Abstract
Background
A phenylalanine (Phe) restricted dietary management is required in phenylketonuria (PKU) to maintain good metabolic control. Nevertheless, five different models of dietary regimes, which differ in their accuracy of Phe documentation, are used. To investigate the effect of the dietary regime on metabolic control, a multicenter evaluation was performed.
Patients/Methods
149 patients (max. 800 mg Phe-intake/day; 108 children aged 1–9 years and 41 adolescents aged 10–15 years) could be included. They were separated according to age and dietary regime, revealed by a questionnaire on dietary habits. Dietary regimes vary from daily strict calculation of all Phe-intake (group 1) to a rather loose regime only estimating Phe-intake and including high protein food (group 5). Data were analyzed with respect to metabolic control (Phe-concentrations, Phe-concentrations above upper recommended limit during 6 months before the interview), Phe-intake (mg/day) and age (years).
Results
Median Phe-concentrations in children did not differ significantly among diet groups (group 1: 161; 2: 229, 3: 236, 4: 249, 5: 288 μmol/l, p = 0.175). However, exact daily Phe calculation led to significantly lower percentage of Phe concentrations above the upper recommended limit (group 1: 17, 2: 50, 3: 42, 4: 50, 5: 75%, p = 0.035). All included patients showed good to acceptable metabolic control. Patients on the dietary regime with the least accuracy, consuming also high protein foods, showed the poorest metabolic control. Median Phe concentrations of all other groups remained within recommended ranges, including from groups not calculating special low protein foods, fruit and vegetables and using a simplified system of recording Phe-intake.
In adolescents no significant differences among diet groups were revealed.
Conclusion
Exact calculation of Phe content of all food is not necessary to achieve good metabolic control in children and adolescents with PKU. Excluding special low protein food, as well as fruit and vegetables from calculation of Phe-intake has no impact on metabolic control. However including protein rich food into the diet and simply estimating all Phe-intake appears insufficient. The simplification of dietary regime may be helpful in enhancing acceptability and feasibility.
Abbreviations: Phe, phenylalanine; PKU, phenylketonuria; Phe > limit, Phe-concentrations above the therapeutic limit; SD, standard deviation
Keywords: Dietary regime, Phenylketonuria, Phenylalanine, Metabolic control, Phe-intake
1. Introduction
Phenylketonuria (PKU) (OMIN 261600) is one of the most common inborn metabolic disorders. The incidence across Europe varies between 1:3.000 and 1:30.000 [1]. PKU is caused by deficient activity of phenylalanine hydroxylase (EC 1.14.16.1) in most tissues, predominantly in the liver. Severe physical and mental disability appears in children with untreated PKU. Postnatal diagnosis by newborn screening, immediate initiation of a lifelong phenylalanine (Phe) restricted diet and supplementation of a Phe free amino acid mixture enriched with micronutrients result in almost normal cognitive development [2], [3]. The individual daily Phe tolerance depends on residual phenylalanine hydroxylase activity and varies significantly among patients [2]. Poor dietary control results in high plasma Phe-concentrations, which lead to neurocognitive impairment and negatively affect attention span, mood [4] and cognitive performance [5]. Consequently, lifelong dietary therapy with good metabolic control is recommended [6].
Across Europe, two different systems are used to achieve a limited Phe-intake: Phe can either be allocated in total daily amounts or by Phe exchange systems, where portion sizes of Phe-containing foods are precalculated for a defined amount of Phe. The latter is used in the United Kingdom, Denmark and Italy. In most other European countries, the exact Phe content of individual serving sizes is calculated and taken into account [7]. Within this system, however, there are differences with respect to its degree of accuracy. So far, only few studies with small patient groups of children and adolescents were performed to examine the influence of dietary regimes on metabolic control [8], [9], [10], [11].
The classical dietary approach consists in the exact calculation of the Phe content of all ingested foods and drinks without any exceptions (dietary regime 1). Patients under dietary regime 2 calculate their Phe ingestion but do not include foods with a very low Phe content, e.g. < 10 mg/100 g food. Dietary regime 3 combines free consumption of low protein foods (< 10 mg Phe/100 g), fruit and vegetables (< 75 mg Phe/100 g) with calculating the exact Phe content of all other foods [9], [10], [11], [12]. Dietary regime 4, the so-called “simplified diet”, consists of estimating the Phe content according to certain principles (free use of five portions of fruit and vegetables; free use of special low protein food including low protein bread, pasta and breakfast cereals; quantities of potatoes, rice and maize are discussed with the parents and then fixed for a period of time) [8]. Despite other instructions some patients only estimate their protein consumption and even consume varying amounts of protein rich foods e.g. meat, milk or eggs (dietary regime 5) [13], [14]. This regime is self-selected by the families and was never recommended, dietary regimes 1–4 have been chosen by the different metabolic centres.
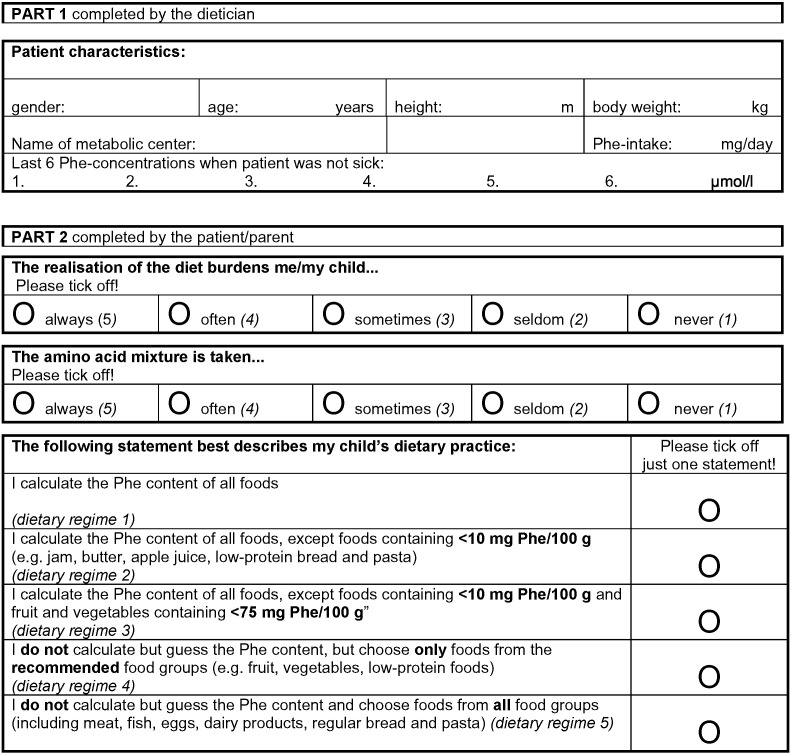
To investigate the influence of the chosen dietary regime on metabolic control, a survey was performed with PKU children and adolescents from ten German speaking metabolic centres, eight from Germany, one from Switzerland and one from Austria using a structured questionnaire (Fig. 1). In addition, the influence of daily Phe-intake and patient's age on metabolic control in relation to the dietary regime was evaluated.
Fig. 1.
Entire questionnaire on dietary habits for patients with PKU.
2. Methods
2.1. Study design
This open, multicentre, retrospective investigation followed the principles of the guidelines in the World Medical Association Declaration of Helsinki of 1975, as revised in 2000 and the harmonized ICH-Guideline for Good Clinical Practice. It was approved by the University of Leipzig's ethics committee (registration-number 372–12–05122012) and registered at the International Clinical Trials Registry Platform (DRKS00004732). Guardians of all included patients gave written informed consent.
The study was open to all German speaking metabolic centres willing to participate. Inclusion was restricted to PKU patients with a maximum Phe-intake of 800 mg/day. Patients older than 15 years, because of higher target reference values, [15] and patients with additional acute or chronic diseases, adhering to additional dietary treatments, or participating in other clinical trials over the past six months were excluded from the analysis.
Data collection was performed during routine clinic visits at the respective metabolic centres between December 2012 and November 2013.
2.2. Patients
A total of 149 PKU patients (77 male/72 female) aged 1–15 (mean ± SD: 7.0 ± 6.6; median = 7) years could be included. They were assigned to the group of children (1–9 years) or the group of adolescents (10–15 years) according to age at clinic visit; this resulted in 108 children (58 male/50 female, mean age ± SD: 4.5 ± 2.8) and 41 adolescents (19 male/22 female, mean age ± SD: 12.2 ± 1.7). All patients were advised to maintain their dried blood Phe-concentrations according to the current recommendations for the German speaking countries (upper therapeutic limit of Phe-concentrations: 240 μmol until the age of ten years, 900 μmol/l thereafter [15] by maintaining the prescribed Phe restricted diet.
2.3. Questionnaire
A two-part questionnaire was developed. The first part recorded patients' characteristics (gender, age, height, body weight, recommended daily Phe-intake, and the last six Phe-concentrations in dried blood prior study entry under routine and thorough dietary conditions, representing the metabolic control over about six months). All data were collected from medical records by trained dieticians and physicians. To verify Phe-intake (recommended versus actual Phe-intake), patients' most recent three-day diet records, documented once a year as part of routine care, were evaluated by the dietician.
In the second part, the patients or their caregivers were asked for information about their degree of accuracy in dietary management with respect to Phe calculation. Additional information about the amino acid mixture consumption and the psychosocial burden implied by the therapy was requested (entire questionnaire see Fig. 1). The second part of the questionnaire was completed anonymously after the clinic visit, and families handed in the questionnaire in a sealed envelope.
2.4. Data analysis
Since guidelines for metabolic control differ according to the patient's age, the entire cohort was divided into two age-groups (children, age 1–9 years, and adolescents, age 10–15 years) before data analysis.
Both age-groups were then assigned to the respective subgroups according to their dietary regime (Fig. 1, Tables 1a and 1b). Psychological burden of diet realisation and adherence to the intake of the Phe free amino acid mixture was expressed as value on a scale of one to five (Fig. 1).
Table 1a.
Comparison (Kruskal–Wallis) of dietary regimes regarding median Phe-concentrations (in μmol/l and in % above therapeutic limit), age, Phe-intake, adherence to amino acid mixture and burden of dietary regime for children.
| Dietary regime |
1 |
2 |
3 |
4 |
5 |
p | ᵡ2 | df |
|---|---|---|---|---|---|---|---|---|
| n | 22 | 25 | 30 | 25 | 6 | |||
| Age [years]: median (range) | 2.5 (1–9) | 4.0 (1–9) | 5.0 (1–9) | 4.0 (1–9) | 4.0 (2–9) | 0.399 | 4.052 | 4 |
| Age [years]: mean (SD) | 3.7 (2.9) | 4.4 (2.8) | 5.1 (2.9) | 4.5 (2.3) | 4.8(3.1) | |||
| Therapeutic range [μmol/l] | 42–240 | 42–240 | 42–240 | 42–240 | 42–240 | |||
| Median Phe-concentration [μmol/l] | 161 | 229 | 236 | 249 | 288 | 0.175 | 6.340 | 4 |
| Median Phe-concentration [μmol/l]: range | 77–829 | 92–544 | 75–744 | 78–531 | 156–529 | |||
| Median Phe-concentration [μmol/l]: interquartile range(IQR) | 96–285 | 161–327 | 176–299 | 178–292 | 210–503 | |||
| Median Phe-concentrations > therapeutic range [%] | 17 | 50 | 42 | 50 | 75 | 0.035a | 10.033 | 4 |
| Median Phe-concentrations > therapeutic range [%]: range | 0–100 | 0–100 | 0–100 | 0–100 | 0–100 | |||
| Median Phe-concentrations > therapeutic range [%]: IQR | 0–38 | 17–75 | 17–83 | 17–75 | 25–100 | |||
| Phe intake [mg]: median (range) | 295 (160–590) | 290 (180–670) | 300 (220–475) | 300 (200–700) | 570 (250–800) | 0.019b | 11.341 | 4 |
| Adherence to amino acid mixture: median (range) | 5 (3–5) | 5 (4–5) | 5 (3–5) | 5 (3–5) | 5 (2–5) | 0.119 | 6.800 | 4 |
| Burden of dietary regime: median (range) | 3 (1–5) | 3 (2–5) | 3 (3–5) | 3 (1–5) | 3 (2–4) | 0.070 | 8.238 | 4 |
Dietary regime 1 vs 2: p = 0.022; 1 vs 3: p = 0.007; 1 vs 4: p = 0.028; 1 vs 5: p = 0.033 (post hoc analyses: Mann–Whitney U)
Dietary regime 1 vs 5: p = 0.008; 2 vs 5: p = 0.007; 3 vs 5: p = 0.008; 4 vs 5: p = 0.006 (post hoc analyses: Mann–Whitney U)
Table 1b.
Comparison (Kruskal–Wallis) of dietary regimes regarding median Phe-concentrations (in μmol/l and in % above therapeutic limit), age, Phe-intake, adherence to amino acid mixture and burden of dietary regime for adolescents.
| Dietary regime |
1 |
2 |
3 |
4 |
5 |
p | ᵡ2 | df |
|---|---|---|---|---|---|---|---|---|
| n | 5 | 10 | 13 | 10 | 3 | |||
| Age [years]: median (range) | 13 (10–15) | 13 (10–15) | 12 (10–13) | 12 (10–15) | 12 (11–15) | 0.657 | 2.433 | 4 |
| Age [years]: mean (SD) | 12.2 (2.2) | 12.5 (2.0) | 11.5 (1.3) | 12.5 (1.8) | 12.7 (2.1) | |||
| Therapeutic range [μmol/l] | 42–900 | 42–900 | 42–900 | 42–900 | 42–900 | |||
| Median Phe-concentration [μmol/l] | 361 | 565 | 532 | 627 | 629 | 0.831 | 1.577 | 4 |
| Median Phe-concentration [μmol/l]: range | 119–810 | 170–1157 | 119–844 | 184–812 | 584–802 | |||
| Median Phe-concentration [μmol/l]: interquartile range(IQR) | 232–713 | 310–716 | 298–655 | 360–757 | 584–802 | |||
| Median Phe-concentrations > therapeutic range [%] | 0 | 20 | 0 | 0 | 17 | 0.393 | 4.178 | 4 |
| Median Phe-concentrations > therapeutic range [%]: range | 0–50 | 0–83 | 0–33 | 0–17 | 0–33 | |||
| Median Phe-concentrations > therapeutic range [%]: IQR | 0–25 | 0–38 | 0–17 | 0–4 | 0–33 | |||
| Phe intake [mg]: median (range) | 500 (190–750) | 355 (200–750) | 350 (200–650) | 420 (290–750) | 550 (500–650) | 0.212 | 5.835 | 4 |
| Adherence to amino acid mixture: median (range) | 5 (4–5) | 5 (4–5) | 5 (1–5) | 5 (4–5) | 5 (5–5) | 0.405 | 4.018 | 4 |
| Burden of dietary regime: median (range) | 3 (2–5) | 3 (1–4) | 3 (1–5) | 3 (1–5) | 4 (2–3) | 0.819 | 1.627 | 4 |
Metabolic control, expressed as mean Phe-concentrations (μmol/l) as well as percent of Phe-concentrations above the therapeutic limit (Phe > limit) was investigated with respect to dietary regime, Phe-intake (mg/day) and age.
Possible associations between the dietary regime and the psychological burden of diet management were investigated, as well as dietary regime and adherence to the intake of the amino acid mixture.
2.5. Statistical analysis
All procedures were performed using IBM SPSS for Windows 20.
Metabolic control was compared between the different dietary groups according to median Phe-concentrations, as well as median Phe > limit. Phe-intake, median Phe-concentrations and median Phe > limit were used as continuous variables and age group as a categorical variable. Primary analytical objectives are dietary regime, as the independent variable, versus median Phe-concentrations and median Phe > limit, as dependent variables. Secondary objectives are dietary regime, as the independent variable, versus psychosocial burden and adherence to the amino acid mixture, as dependent variables.
As normal data distribution could not be assured in Phe-concentrations as well as in Phe > limit, Kruskal–Wallis test was used to search for possible differences in Phe-concentrations, Phe > limit, age, Phe-intake, adherence to amino acid mixture and burden of dietary regime and was followed by Mann–Whitney U test to identify pairs with a significant difference as for post hoc analyses.
Kendall Tau correlation was performed to evaluate the relation between Phe-intake and metabolic control.
Crosstabulation and chi-square test were used to investigate the influence of dietary regime on psychosocial burden of the diet and the adherence to the amino acid mixture intake.
Significance was accepted for p < 0.05. Data are given as median unless otherwise stated.
3. Results
The results for Phe-concentrations, age, Phe-intake, adherence to the amino acid mixture and burden of dietary regime are summarized in Table 1a (children) and 1b (adolescents) and in Figs. 2a (children) and 2b (adolescents). No significant differences of age distribution within the different dietary groups could be revealed, neither among children (p = 0.303), nor among adolescents (p = 0.657).
Fig. 2a.
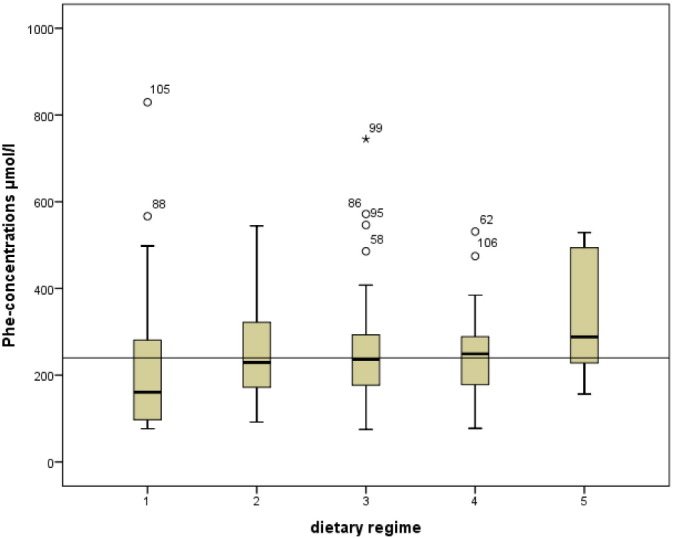
Comparison (Kruskal–Wallis) of metabolic control (expressed as median Phe-concentrations in μmol/l) of dietary regime 1, 2, 3, 4 and 5 in the children's group. Recommended maximum Phe-concentrations: 240 μmol/l.
Fig. 2b.
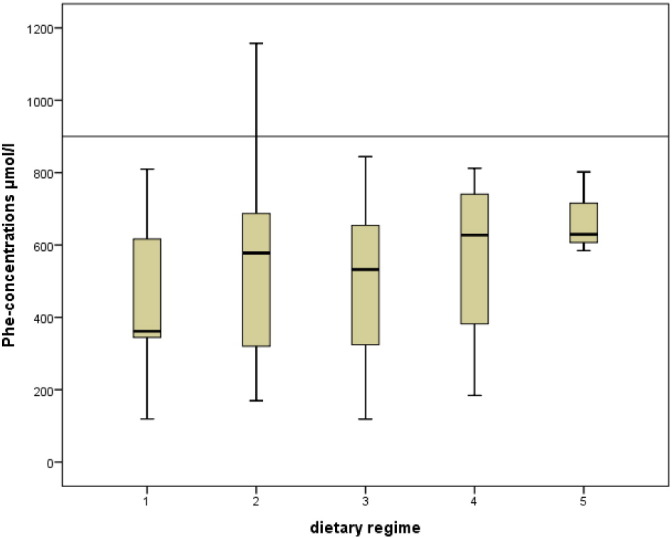
Comparison (Kruskal–Wallis) of metabolic control (expressed as median Phe-concentrations in μmol/l) of dietary regime 1, 2, 3, 4 and 5 in the adolescents' group. Recommended maximum Phe-concentrations: 900 μmol/l.
Median Phe-concentrations of children from dietary regime 1, 2, 3 and 4 were within ideal range (42–240 μmol/l), median Phe concentrations from dietary group 5 were above (288 μmol/l; Table 1a, Fig. 2a). Phe concentrations from dietary regime 1 showed lower median Phe-concentrations (161 μmol/l) than required. Median Phe-concentrations differed slightly and non-significantly among groups (p = 0.175, Table 1a). In contrast, the frequency of Phe-concentrations above the therapeutic limit was significantly different with respect to the dietary regime. Patients on dietary regime 1 had the lowest rate of Phe > limit (p = 0.035; post hoc: dietary regime 1 vs 2: p = 0.022; 1 vs 3: p = 0.007; 1 vs 4: p = 0.028; 1 vs 5: p = 0.033) (Table 1a).
The adolescent cohort displayed very homogeneous results. Median Phe-concentration was far below the upper tolerated limit (900 μmol/l) with all dietary regimes and no significant differences could be detected with respect to the specific dietary regime (p = 0.831, Table 1b). Also, the frequency of Phe > limit did not differ between groups (p = 0.393, Table 1b).
Daily Phe-intake did not correlate with median Phe-concentrations (children: r = − 0.007, p = 0.917; adolescents: r = − 0.032, p = 0.770) or the frequency of Phe > limit (children: r = 0.055, p = 0.444; adolescents: r = 0.195, p = 0.123), neither in children nor in adolescents. Median Phe-intake of dietary groups was significantly different in children (p = 0.019; dietary regime 1 vs 5: p = 0.008; 2 vs 5: p = 0.007; 3 vs 5: p = 0.008; 4 vs 5: p = 0.006), but not in adolescents (p = 0.212).
Children and adolescents equally rated the adherence to the amino acid mixture as “always” and the burden of the dietary regime as “sometimes”. Likewise, the adherence to the intake of the Phe free amino acid mixture and the burden of the dietary regime did not differ between the different dietary regimes.
4. Discussion
The study presented here investigated the influence of dietary regime on metabolic control in 149 children and adolescents with PKU. Data of patients' age, daily Phe-intake, burden of dietary regime and adherence to the intake of amino acid mixture were taken into account.
The recommendation for the upper limit of Phe-concentrations differs substantially among European countries [16]. Whereas the German speaking countries recommend Phe- concentrations in dried blood below 240 μmol/l for children up to the age of 10 and below 900 μmol/l for adolescents up to the age of 15 [15], the recent American guidelines [17] recommend Phe-concentrations below 360 μmol/l in patients of all age groups. A draft of European guidelines has been established recently and recommends Phe-concentrations below 360 μmol/l for children and below 600 μmol/l for adults [18]. All patients included in the present study were treated according to the guidelines for the German speaking countries [15]. These recommendations were also used for this evaluation.
Interestingly, the most accurate version of the diet (dietary regime 1), calculating the Phe content of all ingested food, did not provide a significant benefit with respect to median Phe-concentrations in comparison to all other dietary regimes. However, regarding Phe > limit they yielded better results than all other groups. A reason could be the slightly younger age of the patients, which is associated with very regular food intake (amount and size of meals) in combination with a limited food variety and, therefore, a better calculability of Phe-intake.
Because of irreversible mental disabilities caused by insufficient metabolic control during childhood [15], dietary regime 1 is often recommended [7]. Continuously high Phe-concentrations lead to impaired executive function and diverse psychiatric symptoms in adolescents and adults [5], [17]. In addition, greater fluctuations of Phe in plasma seem to negatively impact neurocognitive development [19], [20], [21]. Interestingly, Phe fluctuations were highest in the children's group on the most accurate version of the dietary regime (dietary regime 1). The potential negative impact in our cohort is certainly limited, since Phe-concentrations mostly fluctuated within the values of the therapeutic range with the exception of few single patients. Therefore, dietary regime 1 seems favourable during early childhood, as the immature brain of toddlers is especially sensitive to insufficient metabolic control [22].
Beyond early childhood, liberalization of dietary regimes appears feasible, and could increase adherence as daily diet restrictions could be easier to follow, especially when children start preschool. Other studies, although investigating small patient groups, could demonstrate the safety of dietary regimes without exact calculation of Phe from all ingested foods and beverages in children with classical PKU. These include investigations of small cohorts with respect to free use of fruits and vegetables in short-term [9], [11], as well as long-term settings [8], [10], [12]. The regimes 2, 3 and 4 revealed similar results, and in total represent a group of 80 patients. Our observations from a larger, real-life cohort thus support the conclusions reached from small interventional studies. These types of dietary regimes provide some benefits with respect to feasibility once children are under supervision of changing caregivers during the day (home, school, and afternoon activities).
Median Phe-concentrations of children following regime 2, 3 or 4 are close to the upper recommended limit. This demonstrates that the maximum recommended intake of natural protein is well fulfilled, providing a maximal possible daily amount of high quality protein [23], [24]. However, the rate of Phe > limit is significantly higher under these regimes. The long-term impact of this finding cannot be estimated at this point, since long-term data throughout childhood, adolescence and adulthood are not yet available. In contrast, pure estimating of Phe-intake and including protein-rich food into the diet (dietary regime 5) cannot be recommended. Interestingly, these patients show significantly higher recommended Phe-intake. High Phe tolerance of patients might bear the danger to lead to lax dietetic training, which may result in poor metabolic control.
In adolescents, patients under dietary regime 1 showed the lowest Phe-concentrations in plasma. The dietary regime 5 shows no negative impact on metabolic control when applying the guidelines for the German speaking countries, accepting Phe-concentrations up to 900 μmol/l. However, a European expert group currently reviews the existing guidelines, and the upper accepted threshold of Phe-concentrations might be lowered to 600 μmol/l in the near future [18]. Therefore, this loose dietary regime cannot be recommended even in adolescents.
Interestingly, the perceived burden of diet realization did not differ among the investigated groups. Neither the dietary regime with the most nor the least accuracy had an impact on the adherence to the intake of Phe free amino acid mixture. However, the questions about the dietary burden and adherence to the intake of the amino acid mixture were mostly answered by the parents, rather than by the patients themselves, and their perceived burden may be larger.
The major shortcoming of the presented data is that the results rely on the accuracy of the patients' reports. In this respect, the relatively small number of patients following dietary regime 5 is interesting. One may speculate that some patients reported to follow a stricter diet than they actually do. In addition, not all patients treated at the participating centres took part, potentially introducing a selection bias, since the families motivated enough to take part in an investigation may be more motivated to adhere to stricter therapy. The observed high rate of participants (5 centres 100%, 4 centres 76–92%, 1 centre 37%) at the different centres argues against marred bias, since rates around 60–70% are regarded to yield representative results in survey studies..
Nevertheless, our data should be reviewed according to the new European guidelines, which are going to be established in the near future.
Taken together, dietary regimes which omit calculating low protein food, fruit and vegetables or, alternatively, a so-called “simplified” dietary regime seem to be the best compromise between ideal metabolic control, acceptability and feasibility. A dietary regime, which is relatively easy to handle during daily routine, could be helpful in maintaining good metabolic control even beyond childhood and adolescence.
5. Conclusions
Exact calculation of Phe content of all food is not necessary to achieve good metabolic control in children and adolescents with PKU. Excluding special low protein food, as well as fruit and vegetables from calculation of Phe-intake has no impact on metabolic control. Including protein-rich foods into the diet and simply estimating all Phe-intake should, however, be strictly avoided. The simplification of dietary control may be helpful in enhancing acceptability and feasibility beyond early childhood.
Conflict of interest, source of funding and authorship
The authors declare that they have no conflicts of interest.
The authors wish to acknowledge that there was no source of funding or financial support for the survey.
The study was designed by RC. The questionnaire was developed by RC, TAG, BS and MU. RC, OU, SK, MU, RS, MC, WS, BH, LF, JM lead the teams of patient recruitment and data collection in the different centres for inborn metabolic diseases. Statistical analyses were performed by RC. RC, TAG and BS interpreted the data and drafted the manuscript. BS provided supervision and guidance throughout the study. All authors critically reviewed and approved the final version submitted for publication.
Acknowledgements
The authors would like to thank the patients and their families for their interest and participation and Holgar Bogatsch (Institute for Medical Informatics, Statistics and Epidemiology of the University of Leipzig) for his statistical advice.
We acknowledge support from the German Research Foundation (DFG) and Universität Leipzig within the program of Open Access Publishing.
Contributor Information
Carmen Rohde, Email: Carmen.Rohde@medizin.uni-leipzig.de.
Alena Gerlinde Thiele, Email: Alena.Thiele@medizin.uni-leipzig.de.
Ulrike Och, Email: och@uni-muenster.de.
Katrin Schönherr, Email: kathrin-schoenherr@med.uni-jena.de.
Uta Meyer, Email: Meyer.Uta@mh-hannover.de.
Stefanie Rosenbaum-Fabian, Email: stefanie.rosenbaum-fabian@uniklinik-freiburg.de.
Cornelia Maddalon, Email: Cornelia.Maddalon@Kispi.uzh.ch.
Sabine Matzken, Email: Sabine.Matzken@paediat.med.uni-giessen.de.
Holger Blessing, Email: holger.blessing@uk-erlangen.de.
Frauke Lang, Email: frauke.lang@web.de.
Monika Jörg-Streller, Email: monika.joerg-streller@uki.at.
Skadi Beblo, Email: Skadi.Beblo@medizin.uni-leipzig.de.
References
- 1.Loeber J.G. Neonatal screening in Europe; the situation in 2004. J. Inherit. Metab. Dis. Aug 2007;30(4):430–438. doi: 10.1007/s10545-007-0644-5. [DOI] [PubMed] [Google Scholar]
- 2.Scriver C.R., Kaufmann S., Eisensmith R.C., Woo S.L. McGraw-Hill; New York: 1998. The hyperphenylalaninemias. In: Scriver RC BASWaVD, editor. The metabolic and molecular basis of inherited disease; pp. 1015–1075. [Google Scholar]
- 3.Enns G.M., Koch R., Brumm V., Blakely E., Suter R., Jurecki E. Suboptimal outcomes in patients with PKU treated early with diet alone: revisiting the evidence. Mol. Genet. Metab. Oct 2010;101(2–3):99–109. doi: 10.1016/j.ymgme.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 4.ten Hoedt A.E., de Sonneville L.M., Francois B., Ter Horst N.M., Janssen M.C., Rubio-Gozalbo M.E. High phenylalanine levels directly affect mood and sustained attention in adults with phenylketonuria: a randomised, double-blind, placebo-controlled, crossover trial. J. Inherit. Metab. Dis. Feb 2011;34(1):165–171. doi: 10.1007/s10545-010-9253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weglage J., Fromm J., van Teeffelen-Heithoff A., Moller H.E., Koletzko B., Marquardt T. Neurocognitive functioning in adults with phenylketonuria: results of a long term study. Mol. Genet. Metab. 2013;110(Suppl):S44–S48. doi: 10.1016/j.ymgme.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Koch R., Burton B., Hoganson G., Peterson R., Rhead W., Rouse B. Phenylketonuria in adulthood: a collaborative study. J. Inherit. Metab. Dis. Sep 2002;25(5):333–346. doi: 10.1023/a:1020158631102. [DOI] [PubMed] [Google Scholar]
- 7.Ahring K., Belanger-Quintana A., Dokoupil K., Gokmen O.H., Lammardo A.M., MacDonald A. Dietary management practices in phenylketonuria across European centres. Clin. Nutr. Jun 2009;28(3):231–236. doi: 10.1016/j.clnu.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann M., Jacobs P., Fingerhut R., Torresani T., Thony B., Blau N. Positive effect of a simplified diet on blood phenylalanine control in different phenylketonuria variants, characterized by newborn BH4 loading test and PAH analysis. Mol. Genet. Metab. Jul 2012;106(3):264–268. doi: 10.1016/j.ymgme.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Rohde C., Mutze U., Weigel J.F., Ceglarek U., Thiery J., Kiess W. Unrestricted consumption of fruits and vegetables in phenylketonuria: no major impact on metabolic control. Eur. J. Clin. Nutr. May 2012;66(5):633–638. doi: 10.1038/ejcn.2011.205. [DOI] [PubMed] [Google Scholar]
- 10.Rohde C., Mutze U., Schulz S., Thiele A.G., Ceglarek U., Thiery J. Unrestricted fruits and vegetables in the PKU diet: a 1-year follow-up. Eur. J. Clin. Nutr. Mar 2014;68(3):401–403. doi: 10.1038/ejcn.2013.272. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald A., Rylance G., Davies P., Asplin D., Hall S.K., Booth I.W. Free use of fruits and vegetables in phenylketonuria. J. Inherit. Metab. Dis. 2003;26(4):327–338. doi: 10.1023/a:1025150901439. [DOI] [PubMed] [Google Scholar]
- 12.Rohde C., Thiele A.G., Mutze U., Kiess W., Beblo S. 12 ed. 2014. Simplifying the diet for patients with phenylketonuria (PKU): unrestricted consumption of fruit and vegetables; pp. 178–180. [Google Scholar]
- 13.MacDonald A., Gokmen-Ozel H., van R.M., Burgard P. The reality of dietary compliance in the management of phenylketonuria. J. Inherit. Metab. Dis. Dec 2010;33(6):665–670. doi: 10.1007/s10545-010-9073-y. [DOI] [PubMed] [Google Scholar]
- 14.Bilginsoy C., Waitzman N., Leonard C.O., Ernst S.L. Living with phenylketonuria: perspectives of patients and their families. J. Inherit. Metab. Dis. 2005;28(5):639–649. doi: 10.1007/s10545-005-4478-8. [DOI] [PubMed] [Google Scholar]
- 15.Burgard P., Bremer H.J., Buhrdel P., Clemens P.C., Monch E., Przyrembel H. Rationale for the German recommendations for phenylalanine level control in phenylketonuria 1997. Eur. J. Pediatr. Jan 1999;158(1):46–54. doi: 10.1007/s004310051008. [DOI] [PubMed] [Google Scholar]
- 16.Blau N. International medical publishers; London, Boston: 2010. Phenylketonuria and BH4 Deficiencies. [Google Scholar]
- 17.Vockley J., Andersson H.C., Antshel K.M., Braverman N.E., Burton B.K., Frazier D.M. Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet. Med. Feb 2014;16(2):188–200. doi: 10.1038/gim.2013.157. [DOI] [PubMed] [Google Scholar]
- 18.van Spronsen F.J. European Guidelines for PKU (Draft) 2014. http://www.endocrinology-and-metabolism-excemed.org/en/endocrinology-and-metabolism/resources/from-the-conferences/espku-conference-2014.html
- 19.Anastasoaie V., Kurzius L., Forbes P., Waisbren S. Stability of blood phenylalanine levels and IQ in children with phenylketonuria. Mol. Genet. Metab. Sep 2008;95(1–2):17–20. doi: 10.1016/j.ymgme.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Cleary M., Trefz F., Muntau A.C., Feillet F., van Spronsen F.J., Burlina A. Fluctuations in phenylalanine concentrations in phenylketonuria: a review of possible relationships with outcomes. Mol. Genet. Metab. Dec 2013;110(4):418–423. doi: 10.1016/j.ymgme.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Hood A., Antenor-Dorsey J.A., Rutlin J., Hershey T., Shimony J.S., McKinstry R.C. Prolonged exposure to high and variable phenylalanine levels over the lifetime predicts brain white matter integrity in children with phenylketonuria. Mol. Genet. Metab. Jan 2015;114(1):19–24. doi: 10.1016/j.ymgme.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith I., Beasley M.G., Ades A.E. Intelligence and quality of dietary treatment in phenylketonuria. Arch. Dis. Child. May 1990;65(5):472–478. doi: 10.1136/adc.65.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donati A., Vincenzi C., Tosti A. Acute hair loss in phenylketonuria. J. Eur. Acad. Dermatol. Venereol. May 2009;23(5):613–615. doi: 10.1111/j.1468-3083.2008.02992.x. [DOI] [PubMed] [Google Scholar]
- 24.Pode-Shakked B., Shemer-Meiri L., Harmelin A., Stettner N., Brenner O., Abraham S. Man made disease: clinical manifestations of low phenylalanine levels in an inadequately treated phenylketonuria patient and mouse study. Mol. Genet. Metab. 2013;110(Suppl):S66–S70. doi: 10.1016/j.ymgme.2013.10.006. [DOI] [PubMed] [Google Scholar]