Abstract
The soaring prevalence of obesity and diabetes is associated with an increase in comorbidities, including elevated risk for cardiovascular diseases (CVDs). CVDs continue to be among the leading causes of death and disability in the United States. While increased nutritional intake from an energy‐dense diet is known to disrupt metabolic homeostasis and contributes to the disease risk, circadian rhythm disruption is emerging as a new risk factor for CVD. Circadian rhythms coordinate cardiovascular health via temporal control of organismal metabolism and physiology. Thus, interventions that improve circadian rhythms are prospective entry points to mitigate cardiometabolic disease risk. Although light is a strong modulator of the neural circadian clock, time of food intake is emerging as a dominant agent that affects circadian clocks in metabolic organs. We discovered that imposing a time‐restricted feeding (TRF) regimen in which all caloric intakes occur consistently within ≤ 12 h every day exerts many cardiometabolic benefits. TRF prevents excessive body weight gain, improves sleep, and attenuates age‐ and diet‐induced deterioration in cardiac performance. Using an integrative approach that combines Drosophila melanogaster (fruit fly) genetics with transcriptome analyses it was found that the beneficial effects of TRF are mediated by circadian clock, ATP‐dependent TCP/TRiC/CCT chaperonin and mitochondrial electron transport chain components. Parallel studies in rodents have shown TRF reduces metabolic disease risks by maintaining metabolic homeostasis. As modern humans continue to live under extended periods of wakefulness and ingestion events, daily eating pattern offers a new potential target for lifestyle intervention to reduce CVD risk.

Keywords: cardiac physiology, cardiometabolic disorders, circadian rhythm, metabolic regulation, mitochondrial electron transport chain, TCP‐1 ring complex chaperonin, time restricted feeding
Abbreviations
- ALF
Ad libitum feeding
- CVD
cardiovascular disease
- ETC
electron transport chain
- ROS
reactive oxygen species
- TRF
time‐restricted feeding
- TRiC
TCP‐1 ring complex
Introduction
A recent World Health Organization (WHO) report revealed that diabetes and obesity‐related diseases have soared in every country, nearly quadrupling over the last 35 years, to 422 million adult cases (http://www.who.int/diabetes/global‐report/en/) (Steinberger et al. 2009; Rodriguez‐Colon et al. 2010). Obesity and diabetes are associated with a number of comorbidities, including elevated risk for CVD (Steinberger et al. 2009; Rodriguez‐Colon et al. 2014; Morris et al. 2016). CVD continues to be among the leading causes of death and disability in the United States. The leading risk factor for cardiac and cardiometabolic diseases are age, shift work, an energy‐dense diet, diabetes and obesity (Durgan & Young, 2010; Azadbakht et al. 2013; Morris et al. 2016). These seemingly unrelated risk factors have one common connection – circadian rhythm disruption. Circadian rhythms are ∼24 h cycles in behaviour, physiology and metabolism that arise from coordinated regulation of numerous pathways in different organs. Almost every organ in animals exhibits a circadian rhythm in gene expression and function. Unbiased gene expression studies are revealing that more than half of the genome shows daily rhythm in expression in a tissue‐specific manner (Hatori et al. 2012; Sherman et al. 2012; Chaix et al. 2014, 2016; Panda, 2016; Zarrinpar et al. 2016). Conversely, genetic perturbation of the circadian clock in model organisms increases the incidence and severity of cardiometabolic diseases (Steinberger et al. 2009; Durgan & Young, 2010; St‐Onge et al. 2016). Similarly, lifestyle perturbation of the circadian clock, as occurs among shift workers or in experimental models of shiftwork in animals, can disrupt the circadian clock and trigger obesity, diabetes, mitochondrial diseases and CVD (Maury et al. 2010; Gill et al. 2015; Morris et al. 2016; St‐Onge et al. 2016). Therefore factors that affect circadian rhythms offer new avenues to understand the aetiology, prevention and treatment of cardiometabolic diseases. While the circadian rhythm in sleep and wakefulness is primarily synchronized to the ambient light–dark cycle, the timing of food intake (and conversely the fasting period) appears to affect the robustness of circadian rhythms in metabolic organs. This has led to the hypothesis that the daily cycle of eating and fasting is a determinant of circadian function in cardiac tissue. Accordingly, consolidating all caloric intake to a few hours without altering the daily intake of quality or quantity of nutrients supports robust circadian rhythms. This newly emerging approach of time‐restriction of caloric intake or time‐restricted feeding (TRF) appears to impart both preventative and therapeutic effects on metabolic diseases in experimental animals. In this review, we will introduce recent work on circadian rhythms and TRF with specific reference to cardiometabolic diseases.
Circadian clocks, rhythms and cardiometabolic diseases
Circadian rhythms in animals emerge from cell‐autonomous, self‐sustaining, ∼24 h transcriptional feedback loops (Panda et al. 2002; Vanin et al. 2012; Harfmann et al. 2015; Chaix et al. 2016). The specific molecular components and mechanisms of circadian rhythms first identified in Drosophila are largely conserved in mammals (Helfrich‐Forster, 2000; Panda et al. 2002; Vanin et al. 2012; Hardin & Panda, 2013). In Drosophila, the transcriptional activators Clock (Clk) and Cycle (Cyc; also known as dBmal1) dimerize and transcriptionally activate the Timeless (Tim) and Period (Per) genes. The formation of the Per–Tim heterodimer, in turn, inhibits the activities of Clk–Cyc (Helfrich‐Forster, 2000; Panda et al. 2002; Hardin & Panda, 2013; Mendoza‐Viveros et al. 2017). The molecular circadian clock generates daily rhythms in a large number of genes and proteins by (a) regulating transcription from cis‐regulatory sites, (b) regulating transcription factors, which then regulate indirect clock targets, (c) affecting post‐transcriptional regulatory processes, and (d) functionally interacting with signalling and transcriptional regulators (Mendoza‐Viveros et al. 2017). As a result, in most animal tissue examined, several hundreds or even thousands of transcripts show daily rhythm in their expression. Functional annotation of these rhythmic transcripts revealed that nutrient metabolism and basic cellular functions are under circadian modulation (Chaix et al. 2016; Zarrinpar et al. 2016).
Importantly, expression and function of many of the clock components are intimately interlinked with cellular metabolism. Binding of Clk–Bmal1 (mammalian homologues of Clk–Cyc) to DNA is influenced by redox state. Furthermore, some of the clock components are post‐translationally modified by phosphorylation, acetylation and glycosylation (Bass & Lazar, 2016), which in turn affect their stability and function. Enzymes mediating these modifications respond to cellular energy states and thereby constitute nodes through which the circadian clock is integrated with energy status. Additionally, several secondary metabolites in mammals affect circadian rhythm through their impact on chromatin modification and by serving as ligands for clock components (Chaix et al. 2016; Longo & Panda, 2016; Manoogian & Panda, 2016; Panda, 2016; Zarrinpar et al. 2016; Mattson et al. 2017). Such reciprocal interaction between metabolism and the circadian system suggested that the circadian system can ‘sense’ metabolic state of the cell and in turn regulate the timing of expression of a large number of genes working in seemingly disparate pathways to optimize physiology.
In mammals, circadian oscillations in thousands of transcripts have been described in tissues of the cardiovascular system, including the atrium, ventricle, aorta and endothelial cells (Rudic et al. 2005; Bray et al. 2008; Koike et al. 2012). Accordingly, circadian mutant mice exhibit compromised cardiovascular functions (reviewed in Paschos & FitzGerald, 2010). For example, over‐expression of the dominant negative circadian clock∆19 mutant (CCM) in mouse cardiomyocytes disrupts normal circadian gene expression and cardiac function. Hearts of CCM mice exhibit increased fatty acid oxidation, lactate release, elevated fractional shortening, bradycardia and a longer R–R interval (Bray et al. 2008). Additionally, circadian mutant mice also exhibit a plethora of subtle cardiovascular defects, which might increase susceptibility to CVDs (Paschos & FitzGerald, 2010). A clock controlled gene – KLF15 – offers a mechanistic link between circadian rhythm and cardiac function. Circadian clock regulates rhythmic expression of KLF15, which in turn regulates circadian expression of Kv channel‐interacting protein 2 (KChIP2), a key component required for transient outward potassium current. In the mouse heart, constitutive over‐expression or knockout of transcription factor KLF15 causes loss of the rhythmic QT interval and enhanced susceptibility to ventricular arrhythmia (Jeyaraj et al. 2012). Whole animal or cardiac specific circadian clock mutant mice also show compromised metabolic homeostasis, which can contribute to CVD risks. In humans, misalignments of circadian rhythms in shift workers lead to increase risks of cardiac diseases (Morris et al. 2016). Overall, these studies have associated the circadian clock to cardiometabolic health and suggest that the maintenance of a robust circadian system may reduce CVD risks.
Time‐restricted feeding paradigm and metabolic health in rodents
Animals and humans have a diurnal rhythm in food intake with the major part of the food consumed during the organism's natural wakeful period. Due to the reciprocal interaction between metabolism and circadian rhythms, this daily eating–fasting rhythm acts synergistically with the molecular circadian clock to drive daily rhythms in anabolic and catabolic metabolism and dependent physiology. Ad libitum access to an energy‐dense high fat diet (HFD) can disrupt the feeding–fasting rhythms in animals with food intake erratically spread over a major part of the 24 h day. Such an erratic eating pattern dampens the normal circadian oscillator in metabolic organs including the liver. In experiments to test the contribution of an HFD vs. erratic eating pattern to HFD‐induced obesity, mice were fed an HFD ad libitum or allowed to eat the same number of calories within a restricted time interval of 8 h. Surprisingly, under such a TRF protocol mice are significantly protected from diet‐induced obesity and associated metabolic diseases. Recently, TRF benefits have been observed in mice fed up to 12 h every day (Hatori et al. 2012; Sherman et al. 2012; Chaix et al. 2014). Some of these benefits in rodents include improved glucose tolerance, reduced triglyceride, reduced cholesterol, reduced systemic inflammation and improved endurance. As hypothesized, TRF sustains a robust circadian clock in the liver and prevents metabolic reprogramming of the hepatic transcriptome that typically occurs in mice fed an HFD ad libitum (Hatori et al. 2012; Sherman et al. 2012; Chaix et al. 2014). Recently it has been shown that feeding mice during the daytime is associated with desynchronization of peripheral clocks and leads to obesity and other metabolic challenges (Yasumoto et al. 2016). Furthermore, additional studies have shown that TRF reduces adiposity in male C57BL/6 mice that are challenged with an HFD and that restricted feeding in middle‐aged C57BL6/J mice alleviates the negative effects of an HFD on metabolic health, including body weight, liver weight and glucose tolerance (Duncan et al. 2016; Sundaram & Yan, 2016). Although TRF yields several metabolic improvements in rodents, its impact on cardiac function, its genetic mechanism, and whether the phenomenon is relevant to non‐rodent species is unclear.
Drosophila as a genetic model organism for cardiovascular and metabolic diseases
Due to parallel genetic functions between flies and vertebrates during cardiogenesis, the Drosophila model has been well established to explore the genetic basis of deterioration of cardiac function that arises due to aging, diet, or genetic mutation (Ocorr et al. 2007; Birse et al. 2010; Wolf & Rockman, 2011; Melkani et al. 2013; Gill et al. 2015). The Drosophila heart is cylindrical in shape, with a conical structure at the anterior end. It resides within the dorsal abdominal cavity, extending from the first to the sixth body‐wall segment along the midline. The primary function of the fly heart is to pump haemolymph. The pumping action is generated by the rhythmic diametric expansion (diastole) and contraction (systole) of the conical/cylindrical structure. By combining a semi‐intact in vivo preparation with high‐speed imaging, cardiac rhythms can be imaged for a sufficient period of time to quantitatively assess defining characteristics (Fig. 1), such as the heart period, and systolic and diastolic diameter (Gill et al. 2015), as well as the variability associated with these measurements from one heartbeat to another (Ocorr et al. 2007; Birse et al. 2010; Wolf & Rockman, 2011; Melkani et al. 2013; Gill et al. 2015). Despite some obvious limitations in modelling many heart diseases and therapeutic interventions, several genetic and non‐genetic risks for heart diseases in humans also increase disease risks in Drosophila. In the adult fly, like in humans, cardiac arrhythmias appear and intensify with age (Ocorr et al. 2007; Birse et al. 2010; Wolf & Rockman, 2011; Melkani et al. 2013; Gill et al. 2015). Likewise, nutritional challenges that compromise cardiac function in humans (e.g. high‐fat or high‐sugar diets) have similar effects in flies (Birse et al. 2010; Na et al. 2013; Gill et al. 2015). In addition, the short life‐span and advanced genetics of Drosophila allow it to serve as an excellent model system for examining gene networks. Drosophila serves as an admirable model system for basic discoveries in metabolic syndrome, circadian rhythms, energy metabolism, mitochondrial homeostasis and cardiac physiology (Ocorr et al. 2007; Birse et al. 2010; Wolf & Rockman, 2011; Melkani et al. 2013; Gill et al. 2015). As in other model organisms and in humans, age, energy‐dense diets and disruptions of circadian rhythm compromise cardiac performance in the fruit fly (Ocorr et al. 2007; Birse et al. 2010; Wolf & Rockman, 2011; Melkani et al. 2013; Gill et al. 2015), suggesting that conserved pathways (including the circadian clock) mediate cardiac muscle function.
Figure 1. Relationship among potential pathways linked with TRF‐induced cardiac benefits and their impact in ameliorating cardiometabolic disorders.
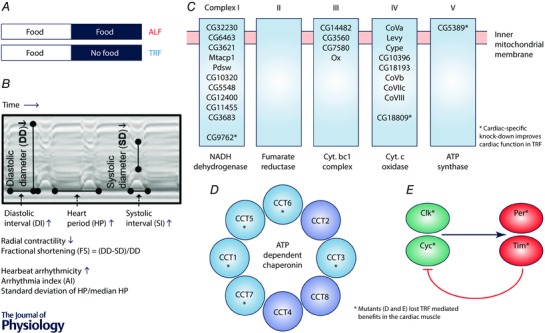
A, as reported before (Gill et al. 2015), flies are maintained on a 12:12 h light–dark cycle. The TRF flies have access to food for 12 h whereas ALF flies have access to food for 24 h. B, cardiac parameters were calculated from mechanical (M)‐mode traces (showing the movement of the heart tube edge (y‐axis) over time (x‐axis)). Age‐associated alterations of cardiac parameters shown with upward and downward arrows. C, TRF down‐regulates expression of mitochondrial ETC components in Drosophila hearts and cardiac‐specific knock‐down of ETC genes CG5389 (Mitochondrial ATP synthase), CG9762 (NADH dehydrogenase) and CG18809 (spliceosome‐associated protein‐18) delays age‐associated cardiac defects. D, up‐regulation of cytoplasmic chaperonin (TCP/TRiC/CCT) was cardiac specific under TRF and mutation of TCP/TRiC/CCT eliminated the TRF benefit. E, mutation of circadian cock genes (Clk, Cyc, Per and Tim) eliminated the TRF benefit. Therefore, circadian genes are required for cardioprotection under TRF. Synchrony between feeding–fasting and light–dark cycles synergistically optimize metabolism by driving anabolic and catabolic processes at appropriate times of the day, which in turn lessens ROS and sustains cytoarchitecture (Gill et al. 2015).
Imposing feeding–fasting rhythms with TRF in Drosophila
Adult flies (Drosophila melanogaster) sleep more during the night and have a short siesta‐like sleep during the day (Helfrich‐Forster, 2000; Klarsfeld et al. 2003; Grima et al. 2004; Picot et al. 2007; Vanin et al. 2012). However, they do occasionally wake up during the night and when food is available ad libitum, as in most experimental situations, they consume a measurable amount of food at night (Gill et al. 2015). As flies age, the circadian organization of activity and rest deteriorates, with increased activity and reduced sleep during the night (Gill et al. 2015). Therefore, the flies offer a tractable model to test the role of the feeding–fasting rhythm on overall health during aging.
To test the role of a daily feeding–fasting cycle on animal health, ad libitum feeding (ALF) and TRF cohorts are carefully maintained. Flies (Oregon‐R) are collected as soon as they emerge and are maintained on ALF for a few days before assigning them to different eating pattern regimens. At ∼2 weeks of age, they are assigned to one of the two paradigms (Fig. 1 A). The ALF group is essentially the standard practice in fly labs where the flies are group housed in vials where a semi‐solid food is available 24 h per day. The flies are maintained on a 12 h light–12 h dark cycle at 22°C in humidified incubators. TRF flies are held in these identical housing conditions and in standard vials with semi‐solid food for 12 h during the daytime and are switched to vials with 1.1% agar (no food) at night. The procedure is repeated every day and the ALF flies are also switched to food vials to control for any unintended consequences of transferring flies between vials every day. As seen in rodents, both ALF and TRF groups consume the same amount of calories every day. Such an experimental set‐up controls for genotype, age, nutrition quality and quantity and allows for systematic analyses of the impact of TRF and ALF on body weight, flight ability, cardiac performance and underlying molecular changes at different ages (Gill et al. 2015).
In Drosophila TRF also exerts beneficial cardiometabolic effects, thus illustrating the relevance of eating pattern on metabolic health across species (Hatori et al. 2012; Sherman et al. 2012; Chaix et al. 2014, 2016; Panda, 2016; Zarrinpar et al. 2016). We have shown that cardiac physiological parameters of 3‐week‐old flies under ALF and TRF were identical. Flies with hearts under ALF showed an age‐dependent increased incidence of cardiac arrhythmias, prolonged systolic and diastolic intervals as well as increased heart period under ALF at 5 weeks of age (Fig. 1 and Table 1). The mean diastolic diameter decreases with age, which results in lowering cardiac contractility (Gill et al. (2015) and Fig. 1 B). These cardiac parameters further deteriorate in 7‐week‐old flies. Interestingly, cardiac contractility was sustained under TRF in both 5‐ and 7‐week‐old flies compared to their ALF counterparts. Additionally, compared to ALF, age‐associated increased cardiac arrhythmias, heart period, and systolic and diastolic intervals were also attenuated under TRF in 5‐ and 7‐week‐old flies. Overall, the cardiac phenotypes of 5‐ or 7‐week‐old TRF flies were similar to those of 3‐ or 5‐week‐old ALF flies. Furthermore, when flies are introduced to TRF later in life (at 5 weeks) they still showed improvement of some cardiac parameters (Gill et al. 2015), compared to age‐matched ALF flies. In addition to attenuation of age‐associated cardiac defects, the TRF regimen suppresses cardiac defects arising from a high fat diet (Gill et al. 2015). Overall, TRF increased the cardiac health‐span of Drosophila.
Table 1.
Summary of altered physiological parameters and cardiac‐specific genes under TRF
| Parameters | ALF | TRF |
|---|---|---|
| Food intake | Ad libitum 24 h | 12 h during the day |
| Activity | Equivalent | Equivalent |
| Sleep | Deteriorates with age (⇊) | Improved (⇈) |
| Flight ability | ⇈ Relative to ALF | |
| Body weight | Vary | Constant |
| Circadian gene expression (head and body) | ⇈ Relative to ALF | |
| Cardiac expression of ETC components (Fig. 1 C) | ⇊ Relative to ALF | |
| Cardiac expression of TCP components (Fig. 1 D) | ⇈ Relative to ALF | |
| Cardiac physiology (age‐ and diet‐induced) | Deteriorates | Sustained |
TRF also improved several health parameters (Table 1) that are known to contribute to cardiovascular disease risks. Flies under TRF were protected from age‐dependent body weight gain, had improved flight index (muscle function), and sustained consolidated nightly sleep even into their middle ages. Such pleiotropic effects reflect TRF impacts on tissues throughout the body including the brain. Accordingly, systematic gene expression studies could identify potential correlative molecular changes.
Cardiac metabolism, proteostasis and cardiometabolic health
Rodent studies have shown the metabolic benefits of TRF and correlative changes in the expression of several established pathways (Hatori et al. 2012; Sherman et al. 2012; Chaix et al. 2014, 2016; Duncan et al. 2016; Sundaram & Yan, 2016; Zarrinpar et al. 2016). However, the impact of TRF on cardiometabolic function and the genetic basis of TRF benefits that attenuate cardiac aging dysfunction are not yet explored in the rodent. In Drosophila, unbiased measurement of global changes in gene expression during 24 h periods in ALF and TRF flies have begun to shed light on probable mechanisms (Fig. 1). The diurnal gene expression pattern of Drosophila head, body and heart yielded several clues. First of all, these gene expression changes under TRF were not similar to the well‐established reporter of caloric restriction (CR) in Drosophila (Farhadian et al. 2012), thus indicating that the underlying mechanism is likely to be different from that of CR. In both head and body, TRF improved the robustness and synchrony of rhythmic transcripts (Gill et al. 2015). Several gene expression studies have shown that hundreds of transcripts display daily rhythm in head and body of ALF flies, with their peak expressions generally distributed throughout day and night. The ratio between peak and trough levels of these transcripts is a measure of robustness or amplitude of oscillation (Gill et al. 2015). TRF improved the amplitude of oscillation of many cycling transcripts in both head and body. The timing of peak expression of these genes (phase) also coalesced at dawn and dusk time. Such changes reflect TRF and might consolidate rhythmic transcripts encoding anabolic and catabolic processes to two distinct parts of the 24 h days. The increased amplitude implies both induction and repression of genes that are tied to nutrient or xenobiotic metabolism are more efficiently controlled under TRF (Gill et al. 2015). Since the circadian clock is necessary for robust and synchronous oscillations in a large number of transcripts (Gill et al. 2015; Longo & Panda, 2016; Mattson et al. 2017), circadian rhythm mutants may fail to drive genome wide expression rhythms and may not respond to TRF (Fig. 1 E). Accordingly, fly strains carrying hypomorphic or loss of function alleles of clk, cyc, per and tim failed to show TRF‐induced benefits on cardiac function (Gill et al. 2015). Many of these mutants also showed compromised cardiac function at birth, which parallels the cardiac defects found in clock mutant mice (Paschos & FitzGerald, 2010).
In addition to rhythmic transcription, changes in tonic expression of genes may also contribute to TRF benefits. Analyses of longitudinal gene expression from the hearts of TRF and ALF Drosophila revealed > 400 transcripts showing either up‐ or down‐regulation throughout the 24 h day. Functional annotation of these transcripts revealed two functional clusters: 19 different genes encoding the mitochondrial electron transport complex were down‐regulated by 10–20% in TRF heart, while 7 out of 8 components of an ATP‐dependent chaperonin were up‐regulated (Fig. 1 C and D). The eukaryotic cytoplasmic chaperonin T‐complex protein (TCP)/TCP‐1 ring complex (TRiC)/chaperonin containing TCP‐1 (CCT) is a barrel‐like structure composed of eight related subunit double repeats (Fig. 1 D). They play a crucial role in proper folding of several proteins relevant for cardiac health including cytoskeletal components (Sternlicht et al. 1993; Kubota et al. 1995; Srikakulam & Winkelmann, 1999; Lundin et al. 2010). Recently, mutation of chaperone CCT7 (Ser525Leu) was associated with enhanced susceptibility for myocardial infarction in humans (Erdmann et al. 2013), possibly due to compromised folding and organization of cytoskeleton proteins. The increase in TCP component mRNA in TRF flies was not accompanied by a significant increase in mRNAs encoding cytoskeletal proteins. Rather, the TRF heart showed a 20–40% reduction in contractile protein mRNAs (Gill et al. 2015). The parallel increase in CCT chaperonins and reduction in cytoskeletal components are likely to indicate optimum cytoskeletal function in cardiac tissues of TRF flies. In Drosophila, P element insertion lines that potentially act as hypomorphic alleles of five different CCT components have been tested in a TRF paradigm (Fig. 1 D). These mutations do not render any TRF benefits, thus suggesting up‐regulation of CCT components is beneficial. Misregulation of cytoskeletal proteins has been associated with cardiac hypertrophy and it will be interesting to test if induction of TCP chaperone during TRF can attenuate several cardiac defects associated with mutation of contractile proteins.
Age‐ and diet‐induced vulnerabilities to proteostasis and mitochondrial function, dampening of rhythms under ALF, as well as the gradual accumulation of lipotoxicity and reactive oxygen species (ROS) may eventually compromise the structural integrity of cardiac muscles (Gill et al. 2015). The high energetic requirements of cardiac muscle, its susceptibility to the ROS byproducts of the electron transport chain (ETC) and its proteostatic requirements for cardiac contractility are likely to render the cardiac muscles vulnerable to changes in cellular homeostasis. The mitochondrial ETC complexes constitute the principal site for production of ATP, heat and reactive oxygen species. A fine balance between ATP and ROS production presumably improves cellular and organismal health (Camara et al. 2010; Feng et al. 2011; Farhadian et al. 2012). TRF modestly reduces expression of 19 ETC components in the heart throughout 24 h and of > 30 components for > 18 h every day (Fig. 1 C). These genes encode components of all five complexes of the ETC, thus suggesting an overall reduction in ETC function may ensue. Reduced ETC function is associated with a health benefit (Camara et al. 2010; Durieux et al. 2011). While higher levels of ETC inhibitors are toxic, low to moderate levels are well tolerated by animals and can have health benefits (Camara et al. 2010). Down‐regulation of three different ETC components (Fig. 1 C) in Drosophila heart sustains cardiac health, even in ALF flies, thus suggesting that reduced ETC gene expression contributes to TRF benefits.
In summary, cardiac benefits linked with the TRF paradigm are mediated by the circadian clock, the TRiC chaperonin and mitochondrial ETC components (Gill et al. 2015). Whether these three functional clusters are part of the same pathway or they act in parallel remains to be investigated (Fig. 1 C, D and E).
Feeding–fasting rhythms, TRF and attenuation of cardiometabolic diseases
In addition to genes/pathways associated with TRF, other physiological processes such as fasting are known to influence genes associated with nutrient sensing that eventually improve physiology (Longo & Panda, 2016; Mattson et al. 2017). Although direct comparison of the TRF gene expression profile with the CR gene expression signature did not indicate CR playing a dominant role, we cannot rule out the benefits of overnight fasting. Daily feeding–fasting rhythms drive signalling pathways that interact with the circadian oscillator to increase the robustness or peak‐to‐trough differences of these transcriptional oscillations (Vollmers et al. 2009; Adamovich et al. 2014). Conversely, continuous food deprivation for 24 h dampens the circadian clock and drastically reduces the number of rhythmic transcripts in the liver (Vollmers et al. 2009). The combination of both a feeding–fasting cycle and a functional circadian clock acts synergistically to support robust rhythm in the expression and function of a large number of genes. These rhythmic outputs subsequently mediate anabolic and catabolic processes that are appropriate for specific phases of the feeding–fasting cycle (Longo & Panda, 2016). This general model of synergistic action between feeding–fasting and the circadian oscillator offers a framework to further examine some of the leading risks for cardiac diseases, including age, energy‐dense diet and non‐genetic circadian disruption.
Human relevance, limitations and future direction
The pleiotropic beneficial effects of TRF in both mammals and insects in mitigating multiple metabolic and cardiac risk factors without altering quality or quantity of nutrition has opened a potential lifestyle modification strategy to combat cardiometabolic diseases. Epidemiological study of 26,902 men (aged 45–82 years) has also shown that an aberrant eating pattern is associated with higher risk of CVD irrespective of dietary composition (Cahill et al. 2013). Results from 16 years of follow‐up reveals that after controlling for diet and lifestyle, late night caloric intake increases heart disease risk in men by 55% (Cahill et al. 2013). This population‐based study also revealed that skipping breakfast increases CVD risk by 27%. Overall, after controlling for genetic factors and dietary composition, an aberrant eating pattern is associated with CVD risks (Cahill et al. 2013). Various population‐based or clinical studies have shown that sleep deprivation or poor sleep quality is associated with increased sympathetic nervous system activity and this increased activity is linked with elevation of hypertension and increased risk for CVD (Nagai et al. 2010; Grandner et al. 2016; St‐Onge et al. 2016). In addition to epidemiological correlative studies, a controlled clinical study has also revealed that chronic circadian misalignment of rest activity and associated daily eating pattern even for a few days can increase cardiovascular disease risk in healthy adults (Morris et al. 2016). These cardiac factors include elevation of systolic and diastolic blood pressure and increased 24 h serum levels of interleukin‐6, C‐reactive protein, resistin and tumour necrosis factor‐α (Morris et al. 2016).
While circadian disruption can occur through misalignment of sleep–wake and erratic eating pattern, assessing the daily eating pattern in humans and whether it can be modified to improve health is an emerging research topic. Such studies will also help to assess how much of the TRF benefits found in rodents and insects can be translated to humans. A recent study using a smartphone app to monitor eating time has revealed more than 50% of adults spread their daily caloric intake over 15 h or longer (Gill & Panda, 2015). Such extended eating in rodents along with obesogenic or high glycaemic diet predispose animals to metabolic diseases. Conversely, in a feasibility study in healthy adults, reducing the eating duration to 10–11 h without overt attempt to reduce calories or change nutrition quality showed weight loss, improved sleep and increased sense of energy (Gill & Panda, 2015). This preliminary observation prompts for more experimental evidence to established relationship among eating pattern, sleep and cardiometabolic health at the molecular level. Even though Drosophila and human hearts have some divergent functions, conserved pathways appear to govern form and function. Therefore, studies in model organisms including flies that allow specific perturbation of lifestyle and genetic makeup are important for mechanistic studies.
Modern humans, due to societal pressures, work schedules and night‐time indoor illumination, stay awake longer, which enables food consumption for longer durations of time. This extended duration itself, in addition to the caloric surplus, can be detrimental to health. Future studies will be useful in testing the effectiveness of TRF in preventing/delaying cardiac dysfunction associated with obesity and metabolic disease. Human nutrition research has primarily focused on two variables: energy intake (food, food type) and expenditure (exercise, thermogenesis, etc.). The daily feeding–fasting rhythm paradigm has translational potential for management of defective metabolism‐induced cardiovascular disease in humans by controlling the timing of dietary intake. These are widely applicable to human health and could be implemented as a community‐based approach to improve human cardiac disease linked with diabetes and obesity.
Additional information
Competing interests
None.
Author contributions
Both authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by National Institutes of Health (NIH) grants RR032100, AG049494 and American Heart Association grant 17260057 to G.C.M. as well as NIH grants DK091618, EY016807, NS066457 and AFAR grant M14322 to S.P.
Acknowledgements
We appreciated comments on the review provided by Dr. Sanford Bernstein (San Diego State University).
Biographies
Girish Melkani is an Assistant Professor (Research) at San Diego State University. His lab research focuses on protein unfolding, and myofibrillar and cardiac biology, addressing key signalling pathways linked with metabolic dysregulation, protein misfolding and myofibrillar based cardiometabolic diseases

Satchidananda Panda is a professor at the Salk Institute for Biological Studies. His lab focuses on transcriptional regulation of circadian rhythms in behaviour, physiology and metabolism. His lab has established time‐restricted feeding (TRF) protocols in mice and Drosophila. Using high resolution genome‐wide temporal gene expression maps in several mouse and Drosophila tissues under various metabolic conditions, his lab is investigating the molecular underpinning of TRF benefits. Using complimentary expertise, the two authors are exploring the impact of daily rhythms on cardiac muscle physiology, circadian rhythm, nutrition, sleep and other metabolic disorders.
References
- Adamovich Y, Rousso‐Noori L, Zwighaft Z, Neufeld‐Cohen A, Golik M, Kraut‐Cohen J, Wan M, Han X & Asher G (2014). Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab 19, 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadbakht L, Kelishadi R, Khodarahmi M, Qorbani M, Heshmat R, Motlagh ME, Taslimi M & Ardalan G (2013). The association of sleep duration and cardiometabolic risk factors in a national sample of children and adolescents: the CASPIAN III study. Nutrition 29, 1133–1141. [DOI] [PubMed] [Google Scholar]
- Bass J & Lazar MA (2016). Circadian time signatures of fitness and disease. Science 354, 994–999. [DOI] [PubMed] [Google Scholar]
- Birse RT, Choi J, Reardon K, Rodriguez J, Graham S, Diop S, Ocorr K, Bodmer R & Oldham S (2010). High‐fat‐diet‐induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila . Cell Metab 12, 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, Jeong WJ, Tsai JY, Bugger H, Zhang D, Rohrwasser A, Rennison JH, Dyck JR, Litwin SE, Hardin PE, Chow CW, Chandler MP, Abel ED & Young ME (2008). Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol 294, H1036–H1047. [DOI] [PubMed] [Google Scholar]
- Cahill LE, Chiuve SE, Mekary RA, Jensen MK, Flint AJ, Hu FB & Rimm EB (2013). Prospective study of breakfast eating and incident coronary heart disease in a cohort of male US health professionals. Circulation 128, 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara AK, Lesnefsky EJ & Stowe DF (2010). Potential therapeutic benefits of strategies directed to mitochondria. Antioxid Redox Signal 13, 279–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P & Panda S (2014). Time‐restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20, 991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A & Panda S (2016). The circadian coordination of cell biology. J Cell Biol 215, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MJ, Smith JT, Narbaiza J, Mueez F, Bustle LB, Qureshi S, Fieseler C & Legan SJ (2016). Restricting feeding to the active phase in middle‐aged mice attenuates adverse metabolic effects of a high‐fat diet. Physiol Behav 167, 1–9. [DOI] [PubMed] [Google Scholar]
- Durgan DJ & Young ME (2010). The cardiomyocyte circadian clock: emerging roles in health and disease. Circ Res 106, 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux J, Wolff S & Dillin A (2011). The cell‐non‐autonomous nature of electron transport chain‐mediated longevity. Cell 144, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P, Takahashi JS & Rosbash M (2001). Stopping time: the genetics of fly and mouse circadian clocks. Annu Rev Neurosci 24, 1091–1119. [DOI] [PubMed] [Google Scholar]
- Erdmann J, Stark K, Esslinger UB, Rumpf PM, Koesling D, de Wit C, Kaiser FJ, Braunholz D, Medack A, Fischer M, Zimmermann ME, Tennstedt S, Graf E, Eck S, Aherrahrou Z, Nahrstaedt J, Willenborg C, Bruse P, Braenne I, Nothen MM, Hofmann P, Braund PS, Mergia E, Reinhard W, Burgdorf C, Schreiber S, Balmforth AJ, Hall AS, Bertram L, Steinhagen‐Thiessen E, Li SC, Marz W, Reilly M, Kathiresan S, McPherson R, Walter U, CardioGram, Ott J, Samani NJ, Strom TM, Meitinger T, Hengstenberg C & Schunkert H (2013). Dysfunctional nitric oxide signalling increases risk of myocardial infarction. Nature 504, 432–436. [DOI] [PubMed] [Google Scholar]
- Farhadian SF, Suarez‐Farinas M, Cho CE, Pellegrino M & Vosshall LB (2012). Post‐fasting olfactory, transcriptional, and feeding responses in Drosophila . Physiol Behav 105, 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T, Liu XS & Lazar MA (2011). A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science 331, 1315–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Le HD, Melkani GC & Panda S (2015). Time‐restricted feeding attenuates age‐related cardiac decline in Drosophila . Science 347, 1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S & Panda S (2015). A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab 22, 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Seixas A, Shetty S & Shenoy S (2016). Sleep duration and diabetes risk: population trends and potential mechanisms. Curr Diab Rep 16, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B, Chelot E, Xia R & Rouyer F (2004). Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature 431, 869–873. [DOI] [PubMed] [Google Scholar]
- Hardin PE & Panda S (2013). Circadian timekeeping and output mechanisms in animals. Curr Opin Neurobiol 23, 724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfmann BD, Schroder EA & Esser KA (2015). Circadian rhythms, the molecular clock, and skeletal muscle. J Biol Rhythms 30, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH & Panda S (2012). Time‐restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high‐fat diet. Cell Metab 15, 848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich‐Forster C (2000). Differential control of morning and evening components in the activity rhythm of Drosophila melanogaster–sex‐specific differences suggest a different quality of activity. J Biol Rhythms 15, 135–154. [DOI] [PubMed] [Google Scholar]
- Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, Lu Y, Eapen BL, Sharma N, Ficker E, Cutler MJ, Gulick J, Sanbe A, Robbins J, Demolombe S, Kondratov RV, Shea SA, Albrecht U, Wehrens XH, Rosenbaum DS & Jain MK (2012). Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 483, 96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A, Leloup JC & Rouyer F (2003). Circadian rhythms of locomotor activity in Drosophila . Behav Processes 64, 161–175. [DOI] [PubMed] [Google Scholar]
- Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK & Takahashi JS (2012). Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 6105, 349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Hynes G & Willison K (1995). The chaperonin containing t‐complex polypeptide 1 (TCP‐1). Multisubunit machinery assisting in protein folding and assembly in the eukaryotic cytosol. Eur J Biochem 230, 3–16. [DOI] [PubMed] [Google Scholar]
- Longo VD & Panda S (2016). Fasting, circadian rhythms, and time‐restricted feeding in healthy lifespan. Cell Metab 23, 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin VF, Leroux MR & Stirling PC (2010). Quality control of cytoskeletal proteins and human disease. Trends Biochem Sci 35, 288–297. [DOI] [PubMed] [Google Scholar]
- Manoogian EN & Panda S (2016). Circadian clock, nutrient quality, and eating pattern tune diurnal rhythms in the mitochondrial proteome. Proc Natl Acad Sci USA 113, 3127–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Longo VD & Harvie M (2017). Impact of intermittent fasting on health and disease processes. Ageing Res Rev (In press; doi: 10.1016/j.arr.2016.10.005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury E, Ramsey KM & Bass J (2010). Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res 106, 447–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkani GC, Trujillo AS, Ramos R, Bodmer R, Bernstein SI & Ocorr K (2013). Huntington's disease induced cardiac amyloidosis is reversed by modulating protein folding and oxidative stress pathways in the Drosophila heart. PLoS Genet 9, e1004024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza‐Viveros L, Bouchard‐Cannon P, Hegazi S, Cheng AH, Pastore S & Cheng HM (2017). Molecular modulators of the circadian clock: lessons from flies and mice. Cell Mol Life Sci 74, 1035–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Purvis TE, Hu K & Scheer FA (2016). Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci USA 113, E1402–E1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J, Musselman LP, Pendse J, Baranski TJ, Bodmer R, Ocorr K & Cagan R (2013). A Drosophila model of high sugar diet‐induced cardiomyopathy. PLoS Genet 9, e1003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Hoshide S & Kario K (2010). Sleep duration as a risk factor for cardiovascular disease – a review of the recent literature. Curr Cardiol Rev 6, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr K, Reeves NL, Wessells RJ, Fink M, Chen HS, Akasaka T, Yasuda S, Metzger JM, Giles W, Posakony JW & Bodmer R (2007). KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of aging. Proc Natl Acad Sci USA 104, 3943–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S (2016). Circadian physiology of metabolism. Science 354, 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Hogenesch JB & Kay SA (2002). Circadian rhythms from flies to human. Nature 417, 329–335. [DOI] [PubMed] [Google Scholar]
- Paschos GK & FitzGerald GA (2010). Circadian clocks and vascular function. Circ Res 106, 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot M, Cusumano P, Klarsfeld A, Ueda R & Rouyer F (2007). Light activates output from evening neurons and inhibits output from morning neurons in the Drosophila circadian clock. PLoS Biol 5, e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Colon S, He F, Bixler EO, Fernandez‐Mendoza J, Vgontzas AN, Berg A, Kawasawa YI & Liao D (2014). The circadian pattern of cardiac autonomic modulation and obesity in adolescents. Clin Auton Res 24, 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Colon SM, Li X, Shaffer ML, He F, Bixler EO, Vgontzas AN, Cai J & Liao D (2010). Insulin resistance and circadian rhythm of cardiac autonomic modulation. Cardiovasc Diabetol 9, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudic RD, McNamara P, Reilly D, Grosser T, Curtis AM, Price TS, Panda S, Hogenesch JB & FitzGerald GA (2005). Bioinformatic analysis of circadian gene oscillation in mouse aorta. Circulation 112, 2716–2724. [DOI] [PubMed] [Google Scholar]
- Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z & Froy O (2012). Timed high‐fat diet resets circadian metabolism and prevents obesity. FASEB J 26, 3493–3502. [DOI] [PubMed] [Google Scholar]
- Srikakulam R & Winkelmann DA (1999). Myosin II folding is mediated by a molecular chaperonin. J Biol Chem 274, 27265–27273. [DOI] [PubMed] [Google Scholar]
- St‐Onge MP, Grandner MA, Brown D, Conroy MB, Jean‐Louis G, Coons M, Bhatt DL; American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; & Stroke Council (2016). Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: A Scientific Statement From the American Heart Association. Circulation 134, e367–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, McCrindle B, Mietus‐Snyder ML (2009). Progress and challenges in metabolic syndrome in children and adolescents: A Scientific Statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation 119, 628–647. [DOI] [PubMed] [Google Scholar]
- Sternlicht H, Farr GW, Sternlicht ML, Driscoll JK, Willison K & Yaffe MB (1993). The t‐complex polypeptide 1 complex is a chaperonin for tubulin and actin in vivo. Proc Natl Acad Sci USA 90, 9422–9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram S & Yan L (2016). Time‐restricted feeding reduces adiposity in mice fed a high‐fat diet. Nutr Res 36, 603–611. [DOI] [PubMed] [Google Scholar]
- Vanin S, Bhutani S, Montelli S, Menegazzi P, Green EW, Pegoraro M, Sandrelli F, Costa R & Kyriacou CP (2012). Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature 484, 371–375. [DOI] [PubMed] [Google Scholar]
- Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD & Panda S (2009). Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci USA 106, 21453–21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf MJ & Rockman HA (2011). Drosophila, genetic screens, and cardiac function. Circ Res 109, 794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto Y, Hashimoto C, Nakao R, Yamazaki H, Hiroyama H, Nemoto T, Yamamoto S, Sakurai M, Oike H, Wada N, Yoshida‐Noro C & Oishi K (2016). Short‐term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metabolism 65, 714–727. [DOI] [PubMed] [Google Scholar]
- Zarrinpar A, Chaix A & Panda S (2016). Daily eating patterns and their impact on health and disease. Trends Endocrinol Metab 27, 69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


