Abstract
Background:
Alzheimer’s disease (AD) is the most common cause of dementia that is an irretrievable chronic neurodegenerative disease. In the current study, we have examined the therapeutic effects of Iris germanica extract on Amyloid β (Aβ) induced memory impairment.
Materials and Methods:
Wistar rats were divided into five groups of 8 per each. Groups were as followed: control group which were normal rats without induction of AD, Aβ group which received Aβ (50 ng/side), iris 100 group which received Aβ + Iris (100 mg/kg), iris 200 group which received Aβ + Iris (200 mg/kg), and iris 400 group which received Aβ + Iris (400 mg/kg). AD was established by intrahippocampal injection of 50 ng/μl/side Aβ1-42. The day after surgery, animals in treatment groups received different doses of the aqueous extract of Iris by gavage for 30 days. Morris water maze test (MWM) was performed to assess the effects of I. germanica on learning and memory of rats with Aβ induced AD.
Results:
Data from MWM tests, including escape latency and traveled distance, demonstrated that I. germanica extract could markedly improve spatial memory in comparison to control. Moreover, the plant had a significantly better effect on the performance of AD rats in the probe test.
Conclusion:
I. germanica extract can successfully reverse spatial learning dysfunction in an experimental model of AD. Further neuro psyco-pharmacological studies are mandatory to reveal the mechanism of action of this natural remedy in the management of AD symptoms.
Keywords: Alzheimer’s disease, Iris germanica, spatial learning, escape latency, traveled distance, beta amyloid, common flag, traditional Persian medicine
Introduction
Today, in 2016, over 47 million people live with dementia worldwide. This population is rapidly growing and is estimated to increase to 131.5 million until the end of 2050 (Prince et al., 2016).
Alzheimer’s disease (AD) is an essential cause of dementia that is an irretrievable chronic neurodegenerative disease (Fodale et al., 2006). Major neuropathological characteristics of AD comprise extracellular insoluble senile amyloid beta (Aβ) accumulation in the form of protein plaques surrounded by dead or dying neurons, extensive neuronal loss, as well as hyperphosphorylation of tau proteins precipitated as intracellular neurofibrillary tangles (NFTs) (Iqbal et al., 2005; Selkoe, 2001). In addition, cholinergic deficiency, dysfunctional apoptosis and synaptic alterations in brain hippocampal areas and the entorhinal cortex, underlie the pathological basis for AD (Law et al., 2001; Majlessi et al., 2012).
Aβ is a pro-inflammatory highly toxic material which results in neuro-inflammation in the brain tissue (Tuppo and Arias, 2005). In the brain, inflammation is associated with stimulation of microglial cells which have a pivotal role in neurodegenerative processes including those occurring in AD (Thameem Dheen et al., 2007).
A growing body of experiments along with clinical evidence support the idea that elevated Aβ levels can be linked with impairment of cognition and can cause pathological events resulting in cognitive defects such as those identified in AD (Bharadwaj et al., 2009; Rodrigue et al., 2009). Oxidative stress markers and neuro inflammation possess a pivotal role in the initiation and development of the disease (Akiyama et al., 2000; Moreira et al., 2009). Although a wide range of studies has been performed for understanding the etiology and pathophysiology of AD, current treatment approaches with acetyl choline esterase inhibitors (AChEIs) are just symptomatic, performed for the management of cognitive symptoms including impairment in perception (Bassil and Grussberg, 2009; Neugroschl and Sano, 2009). Despite the ability of these drugs in alleviating symptoms, they cannot inhibit disease development, the period of their pharmacological effect is restricted, and they also have several adverse effects such as gastrointestinal complications (Mimica and Presecki, 2009). The restrictions of current AD therapeutics have encouraged investigators to discover novel treatments. Current investigations are considering the preventive and/or disease-modifying drugs (Basil and Grussberg, 2009). A wide range of studies is performed to identify drugs that can decrease Aβ accumulation via affecting proteases, which modify Aβ formation, as well as increasing its clearance.
Various medicinal plants, as well as their active components have been used in traditional and complementary therapeutic approaches to improve cognitive performance and managing other complications of AD (Howes et al., 2003; May et al., 2009; Mukherjee et al., 2007). Iris germanica L. which is known as “Irsa” in traditional Persian medicine, can be widely found in various geographical regions all over the world and is cultivated for decorative purposes, as well (Rahman et al., 2003). Iris species are rich sources of isoflavonoids. Various isoflavones like irigenin, nigricin, irilone, and irisolidone were identified in I. germanica rhizome (Ibrahim et al., 2017; Roger et al., 2012; Xie et al., 2013). In addition, benzene derivatives have been isolated from the methanolic extract of I. germanica (Asghar et al., 2010). Moreover, different sterols including sitosterol and stigmasterol have been reported from it (Ibrahim et al., 2012). The rhizomes have been used to manage some neurodegenerative disorders including AD in traditional Persian medicine (Choudhary et al., 2005). Several species of the genus Iris have been reported to exhibit a variety of biological functions including anti-bacterial, anti-ulcer and anti-inflammatory properties (Rahman et al., 2003). To our knowledge, there is no scientific report on the beneficial effect of I. germanica on neurodegenerative disorders. Hence, this study was conducted to examine the effect of I. germanica extract on the spatial performance of rats in an experimental model of AD.
Materials and Methods
Animals
Male Wistar rats with the weight range of 200–250 g were housed as five animals per cage with free access to food and water. Animals were kept in standard animal house condition including 12 h light/dark cycle and room temperature of 20–22 °C. All experimental procedures were performed based on the guidelines of Ethical Committee for the care and use of laboratory animals in Tehran University of Medical Sciences.
Establishment of AD model
Preparation of β-amyloid peptide1–42 (Aβ1–42) and fibrillation
The Aβ1–42, supplied fro Sigma-Aldrich (St. Louis, MO. USA), was dissolved, and the samples were kept at −20 °C until further experiment. Aβ1–42 solutions were prepared with the concentration of 100 ng/μl in phosphate buffered saline (PBS, 0.1 M) and incubated for a five-day period at 37 °C. On the day of the experiment, PBS was inserted to the solution and the final concentration of 50 ng/μl was obtained.
Stereotaxic surgery
Wistar rats were anesthetized via i.p. injection of 5 mg/kg xylazine and 90 mg/kg ketamine, and were fixed under stereotaxic device (Stoelting, USA). A suture was made along the midline. Then, the scalp was retracted, and the bregma area was washed and prepared for injection.
Using a 1-μl Hamilton micro syringe, one microliter of Aβ or vehicle (sham group) was bilaterally injected in the CA1 section of the hippocampus (50 ng/μl/side). The coordinates were based on the Paxinos and Watson rat brain atlas: incisor bar −3.3 mm, 3.8 mm posterior to the bregma, ±3.2 mm lateral to the sagittal suture and 2.7 mm down from top of the skull. Microinjections were applied over a slow period of 60 s (Eftekharzadeh et al., 2012).
Plant material and preparation of Iris aqueous extract
I. germanica rhizomes were purchased from a local herbal store. A botanist authenticated the plant, and a voucher specimen (PMP-228) was deposited in the Herbarium of Department of Pharmacognosy, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.
The rhizomes were cut into small pieces, washed in distilled water, air-dried at room temperature and milled into coarse particles.
To obtain the aqueous extract, 100 g of fine plant powder was soaked in 100 ml distilled water and left for 24 h in the refrigerator, and then boiled gently on the heater with the magnetic stirrer for approximately 6 h. Then, the extract was filtered using filter paper. The obtained filtrate was concentrated with a rotary evaporator and dried in a vacuum oven for 3 days at 40 °C. The extraction ratio was 20%. Finally, the prepared extract was stored in the refrigerator at 4 °C.
Extract administration
Animals were randomly put into five groups of 8 rats per each. Treatment groups were as followed: control group which were normal rats without induction of AD, Aβ group in which received Aβ (50 ng/side), iris 100 which received Aβ (50 ng/side) + Iris (100 mg/kg), iris 200 which received Aβ (50 ng/side) + Iris (200 mg/kg), and iris 400 which received Aβ (50 ng/side) + Iris (400 mg/kg). The condensed aqueous Iris extract was suspended in distilled water. The day after surgery, animals in treatment groups received three different doses (100, 200 or 400 mg/kg) of the extract by gavage for 30 days.
Morris water maze test (MWM)
Thirty days after surgery, MWM training of rats in all experimental groups was started. The maze included a round black-colored water pool (136 cm diameter and 60 cm height), which was filled with 22±2 °C water to a depth of 40 cm. The pool was divided into four equal parts and a Plexiglas hidden platform, with 10 cm diameter, was placed 1 cm under the water surface in the center of the target quadrant, which ws the North-West part. The rats training continued for a 4-day period. Each day of training consisted of one block including four trials. Each of the trials was performed by putting the animal in one of the four quadrants of MWM. Rats had a 90 s period to swim in the pool and find the underwater platform. When an animal was not able to find the platform during this default period, it was guided to the platform by the experimenter. The animals had 20 s rest between two tandem trials and all trials were performed at the same time of the morning each day. The behavior and movement of rats were recorded using a video camera, linked to a computer, above the center of the maze. The test evaluating learning capabilities were performed at the days 31 to 34 after the post-training intrahippocampal injections of the drugs by calculating escape latency (time to reach the platform), traveled distance (the distance to find the platform), and swimming speed parameters. In order to assess post-training probe trial experiments, rats were trained for a period of four days after the recovery from surgery as previously described.
Post-training probe trial tests were performed on day 35 Aβ injection to evaluate the occupancy and crossing of rats in the proximity of the target quadrant (the quadrant included hidden platform during training trials). During the probe trial test, the platform was removed from the pool, and Wistar rats were put in the pool in a part opposite to the target quadrant and permitted to freely swim for 90 s.
Using the Ethovision tracking system (Noldus Information Technology, Wageningen, Netherlands), learning function and spatial memory retention were analyzed.
Histopathological evaluations
For the confirmation of Aβ plaque formation in rat brain, 35 days after Aβ injection, 5 animals (1 rat from each group) were euthanized and their brains were immersed in 4% paraformaldehyde for 24 h and fixative formalin %15 for 24-48 h. Then, an automated processor executed dehydration and paraffin embedding. The brain tissue was segmented and stained by Congo red based on the previous report (Wilcock et al., 2006).
Results
Effects of different treatments on traveled distance, escape latency and swimming speed average for all training days
Figure 1 shows the traveled distance (a), the escape latency (b) and the swimming speed (c) for five treatment groups. Aβ protein resulted in a remarkable elevation of traveled distance (P<0.001) as well as escape latency (P<0.001) in comparison to the control animals (Figure 1a, b). As shown in Figure 1, all parameters, except the swimming speed, improved in groups treated with Iris extract in comparison to Aβ group. Traveled distance was significantly reduced in all groups which received Iris compared with the Aβ injected group (P<0.001 for iris 100 and iris 200 and P<0.0001 for iris 400) (Figure 1a). The same results were attained in analyzing the escape latencies and Iris treated groups showed a significant decrease in escape latency compared to the Aβ group (P<0.001) (Figure 1b). It should be mentioned that, as shown in Figure 1c, no significant differences were observable in swimming speed between the treatment groups (P>0.05).
Figure 1.
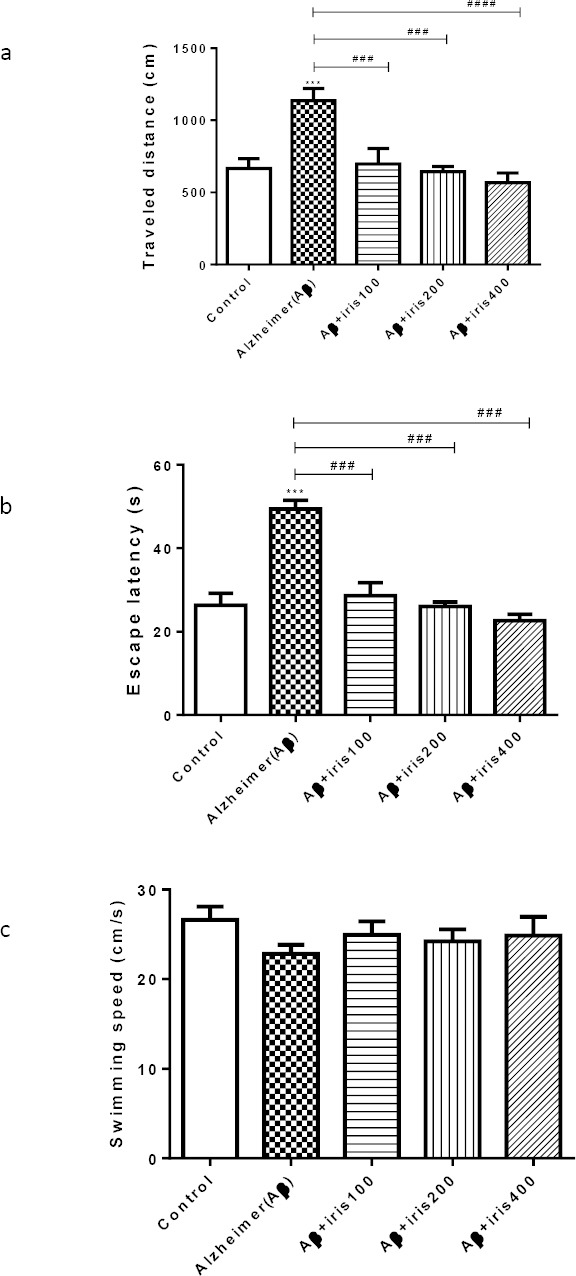
Effect of intrahippocampal injection of Aβ, Aβ plus Iris treated animals on (a) traveled distance, (b) escape latency to platform zone, and (c) swimming speed in MWM. Five groups of animals received bilateral infusion of either phosphate buffered saline (control group), Aβ (50 ng/side), Aβ (50 ng/side) + Iris (100 mg/kg), Aβ (50 ng/side) + Iris (200 mg/kg), Aβ (50 ng/side) + Iris (400 mg/kg). Each point shows the mean ± SEM for 6–8 rats. # Significantly different from Aβ injected group. *Significantly different from control group (P<0.05). **, ##: P<0.01; ***, ###: P<0.001; ****, ####: P<0.0001.
Effects of different treatments on traveled distance, escape latency and swimming speed in training days
Figure 2 shows the results of the behavioral tests for all days in different experimental groups. A statistically significant decrease in the traveled distance was observed on the 3rd and 4th days of the experiment in comparison to the first day in the control group (P<0.01 and P<0.0001 for the 3rd and 4th days, respectively). Herbal treated groups in all doses demonstrated a significant reduction in traveled distance (P<0.0001 in day 4 compared with day 1). No significant differences between traveled distances on the second, third and fourth days were observed, compared to the first day for the Aβ group (Figure 2a).
Figure 2.
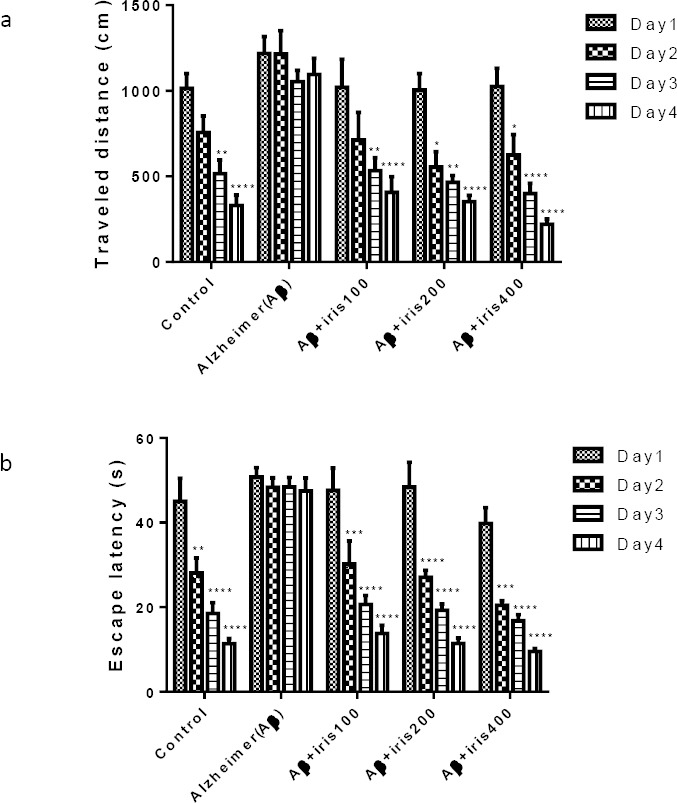
Effect of oral administration of Iris on (a) traveled distance and (b) escape latency to platform zone in MWM for all training days for five groups. Five groups of animals received bilateral infusion of either phosphate buffered saline (control group), Aβ (50 ng/side), Aβ (50 ng/side) + Iris (100 mg/kg), Aβ (50 ng/side) + Iris (200 mg/kg), Aβ (50 ng/side) + Iris (400 mg/kg). Each point shows the mean± SEM for 6–8 rats. * Significantly difference between the first and other training days. (P<0.05); **: P<0.01; ***: P<0.001; ****: P<0.0001.
A similar trend was achieved after the analyzing of escape latency values. Escape latency time significantly reduced on the 3rd and 4th days of the study compared to the first day in the control group (P<0.01 and P<0.0001). Likewise, the treatment group that received Iris 100 mg/kg, 200 mg/kg or 400 mg/kg, demonstrated a remarkable reduction in escape latency on the 4th experimental day compared with the first training day (P<0.0001). Same as the traveled distance, no statistically significant difference was observed between escape latencies on 2nd, 3rd and 4th days compared to the first day for the group that only received Aβ (Figure 2b).
Finally, results of the swimming speed for all of the treatment groups showed no significant effect by neither Aβ nor Iris administration.
Effects of different treatments on post training probe trial test
Figure 3 illustrates the outcomes of post-training probe trial test. Results showed that this parameter significantly reduced in Aβ group compared with the control group (P<0.01). Treatment of animals with I. germanica extract significantly elevated the time spent in the target quadrant in comparison with Aβ injected group (P<0.01)
Figure 3.
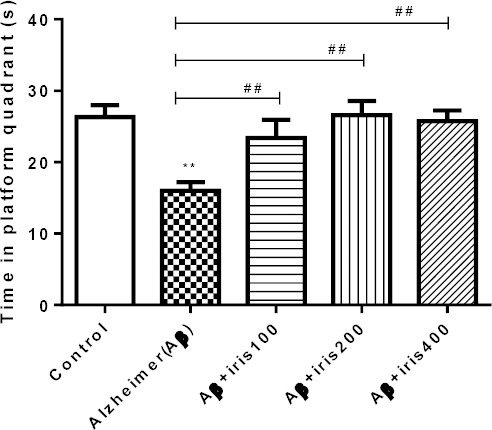
Post-training probe trial tests, which were performed 21 days after drug infusion for all groups. In this test, we removed the platform, left animals for 90 s in the water and measured the time that animals spent in the target quadrant. Five groups of animals received bilateral intrahippocampal infusion of either phosphate buffered saline (control group), Aβ (50 ng/side), Aβ (50 ng/side) + Iris (100 mg/kg), Aβ (50 ng/side) + Iris (200 mg/kg), Aβ (50 ng/side) + Iris (400 mg/kg). Each point shows the mean ± SEM for 6–8 rats. *Significantly different from control group. # Significantly different from Aβ injected groups (P<0.05). **, ##: P<0.01; ***, ###: P<0.001.
Histological tests
For detection of Aβ plaques in the hippocampus, Congo red staining was performed on the tissue. As shown in Figure 4, the staining confirmed the formation of Aβ plaque in brains of the Aβ-injected animals in the hippocampal area. Optical microscope Images showed a significant decrease in the number of Aβ plaques in all three groups treated with Iris germanica extract.
Figure 4.
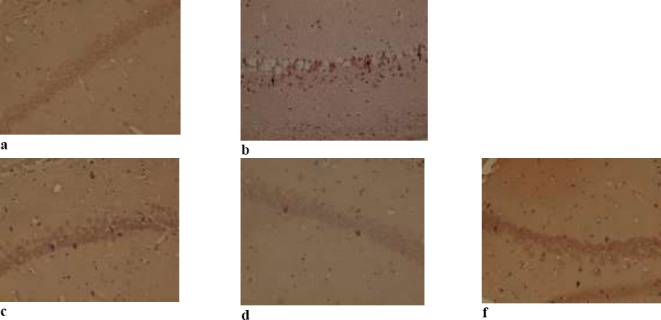
Congo red staining to detect beta-amyloid plaques in the hippocampus in five group: (a) phosphate buffered saline (control group), (b) Aβ (50 ng/side), (c) Aβ (50 ng/side) + Iris (100 mg/kg), (d) Aβ (50 ng/side) + Iris (200 mg/kg), (f) Aβ (50 ng/side) + Iris (400 mg/kg).
Discussion
Alzheimer’s disease (AD) is known as an age-related neurodegenerative disorder that is linked with oncoming loss of cognitive capacity and mild memory impairment (Lustig et al., 2003). The neuropathologic features of the disease including extracellular senile plaques, intracellular neurofibrillary tangles as well as neuronal loss in some brain areas including hippocampal area (Majlessi et al., 2012). One of the key events observed in the neuropathogenesis of AD is Aβ protein deposition and the amyloid plaques formation of in the brain (Wang et al., 2014). Aβ is a 39–43 amino acid peptide that is the result of proteolysis of amyloid precursor protein by β and γ-secretases and the Aβ 1–42 is the most neurotoxic fragment of this peptide (Lambert et al., 1998). In this experiment, intrahippocampal injection of the aggregated Aβ1-42 significantly induced impairments in cognitive ability in the rat. In our study, we have found that aqueous extract of I. germanica L. can improve the cognitive function (learning and memory) in AD model rat induced by Aβ1- 42 that tested by MWM. A wide range of clinical and preclinical studies has suggested the therapeutic role of medicinal plants and their active constituents in the management of neurological disorders including neurodegenerative diseases (Bahramsoltani et al., 2015; Farzaei et al., 2016; Shahpiri et al., 2016).
In a brain affected by AD, the severity of memory dysfunction and cerebral deposition of Aβ is associated with a marked cholinergic dysfunction. The decrease of ACh in specific brain areas leads to learning and memory deficits (Kar et al., 2004). Increased AChE activity and decreased choline acetyltransferase (ChAT) activity may result in the reduction of Ach level. Many studies have suggested that inhibition of neuronal AChE activity in experimental models leads to increase in Ach level and consequently improve the cognitive function (Chattipakron et al., 2007; Nakdook et al., 2010; Ingkaninan et al., 2003). AchE inhibitory activity of I. germanica var. florentina have been demonstrated in vitro (Ullah et al., 2016).
It has been found that I. germanica has potent antioxidant and anti-inflammatory properties (Nadaroglu et al., 2007; Rahman et al., 2003). Inflammation and oxidative damage are two factors that implicate in AD pathogenesis. So, we can suggest that these two mechanisms are involved in the anti-Alzheimer effect of this medicinal plant.
Also, one animal study revealed that Iris extract can lower serum level of cholesterol which is associated with the promotion of amyloidogenesis by regulating β- and γ-secretase activity (Choudhary et al., 2005). Hence, modulating amyloidogenesis mediated by lowering the level of cholesterol is another neuropharmacological mechanism of I. germanica in improving learning and memory abilities of AD (Bodovitz and Klein, 1996; Frears et al., 1999; Wolozin, 2001).
These findings suggest that Iris could attenuate the progression of AD. The mechanism is related to AchE inhibitory, cholesterol lowering, antioxidant and anti-inflammatory properties. Therefore, Iris germanica may be considered as a new therapeutic option for AD.
Conclusion
Iris germanica extract was used to treat experimental model of AD for the first time. The results showed that Iris markedly enhanced the cognitive function of Aβ induced-AD rat in the behavioral test. Further neuropsycopharmacological studies are mandatory to reveal the perfect mechanism of action of this natural remedy in the management of AD symptoms. In addition, the phytochemical investigation is suggested for identifying the main active constituents of I. germanica responsible for the therapeutic effect on AD behavioral symptoms.
Acknowledgement
This study has been partially supported by Tehran University of Medical Sciences (TUMS); Grant No. 93-04-86-27014.
References
- 1.Akiyama H, Barger S, Barnum S, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asghar SF, Habib ur R, Atta ur R, Choudhary MI. Phytochemical investigations on Iris germanica. Nat. Prod. Res. 2010;24:131–139. doi: 10.1080/14786410802435950. [DOI] [PubMed] [Google Scholar]
- 3.Bahramsoltani R, Farzaei MH, Farahani MS, Rahimi R. Phytochemical constituents as future antidepressants:a comprehensive review. Rev. Neurosci. 2015;26:699–719. doi: 10.1515/revneuro-2015-0009. [DOI] [PubMed] [Google Scholar]
- 4.Bassil N, Grossberg GT. Novel regimens and delivery systems in the pharmacological treatment of Alzheimer’s disease. CNS drugs. 2009;23:293–307. doi: 10.2165/00023210-200923040-00003. [DOI] [PubMed] [Google Scholar]
- 5.Bharadwaj PR, Dubey AK, Masters CL, Martins RN, Macreadie IG. Aβaggregation and possible implications in Alzheimer’s disease pathogenesis. J. Cell. Mol. Med. 2009;13:412–421. doi: 10.1111/j.1582-4934.2009.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodovitz S, Klein WL. Cholesterol modulates-secretase cleavage of amyloid precursor protein. J. Biol. Chem. 1996;271:4436–4440. doi: 10.1074/jbc.271.8.4436. [DOI] [PubMed] [Google Scholar]
- 7.Chattipakorn S, Pongpanparadorn A, Pratchayasakul W, Pongchaidacha A, Ingkaninan K, Chattipakorn N. Tabernaemontana divaricata extract inhibits neuronal acetylcholinesterase activity in rats. J. Ethnopharmacol. 2007;110:61–68. doi: 10.1016/j.jep.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Choudhary MI, Naheed S, Jalil S, Alam JM, Atta ur R. Effects of ethanolic extract of Iris germanica on lipid profile of rats fed on a high-fat diet. J. Ethnopharmacol. 2005;98:217–220. doi: 10.1016/j.jep.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Eftekharzadeh B, Ramin M, Khodagholi F. Inhibition of PKA attenuates memory deficits induced by beta-amyloid (1-42), and decreases oxidative stress and NF-kappaB transcription factors. Behav. Brain Res. 2012;226:301–308. doi: 10.1016/j.bbr.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Farzaei MH, Bahramsoltani R, Rahimi R, Abbasabadi F, Abdollahi M. A systematic review of plant-derived natural compounds for anxiety disorders. Curr. Top. Med. Chem. 2016;16:1924–1942. doi: 10.2174/1568026616666160204121039. [DOI] [PubMed] [Google Scholar]
- 11.Fodale V, Quattrone D, Trecroci C, Caminiti V, Santamaria L. Alzheimer’s disease and anaesthesia:implications for the central cholinergic system. Br. J. Anaesth. 2006;97:445–452. doi: 10.1093/bja/ael233. [DOI] [PubMed] [Google Scholar]
- 12.Frears ER, Stephens DJ, Walters CE, Davies H, Austen BM. The role of cholesterol in the biosynthesis of beta-amyloid. NeuroReport. 1999;10:1699–1705. doi: 10.1097/00001756-199906030-00014. [DOI] [PubMed] [Google Scholar]
- 13.Howes MJR, Perry NS, Houghton PJ. Plants with traditional uses and activities, relevant to the management of Alzheimer’s disease and other cognitive disorders. Phytother. Res. 2003;17:1–18. doi: 10.1002/ptr.1280. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim SR, Mohamed GA, Al-Musayeib NM. New constituents from the rhizomes of Egyptian Iris germanica L. Molecules. 2012;17:2587–2598. doi: 10.3390/molecules17032587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibrahim SR, Mohamed GA, Zayed MF, Ross SA. 8-Hydroxyirilone 5-methyl ether and 8-hydroxyirilone, new antioxidant and α-amylase inhibitors isoflavonoids from Iris germanica rhizomes. Bioorg. Chem. 2017;70:192–198. doi: 10.1016/j.bioorg.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Ingkaninan K, Temkitthawon P, Chuenchom K, Yuyaem T, Thongnoi W. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J. Ethnopharmacol. 2003;89:261–264. doi: 10.1016/j.jep.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Iqbal K, Alonso AdC, Chen S. Tau pathology in Alzheimer disease and other tauopathies. Biochim. Biophys. Acta, Mol. Basis Dis. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Kar S, Slowikowski SP, Westaway D, Mount HT. Interactions between beta-amyloid and central cholinergic neurons:implications for Alzheimer’s disease. J. Psychiatry Neurosci. 2004;29:427–441. [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert MP, Barlow A, Chromy BA. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Law A, Gauthier S, Quirion R. Say nO to Alzheimer’s disease:the putative links between nitric oxide and dementia of the Alzheimer’s type. Brain Res. Rev. 2001;35:73–96. doi: 10.1016/s0165-0173(00)00051-5. [DOI] [PubMed] [Google Scholar]
- 21.Lustig C, Snyder AZ, Bhakta M. Functional deactivations:change with age and dementia of the Alzheimer type. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majlessi N, Choopani S, Kamalinejad M, Azizi Z. Amelioration of amyloid β-Induced cognitive deficits by Zataria multiflora Boiss. essential oil in a rat model of Alzheimer’s disease. CNS Neurosci. Ther. 2012;18:295–301. doi: 10.1111/j.1755-5949.2011.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.May BH, Lit M, Xue CC. Herbal medicine for dementia:a systematic review. Phytother. Res. 2009;23:447–459. doi: 10.1002/ptr.2656. [DOI] [PubMed] [Google Scholar]
- 24.Mimica N, Presecki P. Side effects of approved antidementives. Psychiatr. Danubina. 2009;21:108–113. [PubMed] [Google Scholar]
- 25.Moreira PI, Duarte AI, Santos MS, Rego AC, Oliveira CR. An integrative view of the role of oxidative stress, mitochondria and insulin in Alzheimer’s disease. J. Alzheimer’s Dis. 2009;16:741–761. doi: 10.3233/JAD-2009-0972. [DOI] [PubMed] [Google Scholar]
- 26.Mukherjee PK, Kumar V, Mal M, Houghton PJ. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14:289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Neugroschl J, Sano M. An update on treatment and prevention strategies for Alzheimer’s disease. Curr. Neurol. Neurosci. Rep. 2009;9:368–376. doi: 10.1007/s11910-009-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadaroglu H, Demir Y, Demir N. Antioxidant and radical scavenging properties of Iris germanica. Pharm. Chem. J. 2007;41:409–415. [Google Scholar]
- 29.Nakdook W, Khongsombat O, Taepavarapruk P, Taepavarapruk N, Ingkaninan K. The effects of Tabernaemontana divaricata root extract on amyloid β-peptide 25–35 peptides induced cognitive deficits in mice. J. Ethnopharmacol. 2010;130:122–126. doi: 10.1016/j.jep.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M. The World Alzheimer Report, Improving healthcare for people living with dementia. Alzheimer’s Disease International (ADI), London. 2016 http://www.alz.co.uk/research/world-report-2016 .
- 31.Rahman AU, Nasim S, Baig I. Anti-inflammatory isoflavonoids from the rhizomes of Iris germanica. J. Ethnopharmacol. 2003;86:177–180. doi: 10.1016/s0378-8741(03)00055-2. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigue KM, Kennedy KM, Park DC. Beta-amyloid deposition and the aging brain. Neuropsychol. Rev. 2009;19:436–450. doi: 10.1007/s11065-009-9118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roger B, Jeannot V, Fernandez X, Cerantola S, Chahboun J. Characterisation and quantification of flavonoids in Iris germanica L, Iris pallida Lam. Phytochem. Anal. 2012;23:450–455. doi: 10.1002/pca.1379. [DOI] [PubMed] [Google Scholar]
- 34.Selkoe DJ. Alzheimer’s disease:genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 35.Shahpiri Z, Bahramsoltani R, Farzaei MH, Farzaei F, Rahimi R. Phytochemicals as future drugs for Parkinson’s disease:a comprehensive review. Rev. Neurosci. 2016;27:651–668. doi: 10.1515/revneuro-2016-0004. [DOI] [PubMed] [Google Scholar]
- 36.Thameem Dheen S, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr. Med. Chem. 2007;14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- 37.Tuppo EE, Arias HR. The role of inflammation in Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Ullah F, Ayaz M, Sadiq A. Phenolic, flavonoid contents, anticholinesterase and antioxidant evaluation of Iris germanica var;florentina. Nat. Prod. Res. 2016;30:1440–1444. doi: 10.1080/14786419.2015.1057585. [DOI] [PubMed] [Google Scholar]
- 39.Wang Q, Wang C, Shu Z. Valeriana amurensis improves amyloid-beta 1-42 induced cognitive deficit by enhancing cerebral cholinergic function and protecting the brain neurons from apoptosis in mice. J Ethnopharmacol. 2014;153:318–325. doi: 10.1016/j.jep.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Wilcock DM, Gordon MN, Morgan D. Quantification of cerebral amyloid angiopathy and parenchymal amyloid plaques with Congo red histochemical stain. Nat. Protoc. 2006;1:1591–1595. doi: 10.1038/nprot.2006.277. [DOI] [PubMed] [Google Scholar]
- 41.Wolozin B. A fluid connection:cholesterol and Aβ. Proceed. Natl. Acad. Sci. U. S. A. 2001;98:5371–5373. doi: 10.1073/pnas.101123198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie GY, Qin XY, Liu R, Wang Q, Lin BB, Wang GK, Xu GK, Wen R, Qin MJ. New isoflavones with cytotoxic activity from the rhizomes of Iris germanica L. Nat. Prod. Res. 2013;27:2173–2177. doi: 10.1080/14786419.2013.796468. [DOI] [PubMed] [Google Scholar]


