Abstract
Tyrosinemia type 3 (HT3) is a rare inborn error of tyrosine metabolism caused by mutations in the HPD gene encoding 4-hydroxyphenyl-pyruvate dioxygenase, which is transmitted in an autosomal recessive trait. The disorder is characterized by tyrosine accumulation in body fluids and massive excretion of tyrosine derivatives into urine (www.orpha.net). Since it is the least frequent form of tyrosinemia, only few cases with the variable but rather mild clinical features have been described so far.
We report an 11 year old girl presenting with no clinical symptoms and with normal mental development who has been diagnosed with HT3 through metabolic screening on the basis of elevated serum level of tyrosine ranging from 425 to 535 μmol/L (normal values: 29–86 μmol/L), and elevated urinary excretion of p-hydroxyphenyl derivatives confirmed genetically with the homozygous c.479A > G (p.Tyr160Cys) missense change in the HPD gene. The girl has been only presenting with recurrent proteinuria of unknown etiology. A phenylalanine- and tyrosine-restricted diet has never been administered.
Presented case may suggest that high tyrosine concentration itself does not participate directly in neuronal damage described in patients with tyrosinemia type 3.
Keywords: Tyrosinemia type III, Tyrosine metabolism, HPD gene
1. Introduction
Inborn errors of tyrosine (Tyr) metabolism corresponding to four of the five sequential enzymatic reactions of its degradation have been discovered [1], [2]. Three such diseases manifest hypertyrosinemia. These include HT1(type 1), HT2 (type 2), and HT3 (type 3)which is the least frequent form of tyrosinemias. It is caused by mutations in the HPD gene (12q14-qter) encoding 4-hydroxyphenyl-pyruvate dioxygenase (4-HPPD) [3]. The 4-HPPD enzyme, which is present in the mammals' liver and kidney, participates in the oxidation of keto acids of Tyr, and through the reaction involving decarboxylation, oxidation, and rearrangement, the homogenizate is produced [4]. Mutations in the HPD locus are related to two distinct diseases, HT3 transmitted in an autosomal recessive trait, and hawkinsinuria, which is inherited in an autosomal dominant way. Tyrosinemia type 3 is characterized by tyrosine accumulation in body fluids, and massive excretion of tyrosine derivatives (4-hydroxyphenylpyruvic acid [4-HPP], 4-hydroxyphenyllactic acid [4-HPL], and hydrophenylacetic acid) into urine [5].
Only 13 cases with variable, but rather mild, clinical features have been described so far [3,6]. However, 7 of the 8 patients diagnosed beyond the neonatal period have presented mainly with neurologic symptoms. Three of 5 children detected by neonatal screening had psychomotor retardation, one had autism and mental retardation, [3] the others were clinically asymptomatic [7]. Neurologic manifestations, such as mental retardation, ataxia, and seizures are thought to be caused by an elevated blood levels of tyrosine [8].
Although the enzyme is present in the liver and kidney, animal models of HT3 have not demonstrated any evidence of hepato-renal dysfunction in the mutant mice. Fumarylacetoacetase and tyrosine aminotransferases were normal in these animals. Moreover, among 13 reported patients only one had neonatal hepatitis [3]. As some individuals are asymptomatic, the prevalence of HT3 may be underestimated.
2. Case report
We report an 11 year old girl presenting with normal mental development without any neurological symptoms, who has been diagnosed with HT3 on the basis of biochemical findings of elevated urinary excretion of p-hydroxyphenyl derivatives and blood hypertyrosinemia. An elevated blood tyrosine level was detected for the 1st time during the diagnostic procedure of recurrent proteinuria (9–17 mg/L) observed in the girl since the age of 7 years. No abnormalities were found on physical examination. She displayed no food preferences or protein aversions. The results of laboratory tests were within normal values, except for an elevated blood tyrosine (439.9 μmol/L, n = 29–86 μmol/L) and excretion of p-HPP and p-HPL into urine. Control laboratory tests for blood amino-acids and urine organic acids confirmed hypertyrosinemia of 535 μmol/L. Clinical suspicion of HT3 was made and finally confirmed genetically with the next generation sequencing (NGS). The study was approved by the Ethics Committee of the CMHI. Informed consent from the parents/guardians of the patient undergoing large-scale sequencing was obtained.
Known homozygous c.479A > G (p.Tyr160Cys) missense change in the HPD gene (RefSeq NM_002150.2) was revealed by using TruSight One Sequencing Panel (Illumina).
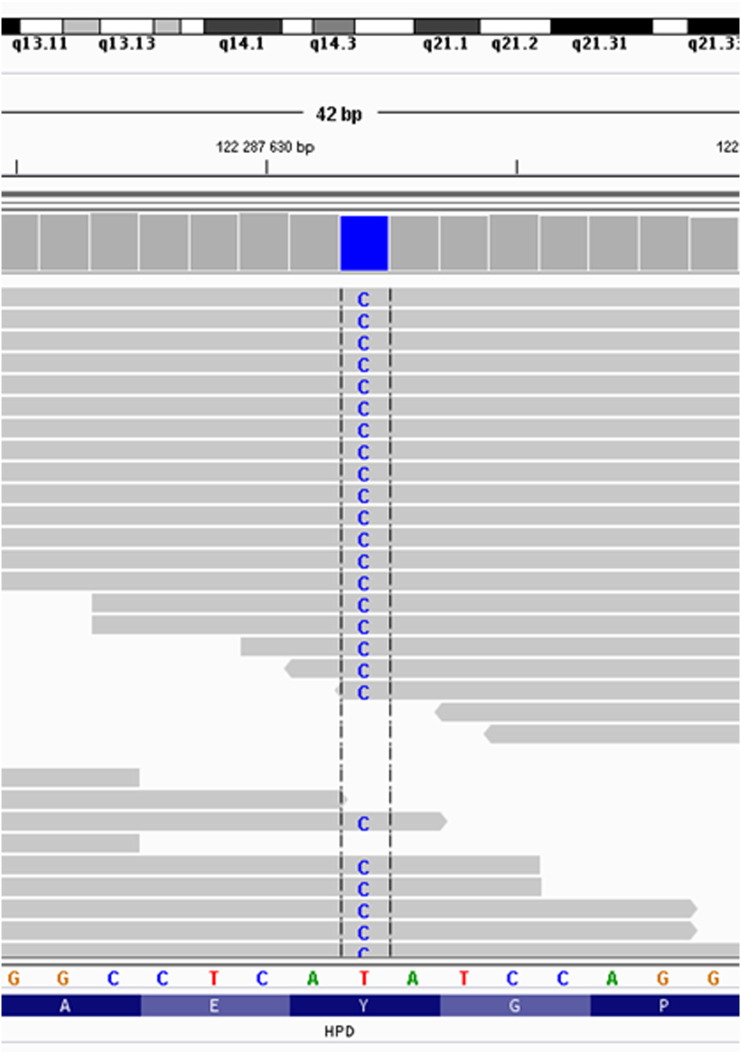
Large physicochemical difference (Grantham dist.: 194) between Tyr and an introduced Cys at protein position 160 indicated possibly pathogenic effect. Identified variant was also predicted as deleterious by four algorithms (MutationAssessor, LRT, Mutation Taster and SIFT). It is a very rare change as it was neither recorded in 1000Genomes nor in our house-made 300 exomes database (Fig. 1).
Fig. 1.
Integrative genomics viewer picture of identified causative c.479A > G (p.Tyr160Cys) variant in HPD. The depth of coverage across the variant was 62/62. The sequencing run achieved 29 433 905 reads, the 10-fold target coverage was 97.5%, and the 20-fold coverage was 94.5%.
The patient has never been following phenylalanine- and tyrosine-restricted diet, which is recommended in HT1 [9, 10].
3. Discussion
Only a few patients with HT3 and variable clinical phenotype have been described in the literature. Since some individuals are asymptomatic, the prevalence of HT3 may be underestimated. The newborn screening for HT3 is not available in Poland where only HT1 is included into the neonatal screening program. Reported patient is homozygotic to c.479A > G (p.Tyr160Cys) missense mutation in the HPD gene. She is not the 1st individual with HT3 and such genotype, however the 1st and the only reported so far without neurologic symptoms [11].
We report an asymptomatic girl being diagnosed due to the recurrent proteinuria. The diagnosis of HT3 in this patient was based on an elevated serum tyrosine levels and the detection of p-hydroxyphenyl derivatives in the urine. It is not clear whether nephrological complications are associated with HT3 as the relevant data are limited due to the rarity of the disease. It is known that in HT1, tubular proteinuria may appear indeed, however, these include phenylalanine and homogentisate which are toxic and cause this dysfunction [12]. In HT3, the conversion of 4-HPP to homogentisic acid by 4HPD does not take place, therefore accumulation of homogentisate is prevented. It is difficult to state then, whether proteinuria in our patient was a manifestation of HT3, or it was an unrelated issue.
Nonetheless, HT3 patients been shown to be more prone to display proteinuria. It may be due to high tyrosine from infancy, while HT1 patients manifest high tyrosine only after diagnosis and NTBC treatment, but the issue has not been resolved yet.
Since in this type of tyrosinemia, the second step in tyrosine degradation does not take place, fumarylacetoacetate (FAA) also does not accumulate, contrary to HT1, where FAA, not tyrosine itself is directly toxic to the liver. It is suggested that FAA through inhibition of DNA glycosylases removing oxidative base lesions in HT1 patients may increase mutagenesis, which may comprise a potential mechanism for development of hepatocarcinoma [13]. Tyrosine, on the other hand, is claimed to cause neurodevelopmental problems. However, this hypothesis seems questionable in the face of asymptomatic HT3 patient. The more, increased excretion of porphyrin precursor, delta-aminolevulinic acid, was detected during neurologic crises in patients with HT1, not tyrosine [14, 15].
Its elevated levels were strictly connected with succinylacetone, a tyrosine metabolite capable of blocking porphyrin synthesis, which accumulates due to primary enzyme defect in HT1 fumarylacetoacetate hydrolase deficiency [13]. Again, since FAA is not accumulated in HT3, the condition should not be associated with neurologic impairment then.
Another proposed hypothesis was that neurological involvement described in patients with tyrosinemia type III might be due to an excessive NO release [16].
Phenylalanine is a large, neutral amino acid (LNAA), which competes for transport across the blood–brain barrier (BBB) via the large neutral amino acid transporter (LNAAT). If phenylalanine is in excess in the blood, it will saturate the transporter blocking other LNAAs transport to the brain [17]. As a result of their decreased brain levels, neurotransmitter synthesis is impaired, which hinders brain development causing intellectual disability. However, the question remains — which tyrosine level is already the toxic one? It is difficult to answer it, since tyrosine levels in patients with neurological symptoms have varied, ranging from 500 to almost 1000 μmol/L. In our patient, the highest level of blood tyrosine was.
535 μmol/L (normal values: 29–86 μmol/L) and she still did not display any neurological symptoms. There may be thus an individual tolerance to different LNAA levels.
In the light of the arguments presented above, phenylalanine- and tyrosine-restricted diet in HT3 seems not to be indispensable, and rather should not be administered in these patients. Our patient has never followed such a diet.
4. Conclusions
Tyrosinemia type 3 may not be associated with neuronal damage, and its course can be asymptomatic. It seem, that a spectrum of symptoms, both hepatic and neurologic ones, in hereditary tyrosinemia is associated with FAA, which accumulation doesn't occur in HT3 because of the inhibition of a second step in tyrosine degradation. It is not clear, whether nephrological complications in our patient are associated with HT3. Moreover, since HT3 may be asymptomatic, its prevalence may be underestimated.
Conflict of interest statement
Edyta Szymanska declares that she has no conflict of interest.
Malgorzata Sredzinska declares that she has no conflict of interest.
Elzbieta Ciara declares that she has no conflict of interest.
Dorota Piekutowska-Abramczuk declares that she has no conflict of interest.
Rafal Ploki declares that he has no conflict of interest.
Dariusz Rokicki declares that he has no conflict of interest.
Anna Tylki-Szymanska declares that she has no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5).
Informed consent was obtained from all patients for being included in the study.
References
- 1.Russo P.A., Mitchell G.A., Tanguay R.M. Tyrosinemia: a review. Pediatr. Dev. Pathol. 2001;4:212–221. doi: 10.1007/s100240010146. [DOI] [PubMed] [Google Scholar]
- 2.Russo P.A., Mitchell G.A., Tanguay R.M. Tyrosinemia: a review. Pediatr. Dev. Pathol. 2001;4:212–221. doi: 10.1007/s100240010146. [DOI] [PubMed] [Google Scholar]
- 3.Heylen E., Scherer G., Vincent M.F. Tyrosinemia type III detected via neonatal screening: management and outcome. Mol. Genet. Metab. 2012;107(3):605–607. doi: 10.1016/j.ymgme.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Moran G.R. 4-Hydroxyphenylpyruvate dioxygenase. Arch. Biochem. Biophys. 2005;433:117–128. doi: 10.1016/j.abb.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Rüetschi U., Cerone R., Pérez-Cerda C. Mutations in the 4-hydroxyphenylpyruvate dioxygenase gene (HPD) in patients with tyrosinemia type III. Hum. Genet. 2000;106(6):654–662. doi: 10.1007/s004390000307. [DOI] [PubMed] [Google Scholar]
- 6.Ellaway C.J., Holme E., Standing S. Outcome of tyrosinaemia type III. J. Inherit. Metab. Dis. 2001;24(8):824–832. doi: 10.1023/a:1013936107064. [DOI] [PubMed] [Google Scholar]
- 7.http://omim.org/search=tyrosinaemia type III.
- 8.Cerone R., Holme E., Schiaffino M.C. Tyrosinemia type III: diagnosis and ten-year follow-up. Acta Paediatr. 1997;86(9):1013–1015. doi: 10.1111/j.1651-2227.1997.tb15192.x. [DOI] [PubMed] [Google Scholar]
- 9.National Organization for Rare Disorders. Physician's Guide to Tyrosinemia Type 1.
- 10.de Laet C., Dionisi-Vici C., Leonard J.V. Recommendations for the management of tyrosinaemia type 1. Orphanet. J. Rare Dis. 2013;11(8):8. doi: 10.1186/1750-1172-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott C.R. The genetic tyrosinemias. Am. J. Med. Genet. C: Semin. Med. Genet. 2006;15:121–126. doi: 10.1002/ajmg.c.30092. [DOI] [PubMed] [Google Scholar]
- 12.Fällström S.P., Lindblad B., Steen G. On the renal tubular damage in hereditary tyrosinemia and on the formation of succinylacetoacetate and succinylacetone. Acta Paediatr. Scand. 1981;70(3):315–320. doi: 10.1111/j.1651-2227.1981.tb16558.x. [DOI] [PubMed] [Google Scholar]
- 13.Bliksrud Y.T., Ellingsen A., Bjørås M. Fumarylacetoacetate inhibits the initial step of the base excision repair pathway: implication for the pathogenesis of tyrosinemia type I. J. Inherit. Metab. Dis. 2013;36(5):773–778. doi: 10.1007/s10545-012-9556-0. [DOI] [PubMed] [Google Scholar]
- 14.de Andrade R.B., Gemelli T., Rojas D.B. Tyrosine impairs enzymes of energy metabolism in cerebral cortex of rats. Mol. Cell. Biochem. 2013;364(1–2):253–261. doi: 10.1007/s11010-012-1225-y. [DOI] [PubMed] [Google Scholar]
- 15.Gibbs T.C., Payan J., Brett E.M., Lindstedt S. Peripheral neuropathy as the presenting feature of tyrosinaemia type I and effectively treated with an inhibitor of 4-hydroxyphenylpyruvate dioxygenase. J. Neurol. Neurosurg. Psychiatry. 1993;56(10):1129–1132. doi: 10.1136/jnnp.56.10.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Eufemia P., Finocchiaro R., Celli M. Increased nitric oxide release by neutrophils of a patient with tyrosinemia type III. Biomed. Pharmacother. 2009;63(5):359–361. doi: 10.1016/j.biopha.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Pietz J., Kreis R., Rupp A. Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. J. Clin. Investig. 1999;8:1169–1178. doi: 10.1172/JCI5017. [DOI] [PMC free article] [PubMed] [Google Scholar]