function of the mechanisms that regulate active left ventricular relaxation. Regression analyses demonstrate a significant correlation between left ventricular blood flow at rest (Fig. 10 A) and during exercise (Fig. 10 B) and IVRT. Thus, with these findings of exercise training‐induced improvement of left ventricular perfusion in old rats, it seems likely that the improvement in left ventricular relaxation is related, in part, to improved microvascular function and improved oxygen delivery to the myocardium.
Figure 10. Relationships between ventricular relaxation and microvascular function in old and young, sedentary and exercise trained rats.
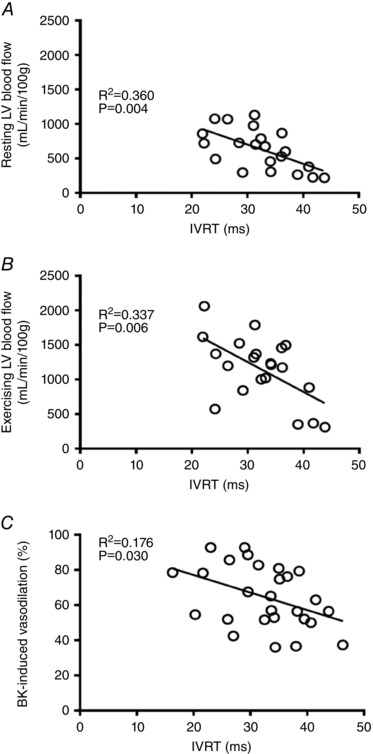
Relationship between isovolumic relaxation time (IVRT) and microvascular function in the left ventricle of old and young, sedentary and exercise trained rats. A, relationship between IVRT and resting left ventricular blood flow (n = 21). B, relationship between IVRT and ventricular blood flow during treadmill exercise (n = 20). C, relationship between IVRT and bradykinin‐induced relaxation in resistance arterioles from the left ventricular free wall (n = 27).
Although various studies have been performed to investigate underlying mechanisms of diastolic dysfunction, the role of coronary blood flow regulation and microvascular dysfunction in development of age‐related diastolic dysfunction that occurs in the absence of comorbidities such as hypertension and coronary artery disease has not been investigated previously. Several studies have demonstrated an association between left ventricular diastolic dysfunction and hypertension‐induced left ventricular hypertrophy (Shapiro & Gibson, 1988; Kyriakidis et al. 1998; Aeschbacher et al. 2001; Dupont et al. 2012). In a study by Shapiro & Gibson (1988) all patients that were diagnosed with left ventricular hypertrophy also demonstrated diastolic dysfunction; however, impaired diastolic function was also observed in hypertensive patients without an increase in left ventricular mass (Dupont et al. 2012). Spontaneous hypertensive rats developed diastolic dysfunction before the onset of hypertension and left ventricular hypertrophy (Aeschbacher et al. 2001; Dupont et al. 2012). Similarly, our results indicate that diastolic dysfunction developed without the presence of hypertension or left ventricular hypertrophy. Our data support the notion that impairment of microvascular function and potentially unmet cardiac demand for oxygen and substrates contribute to development of diastolic dysfunction in aged hearts in the absence of significant comorbidities.
Sugishita's group demonstrated that chronically increased aortic stiffness causes deterioration of endocardial blood flow and coronary flow reserve (Watanabe et al. 1993; Ohtsuka et al. 1994). During diastole, recoil of the aortic wall due to elastic forces propels blood toward the peripheral circulation and into the large coronary arteries. As the aorta stiffens with age, the volume of blood that fills it during systole decreases, contributing to reduced flow into epicardial arteries during diastole, when myocardial flow peaks. In agreement with a number of reports (Lakatta, 2003; Westenberg et al. 2011; Gu et al. 2014), we found that aortic pulse wave velocity increased with ageing and decreased with exercise training. These changes in aortic stiffness have been linked to increased deposition of collagen within the arterial wall and the presence of fragmented elastin fibres in the thickened intima (Wang et al. 2005, 2006, 2012). In the aorta of aged rats, exercise training attenuated aortic stiffening, as determined by reduced PWV, and this diminution of aortic stiffness was marked by reduced collagen concentration and increased elastin content (Gu et al. 2014). The reduction in coronary blood flow at rest in aged rats (Figs 5 A, 6 A, and 7 A and C), may be linked, in part, to aortic stiffening and a reduction in the Windkessel effect in the aged aorta. Exercise training reduced aortic stiffness in aged rats (Figs 4 A and C), potentially increasing the Windkessel effect and increasing flow into the coronary arteries during diastole. A future assessment of coronary artery flow will be necessary to confirm this potential mechanism for increased resting blood flow in old rats following exercise training.
Ageing decreased blood flow to the right ventricular free wall and the endocardium and epicardium of the left ventricular free wall during treadmill exercise; exercise training restored these blood flow responses in old rats (Figs 6 B and D). Given that there were no differences in mean arterial pressure between groups, these results suggest that age‐ and exercise training‐induced changes in regional cardiac flows resulted from adaptations of microvascular reactivity versus changes in perfusion pressure. The age‐induced decreases in regional cardiac blood flows were paralleled by reduced arteriolar vasodilatory responses to bradykinin, confirming earlier reports of age‐induced endothelial dysfunction in coronary arterioles (LeBlanc et al. 2008, 2009; Kang et al. 2009, 2011). Hachamovitch et al. (1989) demonstrated age‐related reductions in epicardial and endocardial left ventricular flows during maximal coronary vasodilatation induced by carbachol; however, these measurements were made in anaesthetized rats and are indicative of reduced coronary reserve, which may be influenced by age‐induced changes in microvascular morphology as well as vascular reactivity. Here, we report for the first time, that exercise training increased regional blood flows during submaximal exercise in the aged left ventricle and improved endothelium‐dependent vasodilatation in coronary arterioles of old rats. Exercise training did not alter diastolic or microvascular function in young rats, a finding that is not surprising given the relative intensity of the training bouts. Training bouts in the young and old rats were matched for absolute intensity and duration. On a relative scale, training bouts occurred in young rats at approximately 50–60% of maximal capacity, whereas old rats were training at closer to 70–80% of maximal capacity (Mazzeo et al. 1984). Because of its continuous activity, the heart in sedentary animals can be considered to be a chronically trained muscle; exercise training‐induced adaptations of cardiac muscle and the coronary circulation require that training bouts occur at a relatively high intensity, i.e. >70% of maximal capacity (Muller et al. 1993, 1994; Parker et al. 1994), such as that which occurred in the old rats in the current study.
In aged skeletal muscle, impairment of endothelial function and dysregulation of microvascular perfusion are significantly reversed by late‐life exercise training (Spier et al. 2004, 2007; Behnke et al. 2012). Exercise training‐induced amelioration of endothelium‐dependent vasodilatation of coronary arterioles is a likely contributor to the improvement of coronary perfusion during exercise that we have documented in this study. We performed regression analysis and found a significant correlation between endothelium‐dependent responsiveness to bradykinin and active ventricular relaxation (IVRT correlates significantly with maximal vasodilatation to bradykinin; Fig. 10 C). These data indicate that greater endothelium‐dependent relaxation is associated with more rapid active ventricular relaxation, and are in keeping with the theory that age‐related endothelial inflammation leads to decreased endothelium‐dependent vasodilatation and localized microdomains of ischaemia during physiological stress such as exercise. In addition to the potential cellular damage produced by repeated ischaemic events could contribute to impairment of ATP availability, inadequate sequestration of calcium, and incomplete actin–myosin association, leading to persistent impairment of active relaxation and diastolic dysfunction.
In coronary and skeletal muscle arterioles of aged rats, age‐related endothelial dysfunction is due, in part, to imbalance between signalling mediated through nitric oxide (NO) and reactive oxygen species (LeBlanc et al. 2008; Kang et al. 2009, 2011). In coronary arterioles, increased scavenging of NO and increased production of hydroxyl radicals are likely mechanisms for age‐induced impairment of both NO‐ and hydrogen peroxide‐mediated endothelium‐dependent vasodilatation. Similarly, in skeletal muscle arterioles, age‐induced impairment of NO‐mediated vasodilatation occurs with age; limited availability of the co‐factor tetrahydrobiopterin and increased superoxide production are likely contributors to the limited bioavailability of NO in aged skeletal muscle arterioles (Delp et al. 2008; Sindler et al. 2009, 2013). In skeletal muscle arterioles from aged rats, this reduction of NO bioavailability is reversed by exercise training (Sindler et al. 2009, 2013). Our current data confirm that endothelium‐dependent vasodilatation declines with age in coronary arterioles and also demonstrate that, as in skeletal muscle arterioles, this decline of endothelial function can be reversed by late‐life aerobic exercise training. The current data indicate that restoration of anti‐oxidant protein expression and improved regulation of the balance between NO and reactive oxygen species may contribute to the reversal of age‐induced endothelial dysfunction by exercise training (Fig. 9).
HFpEF is the predominant form of heart failure found in the elderly (Kitzman et al. 2001). Most elderly HFpEF patients show evidence of diastolic dysfunction; however, non‐cardiac comorbidities including obesity, hypertension, and diabetes mellitus are also highly prevalent in HFpEF (Ather et al. 2012). A recently developed paradigm for HFpEF proposes that these comorbidities produce a peripheral inflammatory state that leads to microvascular endothelial dysfunction, which in the heart leads to stiffening of myocytes and interstitial fibrosis with eventual cardiac remodelling and failure (Paulus & Tschope, 2013). Additionally, there is evidence that exercise oxygen consumption () and exercise tolerance (Kitzman et al. 2010, 2013) are increased and peripheral microvascular dysfunction is attenuated by aerobic exercise training in HFpEF patients (Haykowsky et al. 2012). Induction of HFpEF in a Dahl salt‐sensitive rat model produces endothelial dysfunction and loss of expression of endothelial nitric oxide synthase in the aorta (Adams et al. 2015); however, reversal of this peripheral vascular dysfunction by high intensity exercise training did not reverse diastolic dysfunction. Our current data confirm our previous findings of age‐induced endothelial dysfunction of the coronary resistance vasculature (Kang et al. 2009, 2011; LeBlanc et al. 2009), and suggest that this loss of vasodilatory function in resistance arterioles, which are critical to regulation of coronary blood flow, contributes significantly to development of age‐related diastolic dysfunction (Fig. 10). If diastolic dysfunction in a critical component of HFpEF in the elderly, our data suggest that age‐induced endothelial dysfunction of the coronary resistance vasculature may contribute to development of HFpEF, independently of comorbidities and peripheral vascular disease. Importantly, our results also indicate that exercise training reverses endothelial dysfunction of the coronary resistance vasculature and restores diastolic function in an ageing model that is free of hypertension, obesity and diabetes.
Limitations
We assessed diastolic function using only non‐invasive Doppler imageing. We did not evaluate pressure‐volume loops or study the mechanics of cardiac fibres in our rats. Thus, our measurements of diastolic function are not specific indicators of LV wall stiffness or pressure‐independent relaxation properties of the cardiac muscle; however, we did determine that resting blood pressure and heart rate did not differ between the groups of rats. Thus, although not specific, our measurements are valid indicators of changes in overall diastolic function between rat groups. Our determination that age impairs coronary blood flow and vasodilator responses in parallel with age‐related development of diastolic dysfunction does not establish causality; however, regression analysis revealed significant correlations between IVRT and left ventricular blood flows (Fig. 10 A and B) and endothelium‐dependent vasodilatation (Fig. 10 C). These data suggest that the reactivity of the coronary resistance vasculature is an important determinant of left ventricular diastolic function. Future studies will need to be performed to investigate specific mechanisms of both vascular dysfunction and cardiac remodelling/relaxation properties in the aged heart.
In summary, dysregulation of coronary blood flow and endothelial dysfunction are present in the aged rodent heart, probably contributing to age‐related diastolic dysfunction. Exercise training improves endothelial function of coronary arterioles, increases coronary blood flow at rest and during exercise, and reverses diastolic dysfunction in the aged heart. Thus, exercise training promotes microvascular and diastolic function, even when initiated at an advanced age. Given that diastolic dysfunction is often a component of HFpEF, which is the predominant form of heart failure in the elderly and which has limited therapeutic options, understanding of the mechanisms whereby exercise training improves diastolic function in old age is likely to aid in advancement of treatment of HFpEF in the elderly.
Additional information
Competing interests
None declared.
Author contributions
JMD and BJB designed this study. KH, BJB, MDD and JMD contributed to writing this manuscript. KH did statistical analysis and made figures. BC, JNS, BJB, PG and JMD contributed to the blood flow measurements. KH, JLS and JAB assessed function and immunohistochemistry of arterioles. BJB, MDD, and JMD revised manuscript. JMD obtained financial support. All authors checked and approved final version of the manuscript.
Funding
NIH R01 HL077224 and NIH R01HL90937.
Acknowledgments
The authors thank Xueling Teng and Shige Tsuda for technical support.
Linked articles This article is highlighted by a Perspective by Thorin. To read this Perspective, visit https://doi.org/10.1113/JP274297.
References
- Abdelmoneim SS, Basu A, Bernier M, Dhoble A, Abdel‐Kader SS, Pellikka PA & Mulvagh SL (2011). Detection of myocardial microvascular disease using contrast echocardiography during adenosine stress in type 2 diabetes mellitus: prospective comparison with single‐photon emission computed tomography. Diab Vasc Dis Res 8, 254–261. [DOI] [PubMed] [Google Scholar]
- Adams V, Alves M, Fischer T, Rolim N, Werner S, Schutt N, Bowen TS, Linke A, Schuler G & Wisloff U (2015). High‐intensity interval training attenuates endothelial dysfunction in a Dahl salt‐sensitive rat model of heart failure with preserved ejection fraction. J Appl Physiol (1985) 119, 745–752. [DOI] [PubMed] [Google Scholar]
- Aeschbacher BC, Hutter D, Fuhrer J, Weidmann P, Delacretaz E & Allemann Y (2001). Diastolic dysfunction precedes myocardial hypertrophy in the development of hypertension. Am J Hypertens 14, 106–113. [DOI] [PubMed] [Google Scholar]
- Aroesty JM, Mckay RG, Heller GV, Royal HD, Als AV & Grossman W (1985). Simultaneous assessment of left‐ventricular systolic and diastolic dysfunction during pacing‐induced ischemia. Circulation 71, 889–900. [DOI] [PubMed] [Google Scholar]
- Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH & Deswal A (2012). Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 59, 998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke BJ, Ramsey MW, Stabley JN, Dominguez JM 2nd, Davis RT 3rd, McCullough DJ, Muller‐Delp JM & Delp MD (2012). Effects of aging and exercise training on skeletal muscle blood flow and resistance artery morphology. J Appl Physiol (1985) 113, 1699–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ, Levy D, Anderson KM, Wolf PA, Plehn JF, Evans JC, Comai K, Fuller DL & Sutton MS (1992). Determinants of Doppler Indexes of left‐ventricular diastolic function in normal subjects (the Framingham Heart‐Study). Am J Cardiol 70, 508–515. [DOI] [PubMed] [Google Scholar]
- Borlaug BA & Kass DA (2006). Mechanisms of diastolic dysfunction in heart failure. Trends Cardiovasc Med 16, 273–279. [DOI] [PubMed] [Google Scholar]
- Brenner DA, Apstein CS & Saupe KW (2001). Exercise training attenuates age‐associated diastolic dysfunction in rats. Circulation 104, 221–226. [DOI] [PubMed] [Google Scholar]
- Chan E, Giallauria F, Vigorito C & Smart NA (2016). Exercise training in heart failure patients with preserved ejection fraction: a systematic review and meta‐analysis. Monaldi Arch Chest Dis 86, 759. [DOI] [PubMed] [Google Scholar]
- Chilian WM, Eastham CL & Marcus ML (1986). Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol Heart Circ Physiol 251, H779–H788. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A & Kaley G (2002). Aging‐induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90, 1159–1166. [DOI] [PubMed] [Google Scholar]
- Delp MD, Behnke BJ, Spier SA, Wu G & Muller‐Delp JM (2008). Ageing diminishes endothelium‐dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol 586, 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD, Evans MV & Duan C (1998). Effects of aging on cardiac output, regional blood flow, and body composition in Fischer‐344 rats. J Appl Physiol (1985) 85, 1813–1822. [DOI] [PubMed] [Google Scholar]
- Dupont S, Maizel J, Mentaverri R, Chillon JM, Six I, Giummelly P, Brazier M, Choukroun G, Tribouilloy C, Massy ZA & Slama M (2012). The onset of left ventricular diastolic dysfunction in SHR rats is not related to hypertrophy or hypertension. Am J Physiol Heart Circ Physiol 302, H1524–H1532. [DOI] [PubMed] [Google Scholar]
- Flaim SF, Nellis SH, Toggart EJ, Drexler H, Kanda K & Newman ED (1984). Multiple simultaneous determinations of hemodynamics and flow distribution in conscious rat. J Pharmacol Methods 11, 1–39. [DOI] [PubMed] [Google Scholar]
- Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschope C, Leite‐Moreira AF, Musters R, Niessen HW, Linke WA, Paulus WJ & Hamdani N (2016). Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail 4, 312–324. [DOI] [PubMed] [Google Scholar]
- Groban L ( 2005). Diastolic dysfunction in the older heart. J Cardiothorac Vasc Anesth 19, 228–236. [DOI] [PubMed] [Google Scholar]
- Gu Q, Wang B, Zhang XF, Ma YP, Liu JD & Wang XZ (2014). Chronic aerobic exercise training attenuates aortic stiffening and endothelial dysfunction through preserving aortic mitochondrial function in aged rats. Exp Gerontol 56, 37–44. [DOI] [PubMed] [Google Scholar]
- Hachamovitch R, Wicker P, Capasso JM & Anversa P (1989). Alterations of coronary blood‐flow and reserve with aging in Fischer‐344 rats. Am J Physiol Heart Circ Physiol 256, H66–H73. [DOI] [PubMed] [Google Scholar]
- Hanna MA, Taylor CR, Chen B, La HS, Maraj JJ, Kilar CR, Behnke BJ, Delp MD & Muller‐Delp JM (2014). Structural remodeling of coronary resistance arteries: effects of age and exercise training. J Appl Physiol (1985) 117, 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CJ, Reddy AK, Madala S, Michael LH, Entman ML & Taffet GE (2007). Effects of isoflurane on coronary blood flow velocity in young, old and ApoE−/− mice measured by Doppler ultrasound. Ultrasound Med Biol 33, 512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J & Kitzman DW (2012). Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol 60, 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K‐W, Kim O‐S, Chin JY, Kim WH, Park SH, Choi YJ, Shin JH, Jung KT, Lim D‐S & Lee S‐K (2015). Diastolic dysfunction induced by a high‐fat diet is associated with mitochondrial abnormality and adenosine triphosphate levels in rats. Endocrinol Metab 30, 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang LS, Chen B, Reyes RA, Leblanc AJ, Teng B, Mustafa SJ & Muller‐Delp JM (2011). Aging and estrogen alter endothelial reactivity to reactive oxygen species in coronary arterioles. Am J Physiol Heart Circ Physiol 300, H2105–H2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang LS, Reyes RA & Muller‐Delp JM (2009). Aging impairs flow‐induced dilation in coronary arterioles: role of NO and H2O2 . Am J Physiol Heart Circ Physiol 297, H1087–H1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata T, Daimon M, Miyazaki S, Ichikawa R, Maruyama M, Chiang SJ, Ito C, Sato F, Watada H & Daida H (2015). Coronary microvascular function is independently associated with left ventricular filling pressure in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 14, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzman DW, Brubaker PH, Herrington DM, Morgan TM, Stewart KP, Hundley WG, Abdelhamed A & Haykowsky MJ (2013). Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single‐blind trial. J Am Coll Cardiol 62, 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzman DW, Brubaker PH, Morgan TM, Stewart KP & Little WC (2010). Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single‐blind trial. Circ Heart Fail 3, 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL & Cardiovascular Health Study Research G (2001). Importance of heart failure with preserved systolic function in patients ≥ 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol 87, 413–419. [DOI] [PubMed] [Google Scholar]
- Kyriakidis M, Triposkiadis F, Dernellis J, Androulakis AE, Mellas P, Kelepeshis GA & Gialafos JE (1998). Effects of cardiac versus circulatory angiotensin‐converting enzyme inhibition on left ventricular diastolic function and coronary blood flow in hypertrophic obstructive cardiomyopathy. Circulation 97, 1342–1347. [DOI] [PubMed] [Google Scholar]
- Labovitz AJ, Lewen MK, Kern M, Vandormael M, Deligonal U, Kennedy HL, Habermehl K & Mrosek D (1987). Evaluation of left‐ventricular systolic and diastolic dysfunction during transient myocardial‐ischemia produced by angioplasty. J Am Coll Cardiol 10, 748–755. [DOI] [PubMed] [Google Scholar]
- Lakatta EG (2003). Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation 107, 490–497. [DOI] [PubMed] [Google Scholar]
- Larghat AM, Swoboda PP, Biglands JD, Kearney MT, Greenwood JP & Plein S (2014). The microvascular effects of insulin resistance and diabetes on cardiac structure, function, and perfusion: a cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging 15, 1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc AJ, Chen B, Dougherty PJ, Reyes RA, Shipley RD, Korzick DH & Muller‐Delp JM (2013). Divergent effects of aging and sex on vasoconstriction to endothelin in coronary arterioles. Microcirculation 20, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc AJ, Reyes R, Kang LS, Dailey RA, Stallone JN, Moningka NC & Muller‐Delp JM (2009). Estrogen replacement restores flow‐induced vasodilation in coronary arterioles of aged and ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 297, R1713–R1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc AJ, Shipley RD, Kang LS & Muller‐Delp JM (2008). Age impairs Flk‐1 signaling and NO‐mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol 295, H2280–H2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzeo RS, Brooks GA & Horvath SM (1984). Effects of age on metabolic responses to endurance training in rats. J Appl Physiol Respir Environ Exerc Physiol 57, 1369–1374. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Guth BD, Miura T, Indolfi C, Schulz R & Ross J (1990). Changes of left‐ventricular diastolic function in exercising dogs without and with ischemia. Circulation 81, 1058–1070. [DOI] [PubMed] [Google Scholar]
- Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ & Redfield MM (2015). Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 131, 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JM, Myers PR & Laughlin MH (1993). Exercise training alters myogenic responses in porcine coronary resistance arteries. J Appl Physiol (1985) 75, 2677–2682. [DOI] [PubMed] [Google Scholar]
- Muller JM, Myers PR & Laughlin MH (1994). Vasodilator responses of coronary resistance arteries of exercise‐trained pigs. Circulation 89, 2308–2314. [DOI] [PubMed] [Google Scholar]
- Münzel T, Gori T, Keaney JF, Maack C & Daiber A (2015). Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur Heart J 36, 2555–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel A, Kohlhaas M & Maack C (2014). Mitochondrial reactive oxygen species production and elimination. J Mol Cell Cardiol 73, 26–33. [DOI] [PubMed] [Google Scholar]
- Ohtsuka S, Kakihana M, Watanabe H & Sugishita Y (1994). Chronically decreased aortic distensibility causes deterioration of coronary perfusion during increased left‐ventricular contraction. J Am Coll Cardiol 24, 1406–1414. [DOI] [PubMed] [Google Scholar]
- Ong P & Sechtem U (2016). [Coronary microvascular dysfunction : Clinical aspects, diagnosis and therapy]. Herz 41, 351–360. [DOI] [PubMed] [Google Scholar]
- Parker JL, Oltman CL, Muller JM, Myers PR, Adams HR & Laughlin MH (1994). Effects of exercise training on regulation of tone in coronary arteries and arterioles. Med Sci Sports Exerc 26, 1252–1261. [PubMed] [Google Scholar]
- Paulus WJ & Tschope C (2013). A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62, 263–271. [DOI] [PubMed] [Google Scholar]
- Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A & Frenneaux M (2009a). Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol 54, 402–409. [DOI] [PubMed] [Google Scholar]
- Phan TT, Abozguia K, Shivu GN, Ahmed I, Leyva F, Patel K & Frenneaux M (2009b). Increased atrial contribution to left ventricular filling compensates for impaired early filling during exercise in heart failure with preserved ejection fraction. J Card Fail 15, 890–897. [DOI] [PubMed] [Google Scholar]
- Prasad A, Popovic ZB, Arbab‐Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD & Levine BD (2007). The effects of aging and physical activity on Doppler measures of diastolic function. Am J Cardiol 99, 1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AK, Jones AD, Martono C, Caro WA, Madala S & Hartley CJ (2005a). Pulsed Doppler signal processing for use in mice: design and evaluation. IEEE Trans Biomed Eng 52, 1764–1770. [DOI] [PubMed] [Google Scholar]
- Reddy AK, Madala S, Jones AD, Caro WA, Eberth JF, Pham TT, Taffet GE & Hartley CJ (2009). Multichannel pulsed Doppler signal processing for vascular measurements in mice. Ultrasound Med Biol 35, 2042–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AK, Taffet GE, Li YH, Lim SW, Pham TT, Pocius JS, Entman ML, Michael LH & Hartley CJ (2005b). Pulsed Doppler signal processing for use in mice: applications. IEEE Trans Biomed Eng 52, 1771–1783. [DOI] [PubMed] [Google Scholar]
- Shapiro LM & Gibson DG (1988). Patterns of diastolic dysfunction in left ventricular hypertrophy. Br Heart J 59, 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley RD & Muller‐Delp JM (2005). Aging decreases vasoconstrictor responses of coronary resistance arterioles through endothelium‐dependent mechanisms. Cardiovasc Res 66, 374–383. [DOI] [PubMed] [Google Scholar]
- Sievers R, Parmley WW, James T & Wikmancoffelt J (1983). Energy‐levels at systole vs diastole in normal hamster hearts vs myopathic hamster hearts. Circ Res 53, 759–766. [DOI] [PubMed] [Google Scholar]
- Sindler AL, Delp MD, Reyes R, Wu G & Muller‐Delp JM (2009). Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol 587, 3885–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Reyes R, Chen B, Ghosh P, Gurovich AN, Kang LS, Cardounel AJ, Delp MD & Muller‐Delp JM (2013). Age and exercise training alter signaling through reactive oxygen species in the endothelium of skeletal muscle arterioles. J Appl Physiol (1985) 114, 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW & Muller‐Delp JM (2004). Effects of ageing and exercise training on endothelium‐dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 556, 947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Stallone JN, Dominguez JM 2nd & Muller‐Delp JM (2007). Exercise training enhances flow‐induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and nitric oxide. Am J Physiol Heart Circ Physiol 292, H3119–H3127. [DOI] [PubMed] [Google Scholar]
- Srere PA (1969). Citrate synthase In Methods in Enzymology, pp. 3–11. Elsevier. [Google Scholar]
- Srivaratharajah K, Coutinho T, deKemp R, Liu P, Haddad H, Stadnick E, Davies RA, Chih S, Dwivedi G, Guo A, Wells GA, Bernick J, Beanlands R & Mielniczuk LM (2016). Reduced myocardial flow in heart failure patients with preserved ejection fraction. Circ Heart Fail 9, pii: e002560. [DOI] [PubMed] [Google Scholar]
- Teo LY, Chan LL & Lam CS (2016). Heart failure with preserved ejection fraction in hypertension. Curr Opin Cardiol 31, 410–416. [DOI] [PubMed] [Google Scholar]
- Upadhya B, Taffet GE, Cheng CP & Kitzman DW (2015). Heart failure with preserved ejection fraction in the elderly: scope of the problem. J Mol Cell Cardiol 83, 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heerebeek L & Paulus WJ (2016). Understanding heart failure with preserved ejection fraction: where are we today? Neth Heart J 24, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma N, Eberli FR & Apstein CS (2000). Increased diastolic chamber stiffness during demand ischemia: response to quick length change differentiates rigor‐activated from calcium‐activated tension. Circulation 101, 2185–2192. [DOI] [PubMed] [Google Scholar]
- Wang M, Zhang J, Spinetti G, Jiang LQ, Monticone R, Zhao D, Cheng L, Krawczyk M, Talan M, Pintus G & Lakatta EG (2005). Angiotensin II activates matrix metalloproteinase type II and mimics age‐associated carotid arterial remodeling in young rats. Am J Pathol 167, 1429–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhang J, Telljohann R, Jiang L, Wu J, Monticone RE, Kapoor K, Talan M & Lakatta EG (2012). Chronic matrix metalloproteinase inhibition retards age‐associated arterial proinflammation and increase in blood pressure. Hypertension 60, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, Monticone R & Lakatta EG (2006). Matrix metalloproteinase 2 activation of transforming growth factor‐beta1 (TGF‐beta1) and TGF‐beta1‐type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol 26, 1503–1509. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Ohtsuka S, Kakihana M & Sugishita Y (1993). Coronary circulation in dogs with an experimental decrease in aortic compliance. J Am Coll Cardiol 21, 1497–1506. [DOI] [PubMed] [Google Scholar]
- Westenberg JJ, Scholte AJ, Vaskova Z, van der Geest RJ, Groenink M, Labadie G, van den Boogaard PJ, Radonic T, Hilhorst‐Hofstee Y, Mulder BJ, Kroft LJ, Reiber JH & de Roos A (2011). Age‐related and regional changes of aortic stiffness in the Marfan syndrome: assessment with velocity‐encoded MRI. J Magn Reson Imaging 34, 526–531. [DOI] [PubMed] [Google Scholar]


