Abstract
Background: :
Fucoidan is a complex sulfated polysaccharide extracted from brown seaweed and has a wide variety of biological activities. It not only inhibits cancer cell growth but also inhibits tyrosinase in vitro. Therefore, it is of interest to investigate the effect of fucoidan on B16 murine melanoma cells as the findings may provide new insights into the underlying mechanism regarding the inhibition of melanin formation by fucoidan. In the present study, we aimed to investigate the anti-melanogenic effect of fucoidan and its inhibitory effect on B16 cells.
Materials and Methods:
The influence of fucoidan on B16 melanoma cells and cellular tyrosinase was examined. Cell viability was examined by the cell counting kit-8 assay. Cellular tyrosinase activity and melanin content were determined using spectrophotometric methods and protein expression was analyzed by immunoblotting. Morphological changes in B16 melanoma cells were examined by phase contrast microscopy and apoptosis was analyzed by flow cytometry.
Results:
In vitro studies were performed using cell viability analysis and showed that fucoidan significantly decreased viable cell number in a dose-response manner with an IC50 of 550 ±4.3 µg/mL. Cell morphology was altered and significant apoptosis was induced when cells were exposed to 550 µg/mL fucoidan for 48 h.
Conclusion:
This study provides substantial evidence to show that fucoidan inhibits B16 melanoma cell proliferation and cellular tyrosinase activity. Fucoidan may be useful in the treatment of hyperpigmentation and as a skin-whitening agent in the cosmetics industry.
Keywords: B16 melanoma cells, Fucoidan, Melanogenesis, Tyrosinase
Introduction
Melanin synthesis is the most important function and differentiation characteristic of melanocytes. Melanin protects the skin from ultraviolet radiation damage; however, excess expression or uneven distribution of melanin may cause chloasma, freckles and post-inflammatory hyperpigmentation (de Fine Olivarius et al., 1999; Hearing, 2005; Mizusawa et al., 2011). Therefore, whitening agents have been widely used in cosmetics. The melanin synthesis pathway is a highly complex process regulated by enzymes such as tyrosinase, tyrosinase-related protein-1 and tyrosinase-related protein-2 (Fang et al., 2001; Negroiu et al., 2000; Ye et al., 2010; Lee et al., 2012). Tyrosinase hydroxylates tyrosine to form 3, 4-dihydroxyphenylalanine (DOPA). DOPA is then oxidized to dopamine quinone and dopachrome (Parvez et al., 2006). Tyrosinase is a key enzyme which catalyzes the rate-limiting step of melanin biosynthesis (Cooksey et al., 1997; Hearing, 1999; Kim and Uyama, 2005). Therefore, research on whitening agents is mainly concentrated on tyrosinase inhibitors.
Previous studies have shown that fucoidan possesses diverse biological activities such as antilipidemic (Raghavendran et al., 2005), antithrombotic (Chandía et al., 2008; Zhao et al., 2016), inflammation modulation (Cumashi et al., 2007; Jin and Yu, 2015), antiviral (Hayashi et al., 2008), antitumor (Alekseyenko et al., 2007; Sun et al., 2016) and antioxidant (Wang et al., 2010; Saravana et al., 2016) activities. In our previous study, we found that fucoidan inhibited cell-free tyrosinase activity, and interacted with several residues including copper ions located in the active site (Wang et al., 2012). As fucoidan inhibits tyrosinase and has anti-cancer effects, an investigation on the effect of fucoidan on melanin formation in melanoma may produce some interesting results. Thus, the present study was undertaken to investigate the ability of fucoidan to inhibit cellular tyrosinase derived from melanin-producing B16 melanoma cells and its ability to inhibit melanin formation. The effect of fucoidan on B16 melanoma cells was also determined.
Materials and methods
Materials
Fucoidan (from Fucus vesiculosus), and L-3,4-dihydroxyphenylalanine were purchased from Sigma. Triton X-100 and dimethylsulfoxide were purchased from Sigma. Other compounds used in the study were obtained from Shanghai R&D Center for Standardization of Chinese Medicines (Shanghai, China, purity > 99%).
Cell culture and treatment
B16 murine melanoma cells (Shanghai Institutes for Biological Science, Chinese Academy of Sciences, China) were grown in DMEM medium (Gibco, USA) supplemented with 10% fetal bovine serum (Sigma), 100 units/mL of penicillin (PAA, Austria) and 100 µg/mL of streptomycin (PAA, Austria) at 37°C in a humidified atmosphere of 5% CO2. When the cells reached logarithmic growth phase, various concentrations of fucoidan were added to the cells and cultured 48 h.
Cellular tyrosinase activity assay
Cellular tyrosinase activity was measured as previously described with some modifications (Lee et al., 2006). B16 melanoma cells were seeded in 96-well plates (2×103cells/well) and allowed to adhere for 12 h at 37°C. The cells were incubated with different doses of fucoidan for 48 h and then washed with PBS and lysed with mammalian protein extraction reagent (Pierce, Rockford, IL, USA). The lysates were then centrifuged at 17,500 g for 15 min at 4°C. The protein concentration was determined by the Bradford method (Bio-Rad Laboratories Inc., Hercules, CA, USA). A 200 µL mixture containing 40 µg protein was added to each well of the 96-well plate and incubated for 1 h at 37°C with 2.5 mM 3, 4-dihydroxy-L-phenylalanine (L-DOPA). The absorbance was measured using a microplate reader at 475 nm (BD Bioscience, USA).
Measurement of cellular melanin content
Melanin content was measured as described previously with minor modifications (Jones et al., 2002). The cells were seeded in 60 mm dishes (2×103 cells/well) and allowed to adhere for 12 h at 37°C and then exposed to different concentrations of fucoidan for 48 h, washed with PBS and then lysed in 150 mL of 1 M NaOH containing 10% dimethylsulfoxide for 1 h at 70°C. The samples were placed in wells on a 96-well microplate, and the absorbance was measured at 405 nm with a Microplate Reader. The protein concentration in each sample was determined using the Bio-Rad Protein Assay.
Western blot analysis
A protein suspension was obtained using the method described above. The proteins were separated on SDS-polyacrylamide gels (20 µg per sample) and transferred to polyvinylidene difluoride membranes and after blocking and washing were incubated with primary antibodies. The primary antibodies used were goat polyclonal antibodies against tyrosinase (Product number SC7833), tyrosinase-related protein-1 (TRP1) (Product number SC10443), rabbit polyclonal antibodies against tyrosinase-related protein-2 (TRP2) (Product number AB74073) and the microphthalmia-associated transcription factor (MITF) (product number BS1550). Immunoreactivity was determined with secondary antibodies and enhanced chemiluminescence detection reagents (KGP 1123, Nanjing KeyGEN Biotechnology Co., Ltd., Nanjing, China) using a gel imaging system (ChemiScope 2850, Clinx Science Instruments Co., Let., Shanghai, China). β-Actin was used to adjust protein loading.
Measurement of cell viability
Cell viability was determined using the cell counting kit-8 (CCK8) assay. Briefly, B16 melanoma cells were seeded in 96-well plates at a density of 2×103 cells/well and allowed to adhere for 12 h at 37°C. After 200 (L of substrate containing fucoidan (0, 100, 200, 300, 450, 500, 550 and 600 µg/mL) was added by changing the medium, the cells were incubated for 48 h. Substrate (200 µL) containing 10% CCK8 was added to each well by changing the medium, the cells were incubated for another 2.5 h and the absorbance was measured at 450 nm with a microplate reader (BioTeck, USA).
Morphological changes in B16 melanoma cells
B16 murine melanoma cells in the logarithmic growth phase were inoculated into 6 wells of a glass cover plate for 24 h, and then fucoidan was added to the cells with a final concentration of 0 µg/mL and 550 µg/mL. The cells were then incubated for a further 48 h. Cell morphology and changes in morphology were observed by phase contrast microscopy.
Measurement of apoptosis
B16 murine melanoma cells were incubated with 0 µg/mL and 550 µg/mL fucoidan for 48 h, respectively. The cells were digested by pancreatic enzyme, suspended in medium, washed with precooled PBS, and suspended in binding buffer. Then 5 µL Annexin V-FITC staining fluid and 10 µL PI staining fluid were added to the suspensions and incubated at room temperature away from light for 15 min. Apoptosis was analyzed by flow cytometry (BD Biosciences, USA).
Results
Effect of fucoidan on cellular tyrosinase activity and melanin synthesis in B16 cells
In our earlier report, fucoidan significantly inhibited mushroom tyrosinase in a cell-free system with an IC50 of 11.5 ± 1.3 mg/mL (Wang et al., 2012). In the present study, fucoidan also inhibited tyrosinase. Cellular tyrosinase activity was reduced by fucoidan in a dose-dependent manner (Figure 1). The inhibitory concentration of fucoidan leading to 50% activity loss (IC50) was estimated to be 550 ±4.3 µg/mL. When these results were compared with our earlier report (Wang et al., 2012), it was concluded that the ability of fucoidan to inhibit tyrosinase was different when tyrosinase was free in cells. These results demonstrated that the ability of fucoidan to inhibit cellular tyrosinase was much better than its ability to inhibit free tyrosinase.
Figure 1.
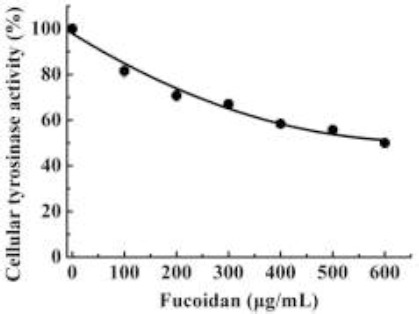
Effect of fucoidan on cellular tyrosinase activity
The effect of fucoidan on melanin production in B16 melanoma cells was determined. Figure 2 shows that fucoidan reduced cellular melanin content in a dose-dependent manner. From Figure 1 and 2, it can be assumed that decreased tyrosinase activity reduced melanin synthesis as tyrosinase is the key enzyme in melanin synthesis. As fucoidan has antitumor activity, we determined whether fucoidan inhibited B16 murine melanoma cells. If the cells were inhibited by fucoidan then reduced melanin synthesis would be the result of both tyrosinase and cell inhibition.
Figure 2.
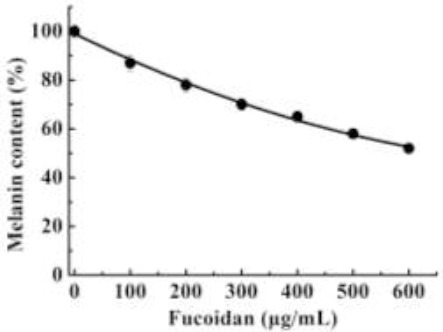
Effect of fucoidan on melanin content
Effect of fucoidan on the expression of MITF, tyrosinase, TRP-1 and TRP-2
Melanin synthesis is stimulated by tyrosinase, TRP-1, and TRP-2, and MITF is the most important transcription factor in the regulation of tyrosinase, TRP1 and TRP2 expression. These proteins were evaluated using western blotting after treatment with different fucoidan concentrations for 48 h. As shown in Figure 3, there was no obvious effect on TRP-1, TRP-2, and MITF expression, but the expression of tyrosinase and MITF were up-regulated.
Figure 3.
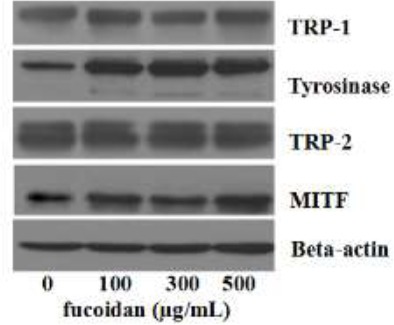
Effect of fucoidan on the expression of tyrosinase, TRP-1, TRP-2, MITF
Effect of fucoidan on cell viability
To determine the effects of fucoidan on cellular melanin synthesis, cell viability was evaluated. The effects of different fucoidan concentrations (0–600 µg/mL) on the growth of B16 melanoma cells were assessed by measuring cell viability using the CCK-8 assay. The results of this experiment are shown in Figure 4. The growth of B16 melanoma cells was affected in a dose-dependent manner following the addition of fucoidan. Fucoidan dose-dependently reduced the number of viable cells present, with cell viability reduced to 46.37±2.6% following the addition of 600 µg/mL fucoidan. The IC50 was 530±3.32 µg/mL. Fucoidan not only inhibited tyrosinase activity, but also had a very good inhibitory effect on melanocytes (Figure 4). These results suggested that fucoidan is a very good whitening agent at the molecular level and cellular level. When the concentration of fucoidan was 500 µg/ml, cell viability was approximately 50.65%, and the cellular tyrosinase activity was 55.7%. These results were similar to those with kojic acid (Chan et al., 2011).
Figure 4.
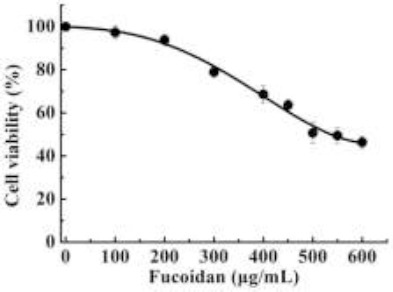
Effect of fucoidan on B16 melanoma cells
Effect of fucoidan on cell morphology
B16 melanoma cells were cultured with fucoidan (550 µg/mL) for 48 h, and then observed under a phase contrast microscope. B16 melanoma cells grew well in the control group. The cells were dense with regular growth. In contrast to the control, fucoidan treatment severely inhibited cell activity within 48 h, produced alterations in cell morphology, and removed the contact vegetative state. Fucoidan showed significant anti-melanocyte activity (Figure 5).
Figure 5.
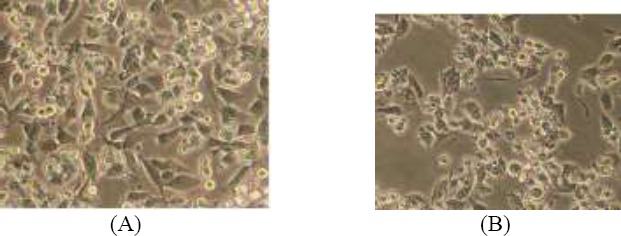
Effects of fucoidan on B16 melanoma cells morphology (100 ×, scale bar 100 μm)(A) control group (B) experimental group (550 μg/ml fucoidan)
Induction of apoptosis in B16 melanoma cells
To determine the inhibitory effect of fucoidan on the proliferation of B16 cells, the rate of apoptosis in cells treated with different concentrations of fucoidan was determined by Annexin V-FITC/ PI staining and flow cytometry. The results showed that in cells incubated with 550 µg/mL fucoidan for 48 h, the apoptosis rate increased from 3.01±1.02% to 13.74±2.47% (Figure 6). This indicated that fucoidan significantly induced cell apoptosis.
Figure 6.
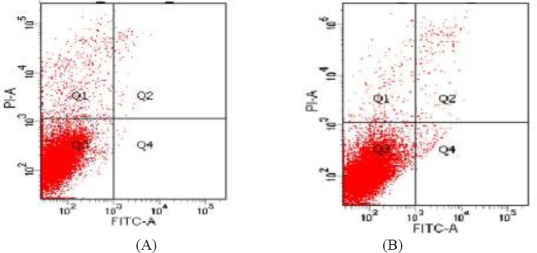
Induction effect of Fucoidan on B16 melanoma cells apoptosis (A) control group (B) experimental group (550 μg/mL fucoidan) Q1: non-viable non-apoptotic cell; Q2: viable apoptotic cell; Q3: Normal growth cell; Q4 : non-viable apoptotic cell; Q2+Q4: apoptosis rate
Discussion
We have previously reported on many tyrosinase inhibitors. A large number of experiments have suggested that inhibition of tyrosinase activity is the key to whitening, and as reported fucoidan is one of these inhibitors. Fucoidan effectively suppressed tyrosinase activity in the form of mixed inhibition (Wang et al., 2012). However, it was reported that polysaccharides or fucoidan had antitumor activity. Fucoidan has shown inhibitory effects on metastasis, angiogenesis and suppression of growth in a variety of cancer cells, such as human breast cancer cells, HT-29 colon cancers and HS-sultan human lymphoma cells (Aisa et al., 2005; Yamasaki-Miyamoto et al., 2009; Kim et al., 2010). Apoptosis may help to protect cells and avoid death. Cunha (2012) demonstrated that melanogenesis stimulation may prevent cell death in melanoma. Pinon et al. (2011) demonstrated that melanogenesis upregulation was beneficial in melanoma cells undergoing apoptosis, and apoptosis was able to delay cell death.
Tyrosinase is known to be a key enzyme, and is responsible for the rate-limiting step in melanin synthesis. It was demonstrated that the inhibitory effect on melanogenesis was associated with a decrease in tyrosinase activity (Cha et al., 2012; Chaabane et al., 2014). Jeong et al. (2015) showed that baicalin, a component of Scutellaria baicalensis decreased melanogenesis and tyrosinase activity. On the other hand, it was reported that tyrosinase was enhanced when melanogenesis increased (Skandrani et al. 2010; Chaabane et al. 2013).
In this study, the results suggested that fucoidan increased the rate of melanoma cell apoptosis. However, melanin was beneficial for apoptosis, and as a result, cells may be induced to regulate the melanin synthesis pathway in order to produce more melanin leading to up-regulation of tyrosinase expression. The up-regulated tyrosinase protein can be stimulated to produce more melanin. But, fucoidan not only has anticancer activity, but is also a tyrosinase inhibitor. In this study, tyrosinase activity was significantly reduced with increased fucoidan dose. This indicated that fucoidan inhibited the biological activity of tyrosinase, and then reduced melanin content.
Conclusions
In conclusion, based on the results obtained from our previous research and the present study, our major findings are as follows: (1) fucoidan inhibited the proliferation of B16 melanoma cells; (2) fucoidan up-regulated tyrosinase expression. In addition, fucoidan also inhibited B16 murine melanoma cellular tyrosinase activity, and the inhibition of B16 murine cellular tyrosinase was greater than that of cell-free mushroom tyrosinase as previously reported. Fucoidan not only induced melanin cell apoptosis but also efficiently inhibited extracellular tyrosinase. Fucoidan may be a very good whitening agent.
Acknowledgement
This study was supported by grants from the Sciences Foundation of Zhejiang Province (LY15C190007), the Zhejiang Provincial Top Discipline of Biological Engineering (ZS2015011), the Shanghai Jiao Tong University Medical engineering cross fund (UG2015QN35) and the SMC-Morningstar Young Scholars Program (15X100090011).
References
- 1.Aisa Y, Miyakawa Y, Nakazato T, Shibata H, Saito K, Ikeda Y, Kizaki M. Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am. J. Hematol. 2005;78(1):7–14. doi: 10.1002/ajh.20182. [DOI] [PubMed] [Google Scholar]
- 2.Alekseyenko TV, Zhanayeva SY, Venediktova AA, Zvyagintseva TN, Kuznetsova TA, Besednova N. N, Korolenko TA. Antitumor and antimetastatic activity of fucoidan, a sulfated polysaccharide isolated from the Okhotsk Sea Fucus evanescens brown alga. Bull. Exp. Biol. Med. 2007;143(6):730–732. doi: 10.1007/s10517-007-0226-4. [DOI] [PubMed] [Google Scholar]
- 3.Cha JY, Yanga HJ, Moon HI, Cho YS. Inhibitory effect and mechanism on melanogenesis from fermented herbal composition for medical or food uses. Food Res Int. 2012;45(1):225–231. [Google Scholar]
- 4.Chaabane F, Pinon A, Simon A, Ghedira K, Chekir-Ghedira L. Phytochemical potential of Daphne gnidium in inhibiting growth of melanoma cells and enhancing melanogenesis of B16-F0 melanoma. Cell Biochem Funct. 2013;31(6):460–467. doi: 10.1002/cbf.2919. [DOI] [PubMed] [Google Scholar]
- 5.Chaabane F, Pinon A, Simon A, Ghedira K, Chekir-Ghedira L. Chloroform leaf extract of Daphne gnidium inhibits growth of melanoma cells and enhances melanogenesis of B16-F0 melanoma. S Afr Bot. 2014;90:80–86. [Google Scholar]
- 6.Chan YY, Kim KH, Cheah SH. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J. Ethnopharmacol. 2011;137(3):1183–1188. doi: 10.1016/j.jep.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 7.Chandía NP, Matsuhiro B. Characterization of a fucoidan from Lessonia vadosa (Phaeophyta) and its anticoagulant and elicitor properties. Int. J. Biol. Macromol. 2008;42(3):235–240. doi: 10.1016/j.ijbiomac.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Cooksey CJ, Garratt PJ, Land EJ, Smit Nico PM. Evidence of the indirect formation of the catecholic intermediate substrate responsible for the autoactivation kinetics of tyrosinase. J. Biol. Chem. 1997;272(42):26226–2635. doi: 10.1074/jbc.272.42.26226. [DOI] [PubMed] [Google Scholar]
- 9.Cunha ES, Kawahara R, Kadowaki MK, Amstalden HG, Noleto GR, Cadena SMSC, Winnischofer SMB, Martinez GR. Melanogenesis stimulation in B16-F10 melanoma cells induces cell cycle alterations, increased ROS levels and a differential expression of proteins as revealed by proteomic analysis. Exp. cell res. 2012;318(15):1913–1925. doi: 10.1016/j.yexcr.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Cumashi A, Ushakova NA, Preobrazhenskaya ME, Piccoli A, Totani L, Tinari N, Morozevich GE, Berman AE, Bilan MI, Usov AI, Ustyuzhanina NE, Grachev AA, Sanderson CJ, Kelly M, Rabinovich GA, Iacobelli S, Nifantiev NE. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 2007;17(5):541–552. doi: 10.1093/glycob/cwm014. [DOI] [PubMed] [Google Scholar]
- 11.de Fine Olivarius F, Wulf HC, Crosby J, Norval M. Isomerization of urocanic acid after ultraviolet radiation is influenced by skin pigmentation. J. Photochem. Photobiol. B. 1999;48(1):42–47. doi: 10.1016/S1011-1344(99)00007-X. [DOI] [PubMed] [Google Scholar]
- 12.Fang D, Kute T, Setaluri V. Regulation of tyrosinase-related protein-2 (TYRP2) in human melanocytes:relationship to growth and morphology. Pigment Cell Res. 2001;14(2):132–139. doi: 10.1034/j.1600-0749.2001.140209.x. [DOI] [PubMed] [Google Scholar]
- 13.Jeong H, Gu GE, Jo AR, Bang JS, Yun HY, Baek KJ, Kwon NS, Park KC, Kim DS. Baicalin-induced Akt activation decreases melanogenesis through downregulation of microphthalmia-associated transcription factor and tyrosinase. Eur Pharmacol. 2015;761:19–27. doi: 10.1016/j.ejphar.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi K, Nakano T, Hashimoto M, Hayashi T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 2008;8(1):109–116. doi: 10.1016/j.intimp.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Hearing VJ. Biochemical control of melanogenesis and melanosomal organization. J. Investig. Dermatol. Symp. Proc. 1999;4(1):24–28. doi: 10.1038/sj.jidsp.5640176. [DOI] [PubMed] [Google Scholar]
- 16.Hearing VJ. Biogenesis of pigment granules:a sensitive way to regulate melanocyte function. J. Dermatol. Sci. 2005;37(1):3–14. doi: 10.1016/j.jdermsci.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Jin JO, Yu Q. Fucoidan delays apoptosis and induces pro-inflammatory cytokine production in human neutrophils. Int. J. Biol. Macromol. 2015;73:65–71. doi: 10.1016/j.ijbiomac.2014.10.059. [DOI] [PubMed] [Google Scholar]
- 18.Jones K, Hughes J, Hong M, Orndorff S. Modulation of melanogenesis by aloesin:a competitive inhibitor of tyrosinase. Pigment Cell Res. 2002;5(5):335–340. doi: 10.1034/j.1600-0749.2002.02014.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim YJ, Uyama H. Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005;62(15):1707–1723. doi: 10.1007/s00018-005-5054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim EJ, Park SY, Lee JY, Park JHY. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010;10:96. doi: 10.1186/1471-230X-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JH, Cho B, Jun HJ, Seo WD, Kim DW, Cho KJ, Lee SJ. Momilactione B inhibits protein kinase A signaling and reduces tyrosinase-related proteins 1 and 2 expression in melanocytes. Biotechnol. Let. 2012;34(5):805–812. doi: 10.1007/s10529-011-0838-7. [DOI] [PubMed] [Google Scholar]
- 22.Lee MH, Lin YP, Hsu FL, Zhan GR, Yen KY. Bioactive constituents of Spatholobus suberectus in regulating tyrosinase-related proteins and mRNA in HEMn cells. Phytochemistry. 2006;67(12):1262–1270. doi: 10.1016/j.phytochem.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Mizusawa K, Kobayashi Y, Sunuma T, Asahida T, Saito Y, Takahashi A. Inhibiting roles of melanin-concentrating hormone for skin pigment dispersion in barfin flounder, Verasper moseri. Gen. Comp. Endocrinol. 2011;171(1):75–81. doi: 10.1016/j.ygcen.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Negroiu G, Dwek RA, Petrescu SM. Folding and maturation of tyrosinase-related protein-1 are regulated by the post-translational formation of disulfide bonds and by N-glycan processing. J. Biol. Chem. 2000;275(41):32200–32207. doi: 10.1074/jbc.M005186200. [DOI] [PubMed] [Google Scholar]
- 25.Parvez S, Kang MK, Chung HS, Cho CW, Hong MC, Shin MK, Bae H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. 2006;20(11):921–934. doi: 10.1002/ptr.1954. [DOI] [PubMed] [Google Scholar]
- 26.Pinon A, Limami Y, Micallef L, Cook-Moreau J, Liagre B, Delage C, Duval RE, Simon A. A novel form of melanoma apoptosis resistance:Melanogenesis up-regulation in apoptotic B16-F0 cells delays ursolic acid-triggered cell death. Exp cell res. 2011;317(12):1669–1676. doi: 10.1016/j.yexcr.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Raghavendran HR, Sathivel A, Devaki T. Effect of Sargassum polycystum (Phaeophyceae)-sulphated polysaccharide extract against acetaminophen-induced hyperlipidemia during toxic hepatitis in experimental rats. Mol. Cell. Biochem. 2005;276(1-2):89–96. doi: 10.1007/s11010-005-3194-x. [DOI] [PubMed] [Google Scholar]
- 28.Saravana PS, Cho YJ, Park YB, Woo HC, Chun BS. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016;153:518–525. doi: 10.1016/j.carbpol.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Skandrani I, Pinon A, Simon A, Ghedira K, Chekir-Ghedira L. Chloroform extract from Moricandia arvensis inhibits growth of B16-F0 melanoma cells and promotes differentiation in vitro. Cell Proliferation. 2010;43:471–479. doi: 10.1111/j.1365-2184.2010.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun J, Sun J, Song B, Zhang L, Shao Q, Liu Y, Yuan D, Zhang Y, Qu X. Fucoidan inhibits CCL22 production through NF-κB pathway in M2 macrophages:a potential therapeutic strategy for cancer. Sci. Rep. 2016;6:35855. doi: 10.1038/srep35855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Zhang Q, Zhang Z, Song H, Li P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010;46(1):6–12. doi: 10.1016/j.ijbiomac.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Wang ZJ, Si YX, Oh S, Yang JM, Yin SJ, Park YD, Lee J, Qian GY. The effect of fucoidan on tyrosinase:computational molecular dynamics integrating inhibition kinetics. J. Biomol. Struct. Dyn. 2012;30(4):460–473. doi: 10.1080/07391102.2012.682211. [DOI] [PubMed] [Google Scholar]
- 33.Yamasaki-Miyamoto Y, Yamasaki M, Tachibana H, Yamada K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J. Agric. Food Chem. 2009;57(18):8677–8682. doi: 10.1021/jf9010406. [DOI] [PubMed] [Google Scholar]
- 34.Ye Y, Chu JH, Wang H, Xu H, Chou GX, Leung AKM, Fong WF, Yu ZL. Involvement of p38 MAPK signaling pathway in the anti melanogenic effect of San-bai-tang, a Chinese herbal formula, in B16 melanoma cells. J. Ethnopharmacol. 2010;132(2):533–535. doi: 10.1016/j.jep.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Zhao X, Guo F, Hu J, Zhang L, Xue C, Zhang Z, Li B. Antithrombotic activity of oral administered low molecular weight fucoidan from Laminaria Japonica. Thromb. Res. 2016;144:46–52. doi: 10.1016/j.thromres.2016.03.008. [DOI] [PubMed] [Google Scholar]


