Abstract
Background
Pompe disease is an autosomal recessive metabolic disorder caused by the deficiency of the lysosomal enzyme acid α-glucosidase. This deficiency leads to glycogen accumulation in the lysosomes of muscle tissue causing progressive muscular weakness particularly of the respiratory system. Enzyme replacement therapy (ERT) has demonstrated efficacy in slowing down disease progression in infants. Despite the large number of studies describing the effects of physical training in juvenile and adult late onset Pompe disease (LOPD). There are very few reports that analyze the benefits of respiratory muscle rehabilitation or training.
Methods
The effectiveness of respiratory muscle training was investigated using a specific appliance with adjustable resistance (Threshold). The primary endpoint was effect on respiratory muscular strength by measurements of MIP and MEP. Eight late-onset Pompe patients (aged 13 to 58 years; 4 female, 4 male) with respiratory muscle deficiency on functional respiratory tests were studied. All patients received ERT at the dosage of 20 mg/kg/every 2 weeks and underwent training with Threshold at specified pressures for 24 months.
Results
A significant increase in MIP was observed during the follow-up of 24 month: 39.6 cm H2O (+ 25.0%) at month 3; 39.5 cm H2O (+ 24.9%) at month 6; 39.1 cm H2O (+ 23.7%) at month 9; 37.3 cm H2O (+ 18.2%) at month 12; and 37.3 cm H2O (+ 17.8%) at month 24. Median MEP values also showed a significant increase during the first 9 months: 29.8 cm H2O, (+ 14.3%) at month 3; 31.0 cm H2O (+ 18.6) at month 6; and 29.5 cm H2O (+ 12.9) at month 9. MEP was then shown to be decreased at months 12 and 24; median MEP was 27.2 cm H2O (+ 4.3%) at 12 months and 26.6 cm H2O (+ 1.9%) at 24 months. The FVC remain stable throughout the study.
Conclusion
An increase in respiratory muscular strength was demonstrated with Threshold training when used in combination with ERT.
Keywords: Pompe disease, Late-onset type II glycogenosis, Respiratory muscles, Muscle training, Enzyme replacement therapy
1. Background
Pompe disease is an inherited autosomal recessive disorder caused by the deficiency of the enzyme acid α-glucosidase (GAA), which leads to an intralysosomal accumulation of glycogen in various tissues, especially skeletal muscle [1]. The disease occurs in approximately 1 per 40,000 births and causes a progressive myopathy. Based on the time of symptom development, the disease is typically classified as early-infantile onset (IOPD) with absence or nearly loss of GAA activity resulting in cardiomyopathy and muscle hypotonia and poor prognosis without therapy or late-onset childhood, juvenile or adult form (LOPD). The late-onset type usually involves the proximal muscles and the respiratory muscles with progressive respiratory muscle weakness, night-time respiratory difficulty, progressive hypoventilation, as well as abnormal laboratory data including polycythemia, rise of carbon dioxide (pCO2) in blood, thus potentially leading to respiratory failure [2]. In some cases, respiratory insufficiency may be present without limb-girdle muscle weakness [3]. The degree of involvement of respiratory muscles is evaluated by pulmonary function tests (forced vital capacity [FVC], maximum inspiratory pressure [MIP], and maximum expiratory pressure [MEP]), sleep assessment, and blood gas analysis [4]. The main treatment option is enzyme replacement therapy (ERT) [5], [6]. Guidelines for physiotherapeutic management of muscular respiratory weakness are not available; however, current recommendations suggest following the guidelines of other neuromuscular disorders [7] in order to maintain muscular and respiratory function, prevent complications and preserve the highest level of autonomy. Studies on muscle training and rehabilitation in patients with Pompe disease recommend aerobic and sub-maximal exercise to stimulate the degradation of the glycogen accumulated in cytosol [8]. Maximal muscular exercise is considered dangerous because it could lead to greater muscle degeneration [9]. Training protocols for neuromuscular diseases have been proposed since disease-specific programs are lacking. These procedures require the use of sub-maximal and aerobic exercise to avoid excessive work and to take advantage of functional activities; cardiopulmonary response should also be monitored [10]. The potential benefits of pulmonary rehabilitation or training of respiratory muscles in late onset Pompe disease are not yet known. There is only a single report available of respiratory muscle training showing an improvement in expiratory and inspiratory strength after a short training period [11].
2. Methods
We performed a longitudinal, observational study that was approved by the Ethics Committee of the Hospital of Cattinara (Trieste, Italy) and conducted between March 2007 and November 2011 all eight patients completed the study protocol of 24 months of training. This study aims at evaluating the effectiveness of inspiratory muscle training using a specific device with adjustable resistance (Threshold, Respironics, Inc., below: threshold) in patients with late-onset Pompe disease (LOPD) receiving ERT. The study included 8 patients and the inclusion criteria were diagnosis with LOPD, determined by lack of GAA enzyme activity performed on skin fibroblasts (the gold standard for Pompe disease diagnosis) and muscular weakness on pulmonary function tests. All patients were treated with ERT for at least one year before the beginning of the study. No comorbidities except a diagnosis of asthma were present in one patient in the study group. This criterion was included in order to be sure that the improvement in muscle strength was due to the training protocol, and not ERT. Patients were required to have a degree of autonomy between Grades 4 and 5 on the modified Gardner–Medwin–Walton scale. Exclusion criteria included respiratory failure and lack treatment with ERT. Follow-up visits were scheduled at 3, 6, 9, 12 and 24 months. Pulmonary function tests and respiratory muscle strength were evaluated at each visit, particularly the maximal inspiratory pressure (MIP), measured at residual volume, and the maximal expiratory pressure (MEP), measured from total lung capacity. All measurements were performed with the device ― Micro MPM 8 (Micro Medical Limited, Rochester, England). Forced vital capacity (FVC) in sitting position and clinical examination using the modified Gardner–Medwin–Walton scale to determine the grade of autonomy were also evaluated.
2.1. Training device
The threshold is a device that exerts training of the inspiratory muscles against fixed resistance based on the technique developed by Nickerson and Keens [12]. In this disease training with threshold has been recommended because of its ability to maintain a constant resistance regardless of breathing rate. The positive effects of inspiratory muscle training with threshold are recognized in obstructive pulmonary diseases [13], [14], [15], myasthenia gravis [16], amyotrophic lateral sclerosis [17], diaphragmatic dysfunction [18], congestive heart failure [19], as well as patients weaned from mechanical ventilation [20].
2.2. Training protocol
At the first visit, patients were instructed to perform the training protocol with medical and physiotherapy supervision. In the case of pediatric patients, the family and parents were also educated on the protocol. The following was used for the threshold training protocol:
Training cycle: 1′ inspiratory load set at 30% of best MIP, followed by 2′ deep slow breathing. The patient was instructed to perform 15 training cycles per day for 45 min (15′ at 30% of MIP and 30′ at rest with deep slow breathing) 7 days/week.
Inspiratory load varied according to the MIP percentage at each control visit. The load was adjusted according to the most recent, highest MIP measure. If 30% of best MIP was below the minimum load available for the threshold, the device was regulated at the minimum value. However, the patient was asked to initially maintain the training load for 30″, with the same resting period. If the MIP increased during training, an increase in workload was added to obtain the 30% of the best MIP. Compliance with the training protocol was evaluated under medical supervision on each control visit with the presence of the physiotherapist. The patients were interviewed weekly between control visits to check and support the compliance at home. Furthermore, proper use of the threshold was evaluated during each administration of ERT. The primary endpoint was to determine the efficacy of the training by improvement in MIP as well as pulmonary function tests at each control visit. Standard descriptive statistics were used to analyze the demographic and clinical characteristics of the patients at baseline. The One Way repeated measures ANOVA with the Sigma Stat program was used to compare MIP, MEP, and FVC from baseline to 3, 6, 9, 12 and 24 months. A two-tailed p value < 0.05 was considered significant.
3. Results
Between March 2007 and November 2011 all eight patients completed the study protocol of 24 months of training. All patients were receiving ERT during the study and none were on ventilatory support or bedridden. The demographic and clinical characteristics of the 8 patients at baseline are shown in Table 1. show the values of MIP, MEP, and FVC measured at baseline and at follow-up visits for each patient.
Table 1.
Values of MIP, MEP and FVC measured at baseline and at follow-up visits.
| Patient | PFT | 6–8 months before | Baseline | 3 months | 6 months | 9 months | 12 months | 24 months |
|---|---|---|---|---|---|---|---|---|
| 1 | MIP | 50 | 50 | 53 | 51 | 55 | 50 | 47 |
| MEP | 44 | 43 | 49 | 46 | 47 | 46 | 48 | |
| FVC | 79 | 79 | 81 | 78 | 76 | 80 | 88 | |
| 2 | MIP | 22 | 12 | 17 | 15 | 14 | 15 | 18 |
| MEP | 30 | 10 | 12 | 13 | 12 | 10 | 10 | |
| FVC | 33 | 32 | 31 | 30 | 29 | 27 | 27 | |
| 3 | MIP | 61 | 12 | 15 | 16 | 19 | 15 | 14 |
| MEP | 70 | 13 | 14 | 15 | 18 | 19 | 17 | |
| FVC | 48 | 42 | 46 | 43 | 41 | 38 | 40 | |
| 4 | MIP | 34 | 30 | 35 | 33 | 32 | 31 | 35 |
| MEP | 40 | 18 | 24 | 26 | 20 | 21 | 26 | |
| FVC | 81 | 86 | 75 | 88 | 83 | 80 | 92 | |
| 5 | MIP | 30 | 63 | 74 | 74 | 73 | 70 | 74 |
| MEP | 48 | 43 | 51 | 49 | 48 | 45 | 38 | |
| FVC | 62 | 51 | 65 | 60 | 55 | 58 | 57 | |
| 6 | MIP | 36 | 35 | 58 | 59 | 55 | 56 | 50 |
| MEP | 68 | 41 | 48 | 49 | 45 | 43 | 41 | |
| FVC | 25 | 23 | 25 | 21 | 17 | 19 | 21 | |
| 7 | MIP | 33 | 24 | 29 | 33 | 35 | 31 | 31 |
| MEP | 62 | 24 | 21 | 29 | 26 | 15 | 12 | |
| FVC | 30 | 23 | 29 | 20 | 25 | 24 | 28 | |
| 8 | MIP | 28 | 27 | 36 | 35 | 30 | 31 | 29 |
| MEP | 34 | 17 | 20 | 21 | 20 | 19 | 21 | |
| FVC | 40 | 35 | 37 | 32 | 34 | 31 | 30 |
3.1. Pulmonary function
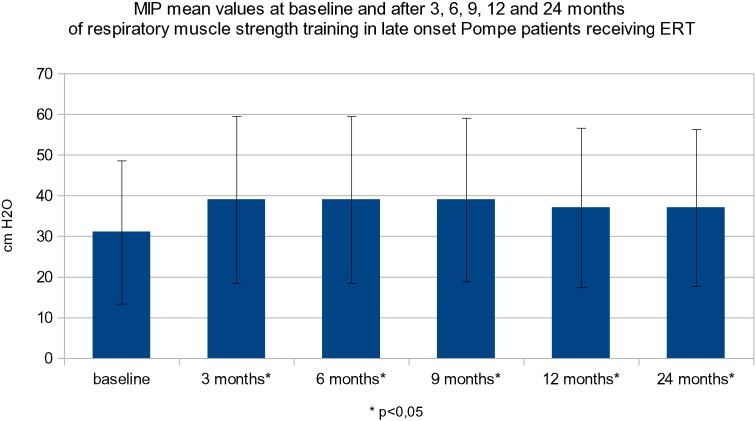
At baseline, the mean (SD) MIP was 31.6 cm H2O (SD: ± 17.7). The average improvement from baseline in MIP at 3 months (mean: 39.6 cm H2O [SD: 20.5]) was + 25.2%, at 6 months (mean: 39.5 cm H2O; [SD: ± 20.5]) was + 24.9%, at 9 months (mean: 39.1 cm H2O [SD: ± 20.1]) was + 23.7%, at 12 months (mean 37.3 cm H2O; [SD: ± 19.6]) was + 18.2%, and at 24 months (mean: 37.2 cm H2O; [SD: ± 19.3]) was + 17.8% in our group of patients (Fig. 1).
Fig. 1.
MIP mean values at baseline and after 3, 6, 9, 12 and 24 months of respiratory muscle strength training in late onset Pompe patients receiving ERT.
The difference in MIP between baseline and follow-up visits was statistically significant (p 0008). At 3 and 24 months, MIP values were comparable, demonstrating that the improvement remained stable over the course of the study (p < 0.05; Table 5). MEP was also evaluated. At baseline, the mean (SD) were 26.1 cm H2O (SD: ± 14). There was a statistically significant increase in MEP from baseline at 3 months by + 14.3% (mean: 29.8 cm H2O; [SD: ± 16.5]), 6 months (+ 18.6%; mean: 31 cm H2O; ([SD: ± 15]), and 9 months + 12.9% (mean: 29.5 cm H2O; [SD: ± 14.7]). However, there was no statistically significant improvement compared to baseline at 12 months + 4.3% (mean: 27.2 cm H2O [13.8]) and at 24 months + 1.9% (mean: 26.6 175 cm H2O; [SD: ± 14.2]) (Fig. 2). To check the real benefit of Threshold training the MIP and MEP values were also taken in a period of 6 to 8 month before the beginning of the study. In this period the patient were only under enzyme treatment. The MIP mean was 33.2 cm H2O instead of 31.6 cm H2O of the baseline values. These data are not statistically different. This means that the MIP and MEP values are stable before the beginning of the study. FVC was also evaluated. At baseline, the percentage of the predicted value of FVC had a mean (SD) of 46.3%(SD: ± 24.2). FVC remained stable at 3 months (48.6%), 6 months (46.5%), 9 months (45%), 12 months (44.6%) and 24 months (47.8%). There were no detectable improvements during the study. (Fig. 3). Patients were assessed for dyspnea grade and clinical examination using the modified Gardner–Medwin–Walton scale for the grade of autonomy at baseline and after each control visit. At baseline, the modified Gardner–Medwin–Walton scale was between Grades 4 and 5. No modification of dyspnea symptoms was observed. Grade values remained stable throughout the study.
Fig. 2.
MEP mean values at baseline and after 3, 6, 9, 12 and 24 months of respiratory muscle strength training in late onset Pompe patients receiving ERT.
Fig. 3.
FVC mean values at baseline and after 3, 6, 9, 12 and 24 months of respiratory muscle strength training in late onset Pompe patients receiving ERT.
4. Discussion
Our data show that respiratory muscle training with threshold preserves and improves inspiratory muscle strength in patients with LOPD. Some examples can be found in the literature for training rehabilitation with Threshold. In this study we observed that MIP significantly improved in our study population and this improvement appeared to remain stable in the subsequent follow-up period. At the beginning of the study some individual response are different, this can be explicate with the adapting in training program and procedure for monitoring pulmonary functional tests. This finding indicates that patient compliance was reliable.
Decline in respiratory strength is the most important prognostic factor in patients with LOPD.
Natural progression of respiratory muscle weakness in Pompe disease in the absence of treatment has been estimated at a decrease of 3.2% in MIP per year [21]. ERT may itself stabilize respiratory function [22], [23]. However, we cannot assess the effect of the training alone since all the patients were treated with ERT. Furthermore, evaluation of MEP showed a positive trend even though the patients did not undergo specific training for these muscles. Our data confirm a previous observation of two case reports by Jones et al. [11] who demonstrated a favorable effect of specific individual respiratory muscle training for several weeks in two patients. However, the patients in this study were followed up to 24 months and the observed benefits did not persist during this period. MIP and MEP were chosen as the primary endpoints because respiratory muscle training can cause an effect on these outcomes as well as respiratory function. We found that respiratory strength improvement may last up to 24 months and that FVC remained stable during the study. No difference of dyspnea symptoms was observed during the study. We showed that the increase of MIP and MEP is very pronounced in the first year with a slow decrease in the second year of treatment, especially with MEP. The initial benefit of increased MEP can be attributed to different training effects. It is likely that during exercise, expiratory muscles are also stimulated. In LOPD, the thorax exercise allows for better expansion and mobility of the sternocostal joints preventing their calcification and muscle hypoextensibility/contracture. Some patients presents different MIP and MEP trends at the beginning of this study, one possible explication is that MIP and MEP maneuvers are technique dependent and this can in part explicate this numbers. The early decrease in MEP is presumably because the training with Threshold operates only on inspiratory muscles. In addition, the patients remained at the same grade of autonomy measured by the modified Gardner-Medwin-Walton scale. During the study no correlation between the MIP values and the modified Gardner-Medwin-Walton scale were observed. One of the major challenges encountered in this study was the ability to assess the compliance of patients. Since it is a rare disorder, patients usually have to travel to a specialized center in order to be treated. In fact, only 1/8 patients were living in Trieste; therefore, conducting interviews by telephone was the most appropriate method to promote and evaluate training compliance. Adherence to treatment may be negatively affected by the higher level of resistance applied to the device, thus markedly increasing the intensity of work; it may sometimes lead an effect that is difficult to maintain. The training was explicated into the hospital and the patients were formed to repeat the same exercise at home. We have no certitude of the compliance of exercise at home. Therefore, patients have to be systematically encouraged to continue the training protocol. Our data provide further insights into the beneficial, cost effective, and safe results of inspiratory muscle training in Pompe disease. Since this is a rare disease, it would be difficult to conduct controlled studies. We believe that respiratory muscle training should be established early in the treatment of this infrequent but disabling condition. Traditionally, excessively strenuous resistance exercises have been discouraged in muscle disorders because of the potential for exacerbating muscle lesion and degeneration. Studies evaluating the effect of ERT in LOPD disease showed a favorable pattern of response in muscle strength, including respiratory parameters, but further studies are needed to confirm these and our data.
5. Conclusion
Respiratory muscle training with threshold in combination with ERT showed a positive effect on inspiratory muscle strength in patients with late-onset Pompe disease. We observed a significant increase in respiratory muscle strength in the first year in our study population and this improvement appeared to remain stable in the subsequent follow-up period. A larger randomized controlled trial is needed to confirm these results.
5.1. Consent
Written informed consent was obtained from the patients and patient's parents (in one case of non-adult patient) of kin for the publication of this report.
Abbreviations
- ERT
enzyme replacement therapy
- FVC
forced vital capacity
- GAA
glucosidase acid alpha
- LOPD
late-onset Pompe disease
- MEP
maximal expiratory pressure
- MIP
maximal inspiratory pressure
- pCO2
partial pressure carbon dioxide
- SD
standard deviation
- cm H2O
centimeter of water
- l/s
liters per second
- Micro MPM 8
Micro Medical Limited, Rochester, England
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
Study concept and design: MJ, MC. Acquisition of data: MJ, FC, MK, RC, CL, RDP, GA.
Analysis and interpretation of data: MJ, MK, FC, RC, CL, BB, MC. Drafting of the manuscript: MJ, MK, BB, MC. Critical revision of the manuscript: all authors. Statistical analysis: MJ, MK, MC. Study supervision: BB, MC. All authors read and approved the final manuscript.
Acknowledgments
The authors would like to thank to the patients and their families for the collaboration.
Contributor Information
Jevnikar Mitja, Email: mitja.jevnikar@gmail.com.
Kodric Metka, Email: metka.kodric@gmail.com.
Cantarutti Fabiana, Email: bianafa@inwind.it.
Rossella Cifaldi, Email: rossella.cifaldi@gmail.com.
Cinzia Longo, Email: cinzia.lng@gmail.com.
Della Porta Rossana, Email: rossana.dellaporta@gmail.com.
Bembi Bruno, Email: bembi.bruno@aoud.sanita.fvg.it.
Confalonieri Marco, Email: marco.confalonieri@aots.sanita.fvg.it.
References
- 1.Raben N., Plotz P., Byrne B.J. Acid a-glucosidase deficiency (glycogenosis type II, Pompe disease) Curr. Mol. Med. 2002;2:145–166. doi: 10.2174/1566524024605789. [DOI] [PubMed] [Google Scholar]
- 2.Kishnani P.S., Steiner R.D., Bali D., Berger K., Byrne B.J., Case L.E. Pompe disease diagnosis and management guideline. Genet. Med. 2006;8:267–288. doi: 10.1097/01.gim.0000218152.87434.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pellegrini N., Laforet P., Orlikowski D., Pellegrini M., Caillaud C., Eymard B., Raphael J.C., Lofaso F. Respiratory insufficiency and limb muscle weakness 280 in adults with Pompe's disease. Eur. Respir. J. 2005 Dec;26(6):1024–1031. doi: 10.1183/09031936.05.00020005. [DOI] [PubMed] [Google Scholar]
- 4.Van der Ploeg A.T. Monitoring of pulmonary function in Pompe disease: a muscle disease with new therapeutic perspectives. Eur. Respir. J. 2005;26:984–985. doi: 10.1183/09031936.05.00112005. [DOI] [PubMed] [Google Scholar]
- 5.Kishnani P.S., Nicolino M., Voit T., Rogers R.C., Tsai A.C., Waterson J. Chinese hamster ovary cell-derived recombinant human acid alpha-glucosidase in infantile-onset Pompe disease. J. Pediatr. 2006;149:89–97. doi: 10.1016/j.jpeds.2006.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van der Ploeg A.T., Clemens P., Corzo D., Escolar D., Florence J., Groeneveld G. A randomized study of alglucosidase alfa in lateonset Pompe's disease. N. Engl. J. Med. 2010;362:1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- 7.Cupler E.J., Berger K.I., Leshner R.T., Wolfe G.I., Han J.J., Barohn R.J., Kissel J.T. AANEM consensus committee on late-onset Pompe disease. Consensus treatment recommendations for late-onset Pompe disease. Muscle Nerve. 2012 Mar;45(3):319–333. doi: 10.1002/mus.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Case L.E., Kishnani P.S. Physical therapy management of Pompe disease. Genet. Med. 2006;8:318–327. doi: 10.1097/01.gim.0000217789.14470.c5. [DOI] [PubMed] [Google Scholar]
- 9.Case L.E., Kishnani P.S. Physical therapy management of Pompe disease. Genet. Med. 2006 doi: 10.1097/01.gim.0000217789.14470.c5. [DOI] [PubMed] [Google Scholar]
- 10.Fowler W.M. Role of physical activity and exercise training in neuromuscular diseases. Am. J. Phys. Med. Rehabil. 2002;81:S187–S195. doi: 10.1097/01.PHM.0000029726.80774.83. [DOI] [PubMed] [Google Scholar]
- 11.Jones H.N., Moss T., Edwards L., Kishnani P.S. Increased inspiratory and expiratory muscle strength following respiratory muscle strenght training (RMST) in two patients with late-onset Pompe disease. Mol. Genet. Metab. 2011;104:417–420. doi: 10.1016/j.ymgme.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Nickerson B.G., Keens T.G. Measuring ventilatory muscle endurance in humans as sustainable inspiratory pressure. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982;52(3):768–772. doi: 10.1152/jappl.1982.52.3.768. [DOI] [PubMed] [Google Scholar]
- 13.Nield M.A. Inspiratory muscle training protocol using a pressure threshold device: effect on dyspnea in chronic obstructive pulmonary disease. Arch. Phys. Med. Rehabil. Jan 1999;80(1):100–102. doi: 10.1016/s0003-9993(99)90315-5. [DOI] [PubMed] [Google Scholar]
- 14.Dornelas de Andrade A., Silva T.N.S., Vasconcelos H., Marcelino M., Rodrigues-Machado M.G., Galindo Filho V.C., Moraes N.H., Marino P.E.M., Amorim C.F. Inspiratory muscular activation durino threshold® therapy in elderly healthy and patients with COPD. J. Electromyogr. Kinesiol. 2005;15:31–639. doi: 10.1016/j.jelekin.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Scherer T.A., Spengler C.M., Owassapian D., Imhof E., Boutellier U. Respiratory muscle endurance training in chronic obstructive pulmonary disease: impact on exercise capacity, dyspnea, and quality of life. Am. J. Respir. Crit. Care Med. Nov 2000;162(5):1709–1714. doi: 10.1164/ajrccm.162.5.9912026. [DOI] [PubMed] [Google Scholar]
- 16.Weiner P., Gross D., Meiner Z., Ganem R., Weiner M., Zamir D., Rabner M. Respiratory muscle training in patients with moderate to severe myasthenia gravis. Can. J. Neurol. Sci. Aug 1998;25(3):236–241. doi: 10.1017/s0317167100034077. [DOI] [PubMed] [Google Scholar]
- 17.Cheah B.C., Boland R.A., Brodaty N.E., Zoing M.C., Jeffery S.E., McKenzie D.K., Kiernan M.C. Inspirational—inspiratory muscle training in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Oct-Dec 2009;10(5–6):384–392. doi: 10.3109/17482960903082218. [DOI] [PubMed] [Google Scholar]
- 18.Kodric M., Trevisan R., Torregiani C., Cifaldi R., Longo C., Cantarutti F., Confalonieri M. Inspiratory muscle training for diaphragm dysfunction after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2013;145:819–823. doi: 10.1016/j.jtcvs.2012.07.087. [DOI] [PubMed] [Google Scholar]
- 19.Weiner P., Waizman J., Magadle R., Berar-Yanay N., Pelled B. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin. Cardiol. Nov 1999;22(11):727–732. doi: 10.1002/clc.4960221110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin A.D., Smith B.K., Davenport P.D., Harman E., Gonzalez-Rothi R.J., Baz M., Layon A.J., Banner M.J., Caruso L.J., Deoghare H., Huang T.T., Gabrielli A. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Crit. Care. 2011;15(2):R84. doi: 10.1186/cc10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van der Beek N.A., Van Capelle C.I., Van der Velden-van Etten K.I., Hop W.C., Van den Berg B., Reuser A.J., an Doorn P.A., van der Ploeg A.T., Stam H. Rate of progression and predictive factors for pulmonary outcome in children and adults with Pompe disease. Mol. Genet. Metab. 2011 Sep-Oct;104(1–2):129–136. doi: 10.1016/j.ymgme.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Wochenschr W.M., Schneider I., Hanisch F., Müller T., Schmidt B., Zierz S. Jan 2013. Respiratory function in late-onset Pompe disease patients receiving long-term enzyme replacement therapy for more than 48 months; p. 1. (Epub 2012 Nov 19) [DOI] [PubMed] [Google Scholar]
- 23.Bembi B. Long-term observational, non-randomized study of enzyme replacement therapy in late-onset glycogenosis type II. J. Inherit. Metab. Dis. 24 August 2010 doi: 10.1007/s10545-010-9201-8. [DOI] [PubMed] [Google Scholar]