Abstract
Background:
Chinese herbal decoction (CHD) has been extensively used in the treatment of atrophic gastritis (AG) in China and other Far Eastern countries. We conducted a systematic review and meta-analysis to estimate the efficacy and safety of CHD in AG.
Materials and Methods:
Pubmed, Embase, Cochrane central register of controlled trials (central), VIP, China National Knowledge Infrastructure, Sinomed, Wanfang data were searched (up to December 2015). Randomized controlled trials recruiting patients with AG comparing CHD (alone or with western medicine (WM)) with WM were eligible. Dichotomous data were pooled to obtain relative risk (RR), with a 95% confidence interval (CI).
Results:
Forty-two articles including 3,874 patients were identified. CHD, used alone or with WM, had beneficial effect over WM in the improvement of clinical manifestations (RR=1.28; 95% CI 1.22-1.34) and pathological change (RR=1.42; 95% CI 1.30-1.54) for AG patients. However, the H. pylori eradication effect of CHD was not supported by the existing clinical evidence, because of the significant study heterogeneity (I2>50%) and inconsistency between the primary results and sensitivity analysis.
Conclusions:
CHD, if prescribed as a complementary therapy to WM, may improve the clinical manifestations and pathological change for AG patients. But its monotherapy for H. pylori eradication is not supported by enough clinical evidence.
Keywords: atrophic gastritis, Helicobacter pylori, Chinese herbal decoction, meta-analysis
Introduction
Atrophic gastritis (AG) is defined as the non-metaplastic and metaplastic atrophy of gastric mucosa which is replaced by connective tissue or glandular structures inappropriate for location, such as intestinal-type epithelium and pyloric-type glands (Rugge et al., 2011). Epidemiological surveys revealed that the global incidence of AG is about 0-10.9% (Adamu et al., 2010), and the prevalences are higher in Far Eastern countries (such as China, Japan, and Korea) than those in the western ones (Aoki et al., 2005; Weck and Brenner,2006; Weck et al., 2007). The persistent H. pylori-related inflammatory condition is one of the most important pathogeneses of AG (Eid and Moss, 2002), making the risk for intestinal-type gastric cancer 5.13 to 24.71-fold higher in gastritis patients than in normal people (Kato et al., 1992). H. pylori eradication therapies, such as the one-week combined use of moxifloxacin, tetracycline and lansoprazole, are recommended by the western medicine (WM) system to control AG (Taş et al., 2011). However, some recent studies reported that the clinical eradication rate of H. pylori has decreased to an unacceptable low level of 25%-80% (Gisbert et al., 2007; Graham and Fischbach,2010; Gumurdulu et al., 2004). The main causes of eradication failure are the poor compliance of patients, emerging resistant H. pylori strains and adverse drug reactions (Graham and Fischbach, 2010; Megraud, 2004; Safavi et al., 2015). The unsatisfactory efficacy and safety in WM emphasize the need for more alternative approaches to the managing AG.
Traditional Chinese Medicine (TCM) has been widely used for treating gastritis in China and other Far Eastern countries for tens of centuries (Chen et al., 2003; Qin et al., 2013; Tang et al., 2016; Xia,2004). Nowadays, Chinese physicians often prescribe TCM combined with WM, in the belief that patients will benefit from both the western and Chinese traditional therapies (Lu and Chen, 2015). Among the widely-used herbal decoctions, tens of herbs (e.g. Abrus cantoniensis Hance, Saussurea lappa (Decne.) Sch. Bip, Eugenia caryophyllata Thunb) showed potent anti-H. pylori activity (MICs: ∼40μg/ml) (Li et al., 2005; Safavi et al., 2015). Certain herbal extracts were also proved to effectively exhibit anti-inflammatory activity and reduce gastric symptoms by suppressing the production of nitric oxide, prostaglandin E(2), cyclooxygenase-2, TNF-α, IL-6 and interleukin 1β (Meng and Yang, 2010; Song et al., 2009). Clinical trials showed that some Chinese decoctions or herbal extracts were effective in alleviating AG symptoms and eradicating H. pylori (Meng and Yang,2010; Song et al., 2009), without increasing the incidence of adverse effects or producing resistant colonies (Higuchi et al., 1999). Although TCM may be a promising supplement to WM, there is no evidence from large-scale, multicenter clinical trials on its clinical use. Hence, we performed a systematic review and meta-analysis to evaluate the efficacy and safety of Chinese herbal decoction (CHD), the most essential and traditional part of TCM, for AG treatment.
Material and methods
Search strategy and study selection
A comprehensive retrieval was conducted in seven electronic databases of PubMed (1966 to December 2015), Embase (1980 to December 2015), Cochrane central register of controlled trials (central) (Issue 7, 2015), Sinomed (up to 2015), VIP Information (up to 2015), China National Knowledge Infrastructure (up to 2015) and Wangfang Data (up to 2015), without language restriction. AG of H. pylori origin, rather than autoimmune origin, was included in this study. Only randomized controlled trials (RCTs) were eligible for inclusion in this review. Trials should compare CHD (used alone or plus WM) versus WM. In WM group, patients with gastrointestinal symptoms (gastrectasia, stomach-ache, dyspepsia, etc.) should be alleviated by medications such as proton pump inhibitor; and patients with H. pylori should be treated with eradication therapy, such as triple therapy. AG should be diagnosed according to case history and pathological diagnosis (atrophy of the gastric mucosa, intestinal metaplasia, atypical hyperplasia, inflammatory cell infiltration, exposed sub-mucosal vessels). Diagnosis of H. pylori should be based invasively on endoscopic biopsy check with a rapid urease test, histological examination, or microbial culture; or noninvasively on a blood antibody test, stool antigen test, or carbon urea breath test. Efficacy is assessed by improvement of clinical manifestations (alleviation of gastrectasia, stomach-ache, dyspepsia, etc.), pathological diagnosis (alleviation of atrophy of the gastric mucosa, intestinal metaplasia, atypical hyperplasia, inflammatory cell infiltration, and exposed submucosal vessels), and the eradication of H. pylori. The authors of related studies were contacted to provide additional information on trials where required. Search terms used in this study were traditional Chinese medicine, herbal medicine, atrophic gastritis, randomized controlled trial, phytotherapy (both as medical subject heading (MeSH) and free text terms), or the following free text terms: herbal, Chinese medicine, traditional medicine, and the names of widely used formulae, such as Ban-Xia-Xie-Xin decoction, Wei-Su-Chong-Ji decoction, Xiao-Jian-Zhong decoction, Hou-Bu-Wen-Zhong decoction, etc. We also searched the reference lists of the original reports, reviews, and letters to the editor, case reports and meta-analyses of studies to identify studies which had not yet been included in the computerized databases. The last search was performed on 1st December 2015. Two reviewers (WJF, XYZ) independently assessed the eligibility of each study to be included in our meta-analysis using predesigned eligibility forms according to eligible criteria, and this was checked by another author (BY). Any disagreement was resolved by consensus between the two reviewers (SJS, ZM), adjudicated with the support of a third reviewer (BY).
Data collection process and data items
Data extraction were performed by three reviewers (WJF, XYZ, BY) with a Microsoft Excel spreadsheet (XP professional edition; Microsoft, Redmond, Washington, USA), and any disagreement was resolved by discussion. We consulted authors of the original studies through emails to get information if any problem occurred. The following data were collected: study design, sample size, therapeutic duration, criteria for efficacy judgment, intervention and control, eradication rate of H. pylori, clinical manifestation improvement, pathological improvement, adverse events.
Assessment of risk of bias
The studies were appraised independently by two authors (WJF, XYZ). Considering the different features of CHD from WM, we appropriately modified the Jadad scale, as some previous meta-analyses did. (Xu. et al.,2011; Zou et al,. 2011) The modified Jadad scale was as follows: (1) was the study described as randomized? (2-properly with detailed description of randomization, 1-randomized but detail not reported, 0-inappropriate randomization); (2) was allocation concealment used? (2-properly used, 1-unclear, 0-not used); (3) was the blind method used? (2-double-blind, 1-single-blind, 0-open-label); (4) were dropout and follow-up reported? (1-numbers and reasons reported, 0-not reported); and (5) was the treatment based on TCM symptom types (also called Bianzheng Lunzhi in Chinese (Karchmer,2013))? (2-properly with detailed description, 1-mentioned but detail not reported, 0-not mentioned or inappropriate). A study with a quality score ≤2 was considered as a study at high risk of bias, a study with a quality score ≥ 5 was considered as a study at low risk of bias, and the left were at moderate risk of bias.
Summary measures and synthesis of results
We undertook separate synthesis for each comparison. Dichotomous data were summarized as relative risk (RR) with 95% Confident Intervals (CIs), and a random effects model (DerSimonian and Laird,1986) was used whether heterogeneity was found in order to gain a more conservative outcome. When the authors reported dichotomous data (effective or ineffective), we retrieved them directly. In studies where multiple strata were given to define improvement, we converted these outcomes into dichotomous data to permit the overall analysis. Since the included study use the same validated criteria for the judgement of cure, we grouped together cure, significant improvement, and improvement as effective and no improvement, deterioration, as ineffective. Publication bias was examined using funnel plot and Egger’s tests. Heterogeneity between studies was tested using the inconsistency index (I2) statistic with a cutoff of 50%. The statistical analysis was carried out with RevMan 5.0 software (Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration, 2008) and Stata/SE version 10.0 (StataCorp, College Station, Texas, USA).
Sensitivity analysis
Because the poor-quality of RCT design might lead to exaggerated estimates of intervention benefit (Kjaergard et al.,2001), sensitivity analyses were performed to evaluate the robustness of outcomes and identify sources of heterogeneity. We conducted predesigned sensitivity analyses among studies of low to moderate risk of bias (modified Jadad score ≤2).
Results
Study characteristics and risk of bias
The search strategy was shown in the flow diagram (Figure 1). We included 42 RCTs, involving 21 studies (n=2,024) comparing CHD monotherapy with WM and 21 studies (n=1,850) comparing integration of CHD and WM (Int. CHD-WM with WM). According to the modified Jadad scale, totally 25 RCTs are at moderate risk of bias, 18 are at high risk of bias (see Supplementary Table 1). Study design details of each RCTs were shown in Supplementary Table 2, and the relationship among Chinese PinYin names, Chinese names, English names and Latin names of herbs mentioned in Supplementary Table 2 were demonstrated in Supplementary Table 3.
Figure 1.
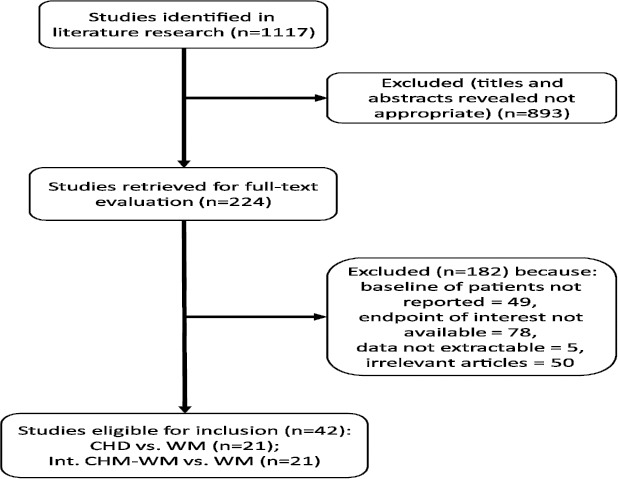
Flow diagram of RCTs included (Note: RCT, randomized controlled trial)
Supplementary Table 1.
Modified Jadad score of the included
| 1.RCTs CHD vs. WM | |||||||
|---|---|---|---|---|---|---|---|
| Trial | Year | Randomized | Allocation concealment | Blind | Dropout | Treatment based on TCM syndromes | Modified Jadad score |
| Wei BQ | 2003 | 1 | 0 | 0 | 0 | 2 | 3 |
| Gong DH | 2004 | 1 | 0 | 0 | 0 | 2 | 3 |
| Li JR | 2005 | 1 | 0 | 0 | 0 | 2 | 3 |
| Wang HY | 2005 | 1 | 0 | 0 | 0 | 1 | 2 |
| Wei YQ | 2005 | 1 | 0 | 0 | 0 | 2 | 3 |
| Kan SY | 2006 | 1 | 0 | 0 | 0 | 2 | 3 |
| Meng ZJ | 2006 | 1 | 0 | 0 | 0 | 2 | 3 |
| Zhao HB | 2006 | 1 | 0 | 0 | 0 | 0 | 1 |
| Chen YJ | 2007 | 1 | 0 | 0 | 0 | 1 | 2 |
| Luo LB | 2007 | 1 | 0 | 0 | 0 | 2 | 3 |
| Zhao M | 2007 | 1 | 0 | 0 | 0 | 2 | 3 |
| Ou WE | 2008 | 1 | 0 | 0 | 0 | 2 | 3 |
| Wang ZM | 2008 | 1 | 0 | 0 | 0 | 2 | 3 |
| Xiao LD | 2008 | 1 | 0 | 0 | 0 | 0 | 1 |
| Shi CH | 2009 | 1 | 0 | 0 | 0 | 2 | 3 |
| Shu H | 2009 | 1 | 0 | 0 | 0 | 2 | 3 |
| Su XH | 2009 | 2 | 0 | 0 | 0 | 2 | 4 |
| Li LH | 2010 | 1 | 0 | 0 | 0 | 0 | 1 |
| Meng L | 2010 | 1 | 0 | 0 | 0 | 1 | 2 |
| Zhang YM | 2011 | 1 | 0 | 0 | 0 | 2 | 3 |
| Zhu XP | 2013 | 1 | 0 | 0 | 0 | 2 | 3 |
| 2. Int. CHD-WM vs. WM | |||||||
| Trial | Year | Randomized | Allocation concealment | Blind | Dropout | Treatment based on TCM syndromes | Modified Jadad score |
| Wang HB | 2003 | 1 | 0 | 0 | 0 | 0 | 1 |
| Yue WJ | 2003 | 1 | 0 | 0 | 0 | 2 | 3 |
| Zhou AX | 2006 | 1 | 0 | 0 | 0 | 2 | 3 |
| Shen Y | 2007 | 1 | 0 | 0 | 0 | 0 | 1 |
| Xiang SY | 2007 | 1 | 0 | 0 | 0 | 0 | 1 |
| Zhang DF | 2008 | 1 | 0 | 0 | 0 | 0 | 1 |
| Gao Z | 2009 | 1 | 0 | 0 | 0 | 1 | |
| Huang GC | 2009 | 1 | 0 | 0 | 0 | 1 | 2 |
| Lei CH | 2009 | 1 | 0 | 0 | 1 | 2 | 4 |
| Ma J | 2009 | 1 | 0 | 0 | 0 | 2 | 3 |
| Song FL | 2009 | 1 | 0 | 0 | 0 | 2 | 3 |
| Liu HR | 2010 | 2 | 0 | 0 | 0 | 0 | 2 |
| Kuang YJ | 2011 | 1 | 0 | 0 | 0 | 2 | 3 |
| Wang XF | 2011 | 1 | 0 | 0 | 0 | 2 | 1 |
| Li SQ | 2012 | 1 | 0 | 0 | 0 | 2 | 3 |
| Zhang WH | 2012 | 1 | 0 | 0 | 0 | 0 | 1 |
| Han XF | 2013 | 1 | 0 | 0 | 0 | 2 | 3 |
| Wang J | 2013 | 1 | 0 | 0 | 0 | 2 | 1 |
| Shan Q | 2014 | 1 | 0 | 0 | 0 | 2 | 3 |
| Fu HK | 2014 | 1 | 0 | 0 | 0 | 2 | 3 |
| Liu DX | 2014 | 1 | 0 | 0 | 0 | 0 | 1 |
Supplementary Table 2.
Study design details of the included RCTs
| 1: CHD vs. WM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | DOI/ Website | Duration of clinical trail | Year of publication | Age [range] | Participants (male/female) | Number of Intervention/ Control | Intervention | Control | Duration of therapy | Adverse events |
| Zhu XP | 10.3969/j.issn.1003-5699.2007.03.015 | 2003-2013 | 2013 | [23, 67] | 60 (43/17) | 30/30 | Chai-Hu-Shu-Gan Decoction [ChaiHu 20g, DanShen 20g, FuLing 20g, NanShaShen 15g, BaiZhu 14g, TaiYangHua 14g, ZeXie 14g, BaiShao 12g, ChiShao12g, RouDoukou 12g, TanXiang 6g, HuangLian 6g, BanXia 6g, ZhiGanCao 6g] | Domperidone, Bismuth Biskalcitrate, Triple therapy | 28 days | Not reported |
| Zhang YM | 10.3969/j.issn.1006-0979.2011.08.004 | 2000-2010 | 2011 | [28, 69] | 120 (63/57) | 60/60 | Tianqi 5g, BanXie 6g, GanCao 10g, HuangQin 10g, ZhiKe 10g, BaiShao 12g, PuGongYing 15g, DangGui 15g, NanShaShen 15g, MaiDong 15g, BaiZhu 15g, DangShen 15g, DanShen 30g, HuangQi 45g | Colloidal Bismuth Pectin, Metronidazole, Domperidone, Amoxicillin | 60 days | Not reported |
| Li LH | http://www.cnki.com.cn/Article/CJFDTOTAL-SCZY201011038.htm | 2006-2009 | 2010 | [33, 72] | 55 (30/25) | 30/25 | HuangQi 30g, TaiZiShen 30g, BaiZhu 20g, ShanYao 30g, NanShaShen 15g, YuZhu 15g, BaiHe 15g, MaiDong 20g, NvZhenZi 20g, LiChang 20g, DanShen 30g, PuHuang 15g, GanCao 10g. | Vitacoenzyme Tablets, Colloidal Bismuth Pectin, Compound Pepsin | 3 months | Not reported |
| Meng L | http://www.cnki.com.cn/Article/CJFDTotal-LZXB201004078.htm | 2004-2008 | 2010 | [41, 72] | 84 (57/27) | 56/28 | Shan-Jia-Yu-Wei Decoction [PaoShanJia 6g, ZaoCi 10g, TongHuaGen 10g, ChaiHu 10g, FoShou 15g, ZhiKe 15g, TaoRen 10g, HongHua 10g, JiNeiJin 30g, JiaoSanXian 30g, HuangLian l0g, WuZhuYu 3g, PuGongYing 10g, FuLing 30g, BaiZhu 30g, BaiJi 30g, BaiShao 15g, GanCao 6g] | Vitacoenzyme Tablets, Domperidone | 60 days | Not reported |
| Shi CH | 10.3969/j.issn.1671-038X.2009.06.018 | 2006-2009 | 2009 | [17, 62] | 76 | 46/30 | Jian-Pi-Yi-Wei Decoction [TaiZiShen 25g, BaiZhu 10g, MuXiang 10g, ShaRen 10g, BeiMu10g, PuHuang 15g, LianQiao 12g, MoYuGu 15g, MaiDong 10g, NanShaShen 20g] | Vitacoenzyme Tablets or Gefarnate. Patients with H.pylori infection: Tinidazole, Amoxicillin, Omeprazole, patients with bile regurgitation: Talcid, patients with gastrectasia: Domperidone | 90 days | Not reported |
| Su XH | http://d.g.wanfangdata.com.cn/Periodical_cczyxyxb200906025.aspx | 2000-2008 | 2009 | No data | 120 (60/60) | 62/58 | Gan-Cao-Xie-Xin Decoction [GanCao 10g, BanXie 12g, HuangQi 15g, HuangLian 10g, DangShen 30g, HuangQi 30g, ShanYao 15g, ChaiHu 15g] | Amoxicillin, Clarithromycin, Domperidone | 3 months | Not reported |
| Shu H | 10.3969/j.issn.1005-7072.2009.02.028 | 2004-2007 | 2009 | [24, 65] | 58 (23/35) | 30/28 | Shen-Shi-Yang-Wei Decoction [DangShen 20g, HuangQi 30g, BaiZhu 15g, DanShen 30g, DangGui20g, ChiShao 15g, GanCao 6g] | Domperidone, Vitacoenzyme Tablets. Patients with H. pylori: De Nol, Amoxicillin | intervention: 69days, control: less than 4 weeks | Not reported |
| Wang ZM | 10.3969/j.issn.1009-5519.2008.12.111 | 2005-2007 | 2008 | [34, 65] | 78 (46/32) | 45/33 | BanXie 12g, HuangLian 6g, HuangQi 12g, GanJiang 12g, TaiZiShen 20g, GanCao 9g, ChaiHu 12g, ZhiKe 15g, XiangFu 10g, ChuanXiong 12g, BaiShao 18g, MuXiang 12g, ShaRen 10g, FoShou 12g, FoShou 12g | Hydrotalcite tablet, Mosapride | 3 months | Not reported |
| Ou WE | 10.3969/j.issn.1003-7705.2008.02.015 | 1994-2008 | 2008 | No data | 172 (106/66) | 86/86 | YanHuSuo 15g, DanShen 15g, ShanZha 15g, Tianqi 3g, PuHuang 10g, DangGui 20g, BaiJi 20g, RuXiang 10g, WuYao 10g, BaiHe 30g, BaiShao 15g, ShiHu 15g, NanShaShen 15g, GanCao 5g | Vitacoenzyme Tablets, Domperidone | 3 months | one case reported urticaria in intervention |
| Xiao LD | 10.3969/j.issn.1673-7717.2008.05.051 | 2006-2007 | 2008 | [19, 77] | 60 (36/ 24) | 30/30 | Wei-Yan Decoction [PuGongYing 30g, BaiHuaSheSheCao 30g, HuangLian 6g, HuangQi 10g, DanShen 20g, HuangQi 15g, BaiZhu 15g, FuLing 15g, BanXie 10g, ChenPi 10g, WaLengZi 30g, MoYuGu 30g, JiangHuang 15g, GanJiang 8g] | Omeprazole, Clarithromycin, Amoxicillin | intervention: 20 days, control: 7 days | Not reported |
| Chen YJ | 10.3969/j.issn.1006-6233.2007.03.030 | 2000-2006 | 2007 | [23, 70] | 92 (49/43) | 50/42 | Jian-Pi-Yang-Ying-He-Wei Decoction [TaiZiShen, WuZhiMaoTao, ShiHu, FoShou, YanHuSuo, NanShaShen, BaiJi, DanShen, MaiDong] | Marzulene S | 24 weeks | Not reported |
| Luo LB | 10.3969/j.issn.1000-7369.2007.09.032 | 2002-2006 | 2007 | [25, 73] | 82 (55/27) | 42/40 | Man-Wei-Ning Decoction [DangShen 15g, WuZao 15g, ZhiKe 10g, DangGui 10g, BaoShao 20g, HuangQi 20g, YanHuSuo 12g, GanCao 6g, TianQi 4g] | Bismuth Potassium Citrate Capsules | 8 weeks | six cases reported nausea, vomit or constipation in control |
| Zhao M | 10.3969/j.issn.1000-7369.2007.09.029 | 2007 | 2007 | [31, 50] | 64 | 32/32 | (1) For TCM syndrome of Weakness of Spleen and Stomach: HuangQi 15g, DangShen 15g, BaiZhu 10g, FuLing 10g, BaiShao 10g, GanCao 10g, ShengJiang 10g, DaZao 6g, ShaRen 6g, (2) for TCM syndrome of Coke and Blood Stasis: BanXie 10g, ChenPi 6g, HouPu 12g, ZhiKe 10g, WuLingZhi 12g, ChuanXiong 10g, DanShen 20g, YanHuSuo 20g, (3) for TCM syndrome of Deficiency of Stomach-Ying: NanShaShen 15g, MaiDong 12g, DiHuang 12g, DangGui15g, ShiHu 10g, BaiShao 10g, WuMei 15g, GanCao 6g. | Amoxicillin, Chinese Goldthread Rhizome, Colloidal Bismuth Pectin1, Domperidone | 8 weeks | Not reported |
| Meng ZJ | 10.3969/j.issn.1005-5304.2006.04.028 | 1998-2005 | 2006 | [27, 70] | 135 | 75/60 | Wei-Shu Decoction [HuangQi 45g, DangShen 15g, BaiZhu 15g, ZhiKe 10g, MaiDong 15g, NanShaShen 15g, DangGui15g, BaiShao 12g, HuangQi 10g, PuGongYing 15g, BanXie 6g, DanShen 30g, TianQi 5g, GanCao 10g] | Amoxicillin, Metronidazole, Colloidal Bismuth Pectin1, Domperidone | 8 weeks | Not reported |
| Kan SY | 10.3969/j.issn.0257-358X.2006.02.008 | 1997-2005 | 2006 | [31, 64] | 110 (65/45) | 70/40 | Yang-Ying-Rong-Wei-Wan [DiHuang, BeiShaShen, DangGui, ShouWuTeng, HuangLian, BaiShao, GouQiZi, MaiDong, BanXie, JiangHuang, BianDou, GanCao, MaiYa] | Vitacoenzyme Tablets | 90 days | Not reported |
| Zhao HB | 10.3969/j.issn.1000-3649.2006.09.037 | 2001-2005 | 2006 | [18, 58] | 90 (52/38) | 49/41 | Wei-Ling-San [CaoGuo 70g, WuLingZhi 70g, RuXiang 65g, MoYao 65g, GuaLou 85g, BaiHuaSheSheCao 85g, HuangLian70g, BanXie 50g] | Sucralfate, Metronidazole | 2 months | Not reported |
| Li JR | 10.3969/j.issn.1000-1719.2005.02.018 | 2004 | 2005 | [31, 51] | 72 | 36/36 | (1) For TCM syndrome of Weakness of Spleen and Stomach: HuangQi 15g, DangShen 15g, BaiZhu 10g, FuLing 10g, BaiShao 10g, GuiZhi 6g, GanCao 10g, ShengJiang 10g, DaZao 6, ShaRen 6g, (2) for TCM syndrome of Coke and Blood Stasis: BanXie 10g, ChenPi 6g, FuLing 10g, HouPu 12g, BaiZhu 15g, WuLingZhi 12g, ChuanXiong 10g, YanHuSuo 20g, SanLeng 10g, JiangHuang 10g, GanCao 10g, DanShen 20g, YiYiRen 10g, ShaRen 6g, (3) for TCM syndrome of Deficiency of Stomach-Ying: NanShaShen 15g, MaiDong 12g, DiHuang 12g, DangGui15g, BaiShao 10g, ShiHu 10g, WuMei 15g, GanCao 6g | Amoxicillin, Chinese Goldthread Rhizome, Colloidal Bismuth Pectin1, Domperidone | 8 weeks | Not reported |
| Wang HY | 10.3969/j.issn.1000-1719.2005.12.037 | 2005 | 2005 | [26, 63] | 130 (7 /56) | 100/30 | Shen-Ji-Yang-Wei Decoction [HuangQi 15g, NanShaShen 10g, BaiShao 15g, YanHuSuo 10g, DanShen 10g, BaiJi 8g, BeiMu15g, GanCao 5g] | Omeprazole, Amoxicillin, Metronidazole | 4 weeks | Not reported |
| Wei YQ | 10.3969/j.issn.1004-745X.2005.12.067 | 1990-2003 | 2005 | [31, 65] | 90 (54/36) | 54/36 | TaiZiShen 50g, HuangQi 50g, FuLing 30g, BaiZhu 12g, DanShen 15g, JiangHuang 15g, MuGua 12g, WuMei 9g, ChaiHu 9g, BaiHuaSheSheCao 30g | Bismuth Potassium Citrate Capsules, Amoxicillincapsule, Vitacoenzyme Tabletstablet, Domperidone tablet. | 6 months | Not reported |
| Gong DH | 10.3969/j.issn.1672-951X.2004.12.008 | 1998-2004 | 2004 | [16, 67] | 100 (58/42) | 50/50 | HuangQi 15g, BaiZhu 10g, FuLing 10g, NanShaShen 15g, MaiDong 15g, E’Jiao 10g, PuGongYing 15g, BaiHuaSheSheCao 15g, WuLingZhi 10g, PuHuang 10g, ZhiKe 10g, ShanZha 10g | Vitacoenzyme Tabletstablet, Folic Acidtablet, domperidone | 2-4 months | Not reported |
| Wei BQ | 10.3969/j.issn.0256-7415.2003.12.015 | 1998-2002 | 2003 | No data | 176 (95/81) | 96/80 | Wei-Ling Decoction [DangShen15g, FuLing, DanShen 15g, XiangFu 15g, JiaoSanXian 15g, PuGongYing 15g, GaoLiangJiang 10g, NvZhenZi 10g, BaiShao 10g, GuiZhi 10g, GanCao 6g, HuangQi 30g, BaiZhu 12g] | Patients with gastrectasia: Cisapride or Domperidone; patients with stomach-ache: Compound Belladonna Mixture, patients with anemia: Vitamin B and Folic Acid; patients with H.pylori infection: Amoxicillin, metronidazole, Colloidal Bismuth Pectin | 45 days | Not reported |
| 2: Int. CHD-WM vs. WM | ||||||||||
| Autdor | DOI/ Website | Duration of clinical trail | Year of publication | Age [range] | Participants | Number of Intervention/ Control | Intervention | Control | Duration of tderapy | Adverse events |
| Fu HK | http://lib.cqvip.com/qk/93943A/201403/48935322.html | 2010-2012 | 2014 | [25, 69] | 50 (19/31) | 25/25 | WM: Omeprazole, Amoxicillin, Clarithromycin CHD: DangShen 10g, DiHuang 10g, WuLingZhi 10g, PuHuang 10g, TaiZiShen 20g, GanCao 6g, CHD: HuangQi 30g, BaiZhu 12g, NanShaShen 15g, MaiDong 15g | Omeprazole, Amoxicillin, Clarithromycin | N/R | Not reported |
| Liu DX | 10.3969/j.issn.1004-437X2014.01.055 | 2010-2012 | 2014 | [33, 36] | 80 (59/21) | 40/40 | WM: Omeprazole, Vatacoenayme, Tinidazole, Clarithromycin CHD: HuangQi 15g, DangShen 10g, BaiShao 10g, NanShaShen 10g, DangGui10g, PuHuang 10g, WuLingZhi 10g, PuHuang 10g, ZhiGanCao 5g | Omeprazole, Vatacoenayme, Tinidazole, Clarithromycin | 2 months | Not reported |
| Shan Q | 10.3969/j.issn.1004-7484(x).2014.06.038 | 2011-2013 | 2014 | [38, 42] | 112 (31/27) | 54/58 | WM: Thiazole, PPI, Triple therapy or Quadruple therapy CHD: for patients with stagnation of liver-QI and stomach-QI: Chai-Hu-Shu-Gan-San; for patients with epigastralgia: Hua-Gan-Jian-He-Zuo-Jin-Wan; for patients with damp heat in the spleen and the stomach: Huang-Lian-Wen-Dan Decoction; for patients with weakness of the spleen and the stomach: Liu-Jun-Zi Decoction; for patients with Stomach yin deficiency: NanShaShen, MaiDong Decoction; patients with stomach and blood stasis: DanShen Decoction | Thiazole, PPI, Triple therapy or Quadruple therapy | 6 months | Not reported |
| Wang J | 10.3969/j.issn.1009-4393.2013.6.109 | 2011-2012 | 2013 | [22, 79] | 128 (74/54) | 64/64 | WM: Vatacoenayme, Bismuth Biskalcitrate, Amoxicillin, Tinidazole, Domperidone CHD: DangShen 20g, BaiZhu 15g, HuangQi 20g, BaiShao 15g, FuLing 15g, DanShen 20g, DangGui 12g, YanHuSuo 15g, ShaRen 10g, TianQi 3g, E’Zhu 10g, MuXiang 10g, ChaiHu 10g | Vatacoenayme, Bismuth Biskalcitrate, Amoxicillin, Tinidazole, Domperidone | 2 months | Not reported |
| Han XF | http://d.wanfangdata.com.cn/Periodical/jkbd-x201308126 | 2010-2012 | 2013 | [37, 78] | 40 (19/27) | 20/20 | WM: Vatacoenayme, Amoxicillin CHD: Yi-Wei-Suo-Xing-Wei-Yan Decoction: DangShen 30g, PuGongYing 30g, ChuanXiong 10g, HuangQi30g, E’Zhu 10g, JiNeiJin 15g, TianQi 3g, DanShen 20g, DaHuang 5g, HouPu 5g, YanHuSuo 15g | Vatacoenayme, Amoxicillin | 4 months | Not reported |
| Li SQ | doi: 10.3969/j.issn1007-8231.2012.11.067 | 2011 | 2012 | [30, 65] | 59 (41/17) | 29/29 | WM: Vatacoenayme, Colloidal Bismuth, Amoxicillin, Tinidazole, Domperidone CHD: HuangQi 30g, DangShen 20g, BaiZhu 10, FuLing 10g, BaiShao 15g, MaiDong 15g, ShiHu 15g, DiHuang 30g, E’Zhu 15g, FoShou 15g, QingDai 3g, ZhiGanCao 9g | Vatacoenayme, Colloidal Bismuth, Amoxicillin, Tinidazole, Domperidone | 4 weeks | Not reported |
| Zhang WH | http://d.wanfangdata.com.cn/Periodical/jkbd-z201208150 | 2005-2010 | 2012 | [28, 72] | 120 | 68/52 | WM: Tinidazole, Clarithromycin, Domperidone, Vatacoenayme CHD: NanShaShen 10g, MaiDong 10g, ShiHu 10g, ZeXie 10g, BaiZhu 10g, BaiShao 10g, TaiZiShen 15g, DanShen 15g, HuangQi 12g, ShanYao 15g, FoShou 6g, MuXiang 6g, ShaRen 5g, ShengMa 6g, Dihuang 10g CHD: Wen-Yang-Hua-Tan Decoction: HuangQi 50g, YiYiRen 20g, DangShen 20g, BaiZhu 15g, FuLing 15g, GuiPi 10g, DingXiangZhi 10g, HuoXiang 10g, PeiLan 10g, ShaRen 5g | Tinidazole, Clarithromycin, Domperidone, Vatacoenayme | 2 months | Not reported |
| Wang XF | http://www.cqvip.com/QK/87361X/201103/1003588945.html | 2009-2010 | 2011 | [19, 72] | 136 (78/58) | 68/68 | WM: Vatacoenayme, Omeprazole, Metronidazole, Amoxicillin CHD: Wen-Yang-Hua-Tan Decoction: HuangQi 50g, YiYiRen 20g, DangShen 20g, BaiZhu 15g, FuLing 15g, ShengJiang 10g, GuiZhi 10g, DingXiangZhi 10g, HuoXiang 10g, PeiLan 10g, ShaRen 5g | Vatacoenayme, Omeprazole, Metronidazole, Amoxicillin | 6-8 weeks | control: 12 patients with gastrointestinal adverse reaction, such as nausea, vomiting, abdominal distention; intervention: six patients with minor gastrointestinal adverse reaction |
| Kuang YJ | doi:10.3969/j.issn.1009-4393.2011.19.101 | 2008-2010 | 2011 | [21, 67] | 120 (76/44) | 60/60 | WM: Bismuth Biskalcitrate, Omeprazole, Clarithromycin, Amoxicillin, Domperidone CHD: TianQi 3g, ZhiGanCao 5g, WuZhuYu 5g, ShaRen 6g, HuangLian 6g, NanShaShen 6g, DangGui 10g, BaiZhu 10g, HouPu 10g, HuangQi 15g, FuLing 15g, DangShen 20g, ShanYou 20g | Bismuth Biskalcitrate, Omeprazole, Clarithromycin, Amoxicillin, Domperidone | N/R | no patient with adverse reaction both in control and intervention |
| Liu HR | http://www.cqvip.com/QK/71135X/201107/34896346.html | 2003-2007 | 2010 | [30, 63] | 100 (59/41) | 50/50 | WM: Omeprazole, Clarithromycin, Tinidazole; CHD: Wei-Shu-Jian-Ji [ChaiHu, BaiShao, GanCao, ZhiKe, DaHuang, PuGongYing, FuLing, BaiZhu, ChuanLianZi, YanHuSuo, LiYa, DanShen] | Omeprazole, Clarithromycin, Tinidazole | 6 months | Not reported |
| Gao Z | http://www.cqvip.com/Main/Detail.aspx?id=31422276 | 1999-2007 | 2009 | [25, 72] | 83 (54/29) | 46/37 | WM: Colloidal Bismuth Pectin Vitacoenzyme Compound Pepsin; CHD: Zi-Yin-Hua-Yu-Ning-Wei Decoction [TaiZiShen 20g, BaiShao 20g, ShiHu 15g, WuMei 15g, GanCao 10g, E’Zhu 10g, ZiSuGeng 10g, TianQi 6g, HuangYaoZi 12g, ChuanLianZi 9g] | Colloidal Bismuth Pectin Vitacoenzyme Compound Pepsin; | 12 months | Not reported |
| Lei CH | http://www.cnki.com.cn/Article/CJFDTotal-SYBD200904007.htm | 2009 | 2009 | [33, 65] | 68 | 34/34 | WM: Metronidazole, Folic Acid; CHD: Si-Jun-Zi Decoction [RenShen 9g, BaiZhu 9g, FuLing 9g, WuLingZhi 8g, ChuanXiong 8g, BaiHuaSheSheCao 10g, GanCao 6g] | Metronidazole, Folic Acid | 24 weeks | Not reported |
| Ma J | http://www.cqvip.com/Main/Detail.aspx?id=30648302 | 2007-2008 | 2009 | [30, 65] | 60 (31/29) | 30/30 | WM: Omeprazole, Clarithromycin, Amoxicillin; CHD:Jian-Pi-Yi-Wei Decoction [HuangQi 20g, Dangshen 20g, BaiZhu 15g, GuiZhi 10g, DanShen 15g, ChiShao15g, ShanZha 10g, BaiShao 15g, E’Zhu 10g, GanCao 5g] | Omeprazole, Clarithromycin, Amoxicillin, Vitamin E, Carotene | 3 months | Not reported |
| Huang GC | 10.3969/j.issn.1672-2779.2009.04.071 | 2004-2007 | 2009 | [30, 81] | 85 (58/27) | 45/40 | WM: Colloidal Bismuth Pectin capsule; CHD: Ban-Xia-Xie-Xin Decoction [BanXie 10g, Dangshen 20g, HuangLian6g, HuangQi 10g, GanJiang 5g, GanCao 6g, PuGongYing 15g, ZhiKe 10g, MoYuGu 15g, BaiHuaSheSheCao 15g] CHD: for TCM syndrome of Weakness of Spleen and Stomach: Dangshen, HuangQi, ShanYao, GanCao, BaiZhu, GanJiang, NanShaShen, DaZao; for TCM syndrome of Liver Stomach Disharmony: ChaiHu, MuXiang, FoShou, BaiShao, NanShaShen, MaiDong, YuZhu, HuangLian, ShiHu, GanCao, YanHuSuo, Reed Rhizome; for TCM syndrome of Stagnation of Phlegm: ChenPi, FuLing, BanXie, BaiZhu, HouPu, HuangQi, GanJiang, GanCao; for TCM syndrome of Damp-Heat in Spleen and Stomach: HuangLian, HuangQi, PuGongYing, BanXie, ZhuRu, FuLing, YiYiRen, ZeXie, FoShou, ChenPi, ZhiKe; for TCM syndrome of Stomach Collateral Stasis: YanHuSuo, MoYao, WuLingZhi, CaoGuo, DanShen, ChiShao, E’Zhu, PuHuang, TaoRen. | Colloidal Bismuth Pectin capsule | 12 weeks | Not reported |
| Song FL | http://lib.cqvip.com/qk/91070A/200903/29592636.html | 2005-2007 | 2009 | [33, 65] | 68 (41/27) | 34/34 | WM: Metronidazole, Folic Acid; CHD: Jia-Wei-Si-Jun-Zi Decoction [Dangshen 9g, BaiZhu 9g, FuLing 9g, WuLingZhi 8g, ChuanXiong 8g, BaiHuaSheSheCao 6g, GanCao 6g] | Metronidazole, Folic Acid | 24 weeks | Not reported |
| Zhang DF | 10.3969/j.issn.1001-6910.2008.03.016 | 1999-2006 | 2008 | [45, 70] | 70 (41/29) | 36/34 | WM: Omeprazole capsule, Amoxicillin capsule, Metronidazole tablet, Folic Acid; CHD: Dan-shen-Yin plus Chai-Shao-Liu-Jun-Zi Decoction [HuangQi 3g, RenShen 6g, FuLingg, BaiZhu 6g, DanShen 3g, DangGui12g, TanXiang 6g, ShaRen 6g, BanXie 6g, MuXiang 6g, BaiShao 12g, ChaiHu 10g, HuangQi 12g, GanCao 3g] | Omeprazole capsule, Amoxicillin capsule, Metronidazole tablet, Folic Acid | 60-150 days | Not reported |
| Shen Y | 10.3969/j.issn.1001-9448.2007.03.068 | 2000-2006 | 2007 | [17, 75] | 132 (82/50) | 69/63 | WM: Bismuth Potassium Citrate Capsules, Domperidone, Weilesheng tablet, Cimetidine, metronidazole, Amoxicillin CHD: Dan-Shen-Yin plus Shi-Xiao-San [DanShen 30g, YanHuSuo 10g, TanXiang 10g, ShaRen 5g, ZiSuGeng 10g, JuLuo 10g, DaHuang 5g, GanCao 3g, PuHuang 10g, WuLingZhi 10g] | Bismuth Potassium Citrate Capsules, Domperidone, Weilesheng tablet, Cimetidine, metronidazole, Amoxicillin | 3 months | two cases reported rash in intervention |
| Xiang SY | 10.3969/j.issn.1671-4040.2007.05.007 | 2007 | 2007 | [37, 68] | 130 (72/58) | 80/50 | WM: Sucralfate tablet, Mosapride, Folic Acid; CHD: Fu-Fang-Wu-Shen Decoction [Dangshen 15g, NanShaShen 12g, XuanShen 10g, DanShen 10g, KuShen 10g, MaiDong 15g, HuangJing 10g, ChuanXiong 10g, HuangLian 10g, HuangQi 12g, ShiHu 15g, JinFeiCao 10g] | Sucralfate tablet, Mosapride, Folic Acid | 3 months | Not reported |
| Zhou AX | http://192.168.89.197:8012/ArticleDetail.aspx?id=21906480 | 2004-2006 | 2006 | [29, 71] | 60 (30/30) | 30/30 | WM: one chosen from Omeprazole, Lansoprazole, Bismuth Potassium Citrate, plus one chosen from Clarithromycin, Amoxicillin, Metronidazole CHD: Bu-Yi-Ha-Yu Decoction [HuangQi 30g, TaiZiShen 30g, NanShaShen 20g, MaiDong 20g, DangGui20g, DiHuang 30g, E’Zhu 10g, HongHua 6g, MoYao 10g, BaiHuaSheSheCao 30g] | One chosen from Omeprazole, Lansoprazole, Bismuth Potassium Citrate, plus one chosen from Clarithromycin, Amoxicillin, Metronidazole. | 2 months | Not reported |
| Wang HB | 10.3870/j.issn.1004-0781.2003.11.010 | 1999-2000 | 2003 | 54 patients >40, 11 patients <40: | 65 | 35/30 | WM: Colloidal Bismuth Pectin, Amoxicillin, Furazolidone; CHD: Wei-Fu-Jian-Ji [TaoRen 10g, HongHua 10g, ChuanXiong 10g, ChiShao10g, PuGongYing 10g, MuXiang 10g, GanCao 10g, HuangQi 20g, Dangshen 15g] | Colloidal Bismuth Pectin, Amoxicillin, Furazolidone | 4 weeks | Not reported |
| Yue WJ | 10.3760/cma.j.issn.1008-6706.2003.11.055 | 1998-2003 | 2003 | [31, 79] | 85 (49/36) | 58/27 | WM: Colloidal Bismuth Pectin capsule, Tinidazole tablet, Vitamin C tablet, Folic Acid tablet; CHD: Xiang-Sha-Liu-Jun-Zi Decoction [XiangFu 15g, ShaRen 10g, RenShen 15g, BaiZhu 20g, FuLing 15g, GanCao 10g, ChenPi 10g, BanXie 10g] CHD: Chu-Pi-Yang-Wei Decoction: [Dangshen l5g, FuLing 15g, E’Zhu l5g, WuZhuYu 6g, MuXiang 9g, GanCao 6g, HuangJing 15g, ShanYao 30g, ShaRen 6g, HuangLian 6g, BaiJiangCao 30g] | Colloidal Bismuth Pectin capsule, Tinidazole tablet, Vitamin C tablet, Folic Acid tablet | 6 weeks | Not reported |
Supplementary Table 3.
List of traditional Chinese herbs used in included studies
| Pinyin Name | Chinese Name | Latin Name | English Name |
|---|---|---|---|
| BaiHe | 百合 | Lilium brownii var. viridulum | Greenish lily bulb |
| BaiHuaSheSheCao | 白花蛇舌草 | Hedyotis diffusa Spreng. | Spreading hedyotis herb |
| BaiJi | 白及 | Bletilla striata (Thunb.) Rchb.f. | Common bletilla tuber |
| BaiJiangCao | 败酱草 | Hlaspi arvense L | Dahurian patrinia herb |
| BaiShao | 白芍 | Paeonia lactiflora Pall. | White peony root |
| BaiZhu | 白术 | Atractylodes macrocephala Koidz | Largehead atractylodes rhizome |
| BanXie | 半夏 | Pinellia ternata (Thunb.) Breit. | Ternate pinellia |
| BeiMu | 贝母 | Fritillaria cirrhosa D.Don | Fritillaria |
| BeiShaShen | 北沙参 | Glehnia littoralis F.Schmidt ex Miq. | Coastal glehnia root |
| BianDou | 扁豆 | Lablab purpureus (L.) Sweet | Dolichos lablab |
| CaoGuo | 草果 | Amomum tsao-ko Crevost & Lemarié | Tsao-ko amomum fruit |
| ChaiHu | 柴胡 | Bupleurum chinense DC. | Chinese thorowax root |
| ChenPi | 陈皮 | Clausena lansium (Lour.) Skeels | Tangerine peel |
| ChiShao | 赤芍 | Paeonia anomala subsp. veitchii (Lynch) D.Y.Hong & K.Y.Pan | Red peony root |
| ChuanXiong | 川芎 | Cortia striata (DC.) Leute | Szechwan lovage rhizome |
| ChuanLianZi | 川楝子 | Melia toosendan Siebold & Zucc | Szechwan chinaberry fruit |
| DaHuang | 大黄 | Rheum palmatum L. | Rhubarb |
| DangGui | 当归 | Angelica sinensis (Oliv.) Diels | Chinese angelica |
| DangShen | 党参 | Codonopsis pilosula (Franch.) Nannf. | Codonopsis pilosula |
| DanShen | 丹参 | Salvia miltiorrhiza Bunge | Dan-shen root |
| DaZao | 大枣 | Ziziphus jujuba Mill | Common jujube |
| DiHuang | 地黄 | Rehmannia glutinosa (Gaetn) Libosch. ex Fisch. et Mey | Rehmannia root |
| DingXiangZhi | 丁香枝 | Syzygium aromaticum (L.) Merr.Et Perry | Clovetree twig |
| E’Jiao | 阿胶 | Colla Corii Aaini | Ass-hide gelatin |
| E’Zhu | 莪术 | Curcuma zedoaria (Christm.) Roscoe | Rhizoma curcumae |
| FoShou | 佛手 | Citrus medica var. sarcodactylus (Siebold ex Hoola van Nooten) Swingle | Finger citron fruit |
| FuLing | 茯苓 | Poria cocos Wolf [Fungi] | Indian buead |
| GanCao | 甘草 | Glycyrrhiza uralensis Fisch. | Liquorice root |
| GanJiang | 干姜 | Zingiber officinale Roscoe | Dried ginger |
| GaoLiangJiang | 高良姜 | Alpinia officinarum Hance | Lesser galangal rhizome |
| GouQiZi | 枸杞子 | Lycium barbarum L. | Barbury wolfberry fruit |
| GuaLou | 瓜蒌 | Trichosanthes kirilowii Maxim | Mongolian snakegourd fruit |
| GuiZhi | 桂枝 | Cinnamomum cassia (L.) J.Presl | Cassiabarktree twig |
| HongHua | 红花 | Carthamus tinctorius L. | Safflower |
| HouPu | 厚朴 | Citrus grandis (L.) Osbeck | Officinal magnolia bark |
| HuangJing | 黄精 | Polygonatum sibiricum F.Delaroche | Siberian solomonseal rhizome |
| HuangLian | 黄连 | Coptis chinensis Franch. | Chinese goldthread rhizome |
| HuangQi | 黄芪 | Astragalus membranaceus (Fisch.) Bunge | Astragalus membranaceus |
| HuangQin | 黄芩 | Scutellaria Linn. | Radix scutellariae |
| HuangYaoZi | 黄药子 | Dioscorea bulbifera L. | Airpotato yam rhizome |
| HuoXiang | 藿香 | Agastache rugosa (Fisch. & C.A.Mey.) Kuntze | Agastache rugosus |
| JiangHuang | 姜黄 | Curcuma longa L. | Turmeric rhizome |
| JiaoSanXian | 焦三仙 | Hordeum vulgare L Plus Crataegus pinnatifida Bunge Plus Massa Medicata Fermentata | Stir-baking fructus hordei germinatus et crataegt et massa fer-mentata medicinalis |
| JiNeiJin | 鸡内金 | Gallus gallus domesticus Brisson | Corium stomachichum galli |
| JinFeiCao | 金沸草 | Inula japonica Thunb. | Inula flower |
| JuLuo | 桔络 | Citrus reticulata Blanco | Tangerine pith |
| KuShen | 苦参 | Sophora flavescens Aiton | Lightyellow sophora root |
| LianQiao | 连翘 | Forsythia suspensa (Thunb.) Vahl | Weeping forsythia fruit |
| LiChang | 鳢肠 | Eclipta prostrata (L.) L. | Eclipta prostrata |
| LiYa | 粟芽 | Setaria italica (L.) P.Beauv. | Foxtail millet sprout |
| MaiDong | 麦冬 | Ophiopogon japonicus (Thunb.) Ker Gawl. | Dwarf lilyturf root tuber |
| MaiYa | 麦芽 | Hordeum vulgare L | Malt |
| MoYao | 没药 | Commiphora myrrha (Nees) Engl. | Myrrh |
| MoYuGu | 墨鱼骨 | Sepia esculenta Hoyle | Cuttlebone |
| MuGua | 木瓜 | Chaenomeles chinensis (Dum.Cours.) Koehne | Common floweringquine fruit |
| MuXiang | 木香 | Rosa banksiae R.Br. | Costusroot |
| NanShaShen | 南沙参 | Adenophora tetraphylla (Thunb.) Fisch. | Upright ladybell root |
| NvZhenZi | 女贞子 | Ligustrum lucidum W.T.Aiton | Glossy privet fruit |
| PaoShanJia | 炮山甲 | Squama Manis | Parched pangolin scales |
| PeiLan | 佩兰 | Eupatorium fortunei Turcz. | Eupatorium fortunei |
| PuGongYing | 蒲公英 | Taraxacum mongolicum Hand.-Mazz. | Mongolian dandelion herb |
| PuHuang | 蒲黄 | Typha angustifolia L. | Cattail pollen |
| QingDai | 青黛 | Baphicacanthus cusia (Nees) Bremek | Indigo naturalis |
| RenShen | 人参 | Panax ginseng C.A.Mey. | Ginseng |
| RouDoukou | 肉豆蔻 | Myristica fragrans Houtt. | Fructus amomi rotundus |
| RuXiang | 乳香 | Boswellia carteri Birdw. | Frankincense |
| SanLeng | 三棱 | Sparganium stoloniferum (Buch.-Ham. ex Graebn.) Buch.-Ham. ex Juz | Common burreed tuber |
| ShanYao | 山药 | Dioscorea oppositifolia L. | Common yam rhizome |
| ShanZha | 山楂 | Crataegus scabrifolia (Franch.) Rehder | Chinese hawthorn fruit |
| ShaRen | 砂仁 | Amomum villosum Lour. | Villous amonmum fruit |
| ShengJiang | 生姜 | Zingiber officinale Roscoe | Fresh ginger |
| ShengMa | 升麻 | Cimicifuga foetida L. | Rhizoma cimicifugae |
| ShiHu | 石斛 | Dendrobium catenatum Lindl | Noble dendrobium stem herb |
| ShouWuTeng | 首乌藤 | Fallopia multiflora (Thunb.) Harald | Tuber fleeceflower stem and leaf |
| TaiYangHua | 太阳花 | Portulaca grandiflora | Portulaca grandiflora |
| TaiZiShen | 太子参 | Pseudostellaria heterophylla (Miq.) Pax | Pseudostellaria root |
| TanXiang | 檀香 | Gaultheria fragrantissima Wall | Sandalwood |
| TaoRen | 桃仁 | Prunus persica (L.) Batsch | Peach seed |
| Tianqi | 田七 | Panax pseudoginseng var. notoginseng (Burkill) G.Hoo & C.L.Tseng | Pseudo-ginseng |
| TongHuaGen | 通花根 | Tetrapanax papyrifer (Hook.) K.Koch | Ricepaperplant |
| WaLengZi | 瓦楞子 | Concha Arcae | Arc shell |
| WuLingZhi | 五灵脂 | Faeces Trogopterpri | Trogopterus dung |
| WuMei | 乌梅 | Prunus mume (Siebold) Siebold & Zucc. | Smoked plum |
| WuYao | 乌药 | Lindera aggregata (Sims) Kosterm. | Combined spicebush root |
| WuZao | 乌枣 | Diospyros lotus L. | Smoked jujube |
| WuZhiMaoTao | 五指毛桃 | Ficus simplicissima Lour. | Radix fici simplicissimae |
| WuZhuYu | 吴茱萸 | Tetradium ruticarpum (A.Juss.) T.G.Hartley | Medicinal evodia immature fruit |
| XiangFu | 香附 | Cyperus rotundus L. | Nutgrass galingale rhizome |
| XuanShen | 玄参 | Scrophularia oldhamii Oliv. | Figwort root |
| YanHuSuo | 延胡索 | Corydalis yanhusuo | Yanhusuo tuber |
| YiYiRen | 薏苡仁 | Coix lacryma-jobi L. | Ma-yuen jobstears seed |
| YuZhu | 玉竹 | Polygonatum odoratum (Mill.) Druce | Fragrant solomonseal rhizome |
| ZaoCi | 皂刺 | Gleditsia sinensis Lam. | Spina gleditsiae |
| ZeXie | 泽泻 | Alisma plantago-aquatica L. | Rhizoma alismatis |
| ZhiGanCao | 炙甘草 | Glycyrrhiza uralensis Fisch. | Prepared liquorice root |
| ZhiKe | 枳壳 | Citrus × aurantium L. | Submature bitter orange |
| ZhuRu | 竹茹 | Sinocalamus beecheyanus (Munro) McClure | Bamboo shavings |
| ZiSuGeng | 紫苏梗 | Perilla frutescens (L.) Britton | Caulis perillae acutae |
Eradication of H. pylori
There were 12 trials reporting the eradication rate of H. pylori. Remission of H. pylori infection was not achieved in 121 (21.8%) of 554 patients randomized to receive CHD (alone or integrated with WM), compared with 180 (38.2%) of 471 patients received WM (RR=1.29; 95% CI 1.11-1.50) with significant heterogeneity between studies (I2=71%) (Figure 2). There was statistically significant funnel plot asymmetry (Egger’s test p=0.044), suggesting evidence of publication bias or other small study effects.
Figure 2.
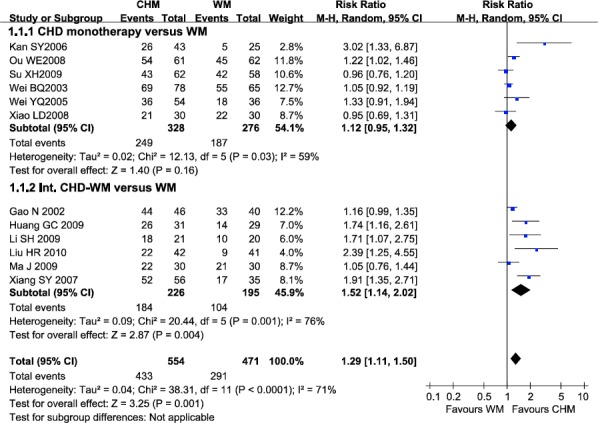
Efficacy of CHD compared with WM in eradication of H. pylori
Note: CHD, Chinese herbal decoction; WM, western medicine; Int. CHD-WM, integrated Chinese herbal decoction and western medicine; 95% CI, 95% confidence interval. Each point on the figure represents a relative risk (RR). The diamond represents the pooled estimate of effect, as calculated according to the random effects model. RR<1 means numerically lower response rate than WM, and RR>1 numerically higher response rate than WM. 95% CI doesn’t include the number 1 means statistical difference between the two groups.
In subgroup of CHD monotherapy versus WM, six trials reported the eradication rate of H. pylori. There was no significant difference between CHD and WM in H. pylori eradication (RR=1.12, 95% CI 0.95-1.32) (Figure 2), with significant heterogeneity between studies (I2=59%). However, the pooled data suggested that CHD with WM had beneficial effect over WM (RR=1.52, 95% CI 1.14-2.02), with significant heterogeneity between studies (I2=76%).
Clinical manifestations improvement
In total, 38 trials compared CHD (alone or integrated with WM) with WM involving 3,812 patients reported clinical manifestations improvement rate. There are 173 (8.3%) of 2,091 assigned to CHD (alone or integrated with WM) who failed to improve clinical manifestations, compared with 503 (28.8%) of 1,747 patients allocated to WM (RR=1.28; 95% CI 1.22-1.34), without significant heterogeneity between studies (I2=44%) (Figure 3). Evidence of publication bias was observed (Egger’s test p=0.000).
Figure 3.
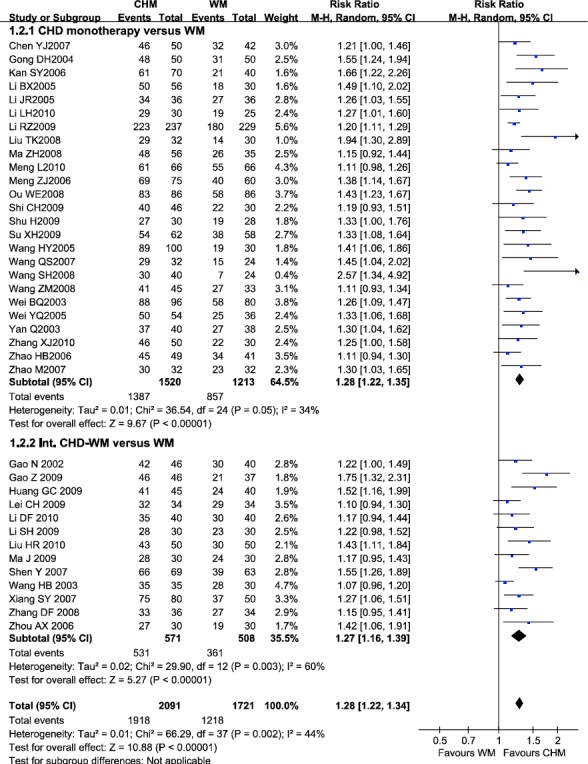
Efficacy of CHD compared with WM in clinical manifestations improvement
Note: CHD, Chinese herbal decoction; WM, western medicine; Int. CHD-WM, integrated Chinese herbal decoction and western medicine; 95% CI, 95% confidence interval. Each point on the figure represents a relative risk (RR). The diamond represents the pooled estimate of effect, as calculated according to the random effects model. RR<1 means numerically lower response rate than WM, and RR>1 numerically higher response rate than WM. 95% CI doesn’t include the number 1 means statistical difference between the two groups.
In subgroup of CHD monotherapy versus WM, 25 studies reported the clinical manifestations improvement rate. The pooled data suggested that CHD had beneficial effect over WM (RR=1.28, 95% CI 1.22-1.35), without significant heterogeneity between studies (I2=34%). While Int. CHD-WM was found to be beneficial over WM alone (RR=1.27, 95% CI 1.16-1.39), with significant heterogeneity between studies (I2=60%). There was statistically significant funnel plot asymmetry in the two subgroups (Egger’s test p=0.0017, p=0.0019, respectively), suggesting evidence of publication bias.
Pathological improvement
Totally, 20 trials compared CHD (alone or integrated with WM) with WM in 1,959 patients reported pathological improvement. 154 (14.9%) of 1034 patients using CHD (alone or integrated with WM) failed to improve pathological change, compared with 360 (46.0%) of 925 patients using WM (RR=1.42; 95% CI 1.30-1.54), without significant heterogeneity between studies (I2=48%) (Figure 4). Evidence of publication bias was observed (Egger’s test p=0.002).
Figure 4.
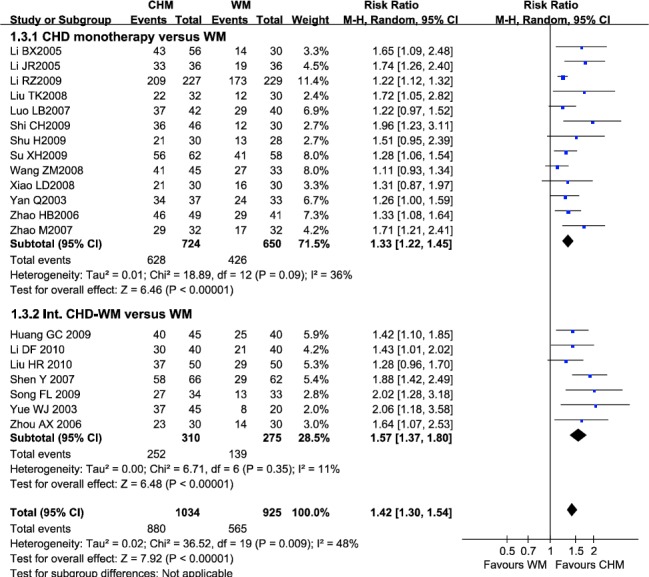
Efficacy of CHD compared with WM in pathological improvement
Note: CHD, Chinese herbal decoction; WM, western medicine; Int. CHD-WM, integrated Chinese herbal decoction and western medicine; 95% CI, 95% confidence interval. Each point on the figure represents a relative risk (RR). The diamond represents the pooled estimate of effect, as calculated according to the random effects model. RR<1 means numerically lower response rate than WM, and RR>1 numerically higher response rate than WM. 95% CI doesn’t include the number 1 means statistical difference between the two group Adverse events
In subgroup of CHD monotherapy versus WM, 13 studies reported pathological improvement rate. The result suggested that CHD had beneficial effect over WM (RR=1.33, 95% CI 1.22-1.45), without significant heterogeneity between studies (I2=36%). In subgroup of Int. CHD-WM versus WM, Int. CHD-WM was found to be beneficial over WM (RR=1.57, 95% CI 1.37-1.80), without significant heterogeneity between studies (I2=11%). Statistically significant funnel plot asymmetry was only found in subgroup of CHD monotherapy versus WM (Egger’s test p=0.016), suggesting evidence of publication bias or other small study effects.
Only minor side effects, such as urticarial, rash, and slight gastrointestinal discomfort, were found in CHD group (shown in Supplementary Table 2). There are no statistical differences in side effects between CHD (alone or with WM) and WM.
Sensitivity analysis
In order to evaluate the robustness of outcomes and identify sources of heterogeneity, we conducted prespecified sensitivity analyses. Totally 25 RCTs are at moderate risk of bias, 18 are at high risk of bias. In subgroup of CHD monotherapy the number is 15 (moderate) and six (high), while in subgroup of Int. CHD-WM the number is 10 (moderate) and 11 (high) (see Supplementary Table 1). The results were similar in direction and magnitude to the primary results expect the eradication rate of H. pylori, suggesting the robustness of most results in this study. However heterogeneity between trials still existed in the some outcomes (Table 1).
Table 1.
Sensitivity analyses of efficacy of CHM compared with WM in AG
| Number of studies | Number of subjects | RR | 95% CI | I2 value | |
|---|---|---|---|---|---|
| Eradication rate of H. pylori | |||||
| CHM (alone or integrated with WM) versus WM | 5 | 544 | 1.16 | [0.96, 1.41] | 66% |
| CHM versus WM | 5 | 544 | 1.16 | [0.96, 1.41] | 66% |
| Int. CHM-WM versus WM | 0 | 0 | N/A | N/A | N/A |
| Clinical manifestations improvement | |||||
| CHM (alone or integrated with WM) versus WM | 20 | 2,213 | 1.27 | [1.22, 1.33] | 12% |
| CHM versus WM | 16 | 1,939 | 1.29 | [1.23, 1.36] | 10% |
| Int. CHM-WM versus WM | 4 | 274 | 1.18 | [1.07, 1.31] | 0% |
| Pathological improvement | |||||
| CHM (alone or integrated with WM) versus WM | 12 | 1,284 | 1.44 | [1.27, 1.63] | 60% |
| CHM versus WM | 9 | 1,092 | 1.35 | [1.20, 1.52] | 53% |
| Int. CHM-WM versus WM | 3 | 192 | 1.87 | [1.42, 2.45] | 0% |
Discussion
Herbal decoction is a concentrated herbal tea in which raw roots, berries and barks are lightly simmered for hours to extract the useful constituents. Compared with Chinese herbal patent medicines, which is the ready-made pills or capsules of herbal extracts as products of modern pharmaceutical industry, CHD is considered to have more advantages such as flexibility in treatment and strictly following the basic TCM theory of Bianzheng Lunzhi strictly. Our study is the first systematic review and meta-analysis evaluating the efficacy and safety of all kinds of decoctions in the treatment of AG according to TCM symptom types. The results demonstrated that: 1) CHD may be more effective than WM in ameliorating clinical manifestations of AG; 2) CHD may be more effective than WM in reverting the precancerous lesions of AG; 3) CHD with WM may be more effective than WM in reverting the precancerous lesion of AG. Evidence from sensitivity analyses revealed that the primary results were relatively stable. However, similar conclusions cannot be drawn in the H. pylori eradication rate because of the significant heterogeneity between studies (I2>50%) and low robustness confirmed by sensitivity analysis. The source of the significant heterogeneity was failed to be identified by sensitivity analysis and subgroup analysis.
Our findings supported the clinical use of CHD for the alleviation of AG-related symptoms and pathologic change, which is consistent with the evidence from previous experimental studies. Pathologic changes and clinical symptoms of AG are mainly caused by H. pylori-related chronic inflammation in human gastric epithelial cells. Some herbs inhibits the generation of reactive oxygen species (ROS) prostaglandin E (2), cyclooxygenase-2 (COX-2), and interleukin (IL)-8 (Wang et al., 2012; Yu et al.,2013; Zaidi et al., 2012), and the strong anti-inflammatory activity can effectively protect gastric epithelial cells from gastric ulcer and cancer. Some herbs, such as Abrus cantoniensis Hance, have potent anti-H. pylori activity (Li et al.,2005; Safavi et al., 2015). However, the clinical efficacy of CHD to eradicate H. pylori in AG patients could not be concluded in the present study. We hypothesized the clinical and pathologic improvement of AG patients were more likely to be caused by the strong activity of CHD to inhibit H. pylori-related inflammation, than the eradication of H. pylori itself. Hence, we recommended that CHD be used as an adjunctive therapy to WM, but not used as an alternative to antibiotics for H. pylori eradication.
We included various decoctions for treating different TCM symptom types related to AG, and used the modified Jadad scale with a new scoring item of Bianzheng Lunzhi. This study design made our research strictly follow the TCM therapeutic theory. The basic therapeutic theory of Bianzheng Lunzhi is fundamentally different from that of WM. In the Bianzheng Lunzhi theory, a TCM physician should take the body, mind and spirit into account to decide which symptom type (not a “disease”) each patient belongs to (Chen et al., 2003). Based on TCM syndrome differentiation, diseases should be further classified into different clinical types for therapy. Hence, different kinds of decoctions can be used, and dosage and/or formula in a certain decoction can be added or subtracted according to individual’s symptom types and changing states of disease. The personalized therapy according to the symptom type differentiation is the guarantee of its efficacy and should be integrated into clinical trial design (Flower et al., 2012). Unlike previous studies focusing on a certain herb or decoction, our study adequately considered this individual-based therapeutic features, and made an overall evaluation of all kinds of prescriptions such as Chai-Hu-Shu-Gan decoction, Shan-Jia-Yu-Wei decoction, Jian-Pi-Yi-Wei decoction, and Gan-Cao-Xie-Xin decoction for various TCM symptom types.
Limitations of this review are as follows. Firstly, all the 42 articles that met the eligible criteria were at moderate to high risk of bias. Although sensitivity analyses excluding studies at high risk of bias found that the results were relatively stable, potential bias would exaggerate the efficacy to some extent (Kjaergard et al., 2001). Secondly, heterogeneity was observed in some results, especially the results of eradication rate of H. pylori. However, source of heterogeneity was failed to be identified by sensitivity analysis and subgroup analysis. Thirdly, publication bias, which might come from language bias, would potentially compromise the validity of some results and led to optimistic outcomes for treatment. Fourthly, our findings provided insufficient precision in the correlation between medical herbs and clinical outcomes. In fact, practitioners of Chinese medicine always prescribe mixtures of plants (decoction) instead of single plant as therapy. Therefore most RCTs regarding traditional Chinese medicine for atrophic gastritis is designed to evaluate the efficacy of decoctions. It is hard for us to evaluate the efficacy of certain plant for gastritis management using the meta-analysis. Last but not least, the herbs mentioned in all the included studies were not validated taxonomically. Although an overall analysis on efficacy of CHD for AG could be performed based on these studies, the inadequate taxonomical information limited the further species-level review on some specific herbs.
Conclusions
We recommended that CHD be prescribed as a complementary therapy to WM for atrophic gastritis, but its monotherapy for H. pylori eradication is not confirmed by existing clinical evidence. The evidence should be further strengthened because studies at low risk of bias were scarce. More large-scale, multicenter, prospective RCTs are needed therefore. We believe this article will stimulate further evaluation of CHD for AG therapy.
Author contributions
Qing-cai Wang and Ming Zhong act as guarantors for the validity of the study report. Study concept and design: Wen-jie Fang. Acquisition of data: Wen-jie Fang, Xin-ying Zhang and Bo Yang. Checking of data: Xin-ying Zhang and Bo Yang. Analysis and interpretation of data: Xin-ying Zhang. Drafting of the manuscript: Wen-jie Fang. Critical revision of the manuscript: Min Chen, Wan-qing Liao and Wei-hua Pan. Statistical analysis: Wen-jie Fang.
Abbreviations
- AG
atrophic gastritis
- TCM
traditional Chinese medicine
- CHD
Chinese herbal decoction
- WM
western medicine
- Int. CHD-WM
integration of Chinese herbal decoction and western medicine
- RCTs
randomized controlled trials
- RR
relative risk
- CI
confidence interval
- I2
inconsistency index
Acknowledgements
We are thankful for the funds provided by 973 Program (2013CB531601 and 2013CB531606), the Severe Infectious Diseases program of the National Health Department (2014ZX09J14106-02A), Institute of Translational Medicine of Changzheng hospital (CZ2016ZH07), the National Natural Science Foundation of China (grant number 81201269, 31270180); Shanghai Science and Technology Committee (grant number 14DZ2272900 and 14495800500).
References
- 1.Adamu MA, Weck MN, Gao L, Brenner H. Incidence of chronic atrophic gastritis:systematic review and meta-analysis of follow-up studies. Eur J Epidemiol. 2010;25:439–448. doi: 10.1007/s10654-010-9482-0. [DOI] [PubMed] [Google Scholar]
- 2.Aoki K, Kihaile PE, Wenyuan Z, Xianghang Z, Castro M, Disla M, Nyambo TB, Misumi J. Comparison of prevalence of chronic atrophic gastritis in Japan, China, Tanzania, and the Dominican Republic. Ann Epidemiol. 2005;15:598–606. doi: 10.1016/j.annepidem.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Chen F, Wei B, Yao W, Luo X. Kang wei granules in treatment of gastropathy related to Helicobacter pylori infection. J Tradit Chin Med. 2003;23:27–31. [PubMed] [Google Scholar]
- 4.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 5.Eid R, Moss SF. Helicobacter pylori infection and the development of gastric cancer. New Engl J Med. 2002;346:65–67. [PubMed] [Google Scholar]
- 6.Flower A, Witt C, Liu JP, Ulrich-Merzenich G, Yu H, Lewith G. Guidelines for Randomizedcontrolled trials investigating Chinese herbal medicine. J Ethnopharmacol. 2012;140:550–554. doi: 10.1016/j.jep.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Gisbert JP, Pajares R, Pajares JM. Evolution of Helicobacter pylori therapy from a meta-analytical perspective. Helicobacter. 2007;12(Suppl 2):50–58. doi: 10.1111/j.1523-5378.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- 8.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 9.Gumurdulu Y, Serin E, Ozer B, Kayaselcuk F, Ozsahin K, Cosar AM, Gursoy M, Gur G, Yilmaz U, Boyacioglu S. Low eradication rate of Helicobacter pylori with triple 7-14 days and quadriple therapy in Turkey. World J Gastroente. 2004;10:668–671. doi: 10.3748/wjg.v10.i5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higuchi K, Arakawa T, Ando K, Fujiwara Y, Uchida T, Kuroki T. Eradication of Helicobacter pylori with a Chinese herbal medicine without emergence of resistant colonies. Am J Gastroenterol. 1999;94:1419–1420. doi: 10.1111/j.1572-0241.1999.01419.x. [DOI] [PubMed] [Google Scholar]
- 11.Karchmer EI. The excitations and suppressions of the times:locating the emotions in the liver in modern Chinese medicine. Cult Med Psychiatry. 2013;37:8–29. doi: 10.1007/s11013-012-9289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato I, Tominaga S, Ito Y, Kobayashi S, Yoshii Y, Matsuura A, Kameya A, Kano T. Atrophic gastritis and stomach cancer risk:cross-sectional analyses. Jap J Cancer Res. 1992;83:1041–1046. doi: 10.1111/j.1349-7006.1992.tb02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med. 2001;135:982–989. doi: 10.7326/0003-4819-135-11-200112040-00010. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Xu C, Zhang Q, Liu JY, Tan RX. In vitro anti-Helicobacter pylori action of 30 Chinese herbal medicines used to treat ulcer diseases. J Ethnopharmacol. 2005;98:329–333. doi: 10.1016/j.jep.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Lu AP, Chen KJ. Improving clinical practice guideline development in integration of traditional Chinese medicine and Western medicine. Chin J Integr Med. 2015;21:163–165. doi: 10.1007/s11655-014-1961-9. [DOI] [PubMed] [Google Scholar]
- 16.Megraud F. H pylori antibiotic resistance:prevalence, importance, and advances in testing. Gut. 2004;53:1374–1384. doi: 10.1136/gut.2003.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng L, Yang ZD. Clinical Observation of Shanjia Yuwei Tang Used to Cure Atrophic Gastritis. J Liaoning Univ of Tradit Chin Med. 2010;12:159–160. in Chinese. [Google Scholar]
- 18.Qin F, Liu JY, Yuan JH. Chaihu-Shugan-San, an oriental herbal preparation, for the treatment of chronic gastritis:a meta-analysis of randomized controlled trials. J Ethnopharmacol. 2013;146:433–439. doi: 10.1016/j.jep.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 19.Rugge M, Pennelli G, Pilozzi E, Fassan M, Ingravallo G, Russo VM, Di MF. Gastritis:the histology report. Digest liver Dis. 2011;43(Suppl 4):S373–384. doi: 10.1016/S1590-8658(11)60593-8. [DOI] [PubMed] [Google Scholar]
- 20.Safavi M, Shams-Ardakani M, Foroumadi A. Medicinal plants in the treatment of Helicobacter pylori infections. Pharm Biol. 2015;53:939–960. doi: 10.3109/13880209.2014.952837. [DOI] [PubMed] [Google Scholar]
- 21.Song FL, Lin YF, Li HR, Lu YP, Yang Z, Gao WY, Gong Y, Liu Y, Chen SQ, Wang CH. Effect of Jiawei Sijunzi decoction based triple therapy on atrophic gastritis and expression of PCNA. Chin J Tradit Chin Med Pharm. 2009;24:367–369. in Chinese. [Google Scholar]
- 22.Tang XD, Zhou LY, Zhang ST, Xu YQ, Cui QC, Li L, Lu JJ, Li P, Lu F, Wang FY, Wang P, Bian LQ, Bian ZX. Randomized double-blind clinical trial of Moluodan for the treatment of chronic atrophic gastritis with dysplasia. Chin J Integr Med. 2016;22:9–18. doi: 10.1007/s11655-015-2114-5. [DOI] [PubMed] [Google Scholar]
- 23.Taş Akbal E, Koçak E, Köklü S. Moxifloxacin-tetracycline-lansoprazole triple therapy for first-line treatment of Helicobacter pylori infection:a prospective study. Helicobacter. 2011;16:52–54. doi: 10.1111/j.1523-5378.2010.00817.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang QS, Cui YL, Dong TJ, Zhang XF, Lin KM. Ethanol extract from a Chinese herbal formula, “Zuojin Pill”, inhibit the expression of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 mouse macrophages. J Ethnopharmacol. 2012;141:377–385. doi: 10.1016/j.jep.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 25.Weck MN, Brenner H. Prevalence of chronic atrophic gastritis in different parts of the world. Cancer Epidemiol Biomarkers Prev. 2006;15:1083–1094. doi: 10.1158/1055-9965.EPI-05-0931. [DOI] [PubMed] [Google Scholar]
- 26.Weck MN, Stegmaier C, Rothenbacher D, Brenner H. Epidemiology of chronic atrophic gastritis:population-based study among 9444 older adults from Germany. Aliment Pharm Therap. 2007;26:879–887. doi: 10.1111/j.1365-2036.2007.03430.x. [DOI] [PubMed] [Google Scholar]
- 27.Xia J. Medicinal herbs used in pairs for treatment of 98 cases of chronic gastritis. J Tradit Chin Med. 2004;24:208–209. [PubMed] [Google Scholar]
- 28.Xu FY, Yang B, Shi D, Li H, Zou Z, Shi XY. Antihypertensive effects and safety of eprosartan:a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2011;2:195–205. doi: 10.1007/s00228-011-1107-3. [DOI] [PubMed] [Google Scholar]
- 29.Yu T, Moh SH, Kim SB, Yang Y, Kim E, Lee YW, Cho CK, Kim KH, Yoo BC, Cho JY, Yoo HS. HangAmDan-B, an ethnomedicinal herbal mixture, suppresses inflammatory responses by inhibiting Syk/NF-kappaB and JNK/ATF-2 pathways. J Med Food. 2013;16:56–65. doi: 10.1089/jmf.2012.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaidi SF, Muhammad JS, Shahryar S, Usmanghani K, Gilani AH, Jafri W, Sugiyama T. Anti-inflammatory and cytoprotective effects of selected Pakistani medicinal plants in Helicobacter pylori-infected gastric epithelial cells. J Ethnopharmacol. 2012;141:403–410. doi: 10.1016/j.jep.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Zou Z, Xu FY, Wang L, An MM, Zhang H, Shi XY. Antihypertensive and renoprotective effects of trandolapril/verapamil combination:a meta-analysis of randomized controlled trials. J Hum Hypertens. 2011;25:203–10. doi: 10.1038/jhh.2010.60. [DOI] [PubMed] [Google Scholar]


