Abstract
Classic concepts about the role of epicardial adipose tissue (EpAT) in heart physiology include its role in cardiac metabolism, mechanical protection of coronaries, innervation and possibly cryoprotection of the heart too. Nevertheless, recent evidence has revealed that epicardial adipose tissue regulates multiple aspects of cardiac biology including myocardial redox state, intracellular Ca2+ cycling, the electrophysiological and contractile properties of cardiomyocytes, cardiac fibrosis as well as coronary atherosclerosis progression. Moreover, it is now understood that the communication between EpAT and the heart is regulated by complex bidirectional pathways, since not only do adipokines regulate cardiac function, but also the heart affects EpAT biology via paracrine ‘reverse’ signalling. Such complex interactions as well as epicardial fat accumulation as a consequence of cardiac disease and epicardium to adipocyte differentiation should be taken into account by the clinical studies investigating EpAT as a risk marker and its potential as a therapeutic target against cardiovascular disease. Further in‐depth exploration of the molecular mechanisms regulating the cross‐talk between the heart and EpAT is expected to enhance our understanding regarding the role of the latter in cardiac physiology and relevant disease mechanisms.
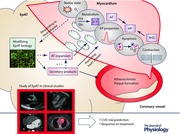
Keywords: adipokines, cardiac biology, epicardial adipose tissue, myocardium, redox state
Abbreviations
- AF
atrial fibrillation
- AT
adipose tissue
- CT
computed tomography
- IHD
ischaemic heart disease
- CVD
cardiovascular disease
- EpAT
epicardial adipose tissue
- MRI
magnetic resonance imaging
- NPR
natriuretic peptide receptor
- PET
positron emission tomography
- PPARG
peroxisome proliferator activator γ
Introduction
Visceral adipose tissue (AT) has a well‐established role in cardiovascular disease (CVD) pathogenesis by determining systemic insulin resistance and releasing active adipokines into the systemic circulation (Antonopoulos et al. 2014). However, further to visceral adiposity, research has recently been focused on the role of ectopic fat depots in CVD pathogenesis. In this aspect, epicardial AT (EpAT) is a fat depot with a potentially important role in cardiac biology, given its close anatomical affinity with the heart. Over the last decade imaging studies have treated EpAT as a quantifiable CVD risk marker (Wang et al. 2009a; Jacobson et al. 2011; Nakanishi et al. 2012; Dabbah et al. 2014; Mahabadi et al. 2014; Stojanovska et al. 2015; Mazurek et al. 2016), while strong translational evidence (Greulich et al. 2011, 2012; Blumensatt et al. 2013; Burgeiro et al. 2016) supports the existence of a cross‐talk between EpAT and the myocardium that is involved in cardiac disease pathogenesis.
Epicardial adipose tissue and cardiac physiology: established knowledge
EpAT is located between the visceral pericardium and the heart, in direct contact with the myocardium. Other terms such as pericardial fat or paracardial fat have been wrongly used in the past to refer to EpAT, but they have a distinct meaning and should not be used interchangeably (Fig. 1). Species‐specific differences in EpAT are marked (Marchington & Pond, 1990; Chiou et al. 1997); whilst in rodents EpAT is almost absent, in humans it can cover up to 80% of the surface of the heart, found even in sub‐epicardium, infiltrating human myocardium (Cherian et al. 2012). Interestingly, EpAT has a common embryonic origin with the heart from the splachnopleuric mesoderm, and is supplied with blood by the coronary circulation, facts which suggest that EpAT may be important for cardiac physiology (Cherian et al. 2012).
Figure 1. Definitions of epicardial, pericardial and paracardial adipose tissue.

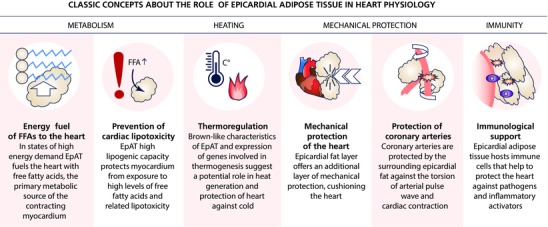
EpAT consists of white adipocytes, preadipocytes, stroma‐vascular cells as well as ganglionic, nerve and immune cells. The exact role of EpAT in cardiac physiology of mammals remains unclear, but evidence suggests that it is multifaceted: its lipogenic capacity suggests that it serves as a local energy store for the heart and protects cardiomyocytes against influx of high free fatty acid levels and lipotoxicity (Marchington & Pond, 1990); its thermogenic capacity implies that it can protect heart against hypothermia (Sacks et al. 2009); it serves as the anatomical site for the ganglia innervating myocardium (Chiou et al. 1997); and it mechanically protects the heart and coronary arteries. These classic concepts about the role of EpAT in cardiac physiology are summarized in Fig. 2.
Figure 2. The classic concepts about the role of epicardial adipose tissue in heart physiology.
Interest in the study of epicardial adiposity by non‐invasive imaging in clinical studies is steadily increasing; echocardiography, computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography/CT (PET/CT) have all been employed for the imaging of EpAT (Antonopoulos et al. 2016b). In agreement with basic science findings, clinical imaging studies have strongly associated EpAT with cardiac disease. Recent evidence from clinical studies using a cross‐modality approach (CT/MRI/proton and phosphorus MR spectroscopy) suggest that obese, diabetic patients have increased ectopic (including epicardial) adiposity and this is associated with impaired myocardial energetics and myocardial mechanics (Levelt et al. 2016). Inflammatory activity of pericoronary EpAT as assessed by PET/CT imaging, despite certain technical limitations, is associated with coronary atherosclerosis (Mazurek et al. 2016). Quantification of EpAT volume by CT imaging has been independently associated with obesity and metabolic syndrome (Wang et al. 2009a), but also with coronary plaque burden, plaque composition and vulnerability (Hassan et al. 2016), the development of coronary atherosclerosis in healthy subjects and the risk of future coronary events in ischaemic heart disease (IHD) patients (Kunita et al. 2014). EpAT volume is also an independent risk factor for atrial fibrillation (AF) development. This is expected given the strong association of obesity with AF risk, but EpAT volume provides prognostic information for AF development independently of body mass index and classic cardiovascular risk factors (Zhu et al. 2015). Periatrial or total EpAT volume also predicts AF recurrence in patients undergoing AF ablation therapy or AF development post‐coronary artery bypass grafting (Kocyigit et al. 2015).
A summary of the studies exploring the value of EpAT as a biomarker for CVD risk is provided in Table 1. These findings could also help explain the ‘obesity paradox’, i.e. the association of obesity with better clinical outcome in patients with established CVD (Antonopoulos et al. 2016b), which has been based solely on the use of body mass index to define obesity. Clinical studies using CT‐based volumetric measurements of fat have shown that increased epicardial fat volume is a strong predictor of the risk of IHD and AF, over and above systemic adiposity indices, contradicting thus the notion of an ‘obesity paradox’. It should be noted though that any findings related to the volumetric analysis of EpAT in humans may be biased, since in clinical studies EpAT volume measurements are rarely adjusted for body or heart size, and pathology studies of autoptic human hearts suggest a constant fat/muscle mass ratio irrespectively of the presence of cardiac disease (Antonopoulos et al. 2016b); for example in a large cohort the association between EpAT volume and AF was lost when adjusted for LA size (Mahabadi et al. 2014). Even so, imaging of EpAT in large clinical cohorts suggests that epicardial adiposity (total/periatrial EpAT, pericoronary EpAT or intramyocardial fat content) may be a more specific and sensitive marker to assess the obesity burden to the heart and its impact on cardiac disease development (Antonopoulos et al. 2016b).
Table 1.
Important clinical studies on epicardial/pericardial adipose tissue volume as a risk marker for cardiovascular disease
| Study | Study population | FU period | Endpoint | Conclusion |
|---|---|---|---|---|
| Coronary artery disease | ||||
| Mahabadi et al. (2013) | 4093 healthy subjects | 8 years | Coronary events | With each doubling of EpAT increased risk for coronary events HR = 1.54 (95% CI: 1.09–2.19) |
| Cheng et al. (2010) | 2571 patients with no CAD | 4 years | MACEs | Doubling of PAT volume associated with increased MACE risk OR = 1.74 (95% CI: 1.03–2.95) |
| Forouzandeh et al. (2013) | 760 patients with acute chest pain | 3.3 years* | MACEs | EpAT volume independently associated with future MACEs |
| Kunita et al. (2014) | 722 CAD patients | 3.7 years | Coronary events | Increased EpAT volume is a risk factor for coronary events |
| Nakanishi et al. (2014) | 517 non‐obese CAD patients | > 1 year | ACS | EpAT volume is a strong predictor of future acute coronary syndromes |
| Ding et al. (2009) | 998 cases‐controls | CAD | For every SD increase in PAT volume, increased HR = 1.33 (95% CI: 1.15–1.54) for CAD | |
| Tamarappoo et al. (2010) | 1,777 CAD patients | 6 months | SPECT–ischaemia | PAT volume is an independent predictor of ischemia at 6 months |
| Atrial fibrillation | ||||
| Mahabadi et al. (2014) | 3467 healthy subjects | 5 years | AF | Left atrial size –but not EpAT volume – is independently associated with AF |
| Zhu et al. (2015) | Meta‐analysis of 10 case–control studies | AF | EpAT volume may be associated with an increased risk of AF. | |
| Nakanishi et al. (2012) | 279 subjects undergoing MDCT | 3.3 years | New AF | Periatrial EpAT volume predicts the development of new‐onset AF in subjects undergoing MDCT |
| Yorgun et al. (2015) | 618 patients (in AF and SR) | n/a | AF | Periatrial and epicardial adipose tissue thickness is independently associated with AF |
| Kocyigit et al. (2015) | 249 AF patients post‐ablation | 29 months | Late AF recurrence | EpAT thickness was an independent predictor for late AF recurrence. |
*Median value. ACS, acute coronary syndrome; CAD, coronary artery disease; CI, confidence interval; EAT, epicardial adipose tissue; HR, hazard ratio; MACE, major adverse cardiac event; MDCT, multiple‐row detector computed tomography; MESA, Multi‐Ethnic Study of Atherosclerosis; n/a, not applicable; OR, odds ratio; PAT, pericardial adipose tissue; SPECT, single photon‐emission computed tomography.
Recent translational evidence on the role of epicardial adipose tissue in cardiac physiology
The growing interest in the links between cardiac physiology and EpAT has led to the detailed investigation of their communication. EpAT is considered to be a type of visceral AT; the latter is an active endocrine organ directly involved in cardiovascular physiology by secretion of active adipokines into the circulation (Antonopoulos et al. 2014), but whether EpAT can participate in such a role has been debated, given its negligible mass compared to other fat depots. EpAT contains smaller adipocytes and lower insulin‐induced glucose uptake compared to subcutaneous AT and expresses low levels of fat‐mobilizing genes, having therefore lower lipid storage and lipolytic capacity compared to other fat depots (Burgeiro et al. 2016). The metabolic activity of EpAT is further altered in the presence of heart failure, and even though the physiological significance of the decreased lipid storage and lipolytic capacity of EpAT is unknown, it has been suggested that it could represent a protective mechanism against cardiac lipotoxicity (Burgeiro et al. 2016). Evidence also suggests that EpAT expresses brown AT‐signature genes, e.g. uncoupling protein 1 (Sacks et al. 2009), and its transcriptome significantly differs from that of subcutaneous or visceral fat, being even site‐specific for the EpAT around the coronaries, atria or ventricles (Gaborit et al. 2015).
Studies have now confirmed the important role of EpAT as a modulator of cardiac disease‐related mechanisms (Greulich et al. 2011, 2012; Antonopoulos et al. 2016a). Secretory products of EpAT affect cardiomyocytes function either via paracrine mechanisms (i.e. passive diffusion through interstitial space and cellular membranes) or in a vasocrine manner (via coronary vasa vasorum) or secretion into the coronary circulation (Cherian et al. 2012). Adipokines secreted by EpAT can exert protective effects on the myocardium; e.g. EpAT‐released orosomucoid inhibits caspase‐3‐mediated apoptosis of cardiomyocytes (Lage et al. 2015) and adiponectin binding to its receptors (AdipoR1/2 and T‐cadherin) exerts beneficial metabolic anti‐oxidant effects on cardiomyocytes (Wang et al. 2009b). Adiponectin reduces O2 − generation from myocardial NADPH‐oxidase (Antonopoulos et al. 2016a) and improves nitric oxide bioavailability and endothelial function in the coronary vasculature (Margaritis et al. 2013; Antonopoulos et al. 2015). Our recent studies have highlighted the importance of Akt and AMP‐kinase intracellular pathways in mediating the effects of adiponectin on the vasculature and human myocardium, respectively (Margaritis et al. 2013; Antonopoulos et al. 2014, 2015, 2016a).
Next to their beneficial effects, EpAT‐released adipokines can also activate monocytes, directly favouring atherogenesis, via their effects on coronary endothelial and vascular smooth muscle cells (Karastergiou et al. 2010). It has been suggested that pericoronary EpAT could even serve as a local storage and supply site for human oxidized LDL to coronary intima, i.e. the hallmark of coronary plaque formation (Uchida et al. 2016). Nevertheless a direct link between pericoronary adipose tissue and atherosclerosis cannot be easily established. For example myocardial bridges, i.e. coronary artery segments not covered by EpAT, are protected against atherosclerosis development (Ishikawa et al. 1997), but total absence of EpAT such as in congenital lipodystrophy does not prevent coronary atherosclerosis development (Chandalia et al. 1995). Other EpAT‐secreted products (such as retinol binding protein‐4 or activin A) can negatively affect cardiac metabolism (Blumensatt et al. 2013); for example activin A secreted from human EpAT induces the expression of miR‐143 in human cardiomyocytes and negatively affects Akt signalling and insulin‐mediated glucose uptake, possibly by regulation of the availability of oxysterol‐binding protein‐related protein 8 in cardiomyocytes (Blumensatt et al. 2013). Interestingly diabetes mellitus is associated with increased infiltration of EpAT by CD14+ monocytes (Greulich et al. 2012), suggesting increased tissue inflammation. This could explain the respective adverse changes in EpAT secretome in animals challenged with high‐fat diet (Greulich et al. 2011) or diabetes development (Greulich et al. 2012; Blumensatt et al. 2013) (e.g. increased activin A, reduced omentin 1 release), which lead to changes in phosphorylation of Akt (at Ser143) or SMAD2 in cardiomyocytes (Greulich et al. 2012; Blumensatt et al. 2013). Such effects of adipokines on cardiomyocytes’ intracellular signalling negatively affect the activity of sarco/endoplasmic reticulum Ca2+‐ATPase and Ca2+ cycling, and promote the contractile dysfunction of cardiomyocytes in diabetic patients (Greulich et al. 2011, 2012). Obviously the net effect of EpAT‐derived mediators on myocardial signalling depends on the biology of the EpAT, as well as the degree of infiltration by immune cells further to the biology of the adipocytes.
Next to the effects on cardiomyocyte metabolism and contractility, EpAT also affects cardiac electrophysiology (Burke et al. 1998; Lin et al. 2012; Verheule et al. 2013; Venteclef et al. 2015). EpAT‐derived adipokines affect myocardial NADPH oxidase activity, which, as we have previously demonstrated, is a critical determinant for the development of AF in experimental models (Reilly et al. 2011) or post‐operatively in patients undergoing cardiac surgery (Reilly et al. 2011; Antoniades et al. 2012). Fibro‐fatty infiltrates of EpAT into subepicardium can disrupt the electro‐mechanical properties of the myocardium, triggering arrhythmias (Burke et al. 1998; Verheule et al. 2013). In experimental obesity models, biatrial electrophysiological, electroanatomical and structural remodelling is caused as a consequence of EpAT expansion into atrial tissue and related profibrotic transforming growth factor (TGF) β signalling (Mahajan et al. 2015). AF per se induces upregulation of adipocyte‐specific genes in atrial tissue (Chilukoti et al. 2015), favouring intra‐atrial fat accumulation, and perpetuating the vicious cycle of fibro‐fatty infiltration in atrial myocardium and AF development. In addition to the direct infiltration of myocardium by fatty tissue, EpAT products can alter the electrophysiological properties of atrial myocytes. Medium from cultured AT negatively affects the action potential duration, L‐type Na+ currents and isoproterenol‐triggered beats favouring arrhythmiogenesis (Lin et al. 2012). Evidence suggests that the EpAT secretome is rich in adipokines with pro‐fibrotic effects, such as thrombospondin 2, vascular endothelial growth factor, activin A, TGF‐β1 and matrix metalloproteinase isoforms, in significantly higher levels compared to other fat depots (Venteclef et al. 2015). The Hatem group (Venteclef et al. 2015) has eloquently shown that EpAT‐secreted adipokines induce extensive fibrosis of rat atria in organo‐culture models and use of an activin A neutralizing antibody reversed these effects, suggesting that activin A has a pivotal role among secreted adipokines with pro‐fibrotic effects and may be even therapeutically targeted (Venteclef et al. 2015).
Overall, accumulating experimental evidence suggests a role of EpAT‐secreted products in aspects of cardiac biology and the regulation of mechanisms of coronary atherosclerosis (Karastergiou et al. 2010; Uchida et al. 2016), ischaemic heart failure (Greulich et al. 2011, 2012; Antonopoulos et al. 2016a) and AF (Burke et al. 1998; Lin et al. 2012; Verheule et al. 2013; Venteclef et al. 2015) (summarized in Fig. 3).
Figure 3. Adipokines and atrial fibrillation development.
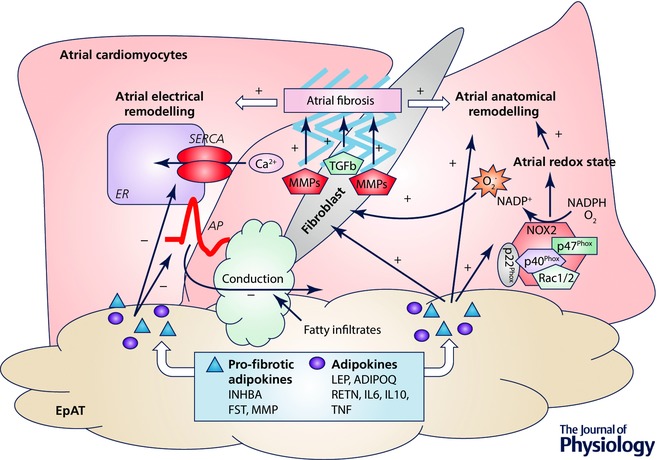
Adipokines have an impact on atrial electrophysiological properties, action potential (AP) duration and sarco/endoplasmic reticulum Ca2+‐ATPase (SERCA) activity of atrial cardiomyocytes, affecting thus arrhythmiogeneicity. Fibrofatty infiltrates into subepicardium also affect per se the electrical conduction properties of atrium. Adipokines can modulate NADPH oxidase activity (mainly NOX2) and myocardial redox state in human atria, which is causally involved in atrial fibrillation development. Through the direct effects of adipokines on extracellular matrix (e.g. matrix metalloproteinases) or via their indirect effects on activation of fibroblasts and modulation of myocardial redox state, and promote of atrial fibrosis. The latter is centrally involved in atrial anatomical and electrical remodelling, which disrupts the electrical conduction properties of atrial tissue and favours atrial fibrillation development. ADIPOQ, adiponectin; FST, follistatin; IL, interleukin; INHBA, activin A; LEP, leptin; MMP, matrix metallopeptidases; RETN, resistin; TGFb, transforming growth factor β; TNF, tumour necrosis factor α; list of adipokines is indicative; +, stimulate/induce; –, decrease/impair.
Evidence for cross‐talk between the heart and epicardial adipose tissue
While the causal role of EpAT biology in obesity‐related cardiac disease has been widely explored leading to the notion of ‘outside‐to‐inside’ signalling, the possibility of a reverse signalling has only recently been investigated (termed ‘inside‐to‐outside’ signalling). Studies have supported that the EpAT gene expression profile is shifted towards a pro‐inflammatory phenotype in the presence of coronary atherosclerosis (Shimabukuro et al. 2013) and heart failure (Burgeiro et al. 2016).
It is now understood that a two‐way interaction between adipocytes and cardiomyocytes occurs, with the latter affecting adipocytes’ gene expression in a paracrine manner (Anan et al. 2011). Proteomic analyses of EpAT demonstrate increased expression of redox‐related proteins compared to subcutaneous fat, suggesting that EpAT has been adaptively evolved to cope with high – local – oxidative stress burden, possibly due to signals received from the adjacent myocardium (Salgado‐Somoza et al. 2010). Recent evidence from our group (Antonopoulos et al. 2016a) supports a cross‐talk between the myocardial redox state and peroxisome proliferator activator γ (PPARG)–adiponectin axis in EpAT. Under conditions of increased myocardial oxidative stress, stress signals (e.g. 4‐hydroxynonenal and possibly others too) released from cardiomyocytes affect PPARG/adiponectin expression in EpAT as a means to locally regulate and lower myocardial oxidative stress by inhibition of NADPH oxidase activity (Antonopoulos et al. 2016a). Besides, not just locally, but also at a systemic level, natriuretic peptide binding to their highly expressed receptors in adipose tissue elicits lipolytic effects. For example, natriuretic peptide receptor type 1 (NPR1) and type 2 (NPR2) signalling stimulates the guanylyl cyclase–cyclic GMP–protein kinase G (PKG) pathway in adipocytes, which increases the expression of hormone‐sensitive lipase (HSL) and lipolysis globally in adipocytes (Antonopoulos et al. 2014). To the contrary, signalling via NPR3, whose expression is up‐regulated in the presence of obesity or diabetes, leads to natriuretic peptide internalization and degradation in lysosomes counteracting the beneficial systemic metabolic effects of natriuretic peptides. The potency of the lipolytic effects of natriuretic peptides on human adipose tissue seems to be highest for type A and lowest for type C natriuretic peptide. Deficiency of natriuretic peptides is causally involved in systemic insulin resistance and diabetes development. Our recent studies on human adipose tissue (Antonopoulos et al. 2014) also suggest that B‐natriuretic peptide is a driver of adiponectin release globally in all human adipose tissue depots, over‐riding any local effects of endogenous adipose tissue inflammation in patients with IHD.
In the cross‐talk between the human heart and EpAT, of particular interest is the adipogenic capacity of epicardial cells. This transformation is apparent in murine models of myocardial injury, where mesothelial lineage cells differentiate to adipocytes following myocardial infarction (Zangi et al. 2017). Indeed epicardial and subepicardial layers host epicardial progenitor derived cells (EPDCs) which can be engaged in adipocyte transformation via pro‐adipogenic factors modulating the epithelial‐to‐mesenchymal transition process (Suffee et al. 2016). Preliminary evidence supports that human atrial myocytes can be the source of such pro‐adipogenic factors and regulate this process of EPDC differentiation to mature adipocytes and epicardial fat accumulation (Suffee et al. 2016). Such observations suggest that EpAT may be actually derived from the adipogenic transformation of epicardium (Yamaguchi et al. 2015). Indeed intramyocardial fat and fibrous tissue infiltrates that are found in AF or cardiomyopathies (such as in arrhythmogenic right ventricular cardiomyopathy) could be the result of this adipogenic process, and thus epicardial adiposity could be the consequence (rather than the cause) of advanced cardiac disease (Yamaguchi et al. 2015).
Therefore modulation of pathways pivotally involved in adipocyte differentiation, adipogenesis or lipolysis by paracrine ‘inside‐to‐outside’ signalling from cardiomyocytes suggests that EpAT expansion and remodelling may be at least partly regulated by cardiac disease‐related mechanisms. It is now understood that EpAT biology is regulated by both systemic factors (e.g. insulin resistance, obesity) and local stimuli from the heart (Antonopoulos et al. 2016a) or the coronaries (Margaritis et al. 2013; Antonopoulos et al. 2015), which can further strengthen or even outweigh any systemic effects. Moreover, the triggered ‘rescue responses’ of EpAT in the presence of cardiac disease, such as the upregulation of adiponectin expression, could be potential therapeutic targets against CVD (Woodward et al. 2016). The cross‐talk between the heart and EpAT is summarized in Fig. 4.
Figure 4. Communication between the cardiomyocytes and epicardial adipose tissue.
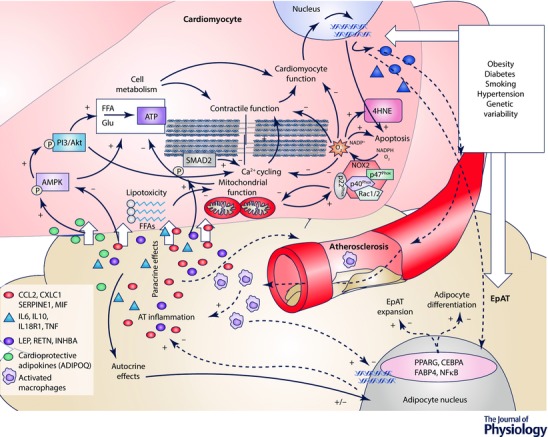
Epicardial adipose tissue (EpAT) and the cardiomyocyte transcriptomic profile are altered in the presence of cardiovascular risk factors or by genetic variability. Nevertheless, further to any systemic effects, a local cross‐talk takes place between cardiomyocytes and EpAT which determines aspects of myocardial biology, cardiac function and coronary atherosclerosis progression. Secreted adipokines (e.g. adiponectin or leptin) differentially affect AMP‐activated kinase (AMPK) and phosphoinositide 3‐kinase (PI3K)/Akt signalling in cardiomyocytes, which are centrally involved in cardiomyocyte metabolism and substrate utilization. Free fatty acid (FFA)‐related lipotoxicity results in mitochondrial dysfunction, impaired oxidative metabolism and increased oxidative stress. NADPH oxidase activity is also enhanced by mitochondrial dysfunction, and alterations in AMPK signalling induced by EpAT‐secreted adipokines. Increased phsopshorylation of SMAD2 (e.g. by activin A) and/or reduced PI3K/Akt signalling by pro‐inflammatory adipokines negatively affect Ca2+ cycling and cardiomyocyte contractility. Increased cardiomyocyte oxidative stress has also direct effects on redox‐sensitive proteins of the contractile apparatus and cell apoptosis. Cardiomyocyte stress due to impaired substrate utilization, contractile dysfunction and increased oxidative burden leads to respective changes in cardiomyocyte transcriptome. Products of increased myocardial oxidative stress, such as 4‐hydroxynonenal (4HNE, an end product of lipid oxidation) and possibly others among the cardiomyocyte secretome may signal back to EpAT and affect key aspects of its biology, such as the differentiation of adipocytes, adipose tissue expansion and its infiltration by inflammatory cells as well as the regulation of transcriptional factors and relevant gene expression profile, which is shifted towards a pro‐inflammatory phenotype. The concept of a bidirectional signalling between the heart and EpAT is represented with continuous (‘outside‐to‐inside’ signalling) and dashed arrows (‘inside‐to‐outside’ signalling) respectively. ADIPOQ, adiponectin; CEBPA, CCAAT/enhancer‐binding protein α; CXCL1, C‐X‐C motif chemokine ligand 1; FABP4, fatty acid binding protein‐4; IL, interleukin; CCL2, C‐C motif chemokine ligand; INHBA, activin A; LEP, leptin; MIF, macrophage migration inhibitory factor; NFκB, nuclear factor κB; PPARG, peroxisome proliferator activator receptor γ; RETN, resistin; SERPINE1, serpin family E member 1; SMAD2, mothers against decapentaplegic homolog 2; TNF, tumour necrosis factor α; list of adipokines is indicative. +, stimulate/induce; –, decrease/impair; short white arrows represent diffusion of adipokines.
Unresolved issues and future perspectives
Despite the well‐acknowledged roles of EpAT in cardiac physiology, several issues remain less‐well clarified. For example it is still not unknown which factors regulate EpAT secretome and the equilibrium between the beneficial and deleterious EpAT‐derived adipokines. Systemic factors such as obesity and insulin resistance are strong determinants of EpAT secretome profile, but the latter is also influenced by local signals derived from the heart. In addition, it is currently debatable whether a pro‐inflammatory phenotype of EpAT is the result or the cause of coronary atherosclerosis and heart disease, given the two‐way interactions between EpAT and the heart. Finally, while the quantification of EpAT volume as a biomarker in cardiac disease has been extensively explored, there are not currently any available imaging modalities to reliably assess EpAT inflammatory status. The complex interactions between the heart and EpAT should be taken into account by the clinical studies investigating the value of EpAT as a risk marker and its potential as a therapeutic target in cardiac disease.
Conclusions
Firm evidence supports that EpAT has a role in cardiac metabolism, mechanical protection of coronaries and thermogenesis, as well as in the regulation of myocardial redox state, Ca2+ currents, electrophysiological and contractile properties of cardiomyocytes, cardiac fibrosis and coronary atherosclerosis progression. Epicardial cells transformation to adipocytes and the paracrine effects of epicardial adipocytes on human cardiomyocyte and fibroblast biology generate a nexus of complex bidirectional actions, which is centrally involved in cardiac disease pathogenesis. In the light of this knowledge, EpAT ‘dysfunction’ should not be considered only as the cause but also as the consequence of cardiac disease since EpAT receives paracrine ‘reverse’ signalling from the adjacent myocardium. Further in‐depth exploration of the molecular mechanisms regulating the cross‐talk between the heart and EpAT is expected to enhance our understanding regarding the role of the latter in cardiac physiology and related disease mechanisms.
Additional information
Competing interests
None.
Author contributions
Both authors wrote the manuscript. Both authors approved the final version of the manuscript, all persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
C.A. is funded by the British Heart Foundation (FS/16/15/32047), the National Institute for Health Research Oxford Biomedical Research Centre and the Novo Nordisk Foundation Grant number NNF15CC0018486.
Biographies
Alexios Antonopoulos (left) is a clinical scientist and cardiologist with expertise in adipose tissue and obesity‐related cardiovascular disease. He received his PhD from Athens Medical School, Greece having specialized in medical genetics and endothelial dysfunction mechanisms. He continued his research as a postdoc clinical scientist in the Division of Cardiovascular Medicine, University of Oxford with a focus on the biology of adipose tissue and the development of non‐invasive imaging modalities for its study in humans.

Charalambos Antoniades (right) is an Associate Professor of Cardiovascular Medicine, Hon. Consultant Cardiologist, BHF Senior Fellow, and leader of the Oxford Group of Translational Cardiovascular Research (Ox‐TCR) in the Division of Cardiovascular Medicine, University of Oxford. Ox‐TCR has established a large bioresource of human vascular, myocardial and adipose tissue, the Oxford Heart Vessel and Fat (OX‐HVF) cohort, which is being used to support hypothesis‐driven research in the field of vascular and myocardial redox state regulation.
References
- Anan M, Uchihashi K, Aoki S, Matsunobu A, Ootani A, Node K & Toda S (2011). A promising culture model for analyzing the interaction between adipose tissue and cardiomyocytes. Endocrinology 152, 1599–1605. [DOI] [PubMed] [Google Scholar]
- Antoniades C, Demosthenous M, Reilly S, Margaritis M, Zhang MH, Antonopoulos A, Marinou K, Nahar K, Jayaram R, Tousoulis D, Bakogiannis C, Sayeed R, Triantafyllou C, Koumallos N, Psarros C, Miliou A, Stefanadis C, Channon KM & Casadei B (2012). Myocardial redox state predicts in‐hospital clinical outcome after cardiac surgery effects of short‐term pre‐operative statin treatment. J Am Coll Cardiol 59, 60–70. [DOI] [PubMed] [Google Scholar]
- Antonopoulos AS, Margaritis M, Coutinho P, Digby J, Patel R, Psarros C, Ntusi N, Karamitsos TD, Lee R, De Silva R, Petrou M, Sayeed R, Demosthenous M, Bakogiannis C, Wordsworth PB, Tousoulis D, Neubauer S, Channon KM & Antoniades C (2014). Reciprocal effects of systemic inflammation and brain natriuretic peptide on adiponectin biosynthesis in adipose tissue of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol 34, 2151–2159. [DOI] [PubMed] [Google Scholar]
- Antonopoulos AS, Margaritis M, Coutinho P, Shirodaria C, Psarros C, Herdman L, Sanna F, De Silva R, Petrou M, Sayeed R, Krasopoulos G, Lee R, Digby J, Reilly S, Bakogiannis C, Tousoulis D, Kessler B, Casadei B, Channon KM & Antoniades C (2015). Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes 64, 2207–2219. [DOI] [PubMed] [Google Scholar]
- Antonopoulos AS, Margaritis M, Verheule S, Recalde A, Sanna F, Herdman L, Psarros C, Nasrallah H, Coutinho P, Akoumianakis I, Brewer AC, Sayeed R, Krasopoulos G, Petrou M, Tarun A, Tousoulis D, Shah AM, Casadei B, Channon KM & Antoniades C (2016a). Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR‐γ/adiponectin signalling. Circ Res 118, 842–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonopoulos AS, Oikonomou EK, Antoniades C & Tousoulis D (2016b). From the BMI paradox to the obesity paradox: the obesity‐mortality association in coronary heart disease. Obes Rev 17, 989–1000. [DOI] [PubMed] [Google Scholar]
- Blumensatt M, Greulich S, Herzfeld de Wiza D, Mueller H, Maxhera B, Rabelink MJ, Hoeben RC, Akhyari P, Al‐Hasani H, Ruige JB & Ouwens DM (2013). Activin A impairs insulin action in cardiomyocytes via up‐regulation of miR‐143. Cardiovasc Res 100, 201–210. [DOI] [PubMed] [Google Scholar]
- Burgeiro A, Fuhrmann A, Cherian S, Espinoza D, Jarak I, Carvalho RA, Loureiro M, Patricio M, Antunes M & Carvalho E (2016). Glucose uptake and lipid metabolism are impaired in epicardial adipose tissue from heart failure patients with or without diabetes. Am J Physiol Endocrinol Metab 310, E550–E564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AP, Farb A, Tashko G & Virmani R (1998). Arrhythmogenic right ventricular cardiomyopathy and fatty replacement of the right ventricular myocardium: are they different diseases? Circulation 97, 1571–1580. [DOI] [PubMed] [Google Scholar]
- Chandalia M, Garg A, Vuitch F & Nizzi F (1995). Postmortem findings in congenital generalized lipodystrophy. J Clin Endocrinol Metab 80, 3077–3081. [DOI] [PubMed] [Google Scholar]
- Cheng VY, Dey D, Tamarappoo B, Nakazato R, Gransar H, Miranda‐Peats R, Ramesh A, Wong ND, Shaw LJ, Slomka PJ & Berman DS (2010). Pericardial fat burden on ECG‐gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging 3, 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian S, Lopaschuk GD & Carvalho E (2012). Cellular cross‐talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. Am J Physiol Endocrinol Metab 303, E937–E949. [DOI] [PubMed] [Google Scholar]
- Chilukoti RK, Giese A, Malenke W, Homuth G, Bukowska A, Goette A, Felix SB, Kanaan J, Wollert HG, Evert K, Verheule S, Jais P, Hatem SN, Lendeckel U & Wolke C (2015). Atrial fibrillation and rapid acute pacing regulate adipocyte/adipositas‐related gene expression in the atria. Int J Cardiol 187, 604–613. [DOI] [PubMed] [Google Scholar]
- Chiou CW, Eble JN & Zipes DP (1997). Efferent vagal innervation of the canine atria and sinus and atrioventricular nodes. The third fat pad. Circulation 95, 2573–2584. [DOI] [PubMed] [Google Scholar]
- Dabbah S, Komarov H, Marmor A & Assy N (2014). Epicardial fat, rather than pericardial fat, is independently associated with diastolic filling in subjects without apparent heart disease. Nutr Metab Cardiovasc Dis 24, 877–882. [DOI] [PubMed] [Google Scholar]
- Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, Ouyang P, Espeland MA, Lohman KK, Criqui MH, Allison M, Bluemke DA & Carr JJ (2009). The association of pericardial fat with incident coronary heart disease: the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 90, 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouzandeh F, Chang SM, Muhyieddeen K, Zaid RR, Trevino AR, Xu J, Nabi F & Mahmarian JJ (2013). Does quantifying epicardial and intrathoracic fat with noncontrast computed tomography improve risk stratification beyond calcium scoring alone? Circ Cardiovasc Imaging 6, 58–66. [DOI] [PubMed] [Google Scholar]
- Gaborit B, Venteclef N, Ancel P, Pelloux V, Gariboldi V, Leprince P, Amour J, Hatem SN, Jouve E, Dutour A & Clement K (2015). Human epicardial adipose tissue has a specific transcriptomic signature depending on its anatomical peri‐atrial, peri‐ventricular, or peri‐coronary location. Cardiovasc Res 108, 62–73. [DOI] [PubMed] [Google Scholar]
- Greulich S, de Wiza DH, Preilowski S, Ding Z, Mueller H, Langin D, Jaquet K, Ouwens DM & Eckel J (2011). Secretory products of guinea pig epicardial fat induce insulin resistance and impair primary adult rat cardiomyocyte function. J Cell Mol Med 15, 2399–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greulich S, Maxhera B, Vandenplas G, de Wiza DH, Smiris K, Mueller H, Heinrichs J, Blumensatt M, Cuvelier C, Akhyari P, Ruige JB, Ouwens DM & Eckel J (2012). Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation 126, 2324–2334. [DOI] [PubMed] [Google Scholar]
- Hassan M, Said K, Rizk H, ElMogy F, Donya M, Houseni M & Yacoub M (2016). Segmental peri‐coronary epicardial adipose tissue volume and coronary plaque characteristics. Eur Heart J Cardiovasc Imaging 17, 1169–1177. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Ishii T, Asuwa N & Masuda S (1997). Absence of atherosclerosis evolution in the coronary arterial segment covered by myocardial tissue in cholesterol‐fed rabbits. Virchows Arch 430, 163–171. [DOI] [PubMed] [Google Scholar]
- Jacobson JT, Hutchinson MD, Cooper JM, Woo YJ, Shandler RS & Callans DJ (2011). Tissue‐specific variability in human epicardial impedance. J Cardiovasc Electrophysiol 22, 436–439. [DOI] [PubMed] [Google Scholar]
- Karastergiou K, Evans I, Ogston N, Miheisi N, Nair D, Kaski JC, Jahangiri M & Mohamed‐Ali V (2010). Epicardial adipokines in obesity and coronary artery disease induce atherogenic changes in monocytes and endothelial cells. Arterioscler Thromb Vasc Biol 30, 1340–1346. [DOI] [PubMed] [Google Scholar]
- Kocyigit D, Gurses KM, Yalcin MU, Turk G, Evranos B, Yorgun H, Sahiner ML, Kaya EB, Hazirolan T, Tokgozoglu L, Oto MA, Ozer N & Aytemir K (2015). Periatrial epicardial adipose tissue thickness is an independent predictor of atrial fibrillation recurrence after cryoballoon‐based pulmonary vein isolation. J Cardiovasc Comput Tomogr 9, 295–302. [DOI] [PubMed] [Google Scholar]
- Kunita E, Yamamoto H, Kitagawa T, Ohashi N, Oka T, Utsunomiya H, Urabe Y, Tsushima H, Awai K, Budoff MJ & Kihara Y (2014). Prognostic value of coronary artery calcium and epicardial adipose tissue assessed by non‐contrast cardiac computed tomography. Atherosclerosis 233, 447–453. [DOI] [PubMed] [Google Scholar]
- Lage R, Moscoso I, Fernandez‐Trasancos A, Cebro M, Couselo M, Fandino‐Vaquero R, Bravo SB, Sierra J, Gonzalez‐Juanatey JR & Eiras S (2015). Differential behaviour of epicardial adipose tissue‐secretomes with high and low orosomucoid levels from patients with cardiovascular disease in H9C2 cells. Mol Cell Endocrinol 416, 77–87. [DOI] [PubMed] [Google Scholar]
- Levelt E, Pavlides M, Banerjee R, Mahmod M, Kelly C, Sellwood J, Ariga R, Thomas S, Francis J, Rodgers C, Clarke W, Sabharwal N, Antoniades C, Schneider J, Robson M, Clarke K, Karamitsos T, Rider O & Neubauer S (2016). Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J Am Coll Cardiol 68, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YK, Chen YC, Chen JH, Chen SA & Chen YJ (2012). Adipocytes modulate the electrophysiology of atrial myocytes: implications in obesity‐induced atrial fibrillation. Basic Res Cardiol 107, 293. [DOI] [PubMed] [Google Scholar]
- Mahabadi AA, Berg MH, Lehmann N, Kalsch H, Bauer M, Kara K, Dragano N, Moebus S, Jockel KH, Erbel R & Mohlenkamp S (2013). Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol 61, 1388–1395. [DOI] [PubMed] [Google Scholar]
- Mahabadi AA, Lehmann N, Kalsch H, Bauer M, Dykun I, Kara K, Moebus S, Jockel KH, Erbel R & Mohlenkamp S (2014). Association of epicardial adipose tissue and left atrial size on non‐contrast CT with atrial fibrillation: the Heinz Nixdorf Recall Study. Eur Heart J Cardiovasc Imaging 15, 863–869. [DOI] [PubMed] [Google Scholar]
- Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JP, Finnie JW, Samuel CS, Royce SG, Twomey DJ, Thanigaimani S, Kalman JM & Sanders P (2015). Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol 66, 1–11. [DOI] [PubMed] [Google Scholar]
- Marchington JM & Pond CM (1990). Site‐specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high‐fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int J Obes 14, 1013–1022. [PubMed] [Google Scholar]
- Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P, Shirodaria C, Sayeed R, Petrou M, De Silva R, Jalilzadeh S, Demosthenous M, Bakogiannis C, Tousoulis D, Stefanadis C, Choudhury RP, Casadei B, Channon KM & Antoniades C (2013). Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation 127, 2209–2221. [DOI] [PubMed] [Google Scholar]
- Mazurek T, Kobylecka M, Zielenkiewicz M, Kurek A, Kochman J, Filipiak KJ, Mazurek K, Huczek Z, Krolicki L & Opolski G (2016). PET/CT evaluation of F‐FDG uptake in pericoronary adipose tissue in patients with stable coronary artery disease: Independent predictor of atherosclerotic lesions' formation? J Nucl Cardiol, doi: 10.1007/s12350-015-0370-6. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Fukuda S, Tanaka A, Otsuka K, Jissho S, Taguchi H, Yoshikawa J & Shimada K (2014). Persistent epicardial adipose tissue accumulation is associated with coronary plaque vulnerability and future acute coronary syndrome in non‐obese subjects with coronary artery disease. Atherosclerosis 237, 353–360. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Fukuda S, Tanaka A, Otsuka K, Sakamoto M, Taguchi H, Yoshikawa J, Shimada K & Yoshiyama M (2012). Peri‐atrial epicardial adipose tissue is associated with new‐onset nonvalvular atrial fibrillation. Circ J 76, 2748–2754. [DOI] [PubMed] [Google Scholar]
- Reilly SN, Jayaram R, Nahar K, Antoniades C, Verheule S, Channon KM, Alp NJ, Schotten U & Casadei B (2011). Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: implications for the antiarrhythmic effect of statins. Circulation 124, 1107–1117. [DOI] [PubMed] [Google Scholar]
- Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, Karas J, Optican R, Bahouth SW, Garrett E, Wolf RY, Carter RA, Robbins T, Wolford D & Samaha J (2009). Uncoupling protein‐1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab 94, 3611–3615. [DOI] [PubMed] [Google Scholar]
- Salgado‐Somoza A, Teijeira‐Fernandez E, Fernandez AL, Gonzalez‐Juanatey JR & Eiras S (2010). Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am J Physiol Heart Circ Physiol 299, H202–H209. [DOI] [PubMed] [Google Scholar]
- Shimabukuro M, Hirata Y, Tabata M, Dagvasumberel M, Sato H, Kurobe H, Fukuda D, Soeki T, Kitagawa T, Takanashi S & Sata M (2013). Epicardial adipose tissue volume and adipocytokine imbalance are strongly linked to human coronary atherosclerosis. Arterioscler Thromb Vasc Biol 33, 1077–1084. [DOI] [PubMed] [Google Scholar]
- Stojanovska J, Kazerooni EA, Sinno M, Gross BH, Watcharotone K, Patel S, Jacobson JA & Oral H (2015). Increased epicardial fat is independently associated with the presence and chronicity of atrial fibrillation and radiofrequency ablation outcome. Eur Radiol 25, 2298–2309. [DOI] [PubMed] [Google Scholar]
- Suffee N, Moris TM, Dilanian G, Farahmand P, Rucker‐Martin C, Dugail I, Pucéat M & Hatem S (2016). Epicardial progenitors are source of adipocyte in human atria. Arch Cardiovasc Dis Suppl 8, 255. [Google Scholar]
- Tamarappoo B, Dey D, Shmilovich H, Nakazato R, Gransar H, Cheng VY, Friedman JD, Hayes SW, Thomson LE, Slomka PJ, Rozanski A & Berman DS (2010). Increased pericardial fat volume measured from noncontrast CT predicts myocardial ischemia by SPECT. JACC Cardiovasc Imaging 3, 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Uchida Y, Shimoyama E, Hiruta N, Kishimoto T & Watanabe S (2016). Pericoronary adipose tissue as storage and supply site for oxidized low‐density lipoprotein in human coronary plaques. PLoS One 11, e0150862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, Amour J, Leprince P, Dutour A, Clement K & Hatem SN (2015). Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo‐fibrokines. Eur Heart J 36, 795–805. [DOI] [PubMed] [Google Scholar]
- Verheule S, Tuyls E, Gharaviri A, Hulsmans S, van Hunnik A, Kuiper M, Serroyen J, Zeemering S, Kuijpers NH & Schotten U (2013). Loss of continuity in the thin epicardial layer because of endomysial fibrosis increases the complexity of atrial fibrillatory conduction. Circ Arrhythm Electrophysiol 6, 202–211. [DOI] [PubMed] [Google Scholar]
- Wang CP, Hsu HL, Hung WC, Yu TH, Chen YH, Chiu CA, Lu LF, Chung FM, Shin SJ & Lee YJ (2009a). Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin Endocrinol (Oxf) 70, 876–882. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tao L, Yuan Y, Lau WB, Li R, Lopez BL, Christopher TA, Tian R & Ma XL (2009b). Cardioprotective effect of adiponectin is partially mediated by its AMPK‐independent antinitrative action. Am J Physiol Endocrinol Metab 297, E384–E391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward L, Akoumianakis I & Antoniades C (2016). Unravelling the adiponectin paradox: novel roles of adiponectin in the regulation of cardiovascular disease. Br J Pharmacol, DOI: 10.1111/bph.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Cavallero S, Patterson M, Shen H, Xu J, Kumar SR & Sucov HM (2015). Adipogenesis and epicardial adipose tissue: A novel fate of the epicardium induced by mesenchymal transformation and PPARγ activation. Proc Natl Acad Sci U S A 112, 2070–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgun H, Canpolat U, Aytemir K, Hazirolan T, Sahiner L, Kaya EB, Kabakci G, Tokgozoglu L, Ozer N & Oto A (2015). Association of epicardial and peri‐atrial adiposity with the presence and severity of non‐valvular atrial fibrillation. Int J Cardiovasc Imaging 31, 649–657. [DOI] [PubMed] [Google Scholar]
- Zangi L, Oliveira MS, Ye LY, Ma Q, Sultana N, Hadas Y, Chepurko E, Später D, Zhou B, Chew WL, Ebina W, Abrial M, Wang Q‐D, Pu WT & Chien KR (2017). An IGF1R‐dependent pathway drives epicardial adipose tissue formation after myocardial injury. Circulation 135, 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Zhang H, Guo L & Hong K (2015). Relationship between epicardial adipose tissue volume and atrial fibrillation: A systematic review and meta‐analysis. Herz 41, 421–427. [DOI] [PubMed] [Google Scholar]


