Abstract
Background:
Present investigation evaluates the antitumor activity of epifriedelinol for the management of cervical cancer by inducing process of apoptosis.
Methods:
Human Cervical Cancer Cell Line, C33A and HeLa were selected for study and treated with epifriedelinol at a concentration of (50-1000 µg/ml). Cytotoxicity of epifriedelinol was estimated by MTT assay and induction of apoptosis was assessed by estimating the activity of caspase 3, 8 and 9 enzyme, apoptosis assay and translocation of cytochrome c. Moreover an expression of several proteins that plays role in the apoptosis process was estimated by western blot method.
Results:
Result of the study suggested that treatment with epifriedelinol significantly decrease the viability count of cancerous cell in a dose perndent manner and also enhances the formation of oligonucleosome in both the cell lines. However activity of caspase enzymes and translocation of cytochrome c were enhanced after treatment with epifriedelinol. It was also observed that epifriedelinol treatment alters the ratio of pro-apoptotic to anti-apoptotic proteins and enhances the expressions of inhibitor of apoptosis proteins (IAP).
Conclusion:
Result of our study proves the anticancer activity of epifriedelinol in cervical cancer by inducing apoptosis as treatment with it enhances the production of oligonucleosomes, translocation of cytochrome c and activity caspase enzymes.
Keywords: Epifriedelinol, cervical cancer, Apoptosis, MTT assay, C33A and HeLa
Introduction
In developing countries women’s are commonly suffering from the cervical cancer. A report was published by Jemal et al., which gives a data of 275100 patients get dead and 529800 new cases of cervical cancer in 2008 (Jemal et al.,2011). There are several methods available for the management of cervical cancer depending on the stage such as management at early stage through surgery and radiation therapy was used for the management of advanced cancer. However in both the cases chemotherapy was used as an adjuvant therapy.
In the recent era, for the effective management of cancer several researches is performing on natural products (Mukherjee et al, 2001). Several reports show that number of herbal drugs was used to manage the cervical cancer by inhibiting growth of cancerous cells and several molecules were developed as the drug for the management of it (Srivastava et al, 2005).
Epifriedelinol was isolated from the roots of Aster tataricus (Asteraceae) and leaves of Vitex peduncularis Wall. ex Schauer (Verbenaceae) (Kannathasan et al, 2015; Ng et al, 2003). Epifriedelinol reported to posse’s strong antibacterial, anti-inflammatory and antioxidant property (Duke and Ayensu, 1985; Ma et al, 2011). Moreover these herbs were traditionally used for the management of cancer. Thus given investigation evaluates the anticancer activity of Epifriedelinol in cervical cancer.
Material and Methods
Cell culture
C-33A and HeLa cancer cell lines of cervical cancer were procured from Shanghai Institutes for Biological Sciences, Shanghai, China. DMEM medium was used for the growth of cell lines of cervical cancer in presence of gentamycin (50µg/ml) and fetal bovine serum 10% (FBS). All these cell lines incubated in humid incubator at 37° C with CO2 incubator.
MTT Assay
MTT assay was performed for the estimation of viability of cell. Trypsin EDTA was used to detach the cancer cells from dish and cells were poured into a 96 well plate in which each well contain 3000 cell and 100 µl of medium. Epifriedelinol (Sigma, USA) at a different concentration of (0, 50, 100, 250, 500 and 1000 µg/ml) incubated with cervical cancer for the duration of 72 hr. Later 1% MTT solution of 50 µl concentrations was poured in to well plate and incubated for the period of 4 hr. then this solution of well was replaced with fresh DMSO solution. Microplate reader was used for the estimation of absorbance of plate at wavelength of 550 nm.
Apoptosis assay
Cell Death Detection ELISA Plus kit was used for the estimation of apoptosis. The method used in this study determined the apoptosis of cell that was associated to DNA fragmentation. Here, C33A and HeLa cells were poured in to plate for the duration of 16 hr with Epifriedelinol. Later to it collect the lysates cells and transfer it to a plate that was coated with streptavidin. In cell lysates a mixture of anti-DNAPOD and anti-histone–biotine was added and then this mixture was incubated for the period of 2 hr. Thereafter it was conjugated to form an immune complex and micro plate reader was used to read the plate at 405 nm.
Estimation of activity of Caspase-3, 8, and 9
All the cancer cell lines (C33A and HeLa) were placed in 96 well plate with and without epifriedelinol and incubate the same for 24 hr. Caspase Assay Kit was used for the estimation of activity of caspase enzymes. PBS was used to was all the cells and centrifuge the same for 5 min at 150 g. caspase-9 (LEHD-pNA), caspase-8 (IETDpNA) and caspase-3 (DEVD-pNA) specified colorimetric substrate peptides contains reaction mixture was mixed with cell lysates and isolated for 2 hr at 37°C. Microplate reader was used to estimate the absorbance at 405 nm wavelength.
Estimation of cytochrome c
Mitochondria releases the cytochrome c after the treatment with epifriedelinol was estimated using cytochrome c ELISA kit. Cells were collected after the 24 hr of treatment and PBS was used to wash the same. Later digitonin cell permeabilization buffer was used to resuspend the cells and incubate it for the period of 5 min. In cytochrome c 96-well plate diluted sample was added and incubate it for 1 hr. Thereafter in each well cytochrome c conjugate was poured and again incubate it for 30 min. Microplate reader was used to estimate the level of cytochrome c at 405 nm.
Analysis of western blot
All the cancer cells were incubated for 2 days after pour it to 100 mm dish and later to it cells were treated with epifriedelinol at different concentration for the period of 1 day. Concentration of protein was estimated using BCA protein kit and for standard bovine serum albumin was used. Gel electrophoresis was used for the estimation of protein. The proteins were incubated at temperature of 4 °C by diluting the same with Bak and Bcl2 antibody. Later wash the same and again incubate with secondary antibody. ECL detection kit was used for determining the binding of antibody through chemiluminescence staining and Image J software was used to perform densitometry analysis.
Statistical analysis
All the values of this study expressed as mean ± SEM and the data was statistically analyzed by one-way ANOVA (Dunnett test) and student t test. p<0.05 was considered statistically significant.
Result
Effect of Epifriedelinol on C33A and HeLa cells
Effect of epifriedelinol on the viability of cell was estimated by MTT assay as shown in Fig. 1. There was significant decrease in the viability of cell (C-33A and HeLa) with the increase in concentration of epifriedelinol than non treated cells. However viability of cell count of HeLa was decreases to 25 % after 72 hr of treatment with epifriedelinol (500 µg/ml) and the count of C-33A cells was decreases to 11% after 72 of treatment with epifriedelinol (250 mg/kg).
Figure 1.
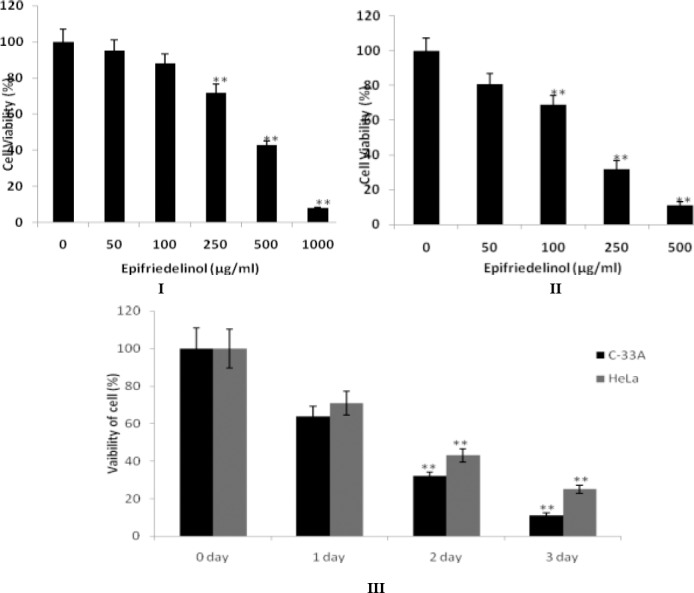
Cytotoxic effects of epifriedelinol on C-33A and HeLa cells. I & II: Epifriedelinol treatment at different concentration for 2 days to C-33A aand HeLa cells. III: Epifriedelinol at a concentration of 250 µg/ml treated to C-33A cells and HeLa cells treated with 500µg/ml concentration of epifriedelinol for 1, 2 and 3 days. Data was expressed as Mean±SEM, **p<0.01 than control group
Moreover IC50 of epifriedelinol was estimated in C-33A and HeLa cells. Result of IC50 of epifriedelinol suggested that it was having more sensitive against C-33A cells than HeLa cells (Table 1).
Table 1.
Effect of epifriedelinol on IC50 value of C-33A and HeLa cells
| Sr. No. | Cell line | 24 hr | 48 hr | 72 hr |
|---|---|---|---|---|
| 1 | HeLa (µg/ml) | 428.4±19.7 | 251.9±11.8 | 123.1±9.6 |
| 2 | C-33A (µg/ml) | 132.8±12.4 | 59.3±6.2 | 42.8±3.7 |
Effect of epifriedelinol on induction of apoptosis
Effect of epifriedelinol on fragmentation of DNA was assessed after 24 hr. It was observe that treatment with epifriedelinol significantly enhances the apoptosis by inducing the fragmentation of DNA to oligonucleosomes in both the cell lines. However formation of oligonucleosomes significantly increases in C-33A and HeLa upto 6 and 3.1 folds respectively (Fig. 2.).
Figure 2.
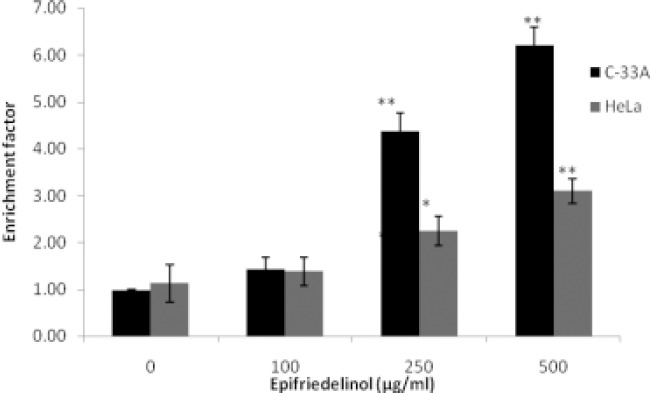
Effect of epifriedelinol on induction of apoptosis in C33A and HeLa cell lines Data was expressed as Mean±SEM, *p<0.05, **p<0.01 than control group
Effect of epifriedelinol on the intrinsic and extrinsic pathway of apoptosis
Effect of epifriedelinol was observed on the pathways of apoptosis in cervical cancer cells as shown in Fig 3. It was observed that treatment with epifriedelinol significantly (p<0.01) enhances the activity of caspase 3, 8 and 9 enzymes in as dose dependent manner in C33A and HeLa cell lines. In addition to it result of assay of cytochrome c suggested that treatment with epifriedelinol increases the cytosolic cytochrome c in a dose dependent manner in both the cell lines (Fig. 4.). Both these finding of this study gives an evidence that epifriedelinol induces the apoptosis by stimulating both intrinsic and extrinsic pathway.
Figure 3.
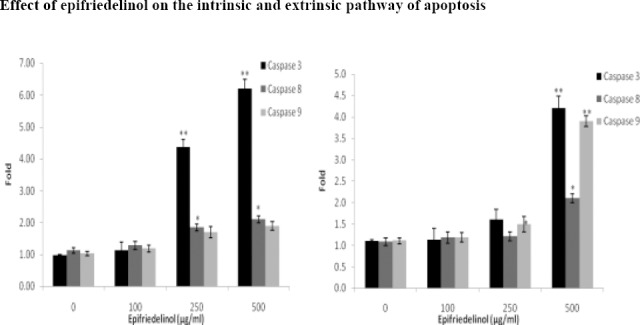
Effect of epifriedelinol on the activity of caspase enzymes in C33A and HeLa cell lines. Data was expressed as Mean±SEM, *p<0.05, **p<0.01 than control group
Figure 4.
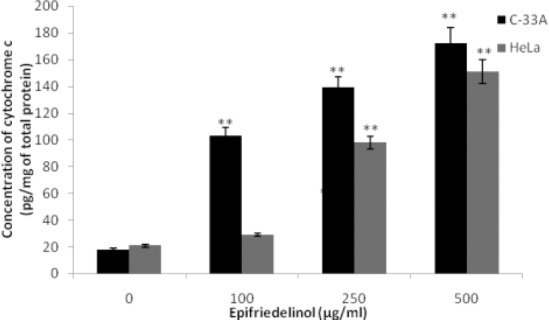
Effect of epifriedelinol on the concentration of cytosolic cytochrome c in both the cell lines
Data was expressed as Mean±SEM, **p<0.01 than control group
Effect of epifriedelinol on the expressions of Bcl-2 Family Proteins
Effect of epifriedelinol on the expressions of Bcl-2 Family Proteins such as Bcl-xL, Bcl-2, Bad, Bim, Bak and Bax for induction of apoptosis in C33A and HeLa cell lines was shown in Fig. 5. It was observed that treatment with epifriedelinol significantly (p<0.01) enhances the expressions of Bad, Bim and Bak proteins in C33A and HeLa cell lines than untreated cells. Whereas, expressions of Bcl-xL and Bcl2 was down regulated in both the cell line after treatment with epifriedelinol than untreated group.
Figure 5.
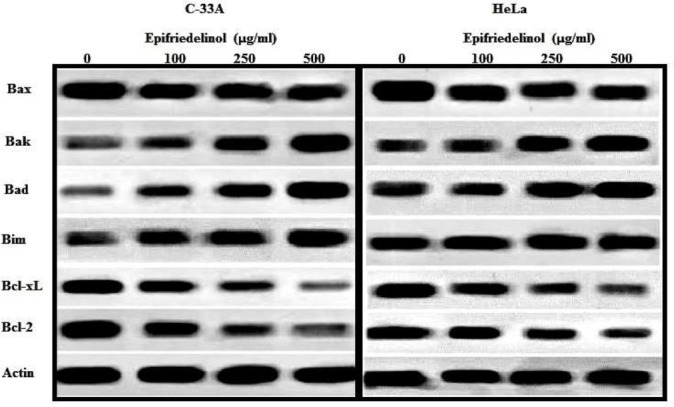
Effect of epifriedelinol on Bcl-2 family proteins of HeLa and C-33A cells by western blot analysis
Effect of epifriedelinol on the expressions of inhibitor of apoptosis proteins (IAP)
Effect of epifriedelinol on the expressions of IAP (survivin and XIAP) was shown in Fig. 6. There was significant decrease in the expressions of survivin and XIAP protein in epifriedelinol treated groups than control group in a dose dependent manner.
Figure 6.
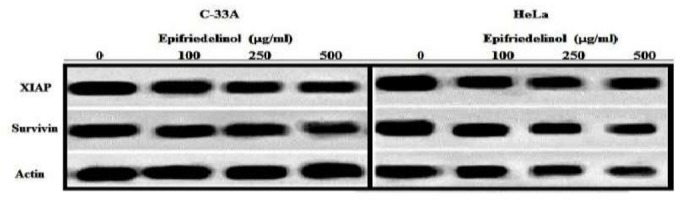
Effect of epifriedelinol on the expressions of IAP family of HeLa and C-33A cells by western blot analysis
Discussion
In developing country, majority of women are suffering from cervical cancer. Several therapies are available for the management of cancer but all of them need adjuvant therapy associated to it for the better management of cancer. This investigation evaluates the effect of epifriedelinol on the cervical cancer by inducing apoptosis. Here two cancerous cell lines C33A and HeLa of cervical cancer were selected and effect of epifriedelinol on the process of apoptosis induction was seen in these cell lines.
Literature suggested that several anticancer drugs induces apoptosis process and thereby alters the growth of cancerous cells (Gimenez-Bonafe et al., 2009). In the reported investigation there was increase in the oligonucleosomes production that characterizes the induction of apoptosis. In addition, treatment with epifriedelinol significantly translocates cytochrome c and also enhances the activity of caspase enzymes.
Bcl-2 regulates the intrinsic pathway of apoptosis and apoptosis process was regulated by the ratio of pro-apoptotic to anti-apoptotic proteins (Frenzel et al., 2009). Here in this study treatment with epifriedelinol significantly down regulates the expressions of anti apoptotic protein and enhances the expressions of pro apoptotic protein. Literature also suggested that cell proliferation in cancer results due to suppression of protein also and IAP protein inhibits the apoptosis (Mita et al., 2008). Moreover this protein enhances the resistance of cancer cell for the apoptosis (Mace et al., 2010). In the recent year molecules are targeted to this protein by the scientist for the design of new strategies to treat the cancer. Result of our study shows that an expression of IAP protein significantly declines after the treatment with epifriedelinol.
Conclusion
Result of our study proves the anticancer activity of epifriedelinol in cervical cancer by inducing apoptosis as treatment with it enhances the production of oligonucleosomes, translocation of cytochrome c and activity caspase enzymes. Moreover it down regulates the expressions of IAP and also disturbs the ratio of pro-apoptotic to anti-apoptotic proteins in the cell.
References
- 1.Duke JA, Ayensu ES. Medicinal Plants of China Reference. Publications Inc; 1985. ISBN d0-917256-20-4. [Google Scholar]
- 2.Frenzel A, Grespi F, Chmelewskij W, Villunger A. Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis. 2009;14:584–596. doi: 10.1007/s10495-008-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gimenez-Bonafe P, Tortosa A, Perez-Tomas R. Overcoming drug resistance by enhancing apoptosis of tumor cells. Curr. Cancer Drug Targets. 2009;9:320–340. doi: 10.2174/156800909788166600. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA. Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 5.Kannathasan K, Senthilkumar A, Venkatesalu V. Crystal structure and antibacterial evaluation of epifriedelinol isolated from Vitex peduncularis Wall. ex Schauer. Arabian Journal of Chemistry. 2015;20 DOI:10.1016/j.arabjc.2015.02.013. [Google Scholar]
- 6.Ma C, Dastmalchi K, Whitaker BD, Kennelly EJ. Two new antioxidant malonated caffeoylquinic acid isomers in fruits of wild eggplant relatives. J Agric Food Chem. 2011;59(17):9645–51. doi: 10.1021/jf202028y. [DOI] [PubMed] [Google Scholar]
- 7.Mace PD, Shirley S, Day CL. Assembling the building blocks:structure and function of inhibitor of apoptosis proteins. Cell Death Differ. 2010;17:46–53. doi: 10.1038/cdd.2009.45. [DOI] [PubMed] [Google Scholar]
- 8.Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin:key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin. Cancer Res. 2008;14:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee AK, Basu S, Sarkar N, Ghosh AC. Advances in Cancer Therapy with Plant Based Natural Products. Curr. Med. Chem. 2001;8:1467–1486. doi: 10.2174/0929867013372094. [DOI] [PubMed] [Google Scholar]
- 10.Ng TB, Liu F, Lu Y, Cheng CH, Wang Z. Antioxidant activity of compounds from the medicinal herb Aster tataricus. Comp Biochem Physiol C Toxicol Pharmacol. 2003;136(2):109–15. doi: 10.1016/s1532-0456(03)00170-4. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava V, Negi AS, Kumar JK, Gupta MM, Khanuja SP. Plant-based anticancer molecules:a chemical and biological profile of some important leads. Bioorg. Med. Chem. 2005;13:5892–908. doi: 10.1016/j.bmc.2005.05.066. [DOI] [PubMed] [Google Scholar]


