Abstract
Background:
Xuefu Zhuyu Tang (XFZYT), first recorded in Correction of Errors in Medical Works by Qing-ren Wang, has been proven reliable and effective for curing various diseases such as atherosclerosis, hypertension, hyperlipidemia, and angina pectoris. It consists of 11 herbs and two of them, Radix platycodonis and Radix cyathulae, have been traditionally considered as guiding herbs and deeply valued by tens of millions of Chinese medicine practitioners. Do Radix platycodonis and Radix cyathulae affect the pharmacokinetics of the effective constituent-paeoniflorin of XFZYT? If yes, in what way? This study aims to answer these questions.
Materials and Methods:
The medicinal solutions of XFZYT, XFZYT without Radix platycodonis (XFZYT-JG), XFZYT without Radix cyathulae (XFZYT-NX), and XFZYT without Radix platycodonis and Radix cyathulae (XFZYT-JG-NX) were prepared and administrated to rats in the normal group and the blood-stasis model group by gavage, respectively. The blood samples of rats in the normal group were obtained 5, 10, 15, 20, 30, 45, 60, 120, and 240 minutes after gavage; whereas the blood samples of rats in the blood-stasis model group were obtained 10, 15, 20, 30, 45, 90, 150, and 240 minutes after gavage. Biological samples were processed; the assays of specificity, precision, linearity, intra-day and inter-day precisions, recovery and stability were conducted; high performance liquid chromatography was performed to detect paeoniflorin content; and DAS software was adopted to generate pharmacokinetic parameters. Mobile phase was composed of acetonitrile and water (16:84), detection wavelength was 230 nm, and riboflavin was set as internal standard substance.
Results:
The pharmacokinetic parameters of the rats in the normal group after oral gavage of XFZYT, XFZYT-JG, XFZYT-NX, and XFZYT-JG-NX were Cmax = (0.363±0.248, 0.065±0.020, 0.099±0.033, 0.099±0.020) mg/L, Tmax = (0.276±0.084, 0.583±0.342, 0.555±0.228, 0.317±0.033)h, t1/2 = (0.501±0.241, 1.021±0.522, 0.853±0.377, 1.227±0.402) h; and AUC0-∞ = (0.381±0.415, 0.13±0.085, 0.166±0.066, 0.185±0.059) mg/L·h.; whereas the pharmacokinetic parameters for the rats in the blood-stasis model group after oral gavage of XFZYT, XFZYT-JG, XFZYT-NX, and XFZYT-JG-NX were Cmax = (0.315±0.153, 0.215±0.044, 0.228±0.056, 0.248±0.09) mg/L, Tmax = (0.5±0, 0.667±0.129, 0.5±0, 0.542±0.102) h, t1/2 = (0.408±0.146, 0.813±0.135, 0.708±0.383, 0.741±0.173) h, and AUC0-∞ = (0.306±0.157, 0.408±0.136, 0.368±0.159, 0.381±0.246) mg/L·h.
Conclusion:
The guiding herbs, Radix platycodonis and Radix cyathulae, significantly increased the absorption amount and rate of paeoniflorin in XFZYT, and accelerated its elimination from the blood.
Keywords: Xuefu Zhuyu Tang, Guiding herb, Radix platycodonis, Radix cyathulae, Paeoniflorin, Pharmacokinetics
Introduction
Xuefu Zhuyu Tang (XFZYT) was first recorded in Yī Lín Găi Cuò (Correction of Errors in Medical Works) written by Qing-ren Wang (1768–1831) (Shoja et al., 2010). Upon an original use of relieving blood stasis in the chest and the principle of Traditional Chinese Medicine (TCM) to “treat different diseases in the same way”, this formula has centuries of clinical use and been proven effective for curing diseases such as atherosclerosis, hypertension, hyperlipidemia, thromboembolism, and angina pectoris (Huang et al., 2007; Shi et al., 2013). Pharmacological researches have elucidated its mechanisms of protecting the cardiovascular and cerebrovascular system (Yu et al., 1988; Zhang et al., 2010; Jiang and Jiang, 2015) and its effects of inducing endothelial progenitor cell angiogenesis, hastening tube formation, regulating blood lipid, and protecting neuro (Gao et al., 2010; Lee et al., 2011; Song et al., 2013; Xing et al., 2016).
The efficacy of herbal medicine has been considered attributable to the synergistic effect of multiple constituents (Ren et al., 2008). To support the traditional use of XFZYT and reveal its substance foundation, high-performance liquid chromatography-mass spectrometry was performed to identify its antiatherogenic constituents (Liu et al., 2004). A total of 103 compounds were unveiled, 35 of which were unambiguously identified (Zhang et al., 2015). However, little attention has been paid to the guiding herbs-Radix platycodonis and Radix Cyathulae in XFZYT. These two herbs embody the fundamental theory of channel tropism in TCM and are highly valued by tens of millions of TCM practitioners.
Channel tropism refers to the selective therapeutic effects of Chinese material medica on a certain part of the human body; while guiding herb is to direct a formula to certain affected channels/areas of the body and may exert obvious/specific therapeutic actions. They are of great significance to the compatibility and clinical utilization of a formula. Hereby, to study the mechanism of guiding herb has important significance for TCM. Major investigations involved indentifying the similarity in the mechanism of action between guiding herb and active targeting carrier; revealing the capacity of guiding herb to regulate qi and blood; interpreting the similarity between channel tropism and systems biology; and validating the guiding effect of a single herb or a certain type of herb (Wang and Li, 2002; Zhao and Liu, 2005; Han, 2008; Liu et al., 2013; Lu, 2004; Chen et al., 2011; Xu et al., 2012).
All those attempts are beneficial to understand the mechanism; however, the pharmacological mechanism of guiding herb in the context of a compound formula has rarely been reported. The effects of Radix platycodonis and Radix cyathulae on the effective constituent in XFZYT are still unsealed, and their pharmacokinetic characteristics have yet been identified. This study, therefore, aimed at evaluating the in vivo effects of Radix platycodonis and Radix cyathulae on paeoniflorin pharmacokinetics of XFZYT. The pharmacokinetic parameters of XFZYT-JG (XFZYT without Radix Platycodonis), XFZYT-NX (XFZYT without Radix Cyathulae), and XFZYT-JG-NX (XFZYT without both Radix Platycodonis and Radix Cyathulae) were measured and compared with that of XFZYT. The results indicated that Radix platycodonis and Radix cyathulae could increase the absorption amount and rate of paeoniflorin of XFZYT in rats, and accelerate its elimination from the blood. To our knowledge, this is the first investigation that provides evidence to reveal the effects of guiding herbs on the pharmacokinetics in the context of a compound formula.
Materials and Methods
Equipment
The following pieces of equipment were used: ultraviolet-visible (UV-Vis) detector (SPD-10Avp) and high performance liquid chromatograph (HPLC, LC-10ATvp) (Shimadzu Corporation, Japan); FA1004 electronic scales (Shanghai liangping Instrument Co., Ltd. Shanghai, China); LG15-W high speed micro centrifuge (Beijing medical centrifuge factory, Beijing, China). Water used in the experiment was purified by a Milli-Q water purification system (Millipore, Billerica, MI, USA).
Chemicals
XFZYT consists of 11 Chinese materia medica, namely 12g Semen Persicae (Tao-Ren), 9g Radix Angelicae Sinensis (Dang-Gui), 4.5g Rhizoma Chuanxiong (Chuan-Xiong), 9g Flos Carthami (Hong-Hua), 6g Radix Paeoniae Rubra (Chi-Shao), 9g Radix Rehmanniae (Di-Huang), 6g Fructus Aurantii (Zhi-Qiao), 3g Radix Bupleuri (Chai-Hu), 4.5g Radix Platycodonis (Jie-Geng), 9g Radix cyathulae (Niu-Xi), and 6g Radix et Rhizoma Glycyrrhizae (Gan-Cao). All herbs, commercially available as dried matter, were purchased from Sichuan BenCaoTang Pharmaceutical Co., Ltd. (Chengdu, China). The voucher specimens were deposited at the herbarium of the Chengdu Qihuang Bio-Tech Institute (Chengdu, China).
Paeoniflorin reference substances (98% purity, batch No.: 121207) were obtained from Chengdu Pufei De Biotech Co., Ltd. (Chengdu, China). Riboflavin reference substances (98% purity, batch No.: 130114) were obtained from Chengdu PureChem-Standard Co., Ltd. (Chengdu, China). Glucan T500 was obtained from Beijing YaAnDa Biotechnology Co., Ltd. (Beijing, China). Chromatographically pure carbinol and acetonitrile were required.
Animals
Forty-eight SPF Sprague-Dawley rats of either sex weighing 180–220g (6 weeks of age) were obtained from Sichuan Academy of Chinese Medicine Sciences (Chengdu, China). Animals were housed in standard plastic cages under automatic 12 h light/dark cycles at 20–24 °C and relative humidity of 40–70%, with free access to purified water. All animals were fed with pellet diet (15–25g/rat/day) provided by Chengdu Dashuo Laboratory Animal Co., Ltd. (Chengdu, China). The experimental procedures were carried out in accordance with the Guidelines for Animal Experimentation and approved by the Institutional Review Committee on Animal Care and Use at Chengdu Qihuang Bio-Tech Institute (Chengdu, China).
Chromatographic Conditions
The experiment was performed on a Diamonsil C18 chromatographic column (250 mm × 4.6 mm, 5 µm) at 30 °C; the mobile phase was composed of acetonitrile and water (16:84); the flow velocity was 1.0 mL·min-1, the detection wavelength was 230 nm, the riboflavin was set as internal standard substance, and the injection volume was 20 µL.
Medicinal Solution Preparation
According to the traditional formulae, the medicinal materials of XFZYT (totally weight of 78 g), XFZYT-JG (73.5 g), XFZYT-NX (69 g), and XFZYT-JG-NX (64.5 g) were accurately weighed, multiplied by 45 to 3 510 g, 3 307.5 g, 3 105 g, and 2 902.5 g, respectively, and immersed in 35 100 mL, 33 075 mL, 31 050 mL, and 29 025 mL of water (v/v, 1:10), respectively, for 30 min at room temperature. XFZYT, XFZYT-JG, XFZYT-NX, and XFZYT-JG-NX were decocted 30 min for 3 times, respectively. After filtration and concentration in a rotatory evaporator for 8 hrs, the aqueous extract of each sample was dried at 45°C in a vacuum drying oven to obtain 426g original extract powder of XFZYT, 1 054g of XFZYT-JG, 948g of XFZYT-NX, and 650 g of XFZYT-JG-NX. The mass fraction of paeoniflorin in original extract power was 2.290 mg·g-1 for XFZYT, 2.620 mg·g-1 for XFZYT-JG, 3.366 mg·g-1 for XFZYT-NX, and 3.120 mg·g-1 for XFZYT-JG-NX. An amount of 4 g extractive powder of each sample was accurately weighed, dissolved in 4.0 mL, 4.6mL, 5.9mL, and 5.4 mL of water, respectively, and swirled to mix thoroughly and obtain the medicinal solutions for oral gavage.
An amount of 1.0 g of Glucan T500 was accurately weighed and dissolved with 20 mL of 0.9% sodium chloride injection to get 5% glucan T500 solution. All solutions were stored at 0°C until analysis.
Stock solution
An amount of 6.63 mg of paeoniflorin reference substance was accurately weighed, set into a brown and dry 50 mL volumetric flask, diluted with methyl alcohol to volume, and then shaken well. A paeoniflorin stock solution with a concentration of 130.0 µg·mL-1 was prepared and stored at 0°C.
An amount of 4.8 mg of riboflavin reference substance was accurately weighed, set into a brown and dry 50 mL volumetric flask, diluted with a 70% methanol aqueous solution to volume, and then shaken well. An internal standard stock solution with a concentration of 94.1 µg·mL-1 was prepared and stored at 0°C. A volume of 1 666.7 µL of internal standard stock solution was diluted with water to 100 mL to obtain internal working standard solution with a concentration of 1.57 µg/mL.
Administration of chemicals
The rats were randomized into two groups, namely the normal group and the blood-stasis model group. Then, 24 animals in each group of either sex were randomly assigned to 4 groups (6 in each group of either sex). The animals were fasting for 12 hrs and allowed free access to water.
Each rat in the blood-stasis model group was injected intravenously (caudal vein) with 5% glucan T500 solution (2 mL·kg-1) (Li Yikui, 1991). Thirty min later, the medical solutions of XFZYT, XFZYT-JG, XFZYT-NX, and XFZYT-JG-NX (5 mL·kg-1) were administrated by oral gavage to the rats in the normal group and the blood stasis group, respectively. Each group was given equal volume of paeoniflorin (11.452 mg·kg-1). Administration was performed once a day.
Blood sample collection
Blood samples were collected from the orbital venous plexus of rats in the normal group 5, 10, 15, 20, 30, 45, 60, 120, and 240 min after administration of gavage; whereas blood samples were collected from the orbital venous plexus of rats in the blood-stasis model group 10, 15, 20, 30, 45, 90, 150, and 240 min after administration of gavage. After allowing the tube to clot for 20 min in a vertical position, the blood samples were centrifuged at 5 000 r·min-1 for 10 min, the serum was then separated and stored in a centrifuge tube at -20°C for assay.
Blood sample processing
To 100 µL of serum, 250 µL of acetonitrile and 20 µL of working internal standard solution were added. The mixture was lightly shaken in vortex and then centrifuged at 5 000 r·min-1 for 10 min. Once centrifuged, the supernatant was transferred and evaporated under nitrogen in a water bath at 40 °C, and 100 µL of water were added to redissolve it.
Statistical analysis
Serum-concentration - time data were analyzed with DAS2.0 software to generate pharmacokinetic parameters. Experimental data were expressed as and statistically analyzed by SPSS13.0 software. One-way ANOVA was applied for the pharmacokinetic parameters of AUC(0-t) and Cmax, and non-parametric test for Ka, Ke, Tmax, and t1/2. Differences were deemed statistically significant at p < 0.05.
Results
Specificity
To paeoniflorin reference substance, internal standard substance solution, blank serum solution and serum solution after treatment were added. An aliquot of the resulting solution was injected into HPLC system, and chromatograms were recorded (Figure 1). The paeoniflorin and endogenous impurities were confirmed separate well; the degree of separation was found to be 1.8; and the retention time was 15.9 min for the paeoniflori, and 9.5 min for the internal standard substance.
Figure 1.
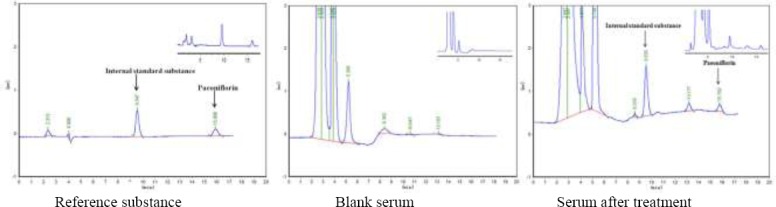
HPLC chromatograms of paeoniflorin and internal standard substance(t/min)
Precision
Paeoniflorin reference substance solution (0.65 mg·L-1) was accurately measured out, an aliquot of the resulting solution was injected for 6 successive times. Results showed that the RSD of paeoniflorin peak area was 0.4%, indicating that the present methods were reliable and reproducible for quantitative determination of analytes.
Linearity and range
To 100 µL of blank serum, paeoniflorin working solution, 20 µL internal standard working solution and 250 µL acetonitrile protein-precipitation were accurately added. The procedure was conducted to obtain a series of blank serum sample solutions with a paeoniflorin concentration of 0.01, 0.02, 0.06, 0.1, 0.2, 0.4, 0.6, and 1.0 µg·mL-1, respectively. The regression equations of serum sample were expressed as Y=1.8925X+0.0029 (weight 1/x2, r =0.9996), where the Y represented peak area ratio between paeoniflorin and riboflavin, and the X represented mass concentration.
Intra-day and inter-day precisions
The serum samples containing paeoniflorin with a mass concentration of 0.01, 0.1, and 1.0 µg·mL-1, respectively, were prepared in quintuplicate and analyzed for six repetitive injections per day for three consecutive days. Results revealed for the blank serum samples containing paeoniflorin at 0.01, 0.1, and 1.0 µg·mL-1, the RSDs of intra-day precision were 3.4%, 3.2%, and 3.2%, respectively; and the RSDs of inter-day precision were 4.2%, 3.1%, and 4.4%, respectively.
Recovery rate
The serum samples containing paeoniflorin with a mass concentration of 0.01, 0.1, and 1.0 µg·mL-1, respectively, were prepared in quintuplicate and measured. The recovery rate was calculated by plotting the ratio of peak area between paeoniflorin and internal standard substance to standard curve. Results revealed that for the serum samples containing paeoniflorin at 0.01, 0.1, and 1.0 µg·mL-1, the recovery rates were 102.5%, 101.3%, and 100.0%, respectively; and the RSDs were 0.8%, 0.9%, and 0.4%, respectively.
Stability
The serum sample solutions were prepared in quadruplicate and stored at -20 °C. The stability of sample solutions was evaluated at room temperature by replicating the injection at 0, 1, 3, and 7 d. All serum samples were shown to be stable within 7 days.
Pharmacokinetics
Chromatograms were recorded and the peak area ratios between paeoniflorin and internal standard substance were plotted to the standard curve equation to calculate paeoniflorin concentration in serum (Figures 2 and 3). Data of concentration-time curve were analyzed. The Cmax and Tmax were obtained directly from the concentration-time curve, while the rest parameters were calculated with DAS 2.0 software (Tables 1 and 2). The results indicated that for the normal rats, the usage of Radix platycodonis and/or Radix cyathulae could increase the absorption of paeoniflorin and accelerate its elimination from the blood; whereas for the blood-stasis rats, the usage of Radix platycodonis or/and Radix cyathulae could increase the absorption of paeoniflorin, sole usage of Radix platycodonis and combining use of Radix platycodonis and Radix cyathulae could accelerate the elimination of paeoniflorin from the blood.
Figure 2.
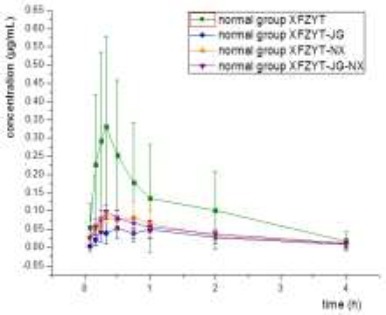
Mean c-t curve of rats in the normal group
Figure 3.
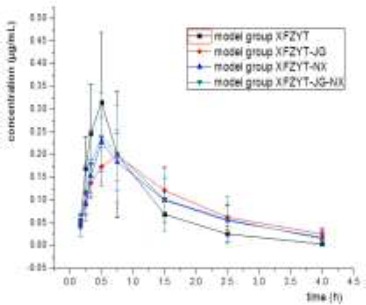
Mean c-t curve of rats in the blood-stasis group
Table 1.
Paeoniflorin pharmacokinetic parameters of rats in normal group after administration of oral gavage(n=6)
| Parameter | Unit | Parameter values (χ̄±s) | ||||
|---|---|---|---|---|---|---|
| XFZYT | XFZYT-JG | XFZYT-NX | XFZYT-JG-NX | |||
| Compartment Model | t1/2 | h | 0.501±0.241② ③ ④ | 1.021±0.522① | 0.853±0.377① | 1.227±0.402① |
| Ke | h-1 | 1.638±0.682② ③ ④ | 0.84±0.418① | 0.972±0.445① | 0.615±0.187① | |
| V1/F | L·kg-1 | 36.521±26.499② ③ ④ | 150.383±73.977① | 93.155±40.472① | 111.528±27.267① | |
| CL/F | L·h-1·kg-1 | 69.846±79.426 | 110.388±43.702 | 79.524±33.226 | 66.105±16.271 | |
| AUC(0-t) | mg·L-1·h | 0.356±0.392 | 0.112±0.06 | 0.149±0.069 | 0.146±0.049 | |
| AUC(0-∞) | mg·L-1·h | 0.381±0.415 | 0.13±0.085 | 0.166±0.066 | 0.185±0.059 | |
| Ka | h-1 | 30.905±31.196③ | 7.549±8.004 | 8.006±8.197①④ | 14.114±10.323③ | |
| Statistical Moment | AUC(0-t)z | mg·L-1·h | 0.408±0.418 | 0.113±0.063 | 0.148±0.069 | 0.146±0.048 |
| AUC(0-∞)z | mg·L-1·h | 0.451±0.447 | 0.129±0.086 | 0.168±0.068 | 0.182±0.052 | |
| MRT(0-t) | h | 0.911±0.435 | 1.397±0.165 | 1.175±0.293 | 1.188±0.419 | |
| MRT(0-∞) | h | 1.214±0.716 | 1.836±0.479 | 1.677±0.407 | 1.955±0.703 | |
| t1/2z | h | 0.724±0.516 | 1.168±0.368 | 1.098±0.373 | 1.304±0.507 | |
| Tmax | h | 0.276±0.084③ | 0.583±0.342 | 0.555±0.228① | 0.317±0.033 | |
| CLz/F | L·h-1·kg-1 | 67.017±86.091 | 111.117±43.201 | 78.495±32.506 | 66.657±16.165 | |
| Vz/F | L·kg-1 | 41.972±30.599② ③ ④ | 177.692±76.789① | 119.686±46.165① | 120.396±43.81① | |
| Zeta | 1.397±0.839 | 0.635±0.163 | 0.688±0.201 | 0.609±0.247 | ||
| Cmax | mg·L-1 | 0.363±0.248② ③ ④ | 0.065±0.02① ④ | 0.099±0.033① | 0.099±0.02① ② | |
z: for the parameter of statistical moment; ①compared with XFZYT, P<0.05; ② compared with XFZYT-JG, P<0.05; ③compared with XFZYT-NX, P<0.05; ④compared with XFZYT-JG-NX, P<0.05.
Table 2.
Paeoniflorin pharmacokinetic parameters of rats in blood-stasis group after administration of oral gavage(n=6)
| Parameter | Unit | Parameter values (χ̄±s) | ||||
|---|---|---|---|---|---|---|
| XFZYT | XFZYT-JG | XFZYT-NX | XFZYT-JG-NX | |||
| Compartment Model | t1/2 | h | 0.408±0.146②③ | 0.813±0.135① | 0.708±0.383 | 0.741±0.173① |
| Ke | h-1 | 1.876±0.605②③ | 0.871±0.136① | 1.289±0.744 | 0.977±0.218① | |
| V1/F | L·kg-1 | 26.538±11.444 | 34.81±8.663 | 31.865±7.669 | 39.643±16.814 | |
| CL/F | L·h-1·kg-1 | 49.408±31.718 | 30.172±7.744 | 40.062±26.515 | 41.177±25.269 | |
| AUC(0-t) | mg·L-1·h | 0.288±0.154 | 0.362±0.125 | 0.338±0.139 | 0.349±0.227 | |
| AUC(0-∞) | mg·L-1·h | 0.306±0.157 | 0.408±0.136 | 0.368±0.159 | 0.381±0.246 | |
| Ka | h-1 | 6.475±3.758 | 3.753±2.423 | 4.543±2.279 | 6.131±2.462 | |
| Statistical Moment | AUC(0-t)z | mg·L-1·h | 0.289±0.157 | 0.364±0.123 | 0.338±0.141 | 0.352±0.226 |
| AUC(0-∞)z | mg·L-1·h | 0.307±0.161 | 0.421±0.128 | 0.369±0.16 | 0.386±0.239 | |
| MRT(0-t) | h | 0.81±0.1 | 1.389±0.157 | 1.229±0.288 | 1.152±0.295 | |
| MRT(0-∞) | h | 0.964±0.13 | 1.971±0.178 | 1.514±0.456 | 1.47±0.415 | |
| t1/2z | h | 0.545±0.112 | 1.271±0.169 | 0.886±0.325 | 0.894±0.299 | |
| Tmax | h | 0.5±0② | 0.667±0.129①③ | 0.5±0② | 0.542±0.102 | |
| CLz/F | L·h-1·kg-1 | 49.974±33.493 | 28.971±6.932 | 40.271±27.6 | 40.008±23.675 | |
| Vz/F | L·kg-1 | 37.508±20.468 | 53.815±16.379 | 42.789±11.053 | 46.495±19.393 | |
| Zeta | 1.316±0.263 | 0.554±0.074 | 0.895±0.402 | 0.842±0.25 | ||
| Cmax | mg·L-1 | 0.315±0.153 | 0.215±0.044 | 0.228±0.056 | 0.248±0.09 | |
z: for the parameter of statistical moment;①compared with XFZYT, P<0.05; ②compared with XFZYT-JG, P<0.05; ③compared with XFZYT-NX, P<0.05; ③compared with XFZYT-JG-NX, P<0.05.
Discussion
XFZYT has been widely used to cure various diseases caused by blood-stasis with reliable and satisfactory efficacy. It has long been considered an embodiment of the channel tropism theory with emphasis on Radix platycodonis and Radix cyathulae as guiding herbs. In the present study, we investigated the in vivo effects of those two herbs on the paeoniflorin pharmacokinetics of XFZYT. Our findings suggest Radix platycodonis and Radix cyathulae can increase the absorption amount and rate of paeoniflorin in XFZYT, and accelerate its elimination from the blood. Herein, we infer that the accelerated elimination may imply an increased distribution of the active constituents from the blood to the tissues; and the channel tropism effect of the guiding herbs may be attributed to their influences on the pharmacokinetic process of the effective constituent.
Selecting the appropriate index component for detection is essential for conducting the pharmacokinetic research in vivo. In this study, paeoniflorin was used as an index component for detection for two reasons: 1) it has been proven effective in inhibiting platelet aggregation, dilating blood vessel, and increasing coronary flow (Yuan and Jing, 2011), which are completely in line with the blood-invigorating and stasis-dissolving function of XFZYT; 2) our preliminary experiments revealed that the content of paeoniflorin of Radix Paeoniae Rubra was comparatively high and detectable; whereas the content of hydroxysafflor yellow A of Flos Carthami was extremely low or even undetectable, and sodium ferulate (the main component of blood-invigorating and stasis-dissolving Radix Angelicae Sinensis and Rhizoma Chuanxiong) was metabolized very fast in vivo and at the minimum limit of detection.
Channel tropism is the core principle of TCM theory and the bridge between TCM theory and clinical practices (Huang and Tang, 2009). The studies of channel tropism and guiding herbs are beneficial for the efficient utilization and modernization of TCM. However, researches on the pharmacokinetic characteristics of guiding herb in the context of a formula were rarely reported. In this study, we applied the method of “decomposing formula” by removing Radix platycodonis from XFZYT (XFZYT-JG), Radix cyathulae from XFZYT (XFZYT-NX), and both Radix platycodonis and Radix cyathulae from XFZYT (XFZYT-JG-NX), respectively. And then by comparing their pharmacokinetic parameters with that of XFZYT, we validated Radix platycodonis and Radix cyathulae’s effects on the pharmacokinetics of paeoniflorin. This approach might provide a valuable reference for further research of such kind.
Conclusions
This study, to the best of our knowledge, reveals for the first time that Radix platycodonis and Radix cyathulae increase the absorption amount and rate of paeoniflorin of XFZYT in rats, and accelerate its elimination from the blood. The study also provides evidence that reveals the effects of the guiding herbs on the pharmacokinetics of the compound in rat.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No. 81102554).
References
- 1.Chen S, Lv G, Huang M, Su J, Fang H, Mou X. Effects of three traditional Chinese medicine with pungent-flavor, warm-nature and meridian tropism in lung on lung-yang deficiency rats induced by compound factors. Chinese Journal of Chinese Materia Medica. 2011;36(11):1512–5. [PubMed] [Google Scholar]
- 2.Gao D, Wu LY, Jiao YH, Chen WY, Chen Y, Kaptchuk TJ, Lu B, Song J, Chen KJ. The effect of Xuefu Zhuyu decoction on in vitro endothelial progenitor cell tube formation. Chinese Journal of Integrative Medicine. 2010;16(1):50–3. doi: 10.1007/s11655-010-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han Y. Treating 40 cases of chronic cardiac insufficiency based on syndrome differentiation together with guiding herb. Shaanxi Journal of Traditional Chinese Medicine. 2008;29(2):135–136. [Google Scholar]
- 4.Huang LM, Tang SH. Research on the origins and content of meridian tropism theory. Journal of Traditional Chinese Medicine. 2009;50:680–682. [Google Scholar]
- 5.Huang Q, Qiao X, Xu X. Potential synergism and inhibitors to multiple target enzymes of Xuefu Zhuyu decoction in cardiac disease therapeutics:a computational approach. Bioorganic & Medicinal Chemistry Letters. 2007;17(6):1779–83. doi: 10.1016/j.bmcl.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 6.Jiang H, Jiang YJ. Prevention and Treatment of Atherosclerosis by Three Different Chinese Medical Compounds:a Mechanism Study. Chinese Journal of Integrative Medicine. 2015;35(10):1244–8. [PubMed] [Google Scholar]
- 7.Lee JJ, Hsu WH, Yen TL, Chang NC, Luo YJ, Hsiao G, Sheu JR. Traditional Chinese medicine, Xue-Fu-Zhu-Yu decoction, potentiates tissue plasminogen activator against thromboembolic stroke in rats. Journal of Ethnopharmacology. 2011;134(3):824–30. doi: 10.1016/j.jep.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Li YK. Pharmacology Experiment of Chinese Material Medica [M] Shanghai: Shanghai Science and Technology Press; 1991. pp. 141–146. [Google Scholar]
- 9.Liu L, Cheng Y, Zhang H. Phytochemical analysis of antiatherogenic constituents of Xue-Fu-Zhu-Yu-Tang using HPLC-DAD-ESI-MS. Chemical and Pharmaceutical Bulletin. 2004;52(11):1295–301. doi: 10.1248/cpb.52.1295. [DOI] [PubMed] [Google Scholar]
- 10.Liu P, Liu S, Chen G, Wang P. Understanding channel tropism in traditional Chinese medicine in the context of systems biology. Frontiers of Medicine. 2013;7(3):277–9. doi: 10.1007/s11684-013-0273-3. [DOI] [PubMed] [Google Scholar]
- 11.Lu SM. Radix platycodonis’s effect on roxithromycin in terms of pharmacokinetics and drug concentration in lung [D] Ya An: Sichuan Agricultural University. 2004 [Google Scholar]
- 12.Ren MT, Chen J, Song Y, Sheng LS, Li P, Qi LW. Identification and quantification of 32 bioactive compounds in Lonicera species by high performance liquid chromatography coupled with time-of-flight mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2008;48(5):1351–60. doi: 10.1016/j.jpba.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 13.Shi WL, Zhang JS, Hu YQ. Prevention and treatment of blood vessels related diseases by xuefu zhuyu decoction (see text):its clinical application and progress in researches of mechanisms. Chinese Journal of Integrative Medicine. 2013;33(5):712–6. [PubMed] [Google Scholar]
- 14.Shoja MM, Tubbs RS, Shokouhi G, Loukas M. Wang Qingren and the 19th century Chinese doctrine of the bloodless heart. International Journal of Cardiology. 2010;145(2):305–6. doi: 10.1016/j.ijcard.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 15.Song X, Wang J, Wang P, Tian N, Yang M, Kong L. 1H NMR-based metabolomics approach to evaluate the effect of Xue-Fu-Zhu-Yu decoction on hyperlipidemia rats induced by high-fat diet. Journal of Pharmaceutical and Biomedical Analysis. 2013;78-79:202–10. doi: 10.1016/j.jpba.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Li XD. Discussion on channel tropism of Chinese material medica and its research methodology. Journal of Jiangxi University of Traditional Chinese Medicine. 2002;14(1):25. [Google Scholar]
- 17.Xing Z, Xia Z, Peng W, Li J, Zhang C, Fu C, Tang T, Luo J, Zou Y, Fan R, Liu W, Xiong X, Huang W, Sheng C, Gan P, Wang Y. Xuefu Zhuyu decoction, a traditional Chinese medicine, provides neuroprotection in a rat model of traumatic brain injury via an anti-inflammatory pathway. Scientific Reports. 2016;6:20040. doi: 10.1038/srep20040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu FQ, Feng YY, Guo L, Guo GL, Yan BL. The effective method for investigation meridian tropism theory in rats. African Journal of Traditional, Complementary, and Alternative Medicines. 2012;10(2):356–67. doi: 10.4314/ajtcam.v10i2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu DY, Wei KB, Wo XD. Clinical and experimental study of xuefu zhuyu tang in treating qi stagnation and the blood stasis type of hyperlipidemia. Chinese Journal of Integrative Medicine. 1988;8(10):601–3. 582. [PubMed] [Google Scholar]
- 20.Yuan SM, Jing H. Insights into the monomers and single drugs of Chinese herbal medicine on myocardial preservation. African Journal of Traditional, Complementary, and Alternative Medicines. 2011;8(2):104–27. doi: 10.4314/ajtcam.v8i2.63195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Jiang ZZ, Yang J, Li YY, Wang YF, Chai X. Chemical material basis study of Xuefu Zhuyu decoction by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Journal of Food and Drug Analysis. 2015;23:811–820. doi: 10.1016/j.jfda.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang YH, Hua HM. The Progress of the Xuefuzhuyu decoction in treating cardiovascular diseases. Information on Traditional Chinese Medicine. 2010;27:118–20. [Google Scholar]
- 23.Zhao RZ, Liu SJ. Theory of channel tropism of Chinese material medica and targeting drug delivery. Journal of Traditional Chinese Medicine. 2005;46(9):643. [Google Scholar]


