Abstract
There is growing evidence that deficits in neuronal plasticity account for some of the neurological problems observed in fetal alcohol spectrum disorders (FASD). Recently, we showed that early alcohol exposure results in a permanent impairment in visual cortex ocular dominance (OD) plasticity in a ferret model of FASD. This disruption can be reversed, however, by treating animals with a Phosphodiesterase (PDE) type 1 inhibitor long after the period of alcohol exposure. Because the mammalian brain presents different types of PDE isoforms we tested here whether inhibition of PDE type 4 also ameliorates the effects of alcohol on OD plasticity. Using in vivo electrophysiology we show that inhibition of PDE4 by rolipram does not restore OD plasticity in alcohol-treated ferrets. This result suggests that contrary to PDE1, PDE4 inhibition does not play a role in the restoration of OD plasticity in the ferret model of FASD.
Introduction
There is growing evidence that deficits in neuronal plasticity account for some of the neurological problems observed in fetal alcohol spectrum disorders (FASD)(Rema and Ebner, 1999;Medina et al., 2003;Medina et al., 2005;Powrozec and Zhou, 2005;Medina and Krahe, 2008). Ocular dominance (OD) plasticity is a well-known and established model of neuronal plasticity in which changes in sensory experience promote functional and anatomical changes in the primary visual cortex (Hubel and Wiesel, 1970;Hubel et al., 1977). In higher mammals, neurons in primary visual cortex receive afferents from the left and right eyes in an alternating columnar fashion. If the eyelid of one eye is closed (monocular deprivation) during a period of development, this eye loses its ability to evoke cortical responses and later its respective ocular dominance columns shrink while the columns representing the normal (open) eye expand (Hubel and Wiesel, 1970;Hubel et al., 1977). This type of plasticity, has been widely used to study the mechanisms that underlie neural plasticity in general and shares common mechanisms with learning and memory (Berardi et al., 2003). We have recently shown that OD plasticity is permanently impaired after early alcohol exposure (Medina et al., 2003;Medina and Ramoa, 2005). After a period of monocular deprivation the visual cortex of alcohol-exposed animals fails to reorganize its connections and the deprived eye remains functional (Medina et al., 2003;Medina and Ramoa, 2005). In this respect this model has a unique role to play to test therapeutic agents that could restore plasticity in models of FASD.
Phosphodiesterase (PDE) inhibitors have been considered promising candidates as plasticity enhancers (Blokland et al., 2006;Lynch, 2002;Rose et al., 2005). They prevent the breakdown of cAMP to 5’-AMP, maintaining activation of protein kinases and thus increasing transcription of the cyclic AMP response-element-binding protein (CREB), leading to the expression of plasticity-related genes (Beavo, 1995;Bito et al., 1996;Chen et al., 2003;Deisseroth et al., 1996;Finkbeiner et al., 1997;Frank and Greenberg, 1994). Recently, we demonstrated that inhibition of PDE type 1 by the alkaloid vinpocetine successfully restored OD plasticity in a ferret model of FASD (Medina et al., 2006). However, so far, it is not known whether this restoration is solely due to PDE type 1 inhibition or if blockade of other isoforms would restore OD visual plasticity as well. Like the PDE1 isoform, PDE4 also regulates intrinsic cAMP levels and is highly expressed in the cerebral cortex and hippocampus (Rose et al., 2005). The importance of this isoenzyme for learning and memory has been demonstrated in genetic and pharmacologic studies (Rose et al., 2005). Mice lacking the subunit PDE4d show better performance in the Morris and in the radial arm mazes [54]. Furthermore, PDE4 inhibitors have been considered potential plasticity enhancers and have been tested in several different models of neurological conditions (Rose et al., 2005;Tully et al., 2003;Dyke and Montana, 1999). In particular, PDE4 inhibition by rolipram [(±)-4-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrrolidone] has been shown to lower the threshold for LTP induction in the hippocampus induction, (Otmakhov et al., 2004), to reinstate LTP duration in aged mice (Barad et al., 1998), and to re-establishing LTP in a transgenic model of Alzheimer’s disease (Gong et al., 2004). In addition, rolipram treatment successfully restored long-term memory in a mouse model of Rubinstein-Taybi syndrome (Bourtchouladze et al., 2003). While Rolipram has been extensively used to improve hippocampal plasticity, its effect on visual cortex plasticity is unknown. In the present study we test whether Rolipram can restore OD plasticity in a ferret model of FASD.
Material and Methods
Ferrets received 3.5 g/Kg alcohol i.p. (25% in saline) or saline as control every other day between postnatal day (P) 10 to P30, which is roughly equivalent to the third trimester equivalent of human gestation (Clancy et al., 2001;Medina et al., 2003;Medina et al., 2005). This alcohol treatment leads to a blood alcohol level (BAL) of approximately 250 mg/dl after 1–5 hours after injection (Medina et al., 2003;Medina and Ramoa, 2005).
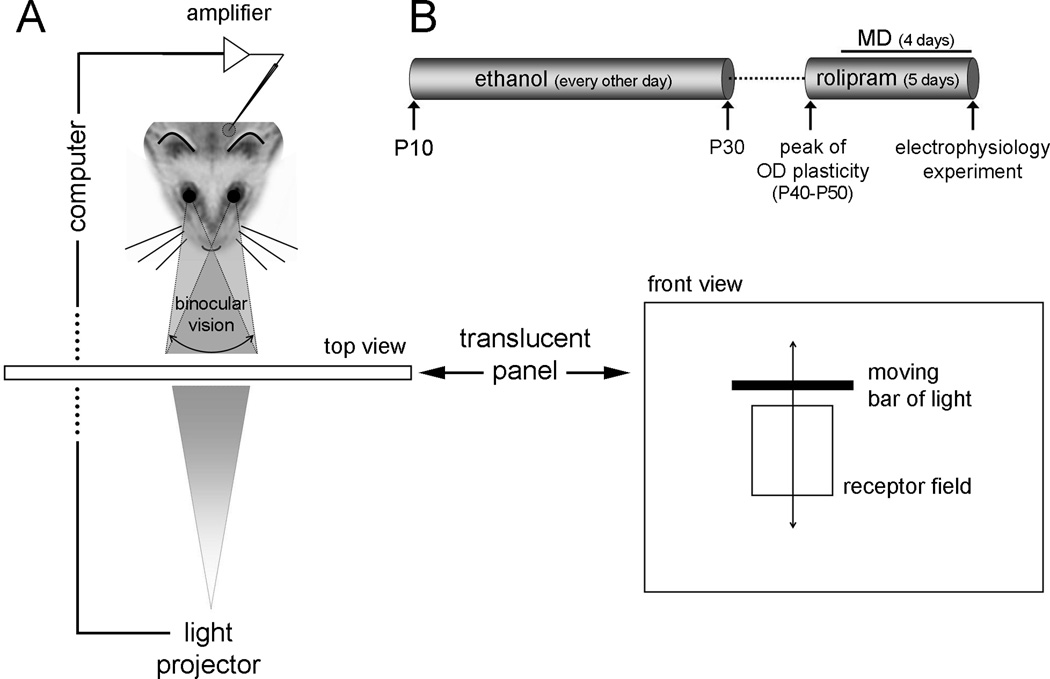
Following a prolonged alcohol-free period (10–15 days), four groups of ferrets had the lid of the right eye sutured closed (monocular deprivation) for four days and were examined for ocular dominance changes at the end of the period of deprivation. One day before the start of the monocular deprivation all animals were administered (i.p.) with Rolipram or vehicle (DMSO solution). Three different doses of Rolipram were used in this study: 0.5, 1.25 and 2.5 mg/Kg. The selection of this dose range is based on previous studies using Rolipram to investigate hippocampal plasticity (Barad et al., 1998;Crowe et al., 2009;Rutten et al., 2007;Zhang and O'Donnell, 2000). The treatment with Rolipram or vehicle was done daily until the electrophysiology experiment (total of 5 days of treatment, Figure 1a). Table I summarizes the number of animals used in our study.
Figure 1.
Experimental design. (A) Ferrets were exposed to 3.5g/Kg of 25% Ethanol i.p. every other day between P10 to P30 (roughly the third trimester equivalent of human gestation). Animals received either Rolipram (0.5, 1.25 or 2.5 mg/Kg) or Vehicle for 4 days during the peak of the critical period of ocular dominance plasticity. After the first Rolipram dose, animals were monocular deprived (right eye) and ocular dominance plasticity (OD) evaluated by extracellular electrophysiology after 4 days. (B) During electrophysiology experiments, animals were deeply anesthetized, positioned in a stereotaxic frame facing a translucent tangential screen and a microelectrode was lowered into their left primary visual cortex. Under computer control, a moving bar of light (0.5° wide and 20° long) was presented to each eye individually at the optimal orientation. One stimulus presentation consisted of the bar of light moving across the receptive field in one direction and back across in the opposite direction. Evoked spikes were collected by a computer (10 stimulus presentations) and all data were from cells with receptive fields in the binocular region of the visual field.
Table 1.
Number of Animals and Cells Used in Each Group
| Group | Number of animals | Number of cells |
|---|---|---|
| Saline | 6 | 190 |
| Ethanol | 4 | 134 |
| Saline + MD + vehicle | 10 | 308 |
| Ethanol + MD + vehicle | 8 | 193 |
| Ethanol + MD + 0.5 mg/kg rolipram | 5 | 127 |
| Ethanol + MD + 1.25 mg/kg rolipram | 6 | 144 |
| Ethanol + MD + 2.5 mg/kg rolipram | 4 | 110 |
| Total | 43 | 1206 |
Quantitative single-unit electrophysiology was performed to assess OD changes in the left hemisphere as described elsewhere (Medina et al., 2003;Mower et al., 2002). Briefly, animals were anesthetized with sodium pentobarbital (35mg/kg, Abbott Laboratories, North Chicago, IL) and single-unit recordings were conducted using a microelectrode lowered into the binocular region of the primary visual cortex. Ocular dominance, spontaneous activity and number of spikes per stimulus were quantitatively determined for each cell by presenting a computer-controlled bar of light to each eye. Each stimulus presentation consisted of the bar of light moving across the receptive field at the optimal orientation in one direction and back across in the opposite direction (Figure 1b). Spikes were collected during the 10 stimulus presentations by a computer using Spike 2 software (Cambridge Electronics Design, Cambridge, UK) and peristimulus histograms were generated. Spontaneous activity was determined by recording activity in the absence of stimulation. At the conclusion of the experiment, ferrets were killed with 0.6ml/kg of a solution of euthasol (Demalva labs, Virginia) containing pentobarbital sodium (390mg/ml) and phenytoin sodium (50mg/ml). To quantify cortical neuron OD, an index was calculated for each cell using the following equation: LE/(LE+RE), where LE stands for response to stimulation of left eye and RE for right eye. An OD index of 1.0 indicates that a neuron is responsive only to the left eye, and an OD index of 0.0 indicates responses only to the right eye. To quantify the changes in ocular dominance after monocular deprivation, we calculated a contralateral bias index (CBI) for each animal as ((P0.00–0.19 – P0.80–1.00) + (P0.20–0.39 – P0.60–0.79)/2 + 100)/200, where PA-B denotes the percentage of cells with OD indices between A and B. A CBI of 0 indicates that the ipsilateral eye dominated the responses in every neuron tested whereas a CBI of 1 indicates that the contralateral eye dominated every neuron. Table I displays the number of cells collected from each experimental group. Comparison of the CBIs displayed on Figure 3 was done by a Univariate ANOVA using Exposure (Saline or Ethanol), and MD (with or without MD) as factors. In addition, a second Univariate ANOVA was done considering each group independently (Saline, Saline+MD, Ethanol, Ethanol+MD, Ethanol+MD+0.5 Rolipram, Ethanol+MD+1.25 Rolipram, Ethanol+MD+2.5 Rolipram). Post hoc analysis was done by Bonferroni test.
Figure 3.

Contralateral bias index scores (CBI) are plotted for the same animal groups displayed in Figure 2. Bars represent means (±SEM), and each open circle represents an individual animal. In Saline and Ethanol treated animals that were allowed normal binocular vision (no MD) most CBIs were higher than 0.5, reflecting a predominance of the experienced (open) eye. In contrast, CBI values of Saline animals monocularly deprived for 4 days were markedly reduced relative to non-deprived animals, indicating a marked predominance of response to stimulation of the non-deprived eye. Monocular deprivation in alcohol exposed animals did not induce such effect, with CBI values similar to non-deprived animals, indicating impaired OD plasticity. Four days of MD in alcohol exposed animals treated with Rolipram failed to restore OD plasticity. For all doses used, CBI values were similar to values observed in monocularly deprived alcohol exposed animals.
Visual stimulation and Spontaneous activity was analyzed by a Univariate ANOVA using Exposure (Saline or Ethanol) and Treatment (0, 0.5, 1.25 or 2.5 mg/Rolipram) as factors. A second ANOVA was done combining all Rolipram treatments into one group. Thus, in this case the factor Treatment was either vehicle or Rolipram. All procedures described in this paper were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University.
Results
Following three days of deprivation, extracellular recordings were conducted in the binocular region of the primary visual cortex of ferrets. To quantify OD of cortical neurons, we calculated an ocular dominance index as described in the methods section. An ocular dominance index of 1.0 indicates that a neuron is responsive only to the left (open) eye, and an ocular dominance index of 0.0 indicates responses only to the right (deprived) eye.
The primary visual cortex of normal ferrets is characterized by a large number of neurons responsive to visual stimulation of both eyes, with the majority of neurons responding to stimulation of the contralateral eye (Issa et al., 1999;Krahe et al., 2005;Medina et al., 2003;Medina and Ramoa, 2005). Figures 2a and 2b show the typical ocular dominance profile in Untreated and Ethanol treated ferrets that received normal visual experience. Note the high proportion of binocular cells and contralateral eye dominance. As expected, monocular deprivation of control animals resulted in a marked shift towards the ipsilateral (open) eye (Figure 2c).
Figure 2.

Ocular dominance (OD) histograms for neurons from all animal groups tested. An index of 0.0 and 1.0 indicate that a neuron responds only to the contralateral (right) or the ipsilateral (left) eye respectively. Intermediate scores represent binocular cells. The primary visual cortex of Saline (A, n=6 ferrets) and Ethanol (B, n=4) treated animals that had normal binocular visual experience (no MD) were characterized by a large number of binocular cells with several neurons dominated by the contralateral eye. Four days of MD of Saline treated animals (C, n=10) induced a marked shift in OD distribution in the direction of the non-deprived ipsilateral eye. In contrast, the same period of MD in animals treated with alcohol induced only a slight OD shift with most neurons still driven by the deprived (contralateral) eye (D, n=8). Rolipram treatment did not restore OD plasticity in alcohol exposed animals (E, n=5; F, n=6; G, n=4). Note that the OD profile of Ethanol treated animals monocularly deprived for four days is very similar to the OD profile of Ethanol-treated animals that received Rolipram treatment. Error bars indicate ±SEM.
Monocular deprivation had relatively little effect on OD in Alcohol treated ferrets (Figure 2d), confirming our previous findings that early alcohol exposure induces a long-lasting impairment in OD plasticity (Medina et al., 2003;Medina and Ramoa, 2005). To test whether a PDE type 4 inhibitor would be able to restore OD plasticity, we assessed the effects of Rolipram on monocular deprived Alcohol treated animals. Surprisingly, Rolipram did not restore OD plasticity in Ethanol treated animals even when higher doses were used. Figures 2e–g show that several neurons remained responsive to the contralateral (deprived) eye after four days of monocular deprivation, regardless of the dose used. Note that the OD profiles in animals treated with Rolipram (Figure 2e–g) are similar to the OD profile observed in animals that only received alcohol (Figure 2d). The CBI values of Alcohol treated animals that received Rolipram confirm these results (Figure 3). A CBI of 0.0 indicates that the ipsilateral eye dominated all cells measured while a CBI of 1.0 means that the contralateral eye dominated all cells (see methods). Figure 3 shows the mean (±SEM) and distribution of CBI scores for the left hemisphere of individual animals from all groups. The ANOVA showed a significant effect of MD (df=1, df error=39, F=23.315, P<0.001) as well as an interaction between MD and exposure type (df= 1, df error=39 F=5.5, P<0.05). As expected, four days of visual deprivation (right eye) induced a shift in CBI values (towards 0.0) in control animals (Oneway ANOVA, df=6, df error=36, F=5.7, P<0.001; Saline vs. Saline+MD, P<0.001, Bonferroni), but not in Ethanol treated ones (Ethanol vs Ethanol+MD, P=1.0, Bonferroni). Likewise, the majority of Ethanol treated animals that received Rolipram did not show a shift in CBI values, regardless of the doses tested (P=1.0 for all comparisons, Bonferroni).
We also made use of extracellular single-unit recordings to verify whether Rolipram treatment resulted in abnormal visually driven activity. Figure 4 shows the quantitative assessment of responses to visual stimulation (Figure 4a) and levels of spontaneous neuronal activity (Figure 4b) in monocular deprived animals that received Saline, Ethanol or Ethanol + Rolipram treatments. No significant differences in mean maximal responses nor in spontaneous activity were observed between groups (Oneway ANOVA; maximal visual responses, df=4, df error=27, F=0.96, P=0.45; spontaneous activity,df=4, df error=27, F=2.19, P=0.097). However, an Oneway ANOVA considering all Rolipram doses combined indicated a significant reduction in spontaneous activity levels of Rolipram treated animals when compared to levels observed in monocular deprived Saline and Ethanol treated animals that did not receive Rolipram: Ethanol+Rolipram+MD (0.59 ±0.065) vs. Saline+Vehicle+MD (0.78 ±0.099) vs Ethanol+Vehicle+MD (0.73 ±0.12); (df=2, df error=29, F=4.8, P=0.036). Another indication of the action of the drug comes from the fact that all animals that received Rolipram presented vomiting minutes after its administration, a common side effect observed in ferrets (Robichaud et al., 2001) and humans (Bertolino et al., 1988). None of the control animals displayed such behavior. Taken together our results indicate that PDE type 4 inhibitors do not play a role in restoring OD visual plasticity in the ferret model of FASD.
Figure 4.

Visual responses of neurons in the primary striate cortex to a moving bar of light in monocularly deprived animals. (A) Mean maximal response (in spikes per run) of cortical neurons to stimulation at the optimal orientation. (B) Mean spontaneous neuronal activity in the absence of visual stimulation. Error bars indicate ±SEM.
Discussion
Our attempt to restore OD plasticity using a PDE4 inhibitor relies on its effects on the cAMP cascade (Blokland et al., 2006;Ghavami et al., 2006) and on evidence that alcohol exposure during development affects cAMP metabolism (Chen et al., 2006;Karacay et al., 2007). However, while the effects of ethanol on the cAMP metabolism are well described in adult animals, much less is known about its effects during brain development. Recently, it has been shown that an increase in cAMP production can protect the developing brain from alcohol-triggered apoptosis. Application of forskolin, which increases cAMP levels trough activation of adenylcyclase (AC), reduces alcohol-induced cell death in cultured cerebellar granule neurons (Karacay et al., 2007). Conversely, reduction of cAMP production by deletion of AC1 and AC8 genes enhances cell death in mice exposed to alcohol (Maas Jr et al., 2005;Karacay et al., 2007). Consistently with these results, Chen and colleagues showed that ethanol lowers cAMP levels, resulting in cell death in hypothalamic cell cultures (Chen et al., 2006). Reduced levels of cAMP can disrupt several cellular functions and, in particular, CREB activation (Bito et al., 1996;Josselyn and Nguyen, 2005;Lamprecht, 2005). In fact, early alcohol exposure can lead to a persistent decrease in pCREB levels in the hippocampus (Roberson et al., 2009), which could account for the problems of spatial learning seen in FASD (Clements et al., 2005;Goodlett and Peterson, 1995;Richardson et al., 2002). Therefore, trying to restore OD plasticity in alcohol exposed animals increasing cAMP levels using a PDE4 inhibitor seems to be a reasonable approach.
Surprisingly, however, our results demonstrate that, contrary to PDE1 (Medina et al., 2006), PDE4 inhibition does not restore OD plasticity in ferrets treated with alcohol. While both isoforms can be found in the visual cortex and act on the cAMP metabolism (Bailey et al., 2004;Beavo, 1995;Bender and Beavo, 2006;Rose et al., 2005;Van staveren et al., 2001), these two types of PDE have distinct mechanisms of activation and can affect different cascades. PDE1 is activated by Ca++ and Calmodulin and its inhibition prevents the breakdown of cAMP to 5’-AMP and of cGMP to 5’-GMP. Since PDE4 inhibition affects only cAMP levels one may suggest that the restorative effects seen with vinpocetine are due to changes in cGMP. Another possibility is related to the fact that, while PDE type 1 is activated by Ca++ and Calmodulin, PDE type 4 is activated by cAMP itself, working in a feedback mechanism that is only triggered when cAMP levels are high. Therefore, a possible explanation for the lack of a restorative effect after Rolipram treatment is that cAMP levels are already low in FASD. Our recent findings that CREB phosphorylation is decreased in the ferret model of FASD support this hypothesis (Krahe et al., 2008). However, we cannot discard the possibility that the range of the Rolipram doses used here was not appropriate. Considering that this is the first study to investigate the role of Rolipram in visual cortex plasticity, our dose choices were based on studies that have used this drug to improve hippocampal plasticity.
The role of rolipram in learning and memory has been extensively tested and results were obtained with a wide range of doses (Barad et al., 1998;Crowe et al., 2009;Rutten et al., 2007;Zhang and O'Donnell, 2000). Barad and colleagues showed that rolipram at either 0.03 or 0.8 mg/Kg doses facilitated the potentiation and latency of LTP. However, the lowest (0.03 mg/Kg) dose was more efficient than the higher one (Barad et al., 1998). In contrast, many other studies show that very low doses (such as 0.01 to 0.03 mg/Kg) are not as effective as higher ones (0.2–2.0 mg/Kg)(Crowe et al., 2009;Rutten et al., 2007;Zhang and O'Donnell, 2000). For instance, while both 0.05 and 1.0 mg/Kg doses of Rolipram successfully reverse scopolamine induced learning deficits in the radial arm maze, lower doses (0.01 and 0.025 mg/Kg) fail to show an effect (Zhang and O'Donnell, 2000). Similarly, 0.1 mg/Kg, but not 0.01 or 0.03 mg/Kg doses of Rolipram improve object recognition in a model of acute tryptophan depletion (Rutten et al., 2007). Finally, it has been recently shown that only doses in the range of 0.25 to 2.0 mg/Kg are able to improve passive avoidance learning in chicks (Crowe et al., 2009). Collectively, these findings suggest that, although we cannot restore OD plasticity in the ferret of model of FASD, the Rolipram dosage used in the present study seems to be appropriate. However, while unlikely, we cannot discard the possibility that doses of Rolipram lower than 0.5 mg/Kg could have an effect.
In conclusion, our results suggest that PDE4 inhibition, for the doses used here, does not restore OD plasticity in the ferret model of FASD. These findings could be explained by: i) the possibility that PDE4 is already inactive in alcohol treated animals due to lower cAMP levels; or ii) the need of activation of different signaling pathways, such as the cGMP cascade. Nonetheless, future studies are needed to investigate the role of other PDEs isoforms in the restoration of OD plasticity in animal models of FASD.
Acknowledgements
This work was supported by NIH (NIAAA) grant AA-13023 to A.E.M.
References
- Bailey CD, Brien JF, Reynolds JN. Chronic prenatal ethanol exposure alters the proportion of GABAergic neurons in layers II/III of the adult guinea pig somatosensory cortex. Neurotoxicol Teratol. 2004;26:59–63. doi: 10.1016/j.ntt.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phophodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci U S A. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Ratto GM, Maffei L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003;26:369–378. doi: 10.1016/S0166-2236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Crippa D, di Dio S, Fichte K, Musmeci G, Porro V, Rapisarda V, Sastre-y-Hernandez M, Schratzer M. Rolipram versus imipramine in inpatients with major, "minor" or atypical depressive disorder: a double-blind double-dummy study aimed at testing a novel therapeutic approach. Int Clin Psychopharmacol. 1988;3:245–253. doi: 10.1097/00004850-198807000-00006. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: A Ca++ and stimulus duration dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Blokland A, Schreiber R, Prickaerts J. Improving memory: a role for phosphodiesterases. Curr Pharm Des. 2006;12:2511–2523. doi: 10.2174/138161206777698855. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, Romashko D, Scott R, Tully T. A mouse model of Rubinstein-Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci U S A. 2003;100:10518–10522. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CP, Kuhn P, Chaturvedi K, Boyadjieva N, Sarkar DK. Ethanol Induces Apoptotic Death of Developing -Endorphin Neurons via Suppression of Cyclic Adenosine Monophosphate Production and Activation of Transforming Growth Factor-1-Linked Apoptotic Signaling. Mol Pharmacol. 2006;69:717. doi: 10.1124/mol.105.017004. [DOI] [PubMed] [Google Scholar]
- Chen L, Muzzio IA, Malleret G, Bartsch D, Verbitsky M, Pavlidis P, Yonan AL, Vronskaya S, Grody MB, Cepeda I, Gilliam TC, Kandel ER. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron. 2003;39:655–669. doi: 10.1016/s0896-6273(03)00501-4. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Clements KM, Girard TA, Ellard CG, Wainwright PE. Short-term memory impairment and reduced hippocampal c-Fos expression in an animal model of fetal alcohol syndrome. Alcohol Clin Exp Res. 2005;29:1049–1059. doi: 10.1097/01.alc.0000171040.82077.e. [DOI] [PubMed] [Google Scholar]
- Crowe SF, Neath J, Hale MW. The type 4 phosphodiesterase inhibitors rolipram and YM976 facilitate recall of the weak version of the passive avoidance task in the day-old chick. Pharmacol Biochem Behav. 2009;92:224–230. doi: 10.1016/j.pbb.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- Dyke HJ, Montana JG. The therapeutic potential of PDE4 inhibitors. Expert Opin Investig Drugs. 1999;8:1301–1325. doi: 10.1517/13543784.8.9.1301. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Frank DA, Greenberg ME. CREB: a mediator of long-term memory from mollusks to mammals. Cell. 1994;79:5–8. doi: 10.1016/0092-8674(94)90394-8. [DOI] [PubMed] [Google Scholar]
- Ghavami A, Hirst WD, Novak TJ. Selective phosphodiesterase (PDE)-4 inhibitors: a novel approach to treating memory deficit? Drugs R D. 2006;7:63. doi: 10.2165/00126839-200607020-00001. [DOI] [PubMed] [Google Scholar]
- Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest. 2004;114:1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CR, Peterson SD. Sex differences in vulnerability to developmental spatial learning deficits induced by limited binge alcohol exposure in neonatal rats. Neurobiol Learn Mem. 1995;64:265–275. doi: 10.1006/nlme.1995.0009. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Υhe period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Issa NP, Trachtenberg JT, Chapman B, Zahs KR, Stryker MP. The critical period for ocular dominance plasticity in the ferret's visual cortex. J Neurosci. 1999;19:6965–6978. doi: 10.1523/JNEUROSCI.19-16-06965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Nguyen PV. CREB, synapses and memory disorders: past progress and future challenges. Curr Dug Targets CNS Neurol Disord. 2005;4:481–497. doi: 10.2174/156800705774322058. [DOI] [PubMed] [Google Scholar]
- Karacay B, Li G, Pantazis NJ, Bonthius DJ. Stimulation of the cAMP pathway protects cultured cerebellar granule neurons against alcohol-induced cell death by activating the neuronal nitric oxide synthase (nNOS) gene. Brain Res. 2007 doi: 10.1016/j.brainres.2007.01.059. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahe TE, Medina AE, Bittencourt-Navarrete RE, Colello RJ, Ramoa AS. Protein synthesis independent plasticity mediates rapid and precise recovery of deprived eye responses. Neuron. 2005;48:329–343. doi: 10.1016/j.neuron.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Krahe TE, Wang W, Medina AE. Vinpocetine increase phosphorylation of CREB and restores orientation selectivity in a ferret model of fetal alcohol spectrum disorder. Int J Dev Neurosci. 2008;26:890. [Google Scholar]
- Lamprecht R. CREB: a message to remember. Cell Mol Life Sci. 2005;55:554–563. doi: 10.1007/s000180050314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G. Memory enhancement: the search for mechanism-based drugs. Nat Neurosci. 2002;5:1035–1038. doi: 10.1038/nn935. [DOI] [PubMed] [Google Scholar]
- Maas JW, Jr, Indacochea RA, Muglia LM, Tran TT, Vogt SK, West T, Benz A, Shute AA, Holtzman DM, Mennerick S, Olney JW, Muglia LJ. Calcium-stimulated adenylyl cyclases modulate ethanol-induced neurodegeneration in the neonatal brain. J Neurosci. 2005;25:2376–2385. doi: 10.1523/JNEUROSCI.4940-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AE, Krahe TE. Neocortical plasticity deficits in fetal alcohol spectrum disorders: Lessons from Barrel and Visual Cortex. J Neurosci Res. 2008;86:256–263. doi: 10.1002/jnr.21447. [DOI] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Coppola DM, Ramoa AS. Neonatal alcohol exposure induces long-lasting impairment of visual cortical plasticity in ferrets. J Neurosci. 2003;23:10002–10012. doi: 10.1523/JNEUROSCI.23-31-10002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Ramoa AS. Early Alcohol Exposure Induces Persistent Alteration of Cortical Columnar Organization and Reduced Orientation Selectivity in the Visual Cortex. J Neurophysiol. 2005;93:1317–1325. doi: 10.1152/jn.00714.2004. [DOI] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Ramoa AS. Restoration of Neuronal Plasticity by a Phosphodiesterase Type 1 Inhibitor in a Model of Fetal Alcohol Exposure. J Neurosci. 2006;26:1057–1060. doi: 10.1523/JNEUROSCI.4177-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AE, Ramoa AS. Early alcohol exposure impairs ocular dominance plasticity throughout the critical period. Brain Res Dev Brain Res. 2005;157:107–111. doi: 10.1016/j.devbrainres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Mower AF, Liao DS, Nestler EJ, Neve RL, Ramoa AS. cAMP/Ca2+ response element-binding protein function is essential for ocular dominance plasticity. J Neurosci. 2002;22:2237–2245. doi: 10.1523/JNEUROSCI.22-06-02237.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhov N, Khibnick L, Otmakhova N, Carpenter S, Riahi S, Asrican B, Lisman J. Forskolin-induced LTP in the CA1 hippocampal region is NMDA receptor dependent. J Neurophysiol. 2004;91:1955–1962. doi: 10.1152/jn.00941.2003. [DOI] [PubMed] [Google Scholar]
- Powrozec TA, Zhou FC. effects of prenatal alcohol exposure on the development of the vibrissal somatosensory cortical barrel network. Brain Res Dev Brain Res. 2005;155:135–146. doi: 10.1016/j.devbrainres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Rema V, Ebner FF. Effect of enriched environment rearing on impairments in cortical excitability and plasticity after prenatal alcohol exposure. J Neurosci. 1999;19:10993–11006. doi: 10.1523/JNEUROSCI.19-24-10993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DP, Byrnes ML, Brien JF, Reynolds JN, Dringenberg HC. Impaired acquisition in the water maze and hippocampal long-term potentiation after chronic prenatal ethanol exposure in the guinea-pig. Eur J Neurosci. 2002;16:1593–1598. doi: 10.1046/j.1460-9568.2002.02214.x. [DOI] [PubMed] [Google Scholar]
- Roberson R, Cameroni I, Toso L, Abebe D, Bissel S, Spong CY. Alterations in phosphorylated cyclic adenosine monophosphate response element of binding protein activity: a pathway for fetal alcohol syndrome-related neurotoxicity. Am J Obstet Gynecol. 2009;200:193.e1–193.e5. doi: 10.1016/j.ajog.2008.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud A, Savoie C, Stamatiou PB, Tattersall FD, Chan CC. PDE4 inhibitors induce emesis in ferrets via a noradrenergic pathway. Neuropharmacology. 2001;40:262–269. doi: 10.1016/s0028-3908(00)00142-8. [DOI] [PubMed] [Google Scholar]
- Rose GM, Hopper A, De Vivo M, Tehim A. Phosphodiesterase inhibitors for cognitive enhancement. Curr Pharm Des. 2005;11:3329–3334. doi: 10.2174/138161205774370799. [DOI] [PubMed] [Google Scholar]
- Rutten K, Lieben C, Smits L, Blokland A. The PDE4 inhibitor rolipram reverses object memory impairment induced by acute tryptophan depletion in the rat. Psychopharmacology (Berl) 2007;192:275–282. doi: 10.1007/s00213-006-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T, Bourtchouladze R, Scott R, Tallman J. Targeting the CREB pathway for memory enhancers. Nat Rev Drug Discov. 2003;2:267–277. doi: 10.1038/nrd1061. [DOI] [PubMed] [Google Scholar]
- Van staveren WC, Markerink-van Ittersum M, Steinbusch HW, de Vente J. The effects of phosphodiesterase inhibition on cyclic GMP and cyclic AMP accumulation in the hippocampus of the rat. Brain Res. 2001;888:275–286. doi: 10.1016/s0006-8993(00)03081-x. [DOI] [PubMed] [Google Scholar]
- Zhang H, O'Donnell JM. Effects of rolipram on scopolamine-induced impairment of working and reference memory in the radial-arm maze tests in rats. Psychopharmacology (Berl) 2000;150:316. doi: 10.1007/s002130000414. [DOI] [PubMed] [Google Scholar]