Abstract
Background: :
Kidney tonifying - spleen strengthening method being one of the modalities for treatment of astheno-oligozoospermia is currently commonly used in the clinical setting. To investigate the mechanism of YiShenJianPi (YSJP) Recipe, used in Traditional Chinese Medicine to benefit “the kidney” and strengthen “the spleen”.
Materials and Methods:
Oligoasthenozoospermia, male BALB/c mice were randomly divided into normal control, disease model, positive control, low-dosage and high-dosage groups. Oligoasthenozoospermia was induced by tripterygium glucosides intragastric administration before treatment started. Through using computer-aided sperm analysis to test the changes in sperm quality, utilizing flow cytometry to test the percentage of sperm with normal mitochondrial transmembrane potential (JC-1 + %), utilizing X-ray microscopy to observe epididymal sperm ultra-microstructure placing special emphasis and photographing the differences in mitochondria of the flagellum region.
Results:
Compared with DM, sperm quality of the treated mice was significantly better (P<0.05, respectively). Compared with PC, the LD group had significantly better quality sperms, while the parameters in the HD group were numerically better. Compared with NC, all other groups had significantly lower percentage of sperms with normal mitochondrial membrane potential. In PC, LD and HD groups, the percentage of sperms with normal mitochondrial membrane potential was significantly higher than that of D. The 9+9+2 mitochondrial sheath structure was complete in NC but damaged in DM. In the treatment groups, this structure was fairly clear.
Conclusion:
YSJP improved semen quality with oligoasthenozoospermia by improving sperm mitochondrial membrane potential and restoring sperm mitochondrial ultrastructure.
Keywords: traditional Chinese medicine, oligoasthenozoospermia, semen quality, mitochondrial membrane potential, mitochondrial ultrastructure
Introduction
Male infertility is an unsolved worldwide problem. World Health Organization (WHO) statistics showed that infertility, cardiovascular disease and cancer have become the three major diseases affecting people’s life and health in modern society (Kaminsky and Sperling, 2014). The current situation of male reproductive health is not optimistic (Lu and Xie, 2001). In the past 50 years, sperm count and sperm quality both dropped substantially worldwide; consequently, WHO has lowered the standard for semen quality more than once. The incidence of infertility has increased from 8-10% half a century ago to 12-16% and it keeps increasing. It is estimated that approximately 80 million couples are infertile worldwide, of which 30% are due to male infertility. Various factors can lead to male infertility, while oligoasthenozoospermia is the most common cause (Guo and Zhang, 2011).
Tripterygium glycosides (GTW) are commonly used to create animal models (Ma et al., 2015). Mice treated with GTW had decreased sperm concentration and reduced sperm mobility, as well as sperm ultrastructural changes including damaged mitochondria sheath, cytoplasmic membrane defects and chromatin depolymerisation. This study aimed to investigate the mechanism of YiShenJianPi Recipe, used in Traditional Chinese Medicine to benefit “the kidney” and strengthen “the spleen”, in the treatment of oligoasthenozoospermia. GTW was used to induce oligoasthenozoospermia in mice and the effect of YSJP Recipe on sperm quality, sperm mitochondrial membrane potential and sperm mitochondrial ultrastructure was examined.
Material and Methods
Experimental animals and the treatments
Sixty male BALB/c mice (Grade SPF, body weight 18-20 g) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (license number of SCXK [Jing] 2012-0001). The study was approved by the Animal Ethics Committee of Dongzhimen Hospital of Beijing University of Chinese Medicine. All experimental procedure followed the internationally accepted standards.
The mice were randomly divided into 5 groups (12 mice/group). GTW 40mg/kg (Anhui Xin Long Hai Pharmaceutical Co., Ltd.) was given once daily to the mice except those in the normal control group by intragastric administration for 4 weeks to induce oligoasthenozoospermia. For the next 4 weeks, the disease model group was given distilled water, the positive control group was given levocarnitine 10 mL/kg (Northeast Pharmaceutical Group Shenyang First Pharmaceutical Co., Ltd.), the low-dosage group was given YiShenJianPi Recipe 1.35 mg/kg, and the high-dosage group was given YiShenJianPi Recipe 2.70 mg/kg once daily. Mice in the normal control group were given distilled water for the whole time.
YiShenJianPi Recipe was consisted of: seeds of Cuscuta chinensis 30 g, fruits of Lycium spp. 30 g, fruits of Schisandra spp. 10 g, roots of Codonopsis pilosula 10 g, unprocessed roots of Astragalus membranaceus 10 g, dried fruit skin of Citrus reticulata 10 g, roots or the whole plant of Bupleurum spp. 6 g, rhizome of Cimicifuga foetida 6 g, rhizome of Ligusticum chuanxiong 6 g and the flowers of Carthamus tinctorius 6 g (granules to make the decoction were provided by Beijing KangRenTang Ltd.).
The mice were sacrificed 30 min after the last administration by cervical dislocation. The testicles and the epididymides were collected and subjected to examinations.
Epididymis semen quality
The left epididymis was put into a test tube containing 1.0 mL saline (preheated to 37°C) and cut into small pieces. The sperms were released by shaking the test tube. After incubated at 37°C for 15 min, the upper layer was carefully removed and centrifuged at 1500 rpm for 5 min. The pellet was resuspended in 1 mL BWW high protein capacitation solution and incubated at 37°C for 2 h. Sperm mobility, sperm concentration and the total number of sperms were measured using computer-aided sperm analysis (CASA; WLJU-9000 Weili Full Colour Sperm Quality Examination System, Beijing Weili New Century Science and Technology Development Co., Ltd., China). Sperms were categorised using the WHO 2009 standards (Li and Huang 2008).
Sperm mitochondrial membrane potential
Sperms from the left epididymis were freed in 2.0 mL saline (preheated to 37°C) and incubated at 37°C for 15min. The suspension (1 mL) was filtered through 200 mesh filter until the upper layer was clear. The sperm suspension was adjusted to 2×109/L with saline and subjected to analysis. Mitochondrial membrane potential was measured using the JC-1 kit (Beijing Solarbio® Life Sciences, China) following the manufacturer’s instructions. In brief, sperm suspension 0.5 mL was added to JC-1 (final concentration 10 mg/L), mixed well and incubated at 37°C for 20 min. After centrifuged at 1500 rpm at 4°C for 4 min, the pelleted was washed twice with JC-1 buffer (1×). The cells were resuspended in 0.5 mL JC-1 staining buffer. A total of 50,000 sperms were examined using flow cytometry (BD FACSC Auto™ type II Cell Analyser, BD Biosciences, USA). Excitation wavelength was 488 nm, blue fluorescence from JC-1 polymers indicated normal mitochondrial membrane potential, while green fluorescence from JC-1 monomers indicated mitochondrial membrane potential loss. The percentage of sperms with blue fluorescence (JC-1+ %) was the percentage of sperms with normal mitochondrial membrane potential.
Ultrastructure of the sperms
Tissue samples from the head of the right epididymis (2 for each group) was made into 1 mm3 cubes and fixed in 1.5 mL 2.5% glutaraldehyde fixing solution for 4 h. After washed in 0.1 mol/L PBS (pH 7.2) for 2 h, the samples were fixed again in 2.5% glutaraldehyde fixing solution for 2-4 h. Dehydration was carried out using gradient ethanol (30%, 50% and 70%) 15 min each. After immersed in a mixture of epoxy resin and acetone (1:1) at 37°C for 24 h, the samples were embedded in EPON 812 (145-160), dodecenyl succinic anhydride, methylcyclohexane-1,2-dicarboxylic anhydride and N,N-dimethylformamide at 60°C for 24 h. Semi-thin sections were prepared and stained with toluidine blue to localise the sections of interest using Olympus BX60 microscope (Olympus). Ultrathin sections (50-70 nm) were prepared using LKB-NOVA Ultramicrotome (DAKO, Switzerland). Uranyl acetate and lead nitrate double staining was used. The ultrastructure of the sperms was observed using a transmission electron microscope (Hitachi HT7700, Japan) and the main focus was the changes in the mitochondria at the tail of the sperms.
Statistical analysis
SAS8.2 was used for data analyses. Numerical data were shown as mean ± standard deviation, using the single factor analysis of variance (one - way ANOVA) to compare between the multiple samples, the comparison between the two groups choose nonparametric test, with P < 0.05 for the difference was statistically significant.
Results
Sperm quality
At the end of the study, measurement of sperm quality of the mice in the disease model group showed that oligoasthenozoospermia was successfully included. Compared with the disease model group, the sperm quality of mice receiving treatment was significantly better (P<0.05 for all parameters, Figure 1). Compared with the positive control, mice in the low-dosage group had significantly better quality sperms (P<0.05 for all parameters, Figure 1), while the parameters in the high-dosage group were better but the differences were not significant (P>0.05 for all, Figure 1). The sperm concentration and the total number of sperm of the positive control were numerically lower than that of the YSJP low- and high-dosage groups (P>0.05, respectively; Figure 1), while those of the low-dosage group were numerically higher than the high-dose group (P>0.05, respectively; Figure 1).
Figure 1.
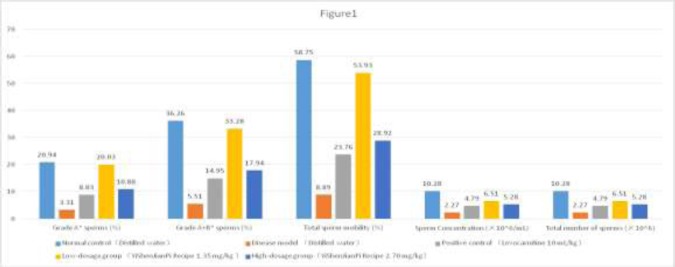
Effect of YiShenJianPi Recipe on semen quality of mice with oligoasthenozoospermia induced by tripterygium glycosides. Values are mean ± standard deviation. N=12for each group. *WHO 2009 standards. †P-value versus disease control <0.05. ‡P-value versus low-dosage group and high-dosage group = 0.0529 and 0.5989, respectively. ¦P-value versus high-dosage group = 0.2049. Ppc, P-value versus positive control.
Sperm mitochondrial membrane potential
The total number of sperms, showing as the blue and green dots in each panel in Figure 3, was similar across the groups, which indicated that it was not affected by tripterygium glycosides and the treatments (levocarnitine 10 mL/kg, YiShenJianPi Recipe 1.35 mg/kg and 2.70 mg/kg). Compared with the normal control, all other groups had significantly lower percentage of sperms with normal mitochondrial membrane potential (i.e., with blue florescent) (Figure 2). For the positive control group, the low-dosage group, and the high-dosage group, the percentage of sperms with normal mitochondrial membrane potential was significantly higher than that of the disease model (Figure 2, Figure 3).
Figure 2.
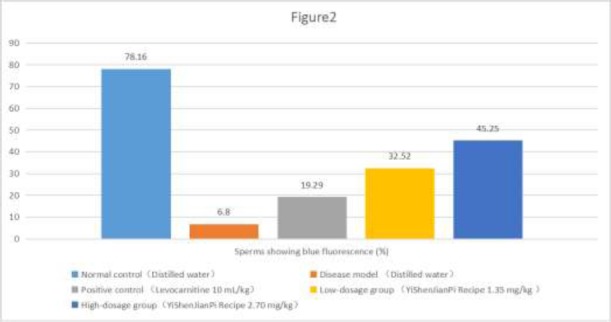
Effect of YiShenJianPi Recipe on sperm mitochondrial membrane potential of mice with oligoasthenozoospermia induced by tripterygium glycosides. Values are mean ± standard deviation. N=12 for each group. *P-value versus low-dosage group and high-dosage group = 0.0141 and 0.0027, respectively. †P-value vs. high-dosage group = 0.0253.
Figure 3.
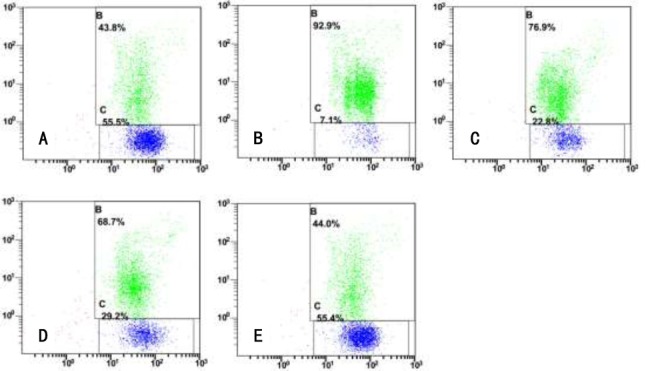
Effect of YiShenJianPi Recipe on sperm mitochondrial membrane potential of mice with oligoasthenozoospermia induced by tripterygium glycosides. (A) Normal control; (B) Disease model; (C) Positive control, treated with levocarnitine 10 mL/kg; (D) Low-dosage group, treated with YiShenJianPi Recipe 1.35 mg/kg; (E) High-dosage group, treated with YiShenJianPi Recipe 2.70 mg/kg.
Blue fluorescence (JC-1 polymer) indicating normal mitochondrial membrane potential of the sperm, green fluorescence (JC-1 monomer) indicating mitochondrial membrane potential loss and red fluorescence indicating impurities in the sample.
Ultrastructure of the sperms
The cross-section of the tail of the sperms in the normal control group showed the complete mitochondrial sheath structure surrounding the dense fibres and the axonemes. There were nine outer fibres, nine axonemes in the middle and two microtubules with clear structure in the centre, which formed the 9+2+2 structure (Figure 4A). For the disease control group, the whole mitochondria in the sperms were obvious swollen, the fibres were loose, and the axonemes were broken, with damaged 9+2+2 structure (Figure 4B). With treatment, compared with the disease control, the mitochondrial sheath structure was fairly complete, the whole mitochondria were less swollen, the arrangement of the fibres and the axonemes were less disrupted, and the 9+2+2 structure was clearly visible (Figures 4C, 4D and 4E).
Figure 4.
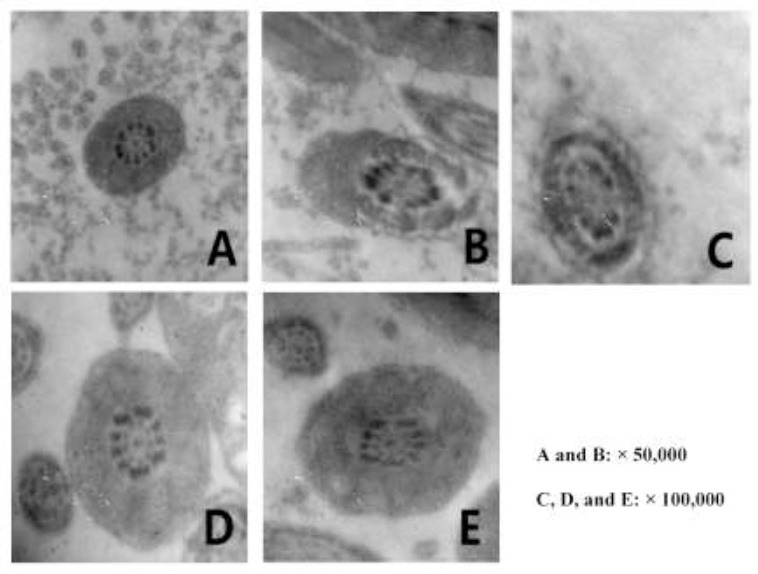
Effect of YiShenJianPi Recipe on sperm mitochondrial ultrastructure of mice with oligoasthenozoospermia induced by tripterygium glycosides. (A) Normal control; (B) Disease model; (C) Positive control, treated with levocarnitine 10 mL/kg; (D) Low-dosage group, treated with YiShenJianPi Recipe 1.35 mg/kg; (E) High-dosage group, treated with YiShenJianPi Recipe 2.70 mg/kg. The magnification is 50, 000 for A and B, and 100,000 for C, D and E.
Discussion
In traditional Chinese medicine, the dried root or the woody part of the root of Tripterygium wilfordii is used to eliminate the wet, enhance the movement of blood and the circulation in the meridian system, reduce swelling and pain, kill parasites and remove any toxic elements (Gao 2007). Large amount of data from experimental and clinical studies have shown that any preparations using T. wilfordii and its various extracts have reproductive toxicity. Long-term intake of such preparations can induce oligozoospermia, asthenozoospermia or even azoospermia (Ma et al., 2011).
Tripterygium glycosides (GTW) are refined fat-soluble extracts from the core of the root of T. wilfordii and with high polarity. They are clinically used to treat rheumatoid arthritis, chronic kidney disease and other autoimmune diseases and demonstrate significant efficacy. However, their application was limited due to serious reproductive toxicity. Experimental studies have shown that GTW can cause epididymal sperm deformity, decreased sperm motility and density and the formation of vacuoles in the sperm mitochondrial sheath (Yamada et al., 2006). It can also cause atrophy of the testicular seminiferous tubules, disarray of the seminiferous epithelial cells, decreased number of spermatocytes at all levels, decreased number of sperms and DNA damage of the testicular cells. It was also found that sperms in the testicles and epididymides were the target of GTW, and the severity of reproductive toxicity was positive correlated with the dose or GTW and exposure time (Li and Peng, 2008). Previous studies carried out by our laboratory established that GTW 40mg/(kg·d) intragastric administration for 4 weeks could create a good model for oligoasthenozoospermia in rats (Ma et al. 2015).
Mitochondria provide the energy for the movement of sperms (Nakata et al., 2015). For mature mammals, there are approximately 70-80 mitochondria per sperm, all are present in the middle section of the tail (Garcia-Rincon et al., 2016; Nakata et al. 2015). It has been found that mitochondrial membrane potential can seriously affect the process of oxidative phosphorylation, with abnormal mitochondrial membrane potential the energy supply to the sperm has been greatly affected (Cassina et al., 2015). Clinically, this can result in decreased sperm motility. There is report showing that sperm mitochondrial membrane potential and the degree of apoptosis were strongly associated with sperm concentration, motility, and vitality, as well as the deformity of the body and the tail of the sperm (Dong et al., 2010). The integrity of the mitochondrial structure and a normal mitochondrial membrane potential are critical to maintain the normal function of the sperm. Decreases in mitochondrial membrane potential can lead to insufficient ATP synthesis and disrupted energy metabolism; further, the loss of membrane potential can cause apoptosis and necrosis of the sperms (Wang and Wu., 2013).
The swing of the tail makes the sperm moving forward, and the complete mitochondrial sheath structure is a guarantee for the energy source that facilitates the tail swinging. Studies showed that damaged mitochondrial structure at the middle of the sperm tail is the key for the loss of motility; other pathological changes, such as changes in the dense fibres of the axoneme inside the tail of the sperm, result in the inefficient usage of ATP leading to the decreased sperm motility or death (Dang et al., 2008; Hu et al., 2006). Therefore, normal mitochondrial membrane potential and normal structure of the mitochondria at the middle of the sperm tail are the basis of normal sperm motility.
In Traditional Chinese Medicine, the essence is considered as the material basis to constitute the human body. The inborn essence is from the parents and stored in “the kidney” and the acquired essence is from the diet and is generated from “the spleen and the stomach”. The two types of essence are interdependent and boosting each other. It is said in the 《mi ben zhong zi jin dan》 that “the path of life starts from having an offspring, and the two key methods of having an offspring are not more than invigorating the Essence for males and nourishing the Blood for females. Resulting in Yang Essence overflowing without exhausting, Yin Blood flowing down without delay, free intermingling of Yin and Yang, combining and congealing of Essence and Blood, an embryo is formed and ready to be born.” Just like what Zhang Jingyue recorded in 《nei jing》: “Essence can create Qi, Qi can create Essence…both Essence and Qi are rooted in mutual growth…with regards to this, water and grain being in the Stomach and Mingmen being in the Kidneys, from the perspective of Essence-Qi then the generation of Kidney Essence is due to the Spleen-Stomach. From the perspective of Fire-Earth, then the Yang Qi of Earth relies on the Mingmen” 《jing yue quan shu》mentions: “The beginning of life is rooted in the source of Essence and Blood; the maintenance of life is due to the nourishment from water and grain. Without Essence and Blood, there can be no foundation for form and body. Without water and grain, there can be no peak in form and body. Thus, the sea of water and grain depends on the pre-birth as its master and the sea of Essence and Blood rely on the post-birth as its resource.” Li Zhongzi mentioned in 《yi zong bi du》: “ The root of the post-birth is in the Spleen and shall be the Earth of the middle palace, the Earth being the mother of the ten thousand things…once there is a body, it must be nourished by nutrition, when grain enters the Stomach, it is sprinkled outwards to the six Entrails resulting in Qi reaching an extreme, regulating the five Viscera resulting in Blood forming, thus human life relies on this to continue, hence root of post-birth is in the Spleen.” Cheng Zhongling of the Qing dynasty recorded in 《yi xue xin wu》: “Both the Spleen and the Kidney are foundational and cannot be disregarded. The ancients shared two oral traditions: “instead of tonifying the spleen why not tonify the Kidney, using the Fire of Mingmen to grow Spleen Earth; or instead of tonifying the Kidney, why not tonify the Spleen, using the Essence from food and direct it towards the Kidney. Most importantly, when Spleen is weak and the Kidney is not deficient, it is urgently required to tonify the Spleen; when Kidney is weak but Spleen is not, then tonifying Kidney should be priority; if both Spleen and Kidney are both deficient, then tonify both together.” In 《fu qing zhu nv ke》” Spleen as post-birth, Kidney as pre-birth; without the pre-birth Qi, Spleen cannot transform food, without the post-birth Qi, Kidney cannot grow. To tonify the Kidney without tonifying the Spleen, then how can Kidney Essence grow”. The health of the Spleen Yang relies on the warmth of the Kidney Yang, ancients said: “tonify Fire to grow Earth”, “Spleen Yang depends on Kidney Yang”. The relationship between Spleen and Kidney is one of post-birth and pre-birth respectively. If the Kidney Yang is warm and the Spleen Yang is healthy, then it can be guaranteed that the activity of the Essence hidden in the Kidney is strong. Ancients said: “Strengthening the Spleen can tonify the Kidney, tonifying the Blood can create Essence, boosting Qi can create and enliven Essence.
With Spleen as post-birth and Kidney as pre-birth, in a clinical setting Kidney deficiency patients commonly also present with Spleen deficiency. Hence it is common to see patients in a clinical setting with infertility arising due to Kidney deficiency and not presenting with Spleen deficiency symptoms at first, through the course of ingesting herbs which tonify the Kidney and replenish the Essence present with Spleen deficiency symptoms due to the nature of the medicine being overly heavy in taste. Hence, through using the Tonifying Kidney-Strengthening Spleen formula, we are able to achieve a desirable effect in a clinical setting in treating Astheno-oligozoospermia.
With the essence stored, “the kidney” is associated with reproductivity and being replenished constantly with acquired essence. Therefore, male fertility is closely related to “the spleen” and “the kidney”. The inborn essence in “the kidney” and the acquired essence from “the spleen and the stomach” jointly promote the formation and maturation of the sperm. Thus, we believe that “spleen and kidney deficiency, essence lost its source” is the cause for oligoasthenozoospermia. Since the origin of the problem is in “the spleen” and “the kidney”, the treatment should be design to benefit “the kidney”, strengthen “the spleen” and replenish the essence. YiShenJianPi Recipe has been adapted from the classic recipe Wuziyanzong Pills, with Cu. chinensis, Lycium spp., Schisandra spp., Co. pilosula and A. membranaceus as the main ingredients. Wuziyanzong Pills can significantly improve sperm mitochondrial membrane potential and improve the ultrastructure of the mitochondria in the tail of the sperm (Wang and Huang et al., 2013), therefore improving sperm motility. In clinical practice, we use the recipe to treat oligoasthenozoospermia with satisfactory outcomes, sperm concentration and sperm mobility of the patients showed significant improvement (Ding et al., 2015). However, in the current study we found that for mice with GTW-induced oligoasthenozoospermia, the improvement in sperm motility in the low-dosage YSJP treatment group was significantly better than that of the high-dosage YSJP treatment group (P<0.05); while our clinical data showed that high-dosage YSJP was more effective than low-dosage YSJP. One possible explanation is that for YSJP decoction to improve spermatogenesis and sperm maturation, an optimal concentration is required, and it is not necessarily that a higher concentration will lead to better efficacy outcome.
During data analysis, we have noticed some limitations of this study. For instance, since we used normal saline rather than cell culture medium to release sperms from the epididymides, the sperm motility might have been affected during this process. In addition, the instrument used for sperm analysis is designed for human sperm analysis and it may not be suitable for the analysis of mice sperm. Also, the effect of experimental error and a too small sample size could not be ruled out.
In conclusion, we have demonstrated that YiShenJianPi Recipe has antagonistic effect on the reproductive toxicity of tripterygium glycosides. It can increase spermatogenesis and improve semen quality by increasing mitochondrial membrane potential of the sperm and restoring the ultrastructure of the mitochondria present in the sperm tail. The current study has provided pathophysiological evidence to support the clinical use of YiShenJianPi Recipe in the treatment of oligoasthenozoospermia.
Acknowledgment
This study was funded by Research Fund of Beijing University of Chinese Medicine (2015-JYB-JSMS062).
References
- 1.Cassina A, Silveira P, Cantu L, Montes JM, Radi R, Sapiro R. Defective Human Sperm Cells Are Associated with Mitochondrial Dysfunction and Oxidant Production. Biology of Reproduction. 2015;5:119. doi: 10.1095/biolreprod.115.130989. [DOI] [PubMed] [Google Scholar]
- 2.Dang LK, Chen S, Wu GT, Jiang L, Wang T. Observation on the ultrastructure changes of sperm tail of infertile male. Journal of Reproductive Medicine. 2008;6:469–473. [Google Scholar]
- 3.Ding J, Shang JW, Yan B, Qu BR, Zhang YS, Wang XJ, Li T. Effect of Yiqi Fuyuan granules on infertility patients with varicocele-below three degrees. Chinese Journal of Human Sexuality. 2015;2:64–67. [Google Scholar]
- 4.Dong ZQ, Xia WJ, Wu M, Yan ZZ. The relationship of mitochondrial membrane potential and the function of the sperm. Chinese Journal of Health Laboratory Technology. 2010;5:1098–1099. [Google Scholar]
- 5.Gao XM. Traditional Chinese Pharmacology (New Century 2nd Edition) Beijing: China Press of Traditional Chinese Medicine; 2007. [Google Scholar]
- 6.Garcia-Rincon J, Darszon A, Beltran C. Speract, a sea urchin egg peptide that regulates sperm motility, also stimulates sperm mitochondrial metabolism. Biochimica et Biophysica Acta. 2016;4:415–426. doi: 10.1016/j.bbabio.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Guo GL, Zhang Y. Analysis of the common causes of male infertility. China Modern Medicine. 2011;28:82–83. [Google Scholar]
- 8.Hu HX, Ma JW, Zhi Y, Ren L, Wang P. Study on ultrastructure of human spermatozoa with transmission electronic microscope. Chinese Journal of Andrology. 2006;5:23–25. [Google Scholar]
- 9.Kaminsky A, Sperling H. Variocele in adolescents. Urologe A. 2014;2:213–217. doi: 10.1007/s00120-013-3384-1. [DOI] [PubMed] [Google Scholar]
- 10.Li F, Peng YF. Advances in research of triptolide-induced impaired male reproductive function. Reproduction & Contraception. 2008;9:571–575. [Google Scholar]
- 11.Li HJ, Huang YF. Practical Andrology. 2nd Edition. Beijing: Science Press; 2008. [Google Scholar]
- 12.Lu RK, Xie YH. Research on male reproductive health should be emphasized. Chinese Journal of Andrology. 2001;1:3–6. [Google Scholar]
- 13.Ma CG, Ji W, Li HG, Guo YK. Reproductive toxicity of tripterygium glycosides tablet and research advance in increasing its efficacy and reducing its toxicity. Journal of Liaoning University of Traditional Chinese Medicine. 2011;12:88–90. [Google Scholar]
- 14.Ma HF, Li HS, Zhao ZJ, Wang B, Zhao B, Mo XW, Liu Y, Cheng MX, Yang MJ. Tripterygium glycosides induced impaired spermatogenesis in a rat model. National Journal of Andrology. 2015;2:179–184. [Google Scholar]
- 15.Nakata K, Yamashita N, Noda Y, Ohsawa I. Stimulation of human damaged sperm motility with hydrogen molecule. Medical Gas Research. 2015;1:2. doi: 10.1186/s13618-014-0023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang TS, Huang JL, Wu DL, Li Q, Liu XG, Hu W. Wuzi Yanzong Pills increases sperm mitochondrial membrane potential and protects its ultrastructure in oligoasthenotspermia model rats. National Journal of Andrology. 2013;5:446–450. [PubMed] [Google Scholar]
- 17.Wang TS, Wu DL, Huang JL, Li Q, Liu XG, Hu W, Chen H, Qiao L, Li DD. Influences of therapy of tonifying kidney and replenishing essence on damage of sperm mitochondrial ultrastructure and membrane potential induced by tripterygium glycosides in rats. Journal of Beijing University of Traditional Chinese Medicine. 2013;3:166–169. [Google Scholar]
- 18.Yamada D, Yoshida M, Williams YN, Fukami T, Kikuchi S, Masuda M, Maruyama T, Ohta T, Nakae D, Maekawa A, Kitamura T, Murakami Y. Disruption of spermatogenic cell adhesion and male infertility in mice lacking TSLC1/IGSF4, an immunoglobulin superfamily cell adhesion molecule. Molecular and Cellular Biology. 2006;9:3610–3624. doi: 10.1128/MCB.26.9.3610-3624.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


