Abstract
Background:
Whole body vibration (WBV) is a form of physical stimulation via mechanical vibrations transmitted to a subject. It is assumed that WBV induces sensory stimulation in cortical brain regions through the activation of skin and muscle receptors responding to the vibration. The effects of WBV on muscle strength are well described. However, little is known about the impact of WBV on the brain. Recently, it was shown in humans that WBV improves attention in an acute WBV protocol. Preclinical research is needed to unravel the underlying brain mechanism. As a first step, we examined whether chronic WBV improves attention in mice.
Material and Methods:
A custom made vibrating platform for mice with low intensity vibrations was used. Male CD1 mice (3 months of age) received five weeks WBV (30 Hz; 1.9 G), five days a week with sessions of five (n=12) or 30 (n=10) minutes. Control mice (pseudo-WBV; n=12 and 10 for the five and 30 minute sessions, respectively) were treated in a similar way, but did not receive the actual vibration. Object recognition tasks were used as an attention test (novel and spatial object recognition – the primary outcome measure). A Balance beam was used for motor performance, serving as a secondary outcome measure.
Results:
WBV sessions of five (but not WBV sessions of 30 minutes) improved balance beam performance (mice gained 28% in time needed to cross the beam) and novel object recognition (mice paid significantly more attention to the novel object) as compared to pseudo WBV, but no change was found for spatial object performance (mice did not notice the relocation). Although 30 minutes WBV sessions were not beneficial, it did not impair either attention or motor performance.
Conclusion:
These results show that brief sessions of WBV improve, next to motor performance, attention for object recognition, but not spatial cues of the objects. The selective improvement of attention in mice opens the avenue to unravel the underlying brain mechanisms.
Keywords: motor performance, attention, novel object recognition, balance beam, chronic treatment
Introduction
Treatment with vibration stimuli delivered to the body via a vibration platform or chair are referred to as whole body vibration (WBV). It provides a mechanical oscillation of a specific amplitude of displacement and frequency, suitable for clinical purposes (Jordan et al., 2005). The contact surface transmits the vibrations throughout the body. One consequence of this type of stimulation is that reflex muscle contractions are induced. The activation of sensory receptors in the muscle (muscle spindles) initiates these reflexes leading to the activation of muscle motor units (Bosco et al., 1999; Burke and Schiller, 1976). Increased muscle activity, blood flow and muscle and skin temperature have been shown to be the consequence of acute WBV protocols (Hazell and Lemon, 2012). WBV can lead to increased muscle strength and improved balance (Russo et al., 2003; Weber-Rajek et al., 2015). Exercise training using WBV has been viewed as a complementary training to standard physical rehabilitation programs, including those related to the treatment of neurodegenerative diseases (Sitjà Rabert et al., 2012). Preclinical research, however, is needed to unravel the underlying brain mechanism. Before mouse models of such diseases can be employed to examine the impact of WBV, more basic knowledge has to be gained on how mice respond to WBV, and which protocols are suitable.
Low intensity WBV can be applied safely to rodents. In young rats, for example, an eight weeks protocol of WBV resulted in increased body weight in the vibration group as compared to the control group (Naghii et al., 2011). Twelve weeks of WBV in old rats resulted in a reduction of body fat mass as compared to controls (Maddalozzo et al., 2008). Despite ample data showing effects of WBV on the body, very little is known about the effects of WBV on the brain. It is assumed that WBV induces sensory stimulation in cortical brain regions through the activation of skin and muscle receptors responding to the vibration. A series of studies in humans showed that two minutes of WBV applied via a chair mounted on a platform improved attention/cognitive interference in healthy (young) adults and adults with Attention Deficit Hyperactivity Disorder (ADHD) (Fuermaier et al., 2014a; Regterschot et al., 2014). Moreover, it was found that repeated WBV treatment (three times three minutes) improved cognitive interference in healthy children (den Heijer et al., 2015). The above mentioned studies show that acute or short-term WBV treatments improve cognitive performance, but it is not known whether this is true for chronic or long-term WBV treatments as well. The rational of this study was to study the effects on attention and motor performance of a five weeks WBV protocol in mice. We selected the CD1 mouse for this study, as it is a commonly used outbred strain. The objective of the current study was to test the hypothesis that attention to a novel object is improved by WBV, and this test served as primary outcome measure. Selective attention can be assessed in mice by way of a novel object recognition task (Levin et al., 2011; Braida et al., 2013). A Balance beam test was used as secondary outcome measure to examine the impact of WBV on motor performance. As no data is available for optimal WBV duration in CD1 mice, sessions of either five or 30 minutes were used.
Materials and Methods
Experimental animals
Young CD1 mice (n = 44; 3 months of age; males; Harlan Netherlands BV, Horst, The Netherlands) were used. All mice were placed individually in home cages, with food and water available ad libitum. They were kept under a 12 hr light / 22 hr dark cycle (lights on at 7:00 am). The air humidity in the room was kept at 40% and temperature was held constant at 21° Celsius. Home cages were cleaned once a week. All procedures concerning animal care and treatment were in accordance with the regulations of the ethical committee for the use of experimental animals of the University of Groningen. These regulations are consistent with the guidelines for the care and use of laboratory animals as described by the U.S. National Institutes of Health. All experiments of this study were approved by the ethical committee of the University of Groningen, The Netherlands.
Whole body vibration procedure
The WBV apparatus was made of an oscillator (LEVELL R.C. Oscillator Type TG200DMP) and power amplifier (V406 Shaker Power Amplifier). A cage (44.5 (Length; L) x 28 (Width; W) x 16 (Height; H) cm) was attached to the oscillator which contained 12 removable compartments (6.5 (L) x = 7.5 (W) x 20 (H) cm). Mice were subjected to low intensity sinusoidal vibrations with a frequency of 30 Hz and amplitude of 1.9 g (this WBV setting improved metabolic health during aging (Reijne et al., 2016); see Figure 1 for exact peak to peak displacements in the cage in X, Y and Z directions. The daily duration of the WBV sessions was either 5 (n=12 per group) or 30 (n=10 per group) minutes a day for five consecutive weeks during the week days (referred to as a chronic treatment). Mice were placed in the compartments following a rotation schedule as the amplitude differed depending on the location (i.e. corners have a higher amplitude; see Figure 1). Control mice (pseudo WBV mice) were similarly placed in the compartments, but did not receive the actual vibration. Animals were randomly assigned to the four different groups (WBV 5 minutes protocol (n=12); Pseudo WBV 5 minutes protocol (n=12); WBV 30 minutes protocol (n=10)); Pseudo WBV 30 minutes protocol (n=10). Between WBV sessions the compartments and plastic box were cleaned with 30% ethanol and towel dried. The timeline of the experiment was five weeks, followed by a Balance beam test and a Novel object recognition (NOR) and a Spatial object recognition (SOR) test in week 6. WBV was continued in week 6, but always performed at the end of the day to prevent that acute effects of the WBV session influenced the test performance.
Figure 1.
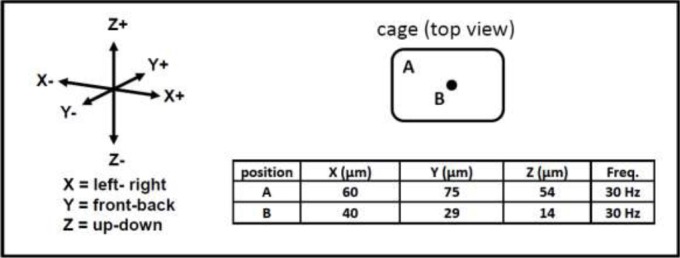
Peak to peak displacement in µm, measured in the corner (position A) and center (position B) of the cage (black dot in cage top view indicates the connection to the underlying oscillator).
Novel object recognition and Spatial object recognition test
Attention for a novel object (Novel object recognition test - NOR) or a replaced object (Spatial object recognition - SOR) in a familiarized environment was tested in a box (LxWxH = 50 cm x 50 cm x 35 cm; Naude et al., 2014; Figure 2). Objects were made of transparent glass in the shape of a beaker (object A) or a conical flask (object B). The test started with a habituation phase in an empty arena (day 1), a training phase (day 2), and a test phase (day 3). The interval between each phase for each individual mouse was exactly 24 hours between day 1, 2 and 3. The activity of the mice during the habituation phase and the degree of exploration during the training phase served as baseline measures. In the habituation phase (day 1) the mice individually underwent three five-minute sessions to get accustomed to the testing environment. Between the sessions the animals were placed back into their home cage for five minutes. In these sessions the test box contained no objects (Figure 2A). In the training phase (day 2) the mice underwent a single 10-minute training session. Two identical transparent glass objects (either A1 and A2 (beakers of 15 cm high), or B1 and B2 (conical flasks of 15 cm high)) were placed symmetrical around the center of the test box.
Figure 2.
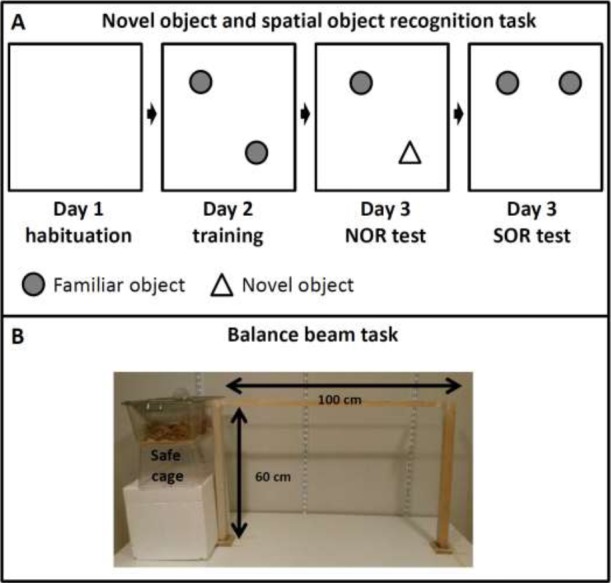
Schematic representation of the Novel object and Spatial object recognition protocol (A) and the Balance beam task (B).
In the test phase (day 3) the mice were reintroduced to the test environment for a 10-minutes NOR testing session and were exposed to two different objects. One object was familiar (A1 or B1), while the other was novel. The objects were placed at the same positions as during the training phase. After 10 minutes the animals were placed back into their home cage for five minutes, before undergoing the next 10-minutes SOR testing session. This time the test environment contained the two familiar objects used in the training phase (A1 and A2, or B1 and B2), but one of the objects (A2 or B2) was moved to a different location. After each session the box was cleaned with 30% ethanol and towel dried to remove olfactory cues. Exploration time (paying attention to an object) was used as outcome measure. Exploration of an object was defined as sniffing or touching the objects with the nose and/or forepaws within 2 cm of the object. All training and testing sessions were videotaped and analyzed using Eline software (Hovens et al., 2015).
Balance beam test
The Balance beam test (Figure 2B), a sensorimotor test which focuses on hind-limb functioning (Carter et al., 1999; Soderling et al., 2003), consisted of a 1-meter long squared wooden strip (diameter 5 mm). The strip was placed horizontally 60 cm above the ground. At the end of the beam a “safe” cage filled with sawdust was placed and served as a target for the mice (see Mazzola et al., 2016 for further details). Animals performed three training trials on the beam (5, 10, and 40 cm away from the safe cage) to get familiarized with the test, and one final test trial at 100 cm. The test trial was recorded on video. Time needed to cross the beam was taken as an outcome measure. The animals were tested twice: once before the start of the WBV protocol and after the WBV protocol was ended.
Statistical Analysis
Statistical analyses to test the hypothesis (H0) that WBV improves attention and motor performance in mice, or whether WBV does not improve these (alternative hypothesis H1) were performed using SPSS. All data was checked for normality (Shapiro-Wilk test). A Student’s t-test was then used to test for significant differences in performance of the different groups (comparisons of intergroup means). A p-value of less than 0.05 (p<0.05) was considered to be statistically significant. Data are presented as mean +/- standard error.
Results
Novel and Spatial object recognition
During the habituation phase, no differences were observed in exploring the arena between the four groups. Next, the exploration time of the different objects during the training phase revealed that the mice had no preference for either object A or B. Relative exploration in the NOR and SOR tests served as primary outcome measure. NOR performance was significantly improved in mice receiving the five weeks of five minutes sessions of WBV as compared to the pseudo WBV control mice (P<0.01; Figure 3A). In contrast, five weeks of sessions of 30 minutes of WBV did not result in any noticeable improvement in performance (Figure 3A). In clear contrast to the NOR performance, no improvements were observed in SOR performance, neither in the five nor in the 30 minutes WBV protocol (Figure 3A).
Figure 3.
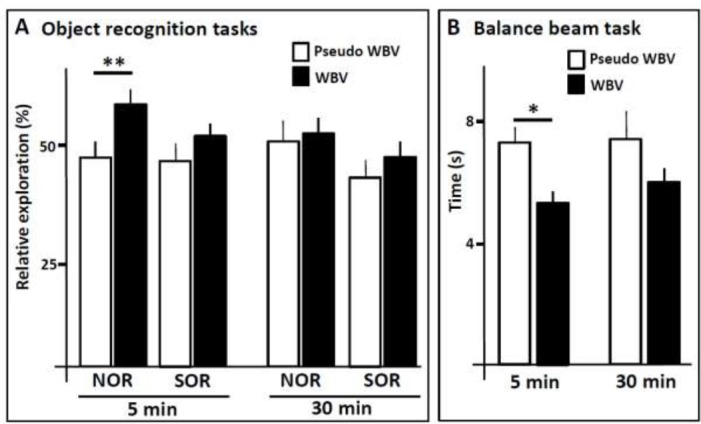
Performance of control (pseudo WBV) and experimental (WBV) mice in the novel object recognition (NOR) and spatial object recognition (SOR) (A), and in the Balance beam (B), after five weeks of WBV with 5 or 30 minute sessions. * P < 0,05; ** P < 0.01. Error bars represent s.e.m.
Balance beam test
The Balance beam served as secondary outcome measure. No significant differences were found in balance beam performance between the four groups prior to the WBV protocol (baseline measurements). The performance of the mice receiving five weeks of five minutes sessions of WBV was significantly improved compared to the mice receiving pseudo WBV (p<0.05; Figure 3B; mice gained 28% in time needed to cross the beam). Mice run between one and two seconds faster over the beam after WBV treatment. Although time needed to cross the beam also decreased in the 30 minutes sessions of WBV (mice gained 19% in time needed to cross the beam), this did not reach statistical significance compared to the pseudo WBV group. Comparisons between the baseline measurements and the performance after the WBV protocol revealed no significant changes in the control groups (pseudo WBV). Like found in the comparison of the pseudo WBV groups with the WBV groups after the five week protocol (which is the most critical comparison because prior experience to the Balance beam can influence subsequent testing), a significant difference was found in the 5 minutes WBV protocol (p<0.05) but not in the 30 minutes protocol between the WBV and pseudo WBV groups.
Discussion
The results show that, although depending on the duration of the WBV sessions, motor performance and attention to object recognition can be significantly improved by WBV. WBV is known to stimulate muscle activity (Bosco et al., 1999; Burke and Schiller, 1976), and the current findings of the Balance beam with improved motor performance after WBV are therefore in full support of these observations. The improved attention in the object recognition task corroborates the found improvements in attention in humans (Fuermaier et al., 2014a,b; Regterschot et al., 2014, den Heijer et al., 2015).
The CD1 mouse has been used in a variety of learning tasks including novel object recognition (see Patil et al., 2012, and references therein), and has no innate deficiency for this task. Both for SOR and NOR the animals were able to recognize the new object (NOR) or the relocated object (SOR), with performance significantly above baseline level (as obtained during during the training phase). Attention to a novel object as measured in the NOR test was shown to be improved by WBV. Recognition of an object depends strongly on the functional integrity of the perirhinal cortex (Winters et al., 2008; Mendez et al., 2015). This may suggest that this brain region is sensitive to WBV. In contrast to NOR, the recognition of a changed spatial configuration of the objects was not affected by WBV in CD1 mice as shown by the SOR test. In the five minutes WBV protocol, the CD1 mice did not improve their SOR performance, which is supposed to be dependent on the hippocampus (Manns and Eichenbaum, 2009; Castilla-Ortega et al., 2012; Mendez et al., 2015). Nevertheless, vibrated CD1 mice showed a tendency to improve, which did not reach significant levels. CD1 mice are believed to have a relatively poor functioning hippocampus (Mulder et al., 2016). CD1 mice have a preference for a striatal-driven over a hippocampal-driven strategy to solve spatial tasks; this preference is most likely due to a poor functioning of the hippocampus. Striatal preference in CD1 mice is even stronger in older mice (age > 18 months; Mulder et al., 2016), which could be related to a further (gradual) loss of hippocampal function due to aging (Kuhn et al., 1996; Gil-Mohapel et al., 2013; Van der Zee, 2015). The striatum is known to be much less sensitive to sensory stimulation as the hippocampus (Manns and Eichenbaum, 2009; Castilla-Ortega et al., 2012; Mendez et al., 2015). It could be that the 5 minutes protocol was not optimally chosen for improving their hippocampus. The 30 minutes WBV protocol revealed a similar picture showing that increasing the WBV session time from 5 to 30 minutes did not help. This could mean that the hippocampus in the CD1 mouse is not sensitive to any WBV setting, or that the optimal setting is shorter than 5 minutes or somewhere between 5 and 30 minutes.
It is important to stress that the 30 minutes WBV sessions did not induce any harm to the mice. Their balance beam performance was almost as good as of the mice receiving the five minutes WBV setting. Likewise in the NOR and SOR, no signs of impairment were found. In all cases 30 minutes of WBV showed a tendency of improvement, but turned out to be much less effective than the five minutes WBV setting in the CD1 mouse. Based on the two session durations used in this study, it cannot be concluded what duration would be optimal, as it could be somewhere between five and 30 minutes. Of note, in C57Bl6 mice (an inbred strain), a 10 minutes WBV protocol resulted in significant improvement in balance beam performance and a variety of learning tasks (unpublished data; Keijser et al., 2011). It could also be that differences in sensitivity for WBV treatment are dependent on the genetic background. If so, this could indicate the need of personalized settings in the human situation to obtain optimal effects of WBV on attention/cognitive (and motor) performance.
In conclusion, these results show that brief sessions of WBV improve, next to motor performance, attention for object recognition, but not recognizing changes in spatial location of the objects. The selective improvement of attention in mice opens the avenue to unravel the underlying brain mechanisms.
Acknowledgments
We thank Dr. Gernot Riedel (Aberdeen University, Scotland) for supporting us with the WBV equipment, and Mandy van der Klij and Bettie Atsma for their valuable contribution to the paper.
References
- 1.Bosco C, Colli R, Introini E, Cardinale M, Tsarpela O, Madella A, Tihanvi J, Viru A. Adaptive responses of human skeletal muscle to vibration. Clin. Physiol. 1999;19:183–187. doi: 10.1046/j.1365-2281.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 2.Braida D, Donzelli A, Martucci R, Ponzoni L, Pauletti A, Langus A, Sala M. Mice discriminate between stationary and moving 2D shapes:Application to the object recognition task to increase attention. Behav. Brain Res. 2013;242:95–101. doi: 10.1016/j.bbr.2012.12.040. [DOI] [PubMed] [Google Scholar]
- 3.Burke D, Schiller HH. Discharge pattern of single motor units in the tonic vibration reflex of human triceps surae. J. Neurol. Neurosurg. Psychiatry. 1976;39:729–741. doi: 10.1136/jnnp.39.8.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. J Neurosci. 1999;19:3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castilla-Ortega E, Pedraza C, Chun J, de Fonseca FR, Estivill-Torrus G, Santin LJ. Hippocampal c-fos activation in normal and LPA1-null mice after two object recognition tasks with different memory demands. Behav. Brain Res. 2012;232:400–405. doi: 10.1016/j.bbr.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 6.den Heijer AE, Groen Y, Fuermaier AB, van Heuvelen MJ, van der Zee EA, Tucha L, Tucha O. Acute effects of Whole Body Vibration on inhibition in healthy children. PloS One. 2015;10(11):e0140665. doi: 10.1371/journal.pone.0140665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuermaier AB, Tucha L, Koerts J, van Heuvelen MJ, van der Zee EA, Lange KW, Tucha O. Good vibrations--effects of whole body vibration on attention in healthy individuals and individuals with ADHD. PloS One. 2014a;9(2):e90747. doi: 10.1371/journal.pone.0090747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuermaier AB, Tucha L, Koerts J, van den Bos M, Regterschot GR, Zeinstra EB, van Heuvelen MJ, van der Zee EA, Lange KW, Tucha O. Whole-body vibration improves cognitive functions of an adult with ADHD. Atten. Defic. Hyperact. Disord. 2014b;6(3):211–220. doi: 10.1007/s12402-014-0149-7. [DOI] [PubMed] [Google Scholar]
- 9.Gil-Mohapel J, Brocardo PS, Choquette W, Gothard R, Simpson JM, Christie BR. Hippocampal neurogenesis levels predict watermaze search strategies in the aging brain. PLoS One. 2013;8:e75125. doi: 10.1371/journal.pone.0075125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazell TJ, Lemon PWR. Synchronous whole-body vibration increases VO2 during and following acute exercise. Eur. J. Appl. Physiol. 2012;112:413–20. doi: 10.1007/s00421-011-1984-2. [DOI] [PubMed] [Google Scholar]
- 11.Hovens IB, van Leeuwen BL, Nyakas C, Heineman E, van der Zee EA, Schoemaker RG. Prior infection exacerbates postoperative cognitive dysfunction in aged rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;15(309) 2:R148–159. doi: 10.1152/ajpregu.00002.2015. [DOI] [PubMed] [Google Scholar]
- 12.Jordan MJ, Norris SR, Smith DJ, Herzog W. Vibration training:an overview of the area, training consequences, and future considerations. J. Strength Cond. Res. 2005;19:459–466. doi: 10.1519/13293.1. [DOI] [PubMed] [Google Scholar]
- 13.Keijser N, Piersma D, Postema F, Venema BJ, Luiten PGM, van der Zee EA. Improved cognitive performance as a result of whole body stimulation in mice and men. The 9th Dutch Endo-Neuro-Psycho (ENP) Meeting. 2011:125. [Google Scholar]
- 14.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat:age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin ED, Bushnell PJ, Rezvani AH. Attention-modulating effects of cognitive enhancers. Pharmacol. Biochem. Behav. 2011;99:146–154. doi: 10.1016/j.pbb.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maddalozzo GF, Iwaniec UT, Turner RT, Rosen CJ, Widrick JJ. Whole-body vibration slows the acquisition of fat in mature female rats. Int J Obes (Lond) 2008;32:1348–54. doi: 10.1038/ijo.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manns JR, Eichenbaum H. A cognitive map for object memory in the hippocampus. Learn. Mem. 2009;16:616–624. doi: 10.1101/lm.1484509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzola PN, Bruinenberg VM, Anjema K, van Vliet D, Dutra-Filho CS, van Spronsen FJ, van der Zee EA. Voluntary exercise prevents oxidative stress in the brain of phenylketonuria mice. JIMD Rep. 2016;27:69–77. doi: 10.1007/8904_2015_498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendez M, Arias N, Uceda S, Arias JL. C-Fos expression correlates with performance on novel object and novel place recognition tests. Brain Res. Bull. 2015;117:16–23. doi: 10.1016/j.brainresbull.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Mulder CK, Gerkema MP, van der Zee EA. Role of aging and hippocampus in time-place learning:link to episodic-like memory? Front. Behav. Neurosci. 2016;19(9):362. doi: 10.3389/fnbeh.2015.00362. doi:10.3389/fnbeh.2015.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naghii MR, Ghanizadeh G, Darvishi P, Ebrahimpour Y, Mofid M, Torkaman G, Asgari AR, Hedayati M. Whole body vibration is a safe exercise training method and induces no impaired alterations on rat plasma parameters. Acta Physiol. Hung. 2011;98:442–8. doi: 10.1556/APhysiol.98.2011.4.7. [DOI] [PubMed] [Google Scholar]
- 22.Naude PJ, Dobos N, van der Meer D, Mulder C, Pawironadi KG, den Boer JA, van der Zee EA, Luiten PGM, Eisel UL. Analysis of cognition, motor performance and anxiety in young and aged tumor necrosis factor alpha receptor 1 and 2 deficient mice. Behav. Brain Res. 2014;258:43–51. doi: 10.1016/j.bbr.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Patil SS, Li K, Heo S, Hoger H, Lubec G. Proteins linked to spatial memory formation of CD1 mice in the multiple T-maze. Hippocampus. 2012;22:1075–1086. doi: 10.1002/hipo.20956. [DOI] [PubMed] [Google Scholar]
- 24.Regterschot GR, van Heuvelen MJ, Zeinstra EB, Fuermaier AB, Tucha L, Koerts J, Tucha O, van der Zee EA. Whole body vibration improves cognition in healthy young adults. PLoS One. 2014;20(9) 6:e100506. doi: 10.1371/journal.pone.0100506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reijne AC, Ciapaite J, van Dijk TH, Havinga R, van der Zee EA, Groen AK, Reijngoud DJ, Bakker BM, van Dijk G. Whole-body vibration reverses aging-induced increases in visceral adiposity and hepatic lipid storage in mice. PLoS One. 2016;11(2):e0149419. doi: 10.1371/journal.pone.0149419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russo CR, Lauretani F, Bandinelli S, Bartali B, Cavazzini C, Guralnik JM, Ferruci E. High frequency vibration training increases muscle power in postmenopausal women. Arch. Phys. Med. Rehabil. 2003;84:1854–1857. doi: 10.1016/s0003-9993(03)00357-5. [DOI] [PubMed] [Google Scholar]
- 27.SitjàRabert M, Rigau Comas D, Fort Vanmeerhaeghe A, Santoyo Medina C, Roquéi Figuls M, Romero-Rodríguez D, Bonfill Cosp X. Whole-body vibration training for patients with neurodegenerative disease. Cochrane Database Syst. Rev. 2012;15:CD009097. doi: 10.1002/14651858.CD009097.pub2. doi:10.1002/14651858.CD009097. [DOI] [PubMed] [Google Scholar]
- 28.Soderling SH, Langeberg LK, Soderling JA, Davee SM, Simerly R, Raber J, Scott JD. Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice. Proc Natl Acad Sci USA. 2003;100:1723–1728. doi: 10.1073/pnas.0438033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van der Zee EA. Synapses, spines and kinases in mammalian learning and memory and the impact of aging. Neurosci. Biobehav. Rev. 2015;50:77–85. doi: 10.1016/j.neubiorev.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Weber-Rajek M, Mieszkowski J, Niespodziński B, Ciechanowska K. Whole-body vibration exercise in postmenopausal osteoporosis. Prz. Menopauzalny. 2015;14:41–47. doi: 10.5114/pm.2015.48679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winters BD, Saksida LM, Bussey TJ. Object recognition memory:neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci. Biobehav. Rev. 2008;32:1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]


