Abstract
Background
In phenylketonuria, dietary treatment prevents most of the severe brain disease. However, patients have to follow a diet restricted in several natural components, what may cause decreased bone density and obesity. Exercise is known to improve both mental functioning and bone density also avoiding obesity, and could optimize aspects of central and peripheral outcome, regardless changes in phenylalanine (Phe) levels. However, the acute effects of exercise on metabolic parameters in phenylketonuria patients are unknown and thereby long-term adaptations are unclear. Therefore, this study aimed to evaluate patients' basal metabolic rate (BMR), and their acute response to an aerobic exercise session on plasma concentrations of Phe, tyrosine (Tyr), and branched-chain amino acids (BCAA), as well as metabolic and hormonal responses.
Methods
Five early- and four late diagnosed phenylketonuria patients aged 21 ± 4 years and 17 sex-, age-, and BMI-matched controls were evaluated for BMR, peak oxygen consumption (VO2peak) and plasma amino acid, glucose, lipid profile and hormonal levels. At least one week later, participants performed a 30-min aerobic exercise session (intensities individually calculated using the VO2peak results). Blood samples were collected in fasted state (moment 1, M1) and immediately after a small breakfast, which included the metabolic formula for patients but not for controls, and the exercise session (moment 2, M2).
Results
Phenylketonuria patients and controls showed similar BMR and physical capacities. At M1, patients presented higher Phe concentration and Phe/Tyr ratio; and lower levels of BCAA and total cholesterol than controls. Besides that, poorly controlled patients tended to stay slightly below the prescribed VO2 during exercise. Both patients and controls showed increased levels of total cholesterol and LDL at M2 compared with M1. Only controls showed increased levels of Tyr, lactate, and HDL; and decreased Phe/Tyr ratio and glucose levels at M2 compared to values at M1.
Conclusions
Acute aerobic exercise followed by a Phe-restricted breakfast did not change Phe concentrations in treated phenylketonuria patients, but it was associated with decreased Phe/Tyr only in controls. Further studies are necessary to confirm our results in a higher number of patients.
Abbreviations: BCAA, branched-chain amino acids; BMI, body mass index; BMR, basal metabolic rate; CTL, control; HDL, high-density lipoprotein; LDL, low-density lipoprotein; N/A, not applicable; NS, non-significant; Phe, phenylalanine; PKU, phenylketonuria; RER, respiratory exchange ratio; Tyr, tyrosine; VCO2, carbon dioxide production; VO2, oxygen consumption; VO2peak, peak oxygen consumption
Keywords: Phenylketonuria, PKU, Aerobic exercise, Basal metabolic rate, Phenylalanine, Natural restricted diet
1. Introduction
Phenylketonuria (PKU, MIM 261600) is an autosomal recessive disease characterized by high levels of phenylalanine (Phe) in plasma and brain, due to the low activity of Phe hydroxylase (PAH, EC 1.14.16.1), which converts Phe into tyrosine (Tyr). Currently, patients are diagnosed by newborn screening programs and are treated with a Phe-restricted diet. Although mental retardation can be prevented with early diagnosis and dietary treatment, some peripheral and neurological problems remain. Regarding brain status, patients have shown non-optimal neuropsychological outcome with decreased capacity especially in executive functions [1], [2], increased risk of depression [3], [4], anxiety, and mood disturbances [5], [6]. On the peripheral level, decreased bone density [7], [8], [9] and increased risk of overweight [10], [11] are also reported in treated PKU patients. As yet, it is not clear whether these problems are due to the high blood Phe concentrations or to the dietary treatment restricting not only Phe, but also other important micronutrients. Moreover, a wide range of protein-rich foods are forbidden thus daily caloric needs are fulfilled with carbohydrates and lipids [10].
Exercise may represent a treatment strategy in PKU since it has been proven to enhance overall health in different populations. In healthy individuals, regular exercise improves mood and cognition [12], decreases depressive symptoms [13], decreases the risk of obesity [14], and improves bone density [15], [16]. Moreover, physical training leads to better neurological outcomes in patients with neurodegenerative diseases [17], [18], and mild cognitive impaired elderly [19]. Therefore, PKU patients might also benefit from the exercise-induced adaptations.
High plasma Phe along with the Phe-restricted diet may lead to different metabolic responses to exercise in continuously treated PKU, what could affect training adaptations [20]. Acutely, exercise increases the metabolic demand and protein turnover, which can affect amino acid levels [21]. In addition, different dietary composition can alter the metabolic response to exercise, leading to specific long-term adaptations [22]. So far, only the study by Grünert et al. [23] has reported the effects of exercise in PKU patients, suggesting aerobic exercise does not importantly affect peripheral Phe levels in these patients. However, that study was not controlled and had evaluated the pre- and post-exercise Phe and Tyr levels as secondary objectives and in a relatively fasted state.
Therefore, the aim of this study was to investigate the acute effects of an aerobic exercise in PKU patients regarding changes in metabolic parameters.
2. Methods
2.1. Study design
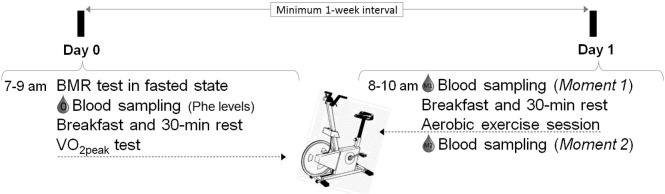
Participants performed two days of interventions (Fig. 1) at the Laboratory of Physical Exercise (LAPEX), Federal University of Rio Grande do Sul (UFRGS), Porto Alegre, Brazil. This study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The local Human Research Ethics Committee has approved the study (N.120292 HCPA-UFRGS), and all participants (or parent/legal tutor for patients younger than 18 years old) signed an informed consent term before starting the tests.
Fig. 1.
Experimental design.
In fasted state, participants went to the lab twice (at day 0 and day 1). In the day 0, participants performed the basal metabolic rate (BMR) test, followed by blood sampling for plasma phenylalanine (Phe) evaluation (0), breakfast and 30-min rest. Then the peak oxygen consumption (VO2peak) test was performed. In the day 1, blood sampling was collected at moment 1 (M1), then participants received breakfast, waited 30-min in rest and performed the aerobic exercise session. Immediately after exercise (moment 2, M2), the last blood sample was collected.
2.2. Participants
Inclusion criteria were: (a) confirmed diagnosis of classical PKU, (b) following treatment regularly in a PKU Center in the south of Brazil, (c) aged > 13 years, (d) not engaged in exercise training, and (e) mentally and physically able to perform exercise. From a total of 16 invited patients, five did not agree to participate due to travel issues and two because of personal reasons. Therefore, nine PKU patients from the Medical Genetics Service — Hospital de Clínicas de Porto Alegre, Brazil, were included. One patient did not follow the fasting requirement at day 1, so he was excluded from the exercise session results. Healthy non-PKU subjects were sex- age- and BMI-matched in an approximately 1:2 ratio to patients, and were not engaged in exercise training. The controls were invited through banners and advertisements in the University community.
Patients were classified as being early diagnosed (if the diagnosis was performed before the end of the first month of life) or late diagnosed (if the diagnosis was performed after the end of the first month of life), and were following treatment since diagnosis. Patients were also classified as “well controlled” if their current Phe levels at M1 were below 700 μmol/L, or as “poorly controlled” if these levels were equal or above 700 μmol/L. The treatment consisted of being on the Phe-restricted diet (low Phe intake along with the metabolic formula).
2.3. BMR test
The BMR was determined in a 10–12 h fasted state between 7.00 and 9.00 am at day 0 in order to assess daily basal caloric expenditure. The participants stayed in supine position for 30 min while their expiratory ventilation was analyzed by an automated open-circuit gas analysis system (MedGraphics Cardiorespiratory Diagnostic Systems, model CPX-D, and using the method Breath by Breath). The expired air fractions of oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured every minute during the last 20 min of the test. The equation proposed by Weir [24], [(3.9 × VO2) + (1.1 × VCO2)], was used to obtain the values in kcal/min, which were then transformed into kcal/kg/day. Respiratory exchange ratio (RER) was also calculated by the quotient between VCO2 and VO2.
2.4. Standard breakfasts
All participants received a standard breakfast (day 0) after the BMR test and, at day 1, 30 min before the exercise session (Fig. 1). The PKU breakfast consisted of a banana, a rice cookie and 200 mL of water mixed with two tablespoons of the metabolic formula (PKU 2 Secunda, Milupa) and one tablespoon of crystal sugar. The controls also received a banana and a rice cookie, but a regular yogurt (200 mL) instead of the metabolic formula. Regarding nutritional facts, PKU patients received a breakfast of 198 kcal and 40 mg of Phe (67% of carbohydrates, 30% of protein — containing approximately 395 mg of tyrosine, 403 mg of isoleucine, 664 mg of leucine, and 470 mg of valine — and 3% of lipids), while controls received a 157 kcal meal containing 340 mg of Phe (80% of carbohydrates, 17% of proteins and 2% of lipids).
2.5. Peak VO2 (VO2peak) test
VO2peak (mL/kg/min) was determined by an incremental stationary cycling exercise test to the point of exhaustion [25], [26], using the gas analyzer described to the BMR test. This test was performed at day 0 of evaluations, 30 min after the standard breakfast.
Regarding standard safety procedures, we monitored for headaches, dizziness, altered vision, heart rate and hematocrit levels during and after exercise bouts (maximum test and aerobic session). A stationary bicycle was used due to its safety even for those who were not used to exercise. A cardiologist followed all the procedures.
2.6. Acute exercise protocol
Using the same ventilatory analyzer described to the BMR and VO2peak tests, all participants performed 30 min of cycling exercise at a prescribed VO2. The prescribed aerobic intensity was calculated for each participant representing the VO2 value at 10% below the second ventilatory threshold, which was assessed by the VO2peak test at least one week earlier. The one-week interval was chosen to avoid possible interferences of the maximum test on the submaximal exercise session, especially for sedentary individuals.
The VO2 was tracked throughout the exercise bout and the participant was asked to keep his/her VO2 within the target zone (prescribed VO2 ± 2 mL/kg/min). When the participant was below or above it, he/she was encouraged to either increase or decrease the load or speed to stay in his/her calculated aerobic zone. Values of prescribed- and averaged actual VO2 during the exercise session were compared and expressed as percentage of the prescribed VO2. Since executive function of early and late diagnosed patients has shown to be affected by current levels of Phe [2], [27], the actual VO2 values were analyzed with regard to Phe control in the group of patients.
2.7. Blood sampling
Blood collections were performed at day 0 after the BMR test in a 10–12 h fasted state (for evaluation of Phe levels only), at day 1 in a 10–12 h fasted condition, i.e. at moment 1 (M1), and immediately after the aerobic exercise bout, i.e. at moment 2 (M2) also having had the light breakfast. The number of samples varied in some evaluations because it was not possible to collect the same amount of blood from all participants.
2.8. Biochemical measurements
Quantitative analysis of Phe, Tyr, and branched-chain amino acid (BCAA, isoleucine, leucine, and valine) levels in plasma was carried out by HPLC with fluorescence detector [28] with an internal variation coefficient less than 3%.
Serum glucose, total cholesterol, HDL and triacylglycerol were determined by specific commercial kits for the automated analyzer Cobas C111. The LDL value was estimated by the difference of total cholesterol and HDL minus triacylglycerol divided by five.
Plasma adiponectin was evaluated using a commercial kit for human adiponectin (Invitrogen KHP0041) ELISA immunoassay. The catecholamines dopamine, noradrenaline and adrenaline were analyzed in plasma by HPLC with electrochemical detection.
2.9. Statistical analysis
Data normality was tested by Shapiro–Wilk test. Control and PKU groups were compared using the independent Student's t-test for basal and M1 analyses, and with two-sided paired t-test for comparisons between M1 versus M2 in each group. Correlations were performed using Spearman's correlation. Categorical variables were compared by Fisher's Exact Test. SPSS 22.0 was employed for all statistical analyses and a p < 0.002 was considered statically significant after applying Bonferroni correction.
3. Results
3.1. Sample characterization
The data of the nine PKU patients and 17 controls are described in the Table 1. Five patients were early diagnosed and four patients were late diagnosed. At day 0, Patients' current Phe concentrations varied between 323 and 761 μmol/L.
Table 1.
Clinical characteristics from control (CTL) and phenylketonuria (PKU) groups at day 0.
| CTL | PKU | p value | |
|---|---|---|---|
| Gender (male:female) | 12:5 | 7:2 | NS |
| Age (years) | 22 ± 4 (17) | 21 ± 4 (9) | NS |
| BMI (kg/m2) | 23 ± 2 (17) | 24 ± 3 (9) | NS |
| Phenylalanine (μmol/L) | 57 ± 14 (17) | 562 ± 141 (9) | < 0.001 |
| BMR (kcal/kg/day) | 21 ± 4 (17) | 23 ± 4 (9) | NS |
| RER during BMR | 0.87 ± 0.07 (17) | 0.82 ± 0.07 (9) | NS |
| VO2peak (mL/kg/min) | 31 ± 6 (17) | 28 ± 8 (9) | NS |
| Workload peak (W) | 216 ± 49 (17) | 203 ± 31 (9) | NS |
BMI, body mass index, BMR, basal metabolic rate, RER, respiratory exchange ratio, VO2peak, peak oxygen consumption, NS, non-significant. Data for numeric variables are expressed as mean ± SD (n). See methodology for details on the statistical analysis.
3.1.1. BMR and VO2peak tests
Two controls, but no PKU patient, experienced discomfort during the protocols. Concerning basal status, PKU patients and controls showed similar values of BMR and RER during the BMR test. In the same way, patients showed similar aerobic capacity and workload peak in the VO2peak test in comparison to controls (Table 1).
3.2. Exercise session
3.2.1. Baseline values at M1
In rest and fasted state, patients showed higher Phe levels and Phe/Tyr ratio, and lower levels of BCAA and total cholesterol in comparison to controls (Table 2). Phe levels ranged between 583 and 1029 μmol/L in the PKU group. Regarding Phe control at M1, five patients showed good control. All early-diagnosed patients had good control of Phe levels, while all late diagnosed patients had poor Phe control. Accordingly, patients showed positive correlation between the age at diagnosis and the Phe levels at M1 (r = 0.97; p < 0.001).
Table 2.
Biochemical parameters at moments 1 (M1) and 2 (M2) in controls (CTL) and phenylketonuria (PKU) patients.
| M1 |
CTL M1 vs PKU M1 |
M2 |
CTL M1 vs CTL M2 |
PKU M1 vs PKU M2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CTL | PKU | p value | CTL | PKU | p value | p value | |||||
| Phe (μmol/L) | 81 ± 24 | (17) | 773 ± 190 | (8) | < 0.001 | 73 ± 15 | (17) | 723 ± 139 | (8) | NS | NS |
| Tyr (μmol/L) | 57 ± 15 | (17) | 62 ± 9 | (8) | NS | 79 ± 20 | (17) | 90 ± 24 | (8) | < 0.001 | NS |
| Phe/Tyr ratio | 1.4 ± 0.2 | (17) | 12.4 ± 2.4 | (8) | < 0.001 | 1.0 ± 0.2 | (17) | 8.3 ± 1.8 | (8) | < 0.001 | NS |
| Tryptophan (μmol/L) | 35 ± 9 | (17) | 29 ± 6 | (8) | NS | 35 ± 8 | (17) | 36 ± 12 | (8) | NS | NS |
| BCAA (μmol/L) | 456 ± 86 | (17) | 332 ± 50 | (8) | 0.001 | 403 ± 63 | (17) | 478 ± 132 | (8) | NS | NS |
| Glucose (mg/dL) | 89 ± 8 | (17) | 83 ± 7 | (8) | NS | 77 ± 9 | (17) | 78 ± 11 | (8) | 0.001 | NS |
| Lactate (mg/dL) | 1.3 ± 0.5 | (17) | 1.3 ± 0.3 | (7) | NS | 3.7 ± 1.6 | (17) | 3.6 ± 1.8 | (7) | < 0.001 | NS |
| Triacylglycerol (mg/dL) | 89 ± 33 | (17) | 76 ± 27 | (8) | NS | 100 ± 39 | (17) | 77 ± 27 | (8) | NS | NS |
| Total cholesterol (mg/dL) | 168 ± 31 | (17) | 121 ± 26 | (8) | 0.001 | 181 ± 34 | (17) | 129 ± 26 | (8) | < 0.001 | < 0.001 |
| HDL (mg/dL) | 54 ± 12 | (17) | 42 ± 6 | (8) | NS | 57 ± 11 | (17) | 45 ± 7 | (8) | < 0.001 | NS |
| LDL (mg/dL) | 97 ± 28 | (17) | 63 ± 19 | (8) | NS | 103 ± 28 | (17) | 68 ± 19 | (8) | 0.001 | 0.001 |
| Dopamine (pg/mL) | 56 ± 15 | (17) | 58 ± 17 | (8) | NS | 57 ± 13 | (17) | 57 ± 11 | (8) | NS | NS |
| Noradrenaline (pg/mL) | 228 ± 77 | (17) | 220 ± 52 | (8) | NS | 229 ± 85 | (17) | 217 ± 53 | (8) | NS | NS |
| Adrenaline (pg/mL) | 49 ± 15 | (17) | 45 ± 12 | (8) | NS | 52 ± 15 | (17) | 43 ± 11 | (8) | NS | NS |
| Adiponectin (ng/mL) | 28 ± 6 | (14) | 37 ± 8 | (8) | NS | 30 ± 9 | (14) | 39 ± 4 | (7) | NS | NS |
Phe, phenylalanine; Tyr, tyrosine; BCAA, branched chain amino acids (isoleucine, leucine, and valine); RER, respiratory exchange ratio; NS, non-significant; N/A, not applicable. Results are expressed as mean ± SD (n), two-sided paired t-test. After applying Bonferroni correction, only results with p < 0.002 were considered significant (see methodology).
3.2.2. Ventilatory measurements during exercise
PKU patients and controls showed similar values of prescribed VO2 (21 ± 6 versus 22 ± 4 mL/kg/min, respectively), and actual VO2 during exercise (18 ± 5 versus 22 ± 4 mL/kg/min, respectively). Despite not statistically different, poorly controlled patients showed the lowest percentage of actual VO2 during exercise in relation to the prescribed value (Fig. 2). Mean RER values during exercise were similar between PKU and controls (0.99 ± 0.09 versus 0.96 ± 0.05, respectively).
Fig. 2.
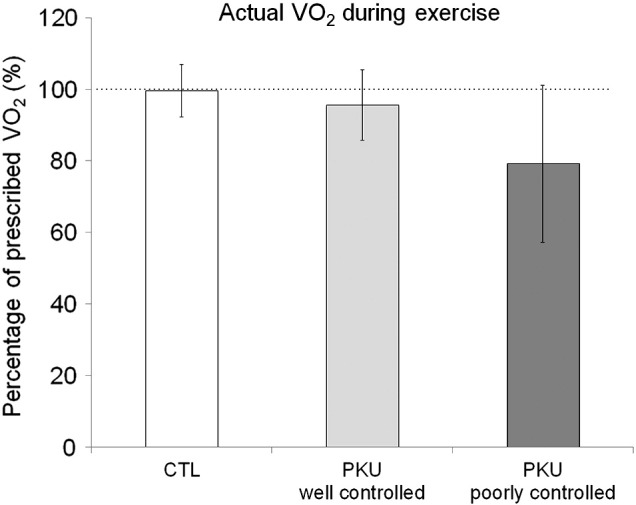
Actual oxygen consumption (VO2) during the 30-min exercise in controls (CTL) and well and poorly controlled phenylketonuria (PKU) patients. Data are expressed as mean ± SD of percentage of prescribed VO2, n = 16 for controls, n = 5 and n = 3 for well and poorly controlled PKU subgroups, respectively.
3.2.3. Biochemical values at M2
Phe concentrations were not different between M1 and M2 in the PKU as well as in the control group. In the PKU group, Phe concentrations ranged between 490 and 984 μmol/L at M2. Phe/Tyr ratio was lower at M2 than at M1 only in controls (Table 2). Total cholesterol, LDL levels were increased at M2 in both PKU patients and controls in comparison with M1 (Table 2). Only in controls, Tyr, lactate, and HDL levels were higher, while glucose levels were lower at M2 in comparison with values at M1. Levels of BCAA, triacylglycerol, dopamine, noradrenaline, adrenaline, and adiponectin were not modified between M1 and M2 in patients and controls.
4. Discussion
To the best of our knowledge, this is the first study to evaluate a controlled aerobic exercise session in PKU patients and healthy individuals. For that, we analyzed patients' basal metabolic status compared to controls, as well as their immediate responses to an aerobic exercise session at the same relative intensity, which was calculated for each participant by a previous VO2peak test. Our data corroborate the study by Grünert et al. [23], where exercise did not change Phe concentrations acutely in PKU patients. Moreover, we showed that metabolic responses to exercise are similar between PKU patients and controls, despite different previous meal.
Our results were measured in a small number of patients of both genders immediately after a 30-min exercise session that was performed after a light breakfast. The small sample size is a limitation of the study, thus making comparisons between subgroups of patients very difficult. That possibly contributed to the fact that most analyses were not significant in the group of patients. To counteract that, we have included a greater number of controls, in a compatible ratio to male and female patients. Due to the nature of the disease, PKU patients and controls received distinct breakfasts, which varied in compositions, thereby possibly causing different biochemical responses to exercise [22], [29]. The different meals represent real dietary habits of PKU and non-PKU individuals, while studying the patients in fasting condition would have caused other biochemical responses that had to be prevented this way [23], [30], [31].
Regarding basal metabolic status, PKU patients showed similar BMR values in comparison to controls. Some studies have found increased basal metabolism in different diseases [32], [33], [34], [35], although the mechanisms are not yet elucidated. Despite that, our results agree with the study by Allen et al. [36], where no differences in BMR were found between early diagnosed PKU children and matched controls.
In rest and fasting (M1), patients showed lower BCAA and total cholesterol levels in comparison with controls. Lower lipoprotein levels and disturbed amino acid concentrations have been already described in PKU children [37], [38], being associated with the composition of the Phe-restricted diet [11].
PKU patients have been encouraged to exercise [11], [39], although its efficacy is not evidence-based. The concern on low physical activity level for PKU patients has risen from data on bone density measurements [7], [8], [9], as well as the eminent weight gain associated to consuming Phe-free products, which are often rich in carbohydrates [10], [11]. Obesity may become a spreading health issue of this era, and exercising regularly may also prevent overweight and its related disorders. Despite that, the PKU patients of the present study showed normal BMI and were so physically active as the controls, seen by the similar values observed on VO2peak and workload peak in the VO2peak test in both groups. However, the three patients who showed the highest Phe values at M1 (poorly controlled), stayed slightly below the prescribed VO2 during the exercise in average. This result did not reach statistical significance probably due to the small sample of patients. However, high Phe levels impair executive functioning [2], [27] that could, in turn, affect exercise performance. In this way, patients that showed bad Phe control before exercise seemed to have difficulties in keeping the prescribed VO2 during cycling, even though being encouraged to do so.
Both patients and controls showed expected responses regarding higher total cholesterol and LDL levels after exercise, since exercise enhances the availably of energetic substrates [40], [41]. Tyr levels were increased after exercise only in the control group. Grünert et al. [23] have shown that Tyr levels increase immediately after aerobic exercise in PKU patients. In that study, patients exercised during 20 min at night (three hours after a dinner) making difficult any comparison with our findings, since in our study patients exercised during 30 min at morning (shortly after a breakfast). Because of the different protein sources of the breakfasts, our patients may have absorbed l-amino acids from the metabolic formula faster than the controls had absorbed casein from the yogurt [42]. Therefore, the effects of the breakfast for patients might have been even more important with regard to amino acids levels at M2. Nevertheless, the aerobic exercise in combination with the amino acid-rich formula did not lead to unexpected metabolic responses in PKU patients. In addition, RER levels were similar between groups during basal condition and exercise. This result suggests that high Phe levels and different dietary composition did not modify substrate utilization in a small sample of treated patients with normal BMI.
5. Conclusions
Acute aerobic exercise followed by a Phe-restricted breakfast did not change Phe concentrations in treated PKU patients, but it is associated to lower Phe/Tyr ratio only in controls. Future studies are needed to confirm our results in a higher number of patients, as well as controlling for dietary intake before exercise.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
PNM, BCT and GHS collected the data. PNM performed the statistical analyses and drafted the manuscript. All authors participated in the study design, contributed to the interpretation of the results and revised the manuscript.
Acknowledgments
This research project has been made possible thanks to a fellowship from PKU Academy under the auspices of EXCEMED — Excellence in Medical Education. Secondary financial support was provided by the “Fundo de Incentivo à Pesquisa e Eventos” (FIPE-HCPA, grant number 12-0292) and “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq, grant number 307540/2014-6).
Contributor Information
Priscila Nicolao Mazzola, Email: pku@priscilamazzola.com.
Bruno Costa Teixeira, Email: brunoct100@hotmail.com.
Gabriel Henrique Schirmbeck, Email: gschirmbeck@gmail.com.
Alvaro Reischak-Oliveira, Email: alvaro.oliveira@ufrgs.br.
Terry G.J. Derks, Email: t.g.j.derks@umcg.nl.
Francjan J. van Spronsen, Email: f.j.van.spronsen@umcg.nl.
Carlos Severo Dutra-Filho, Email: dutra@ufrgs.br.
Ida Vanessa Doederlein Schwartz, Email: ischwartz@hcpa.edu.br.
References
- 1.VanZutphen K., Packman W., Sporri L., Needham M., Morgan C., Weisiger K., Packman S. Executive functioning in children and adolescents with phenylketonuria. Clin. Genet. 2007;72:13–18. doi: 10.1111/j.1399-0004.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- 2.Huijbregts S.C., Gassio R., Campistol J. Executive functioning in context: relevance for treatment and monitoring of phenylketonuria. Mol. Genet. Metab. 2013;110:S25–S30. doi: 10.1016/j.ymgme.2013.10.001. (Suppl) [DOI] [PubMed] [Google Scholar]
- 3.Clacy A., Sharman R., McGill J. Depression, anxiety, and stress in young adults with phenylketonuria: associations with biochemistry. J. Dev. Behav. Pediatr. 2014;35:388–391. doi: 10.1097/DBP.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 4.Sharman R., Sullivan K., Young R.M., McGill J. Depressive symptoms in adolescents with early and continuously treated phenylketonuria: associations with phenylalanine and tyrosine levels. Gene. 2012;504:288–291. doi: 10.1016/j.gene.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Anjema K., van Rijn M., Verkerk P.H., Burgerhof J.G., Heiner-Fokkema M.R., van Spronsen F.J. PKU: high plasma phenylalanine concentrations are associated with increased prevalence of mood swings. Mol. Genet. Metab. 2011;104:231–234. doi: 10.1016/j.ymgme.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 6.ten Hoedt A.E., de Sonneville L.M., Francois B., ter Horst N.M., Janssen M.C., Rubio-Gozalbo M.E., Wijburg F.A., Hollak C.E., Bosch A.M. High phenylalanine levels directly affect mood and sustained attention in adults with phenylketonuria: a randomised, double-blind, placebo-controlled, crossover trial. J. Inherit. Metab. Dis. 2011;34:165–171. doi: 10.1007/s10545-010-9253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miras A., Boveda M.D., Leis M.R., Mera A., Aldamiz-Echevarria L., Fernandez-Lorenzo J.R., Fraga J.M., Couce M.L. Risk factors for developing mineral bone disease in phenylketonuric patients. Mol. Genet. Metab. 2013;108:149–154. doi: 10.1016/j.ymgme.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Al-Qadreh A., Schulpis K.H., Athanasopoulou H., Mengreli C., Skarpalezou A., Voskaki I. Bone mineral status in children with phenylketonuria under treatment. Acta Paediatr. 1998;87:1162–1166. doi: 10.1080/080352598750031158. [DOI] [PubMed] [Google Scholar]
- 9.de Groot M.J., Hoeksma M., van Rijn M., Slart R.H., van Spronsen F.J. Relationships between lumbar bone mineral density and biochemical parameters in phenylketonuria patients. Mol. Genet. Metab. 2012;105:566–570. doi: 10.1016/j.ymgme.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald A., Rocha J.C., van Rijn M., Feillet F. Nutrition in phenylketonuria. Mol. Genet. Metab. 2011;104:S10–S18. doi: 10.1016/j.ymgme.2011.08.023. (Suppl) [DOI] [PubMed] [Google Scholar]
- 11.Rocha J.C., Macdonald A., Trefz F. Is overweight an issue in phenylketonuria? Mol. Genet. Metab. 2013;110:S18–S24. doi: 10.1016/j.ymgme.2013.08.012. (Suppl) [DOI] [PubMed] [Google Scholar]
- 12.Deslandes A.C. Exercise and mental health: what did we learn in the last 20 years? Front. Psychol. 2014;5:66. doi: 10.3389/fpsyt.2014.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rebar A.L., Stanton R., Geard D., Short C., Duncan M.J., Vandelanotte C. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol. Rev. 2015:1–78. doi: 10.1080/17437199.2015.1022901. [DOI] [PubMed] [Google Scholar]
- 14.Wirth A., Wabitsch M., Hauner H. The prevention and treatment of obesity. Dtsch. Arztebl. Int. 2014;111:705–713. doi: 10.3238/arztebl.2014.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strope M.A., Nigh P., Carter M.I., Lin N., Jiang J., Hinton P.S. Physical activity-associated bone loading during adolescence and young adulthood is positively associated with adult bone mineral density in men. Am. J. Mens Health. 2014 doi: 10.1177/1557988314549749. [DOI] [PubMed] [Google Scholar]
- 16.Tveit M., Rosengren B.E., Nilsson J.A., Karlsson M.K. Exercise in youth: high bone mass, large bone size, and low fracture risk in old age. Scand. J. Med. Sci. Sports. 2014 doi: 10.1111/sms.12305. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez S.S., Sandreschi P.F., Silva F.C., Arancibia B.A., da Silva R., Gutierres P.J., Andrade A. What are the benefits of exercise for Alzheimer's disease? A systematic review of past 10 years. J. Aging Phys. Act. 2014 doi: 10.1123/japa.2014-0180. [DOI] [PubMed] [Google Scholar]
- 18.Oguh O., Eisenstein A., Kwasny M., Simuni T. Back to the basics: regular exercise matters in Parkinson's disease: results from the National Parkinson Foundation QII registry study. Parkinsonism Relat. Disord. 2014;20:1221–1225. doi: 10.1016/j.parkreldis.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Nascimento C.M., Pereira J.R., de Andrade L.P., Garuffi M., Talib L.L., Forlenza O.V., Cancela J.M., Cominetti M.R., Stella F. Physical exercise in MCI elderly promotes reduction of pro-inflammatory cytokines and improvements on cognition and BDNF peripheral levels. Curr. Alzheimer Res. 2014;11:799–805. doi: 10.2174/156720501108140910122849. [DOI] [PubMed] [Google Scholar]
- 20.Brown S.P. Lippincott Williams & Wilkins; Baltimore, Maryland, USA: 2001. Introduction to Exercise Science. [Google Scholar]
- 21.Morris C., Grada C.O., Ryan M., Roche H.M., De Vito G., Gibney M.J., Gibney E.R., Brennan L. The relationship between aerobic fitness level and metabolic profiles in healthy adults. Mol. Nutr. Food Res. 2013;57:1246–1254. doi: 10.1002/mnfr.201200629. [DOI] [PubMed] [Google Scholar]
- 22.Brooks G.A. Mammalian fuel utilization during sustained exercise. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998;120:89–107. doi: 10.1016/s0305-0491(98)00025-x. [DOI] [PubMed] [Google Scholar]
- 23.Grunert S.C., Brichta C.M., Krebs A., Clement H.W., Rauh R., Fleischhaker C., Hennighausen K., Sass J.O., Schwab K.O. Diurnal variation of phenylalanine and tyrosine concentrations in adult patients with phenylketonuria: subcutaneous microdialysis is no adequate tool for the determination of amino acid concentrations. Nutr. J. 2013;12:60. doi: 10.1186/1475-2891-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weir J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boulay M.R., Hamel P., Simoneau J.A., Lortie G., Prud'homme D., Bouchard C. A test of aerobic capacity: description and reliability. Can. J. Appl. Sport Sci. 1984;9:122–126. [PubMed] [Google Scholar]
- 26.Dionne I., Van Vugt S., Tremblay A. Postexercise macronutrient oxidation: a factor dependent on postexercise macronutrient intake. Am. J. Clin. Nutr. 1999;69:927–930. doi: 10.1093/ajcn/69.5.927. [DOI] [PubMed] [Google Scholar]
- 27.Jahja R., Huijbregts S.C., de Sonneville L.M., van der Meere J.J., van Spronsen F.J. Neurocognitive evidence for revision of treatment targets and guidelines for phenylketonuria. J. Pediatr. 2014;164:895–899. doi: 10.1016/j.jpeds.2013.12.015. e892. [DOI] [PubMed] [Google Scholar]
- 28.Joseph M.H., Marsden C.A. Amino acids and small peptides. In: LIM C.K., editor. HPLC of Small Peptides. 1986. pp. 13–27. (Oxford) [Google Scholar]
- 29.Coyle E.F., Jeukendrup A.E., Oseto M.C., Hodgkinson B.J., Zderic T.W. Low-fat diet alters intramuscular substrates and reduces lipolysis and fat oxidation during exercise. Am. J. Physiol. Endocrinol. Metab. 2001;280:E391–E398. doi: 10.1152/ajpendo.2001.280.3.E391. [DOI] [PubMed] [Google Scholar]
- 30.van Spronsen F.J., van Rijn M., van Dijk T., Smit G.P., Reijngoud D.J., Berger R., Heymans H.S. Plasma phenylalanine and tyrosine responses to different nutritional conditions (fasting/postprandial) in patients with phenylketonuria: effect of sample timing. Pediatrics. 1993;92:570–573. [PubMed] [Google Scholar]
- 31.van Spronsen F.J., van Dijk T., Smit G.P., van Rijn M., Reijngoud D.J., Berger R., Heymans H.S. Phenylketonuria: plasma phenylalanine responses to different distributions of the daily phenylalanine allowance over the day. Pediatrics. 1996;97:839–844. [PubMed] [Google Scholar]
- 32.Bitz C., Toubro S., Larsen T.M., Harder H., Rennie K.L., Jebb S.A., Astrup A. Increased 24-h energy expenditure in type 2 diabetes. Diabetes Care. 2004;27:2416–2421. doi: 10.2337/diacare.27.10.2416. [DOI] [PubMed] [Google Scholar]
- 33.Raj T., D'Souza G., Elia M., Kurpad A.V. Measurement of 24 h energy expenditure in male tuberculosis patients. Indian J. Med. Res. 2006;124:665–676. [PubMed] [Google Scholar]
- 34.Tarantino G., Marra M., Contaldo F., Pasanisi F. Basal metabolic rate in morbidly obese patients with non-alcoholic fatty liver disease. Clin. Invest. Med. 2008;31:E24–E29. doi: 10.25011/cim.v31i1.3138. [DOI] [PubMed] [Google Scholar]
- 35.Doneda D., Lopes A.L., Oliveira A.R., Netto C.B., Moulin C.C., Schwartz I.V. Gaucher disease type I: assessment of basal metabolic rate in patients from southern. Braz. Blood Cells Mol. Dis. 2011;46:42–46. doi: 10.1016/j.bcmd.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Allen J.R., McCauley J.C., Waters D.L., O'Connor J., Roberts D.C., Gaskin K.J. Resting energy expenditure in children with phenylketonuria. Am. J. Clin. Nutr. 1995;62:797–801. doi: 10.1093/ajcn/62.4.797. [DOI] [PubMed] [Google Scholar]
- 37.Schulpis K.H., Papassotiriou I., Tsakiris S., Vounatsou M., Chrousos G.P. Increased plasma adiponectin concentrations in poorly controlled patients with phenylketonuria normalize with a strict diet: evidence for catecholamine-mediated adiponectin regulation and a complex effect of phenylketonuria diet on atherogenesis risk factors. Metabolism. 2005;54:1350–1355. doi: 10.1016/j.metabol.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 38.Schulpis K.H., Tsakiris S., Karikas G.A., Moukas M., Behrakis P. Effect of diet on plasma total antioxidant status in phenylketonuric patients. Eur. J. Clin. Nutr. 2003;57:383–387. doi: 10.1038/sj.ejcn.1601529. [DOI] [PubMed] [Google Scholar]
- 39.Doulgeraki A., Skarpalezou A., Theodosiadou A., Monopolis I., Schulpis K. Body composition profile of young patients with phenylketonuria and mild hyperphenylalaninemia. Int. J. Endocrinol. Metab. 2014;12:e16061. doi: 10.5812/ijem.16061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazzeo R.S. Catecholamine responses to acute and chronic exercise. Med. Sci. Sports Exerc. 1991;23:839–845. [PubMed] [Google Scholar]
- 41.Chinevere T.D., Sawyer R.D., Creer A.R., Conlee R.K., Parcell A.C. Effects of L-tyrosine and carbohydrate ingestion on endurance exercise performance. J. Appl. Physiol. 2002;93:1590–1597. doi: 10.1152/japplphysiol.00625.2001. [DOI] [PubMed] [Google Scholar]
- 42.Sindayikengera S., Xia W.S. Nutritional evaluation of caseins and whey proteins and their hydrolysates from protamex. J. Zhejiang Univ. Sci. B. 2006;7:90–98. doi: 10.1631/jzus.2006.B0090. [DOI] [PMC free article] [PubMed] [Google Scholar]