Abstract
Key points
Sex differences in left ventricular (LV) mechanics occur during acute physiological challenges; however, it is unknown whether sex differences in LV mechanics are fundamentally regulated by differences in adrenergic control.
Using two‐dimensional echocardiography and speckle tracking analysis, this study compared LV mechanics in males and females matched for LV length during post‐exercise ischaemia (PEI) and β1‐adrenergic receptor blockade.
Our data demonstrate that while basal rotation was increased in males, LV twist was not significantly different between the sexes during PEI. In contrast, during β1‐adrenergic receptor blockade, LV apical rotation, twist and untwisting velocity were reduced in males compared to females.
Significant relationships were observed between LV twist and LV internal diameter and sphericity index in females, but not males.
These findings suggest that LV twist mechanics may be more sensitive to alterations in adrenergic stimulation in males, but more highly influenced by ventricular structure and geometry in females.
Abstract
Sex differences in left ventricular (LV) mechanics exist at rest and during acute physiological stress. Differences in cardiac autonomic and adrenergic control may contribute to sex differences in LV mechanics and LV haemodynamics. Accordingly, this study aimed to investigate sex differences in LV mechanics with altered adrenergic stimulation achieved through post‐handgrip‐exercise ischaemia (PEI) and β1‐adrenergic receptor (AR) blockade. Twenty males (23 ± 5 years) and 20 females (22 ± 3 years) were specifically matched for LV length (males: 8.5 ± 0.5 cm, females: 8.2 ± 0.6 cm, P = 0.163), and two‐dimensional speckle‐tracking echocardiography was used to assess LV structure and function at baseline, during PEI and following administration of 5 mg bisoprolol (β1‐AR antagonist). During PEI, LV end‐diastolic volume and stroke volume were increased in both groups (P < 0.001), as was end‐systolic wall stress (P < 0.001). LV twist and apical rotation were not altered from baseline or different between the sexes; however, basal rotation increased in males (P = 0.035). During β1‐AR blockade, LV volumes were unchanged but blood pressure and heart rate were reduced in both groups (P < 0.001). LV apical rotation (P = 0.036) and twist (P = 0.029) were reduced in males with β1‐AR blockade but not females, resulting in lower apical rotation (males: 6.8 ± 2.1 deg, females: 8.8 ± 2.3 deg, P = 0.007) and twist (males: 8.6 ± 1.9 deg, females: 10.7 ± 2.8 deg, P = 0.008), and slower untwisting velocity (males: 68.2 ± 22.1 deg s−1, females: 82.0 ± 18.7 deg s−1, P = 0.046) compared to females. LV twist mechanics are reduced in males compared to females during reductions to adrenergic stimulation, providing preliminary evidence that LV twist mechanics may be more sensitive to adrenergic control in males than in females.
Keywords: echocardiography, left ventricular mechanics, sex
Key points
Sex differences in left ventricular (LV) mechanics occur during acute physiological challenges; however, it is unknown whether sex differences in LV mechanics are fundamentally regulated by differences in adrenergic control.
Using two‐dimensional echocardiography and speckle tracking analysis, this study compared LV mechanics in males and females matched for LV length during post‐exercise ischaemia (PEI) and β1‐adrenergic receptor blockade.
Our data demonstrate that while basal rotation was increased in males, LV twist was not significantly different between the sexes during PEI. In contrast, during β1‐adrenergic receptor blockade, LV apical rotation, twist and untwisting velocity were reduced in males compared to females.
Significant relationships were observed between LV twist and LV internal diameter and sphericity index in females, but not males.
These findings suggest that LV twist mechanics may be more sensitive to alterations in adrenergic stimulation in males, but more highly influenced by ventricular structure and geometry in females.
Abbreviations
- A
atrial diastolic inflow velocity
- β1‐AR
β1‐adrenergic receptor
- BMI
body mass index
- BSA
body surface area
- DBP
diastolic blood pressure
- E
early diastolic inflow velocity
- EDV
end‐diastolic volume
- EF
ejection fraction
- ESV
end‐systolic volume
- HR
heart rate
- IVST
intraventricular septal wall thickness
- Lengthd
length at end‐diastolic
- LV
left ventriclular
- LVIDd
left ventricular end‐diastolic internal diameter
- LVIDs
left ventricular end‐systolic internal diameter
- MAP
mean arterial pressure
- MVC
maximal voluntary contraction
- PEI
post‐exercise ischaemia
- PWT
posterior wall thickness
cardiac output
- SBP
systolic blood pressure
- SV
stroke volume
- TPR
total peripheral resistance
Introduction
Left ventricular (LV) mechanics are fundamental to ventricular function, as LV twist supports the production of stoke volume (SV) during ejection, and diastolic untwisting drives early filling during diastole (Notomi et al. 2007; Stohr et al. 2011). Previous studies have identified sex differences in LV mechanics, where females have greater LV longitudinal and circumferential strain at rest (Lawton et al. 2011; Augustine et al. 2013). Our group has also identified that females have greater LV twist and faster untwisting than males during large reductions to preload (Williams et al. 2016). It is currently unknown what structural differences or regulatory mechanisms are responsible for these sex differences in LV mechanics. However, it is feasible that differences in LV size or adrenergic stimulation may play a contributing role (Notomi et al. 2007).
Females have been reported to have larger chronotropic responses to periods of acute physiological stress (Fu et al. 2004; Williams et al. 2016), as well as having an increased high frequency power component of heart rate variability (Gregoire et al. 1996; Ramaekers et al. 1998; Barantke et al. 2008), both of which are believed to reflect greater vagal control in females (Shoemaker et al. 2001; Fu et al. 2004). These findings are in contrast to males who commonly have a larger ratio of low‐ to high‐frequency power, which is believed to reflect greater sympathetic (adrenergic) control (Ryan et al. 1994; Gregoire et al. 1996; Kuo et al. 1999; Barantke et al. 2008). These potential sex differences in cardiac adrenergic stimulation are especially relevant to differences in LV mechanics, as altered adrenergic stimulation is reported to impact LV twist (Rademakers et al. 1992; Dong et al. 1999; Notomi et al. 2007). Specifically, the administration of β1 adrenergic receptor (β1‐AR) agonists produces increases in SV and may even double LV twist and peak untwisting velocity (Moon et al. 1994; Akagawa et al. 2007; Notomi et al. 2007). In contrast, β1‐AR blockade results in reductions in LV twist, peak untwisting velocity (Notomi et al. 2007) and strain (Thorstensen et al. 2011). The changes to LV twist predominantly result from alterations to apical rotation, which is likely to be reflective of a greater β‐AR density at the apex compared to the base (Mori et al. 1993; Lyon et al. 2008). However, given that these previous studies have involved exclusively male cohorts, it remains unknown how regional adrenergic control differs between the sexes to ultimately regulate LV twist mechanics. Therefore, the aim of this study was to investigate sex differences in LV mechanics with altered adrenergic stimulation, using activation of the muscle metaboreflex with post‐exercise ischaemia and β1‐AR blockade (bisoprolol) to augment and attenuate adrenergic stimulation, respectively. It was hypothesized that (1) during increases to adrenergic stimulation, LV twist and untwisting velocity would be lower in females than males, and (2) during reductions to adrenergic stimulation, females would have greater twist and faster untwisting than males.
Methods
Ethical approval
All procedures for the study were approved by the University of British Columbia clinical research ethics board (H13‐03472) and conformed to the standards set by the Declaration of Helsinki. Written informed consent was obtained from all participants.
Study participants
Participants from the local university community, between the ages of 19 and 39 years were recruited for the study. Exclusion criteria included the following: a history of cardiovascular, respiratory, or musculoskeletal disease; a body mass index (BMI) greater than 30 kg m−2; a resting blood pressure ≥ 140/90 or < 110/60 mmHg and smoking (or smoking cessation < 12 months). Given the potential influence of sex‐related differences in LV size on LV twist mechanics, males and females were matched for LV length. More specifically, individuals were continually enrolled until a total of 20 males and 20 females were matched for LV length. Those that could not be matched for LV length (within ±0.2 cm) to an individual of the opposite sex were excluded. To minimize the potential variability in LV structure (Arbab‐Zadeh et al. 2014; Weiner et al. 2015), mechanics (Baggish et al. 2008; Weiner et al. 2010a) and adrenergic control (Martin et al. 1991) associated with chronic endurance training, individuals performing > 1 h of moderate‐intensity training five times per week, or ≥ 3 bouts of high intensity training per week were also excluded from the study. Of the 21 males and 26 females enrolled, one male and two females were excluded in the first visit for poor imaging windows. Four females were further excluded at the conclusion of data collection, as a male participant matched for LV length was not enrolled in the study. A total of 20 males and 20 females completed the study and were included in the analysis.
Study design
Participants visited the laboratory on two separate occasions, and were asked to refrain from caffeine, exercise and alcohol for a minimum of 12 h prior to the first visit, and 24 h prior to the second visit. During visit 1, participants were assessed for resting blood pressure, adequate imaging windows and LV length. During visit 2, baseline echocardiographic images were collected following 15 min of quiet rest. Then, participants performed 3 min of isometric handgrip exercise, after which echocardiographic images were collected during the post‐exercise ischaemic (PEI) period. Participants were then administered bisprolol, and a final set of images were collected 2.5 h later. To minimize differences in relative hormone levels and fluid shifts in the second visit, females who were not using combined oral contraceptives were tested in the early follicular phase of their menstrual cycles (days 3–6), and females using combined oral contraceptives were tested during the placebo or pill‐free interval.
Specific methodology
Isometric handgrip and post‐exercise ischaemia
Participants performed three maximal handgrip efforts using their right hand to determine maximal voluntary contraction (MVC), with each trial separated by at least 1 min. An inflatable cuff was placed around the upper right arm, and participants performed isometric handgrip exercise at 35% MVC for 3 min, followed by 3–5 min of PEI to isolate the muscle metaboreflex (Mark et al. 1985). PEI was achieved by inflating the cuff to suprasystolic pressures (240 mmHg) 10 s prior to handgrip release, and handgrip force was continuously recorded and displayed on a screen visible to the participant for visual feedback during the exercise. Collection of echocardiographic images began 30 s following cuff inflation, and the cuff was released when imaging was complete (within approximately 3 min of cuff inflation).
β1‐AR blockade
Following PEI, participants rested for > 15 min, until blood pressure and heart rate (HR) had returned to resting values. Participants were administered an oral 5 mg dose of bisoprolol (β1‐AR antagonist), and returned to rest approximately 2.5 h post‐administration (time of peak plasma concentrations; Leopold, 1986) and a final set of echocardiographic images were collected after 15 min of quiet rest. In the time between bisoprolol administration and imaging, participants remained seated in the laboratory, and refrained from the consumption of food, but were able to drink small quantities of water ad libitum.
Blood pressure and heart rate
Beat‐to‐beat blood pressure data were continually recorded during baseline, handgrip exercise and PEI using finger photoplethysmography (Finometer, Amsterdam, Netherlands). Manual measurements of blood pressure were additionally taken immediately following echocardiographic imaging in each experimental phase. Heart rate was monitored using three‐lead electrogradiography in all phases.
2D and triplane transthoracic echocardiography
Echocardiographic images were acquired with a commercially available ultrasound system (Vivid E9, GE, Fairfield, CT, USA) using M5S 1.5–4.6 MHz and 4 V 1.5–40 MHz transducers, and saved for offline analysis at a later date (EchoPAC v.113, GE). All images were acquired by a single trained sonographer, with participants in the left lateral decubitus position, and at end‐expiration for the assessment of LV structure global function and mechanics in accordance with current guidelines (Lang et al. 2015). LV parasternal long‐axis images were analysed for intraventricular septal thickness (IVST) and posterior wall thickness (PWT), and internal diameter at end‐diastole (LVIDd) and internal diameter at end‐systole (LVIDs). LV length at end‐diastole (LV lengthd) was determined as the mean length from the mitral plane to the apical subendocardium in the apical two‐ and four‐chamber views. Pulsed Doppler recordings were performed in the apical four‐chamber view, and analysed for LV early (E) and atrial (A) diastolic inflow velocities. End‐systolic volume (ESV), end‐diastolic volume (EDV), SV and ejection fraction (EF) were determined using a modified Simpson's technique in triplane recordings of the apical two‐, three‐ and four‐chamber views. All morphological, volume, and Doppler‐derived data represent means of three cardiac cycles. Relative wall thickness was calculated as 2 × PWT/LVIDd, and sphericity index was calculated as LV lengthd/LVIDd. To account for sex‐related differences LV morphology, LV dimensions and volumes were allometrically scaled to body surface area (BSA)0.5 and BSA1.5, respectively (Batterham et al. 1997).
Images for speckle tracking analysis were acquired at a rate of 70–90 frames s−1. Parasternal short‐axis images were acquired at the base with leaflets of the mitral valve visible, for the assessment of basal rotation and circumferential strain. Parasternal short‐axis images were acquired at the apex just proximal to end‐systolic luminal obliteration (van Dalen et al. 2008), for the assessment of apical rotation and circumferential strain. Apical 4‐chamber images were analysed for longitudinal strain.
Speckle tracking and torsional shear analysis
All analyses were performed by a single experienced sonographer who was blinded to the participant sex and the specific experimental condition. Analysis of LV rotation and strain parameters were performed using speckle tracking software (EchoPAC, GE Healthcare), and raw data were time‐aligned and transformed (2D Strain Analysis Tool, Stuttgart, Germany), as previously described (Stöhr et al. 2012; Stembridge et al. 2014; Williams et al. 2016). Images with inadequate tracking in two or more segments were excluded from analysis. Speckle‐tracking data represent averages across all myocardial segments, and averages of three cardiac cycles. Twist data were calculated by subtracting time‐aligned basal data from apical data. Torsion was calculated as LV twist/lengthd. Torsional shear angle was calculated as previously reported by (Aelen et al. 1997) as [(Φapex – Φbase)(r apex + rbase)]/2D where Φ is the rotation, r is the radius and D is LV length at end‐systole. The coefficient of variation of the sonographer for LV twist was 9.2%, in agreement with previous reports (Stembridge et al. 2015; Williams et al. 2016).
LV haemodynamics
Cardiac output () was calculated as SV × HR. Mean arterial pressure (MAP) was calculated as 1/3 systolic blood pressure (SBP) + 2/3 diastolic blood pressure (DBP). Total peripheral resistance (TPR) was calculated as MAP/. End‐systolic wall stress was estimated as a surrogate for LV afterload, and calculated as 0.9SBP × (end‐systolic cavity area/end‐systolic myocardial area) (modified from Haykowsky et al. 2001). End‐systolic cavity area and myocardial area were calculated as π(LVIDs/2)2 and {π[(PWTs + LVIDs + IVSTs)/2]2 − π(LVIDs/2)2}, respectively, under the assumption of a circular ventricular cavity just distal to the papillary muscles.
Statistical analysis and sample size calculation
Independent of analysis used, data are presented as mean ± standard deviation (SD) for clarity of interpretation. Normality of distribution was assessed using the Shapiro–Wilk test. For all dependent variables, normally distributed data were assessed using an independent Student's t test to detect differences between the sexes in each condition. A one‐way repeated measures ANOVA was used to detect within‐group differences, and Fisher's least significant difference test was used to determine pairwise differences when a positive effect was detected. When the normality test failed, a Mann–Whitney test was used to detect sex differences in each condition for non‐parametric data. Friedman's one‐way repeated measures ANOVA on ranks was also used to detect within‐group differences, and Wilcoxon's matched pairs test was used to determine pairwise differences. All statistical analyses were performed using Statistica (version 8.0; StatSoft, Tulsa, OK, USA) with α set a priori to 0.05.
Linear least‐squares regression was used to assess the relationships of LV twist mechanics with LV structure and geometry, and LV volumes in both sexes (inclusive of data from baseline, PEI and β1‐AR blockade). Regression was additionally used to assess the relationship between LV twist and untwisting velocity. Pearson's correlation and Spearman's rank correlation were used to assess the relationships for normally distributed and non‐parametric data, respectively. For clarity of interpretation, all correlation coefficients are presented as r. When a significant relationship was detected in both sexes, slopes of the regression were compared using the extra sum of squares test.
No previous studies have investigated sex differences in LV twist with altered adrenergic stimulation; however, previous work from Dedobbeleer et al. (2013) reported an SD of 2.3 deg in twist during β1‐AR blockade. Utilizing this SD and α = 0.05, it was determined that 20 participants per group would allow us to detect a difference of 2.0 deg in LV twist between the sexes with β = 0.80.
Results
Baseline characteristics, LV structure and haemodynamics
Baseline characteristics are summarized in Table 1. MVC and thus 35% MVC were greater in males (199 ± 52 N) than females (132 ± 34 N; P < 0.001 for both) (Table 1). Males had larger BMI (P = 0.045) and BSA (P < 0.001) than females. As per the study design, LV lengthd was not different between the sexes (P = 0.163). Despite the matching of LV lengthd between the sexes, LVIDd was larger in males (P < 0.001), resulting in sex differences in sphericity index (P = 0.005). However, males had larger LV volumes (P < 0.001) and SV (P = 0.017) than females (Table 2), but allometrically scaled EDV and SV did not differ between the sexes at baseline. In contrast, scaled ESV was smaller in females at baseline (P = 0.034), reflective of a greater EF in females (P = 0.001). Blood pressure and HR did not differ between the sexes. There were additionally no baseline sex differences in relative wall thickness, or in scaled LVIDd, PWT or IVST. E was greater in females (females (F) = 0.94 ± 0.15 m s−1, males (M) = 0.82 ± 0.14 m s−1, P = 0.01); however, A (F = 0.38 ± 0.07 m s−1, M = 0.39 ± 0.11 m s−1) and E/A (F = 2.58 ± 0.70, M = 2.31 ± 0.76) did not differ between the sexes.
Table 1.
Baseline characteristics, LV haemodynamics, structure and geometry
| Males (n = 20) | Females (n = 20) | |
|---|---|---|
| Participant characteristics | ||
| Age (years) | 23 (5) | 22 (3) |
| Height (m) | 1.77 (0.05) | 1.66 (0.07)# |
| Weight (kg) | 72.4 (6.4) | 60.3 (6.4)# |
| BMI (kg m−2) | 23.0 (2.0) | 21.8 (1.5)* |
| BSA (m2) | 1.89 (0.10) | 1.67 (0.12)# |
| MVC (N) | 571 (150) | 377 (98)# |
| Resting haemodynamics | ||
| HR (bpm) | 60 (10) | 62 (8) |
| SBP (mmHg) | 120 (8) | 115 (9) |
| DBP (mmHg) | 73 (9) | 70 (8) |
| MAP (mmHg) | 88 (8) | 85 (7) |
| EF (%) | 55 (3) | 58 (3)# |
| Resting LV structure and geometry | ||
| Lengthd (cm) | 8.45 (0.45) | 8.22 (0.55) |
| Lengthd × BSA−0.5 (cm m−1) | 6.15 (0.29) | 6.37 (0.38)# |
| LVIDd (mm) | 45.1 (3.2) | 40.9 (3.1)# |
| LVIDd × BSA−0.5 (mm m−1) | 32.8 (2.2) | 31.7 (2.2) |
| Sphericity index | 1.88 (0.13) | 2.02 (0.16)# |
| Relative wall thickness | 0.45 (0.06) | 0.45 (0.07) |
Values are means (SD). BMI, body mass index; BSA, body surface area; DBP, diastolic blood pressure; EF, ejection fraction; HR, heart rate; Lengthd, end‐diastolic length; LVIDd, left ventricular internal diameter during diastole; MVC, maximal voluntary contraction; SBP, systolic blood pressure; MAP, mean arterial pressure. * P < 0.05 vs. males; # P < 0.01 vs. males.
Table 2.
LV haemodynamics during altered adrenergic stimulation
| Baseline | Post‐exercise ischaemia | β1‐AR blockade | ||
|---|---|---|---|---|
| HR (bpm) | M | 60 (10) | 63 (10)† | 52 (9)‡ |
| F | 62 (8) | 65 (11) | 50 (8)‡ | |
| MAP (mmHg) | M | 88 (8) | 116 (10)‡ | 81 (8)‡ |
| F | 85 (7) | 107 (10)‡ , # | 75 (11)‡ , # | |
| SBP (mmHg) | M | 120 (8) | 160 (13)‡ | 109 (9)‡ |
| F | 115 (9) | 145 (17)‡ , # | 104 (11)‡ | |
| DBP (mmHg) | M | 73 (9) | 95 (11)‡ | 67 (9)‡ |
| F | 70 (8) | 88 (9)‡ , * | 61 (12)‡ , * | |
| EF (%) | M | 55 (3) | 58 (4)‡ | 55 (3) |
| F | 58 (3)# | 60 (3)* | 60 (3)# | |
| EDV (ml) | M | 113 (20) | 120 (24)‡ | 115 (19) |
| F | 91 (14)# | 96 (16)‡ , # | 92 (13)# | |
| EDV (ml m−3) | M | 43 (7) | 46 (8)‡ | 44 (7) |
| F | 42 (6) | 45 (6)‡ | 43 (6) | |
| ESV (ml) | M | 51 (10) | 51 (12) | 51 (9) |
| F | 38 (6)# | 38 (7)# | 37 (5)# | |
| ESV (ml m−3) | M | 20 (3) | 20 (4) | 20 (3) |
| F | 18 (3)* | 18 (2) | 17 (3)* | |
| SV (ml) | M | 62 (12) | 70 (14)‡ | 63 (12) |
| F | 53 (9)* | 58 (10)‡ , # | 55 (9)# | |
| SV (ml m−3) | M | 24 (4) | 27 (5)‡ | 24 (4) |
| F | 25 (4) | 27 (4)‡ | 25 (4) | |
| (l min−1) | M | 3.66 (0.70) | 4.30 (0.62)‡ | 3.26 (0.67)‡ |
| F | 3.25 (0.41)* | 3.68 (0.50)‡ , # | 2.69 (0.45)‡ , # | |
| (l min−1 m−3) | M | 1.41 (0.24) | 1.66 (0.26)‡ | 1.26 (0.26)‡ |
| F | 1.52 (0.24) | 1.73 (0.32)‡ | 1.26 (0.22)‡ | |
| TPR (mmHg l−1 min−1) | M | 25.1 (5.8) | 27.6 (4.3) | 26.2 (5.7) |
| F | 26.7 (4.9) | 29.5 (4.9)* | 28.8 (7.1) | |
| End‐systolic wall stress (kdyn cm−2) | M | 39.1 (5.3) | 54.7 (8.4)‡ | 33.5 (6.9)‡ |
| F | 37.2 (4.4) | 48.5 (5.2)‡ , * | 32.8 (6.0)‡ |
LV mechanics in response to altered adrenergic stimulation
Table 3 summarizes peak LV mechanics parameters. At baseline, there were no sex differences in LV twist mechanics (twist, torsion, apical rotation, basal rotation and untwisting velocity). However, circumferential strain at the base and longitudinal strain were higher in females compared to males (P = 0.025 and P = 0.015, respectively).
Table 3.
LV mechanics during altered adrenergic stimulation
| Baseline | Post‐exercise ischaemia | β1‐AR blockade | ||
|---|---|---|---|---|
| Twist (deg) | M | 10.2 (2.5) | 11.1 (3.2) | 8.6 (1.9)† |
| F | 11.3 (3.1) | 11.3 (2.4) | 10.7 (2.8)* | |
| Torsion (deg cm−1) | M | 1.20 (0.30) | 1.28 (0.36) | 1.02 (0.23)† |
| F | 1.38 (0.38) | 1.37 (0.30) | 1.33 (0.38)# | |
| Untwisting velocity (deg s−1) | M | −80.3 (25.3) | −77.6 (22.0) | −68.2 (22.1) |
| F | −93.5 (22.6) | −79.4 (28.5) | −82.0 (18.7)* | |
| Apical rotation (deg) | M | 7.8 (1.7) | 8.4 (3.3) | 6.8 (2.1)† |
| F | 8.7 (2.5) | 8.9 (2.3) | 8.8 (2.3)* | |
| Basal rotation (deg) | M | −3.1 (1.8) | −3.8 (1.9)† | −2.5 (1.1) |
| F | −3.3 (2.0) | −3.3 (2.3) | −2.4 (1.7) | |
| Longitudinal strain (%) | M | −17.5 (1.9) | −17.0 (1.7) | −17.2 (1.6) |
| F | −19.0 (1.7)* | −19.5 (1.5)# | −19.0 (1.6)# | |
| Circumferential strain, base (%) | M | −20.3 (3.3) | −20.2 (3.9) | 20.2 (2.5) |
| F | −22.3 (2.1)* | −22.0 (3.0) | −22.3 (3.0)* | |
| Circumferential strain, apex (%) | M | −26.1 (3.7) | −25.5 (3.5) | −25.7 (2.5) |
| F | −25.5 (3.4) | −27.6 (2.7) | −26.0 (2.7) | |
| Torsional shear (deg) | M | 1.92 (0.50) | 2.09 (0.54) | 1.62 (0.37)† |
| F | 2.03 (0.55) | 2.02 (0.44) | 1.91 (0.38)* |
Values are means (SD). All data represent peaks across the cardiac cycle. M, males; F, females. n = 20 females, 20 males for all measures but apical rotation (female n = 19), basal rotation (female n = 19), twist and torsion (female n = 18), torsional shear (female n = 18). * P < 0.05 vs. males; # P < 0.01 vs. males; † P < 0.05 vs. baseline; ‡ P < 0.01 vs. baseline.
Post‐exercise ischaemia
There were no changes from baseline in LV apical rotation, twist, untwisting velocity (Fig. 1) or strain in either group. However, basal rotation was increased in males (P = 0.037). Nonetheless, there were no sex differences in twist during PEI. Longitudinal strain remained higher in females, although circumferential strain at the base was not different between the sexes, and circumferential strain at the apex tended to be higher in females (P = 0.055). There was also no difference between the sexes for torsional shear.
Figure 1. Graphical representation of mean left ventricular (LV) twist mechanics at baseline and during post‐exercise ischaemia and β1‐AR blockade (bisoprolol).
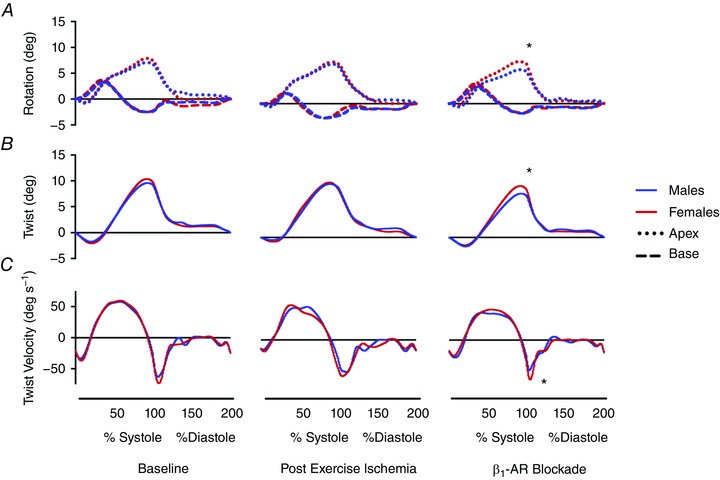
Blue and red lines represent mean data for males and females, respectively. A, dotted and dashed lines represent rotations of the LV apex and base, respectively. B, continuous lines represent LV twist. C, continuous lines represent twist and untwisting velocities. SD values are provided in Table 3. * P < 0.05 males vs. females. [Color figure can be viewed at wileyonlinelibrary.com]
β1‐AR blockade
In females, LV twist mechanics did not differ from baseline, although there was a trend to reduced LV twist (P = 0.063). In males, LV twist and torsion were reduced (P = 0.029 and P = 0.032, respectively), due to a significant reduction to apical rotation (P = 0.036) and a trend to reduction in basal rotation (P = 0.09) (Fig. 1). Untwisting velocity also tended to be reduced in males compared to baseline (P = 0.075). As a result, males had lower LV apical rotation (P = 0.007), twist (P = 0.008) and torsion (P = 0.004), and slower untwisting velocity compared to females (P = 0.046) during β1‐AR blockade. LV strain parameters were not changed from baseline, such that longitudinal strain (P < 0.001) and circumferential strain at the base (P = 0.02) remained higher in females. Torsional shear was significantly reduced in males compared to females (P = 0.022) but there were no sex differences in basal rotation or apical circumferential strain during β1‐AR blockade.
Haemodynamic responses to altered adrenergic stimulation
Post‐exercise ischaemia
Blood pressure increased from baseline in both groups (P < 0.001 for both), and SBP (P = 0.007), DBP (P = 0.031) and MAP (P = 0.006) were greater in males (Table 2). HR increased in males (P = 0.022) and tended to increase in females (P = 0.08); however, HR was not different between the sexes. LVEDV increased (P < 0.001 for both) but ESV was unchanged, resulting in an augmentation of both LVSV and in both sexes (P < 0.001). There were no sex differences in scaled LVEDV or SV, but scaled ESV tended to be lower in females (P = 0.08). Thus, while EF was increased in males (P < 0.001) and tended to increase in females (P = 0.07), EF remained greater in females compared to males (P = 0.025). End‐systolic wall stress increased in both sexes (P < 0.001), and was greater in males compared to females (P = 0.013). Although TPR increased in males (P = 0.014), it was unchanged in females and not different between the sexes. E increased in males (0.87 ± 0.19 m s−1, P = 0.012), and A increased in both sexes during PEI (F = 0.44 ± 0.17 m s−1, M = 0.42 ± 0.10 m s−1, P < 0.05). E/A, however, was unchanged and there were no sex differences in these parameters.
β1‐AR blockade
Blood pressure, HR and were reduced from baseline in both groups (P < 0.001). Both DBP (P = 0.012) and MAP (P = 0.002) were higher in males. However, the reduction to HR was greater in females (−12 ± 6 bpm) compared to males (−8 ± 5 bpm, P = 0.023). LV volumes and EF were not different from baseline in either group. Similar to baseline, scaled LVEDV and SV did not differ between the sexes, but scaled ESV was smaller (P = 0.01) and EF was greater (P < 0.001) in females. End‐systolic wall stress was reduced in both groups (P < 0.001 for both), but was not different between the sexes. TPR was unchanged and did not differ between the sexes. E was unchanged from baseline in both sexes; however, A was reduced in females (0.31 ± 0.06 m s−1, P < 0.001) but not in males (0.35 ± 0.10 m s−1). Thus, E/A was increased in females (2.99 ± 0.16, P < 0.001) but not males (2.56 ± 0.87, P = 0.072).
LV structure and geometry during altered adrenergic stimulation
There were no changes from baseline in absolute or scaled wall thicknesses and LVIDd in either sex, during any stage (Table 4). IVST (P < 0.05) and LVIDd (P < 0.001) were larger in males during all stages, and PWT was larger in males (P < 0.05) except during β1‐AR blockade (P = 0.096). Nonetheless, scaled wall thicknesses and scaled LVIDd were not different between the sexes in any stage. Relative wall thickness and sphericity index were also unchanged in both sexes, during either intervention. Relative wall thickness did not differ between the sexes; however, sphericity index was greater in females at baseline (P = 0.005), during PEI (P = 0.007) and during β1‐AR blockade (P = 0.003). LV lengthd increased in males (P < 0.001) and tended to increase in females (P = 0.07) during PEI, but was unchanged during β1‐AR blockade in either sex. LV lengthd was not different between the sexes during either intervention, but tended to be smaller in females during β1‐AR blockade (P = 0.052).
Table 4.
LV structure and geometry during altered adrenergic stimulation
| Baseline | Post‐exercise ischaemia | β1‐AR blockade | ||
|---|---|---|---|---|
| Lengthd (mm) | M | 84.5 (4.5) | 85.7 (4.8)‡ | 84.7 (4.7) |
| F | 82.2 (5.5) | 83.0 (5.3) | 81.6 (5.2) | |
| LVIDd (mm) | M | 45.1 (3.2) | 45.3 (2.7) | 45.5 (3.3) |
| F | 40.9 (3.1)# | 40.8 (3.9)# | 40.3 (2.8)# | |
| Sphericity index | M | 1.88 (0.13) | 1.89 (0.12) | 1.88 (0.13) |
| F | 2.02 (0.16)# | 2.05 (0.21)# | 2.03 (0.17)# | |
| Relative wall thickness | M | 0.45 (0.06) | 0.44 (0.06) | 0.45 (0.07) |
| F | 0.45 (0.07) | 0.45 (0.07) | 0.48 (0.06) |
Values are means (SD). See Table 1 for abbreviations. * P < 0.05 vs. males; # P < 0.01 vs. males; † P < 0.05 vs. baseline; ‡ P < 0.01 vs. baseline.
Relationships of LV mechanics with structure and geometry
There was a significant relationship between LV twist and untwisting velocity in both males (r = −0.58,
P < 0.001) and females (r = −0.57, P < 0.001), and this was not different between the sexes. In females, there was a significant relationship for LVIDd with LV apical rotation (r = −0.30, P = 0.02), and twist (r = −0.35, P = 0.013) (Fig. 2). Additionally, there were significant relationships for sphericity index with apical rotation (r = 0.32, P = 0.019) and twist (r = 0.35, P = 0.012), in females but not males. There were no relationships for LV lengthd with twist or rotation in either group. No relationships between LVEDV or SV with LV apical rotation, basal rotation or twist were observed for either sex.
Figure 2. Relationships for LV twist mechanics with chamber structure and geometry.
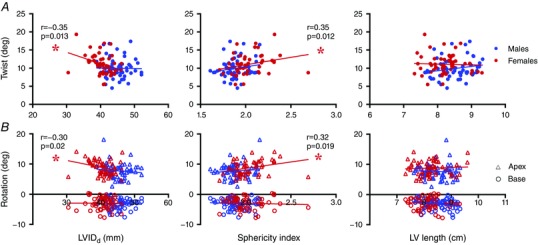
Data include measures during baseline, post‐exercise ischaemia and β1‐AR blockade. Blue and red represent data for males and females, respectively. A, filled circles represent LV twist. B, open triangles and circles represent LV rotation at the apex and base, respectively. *Significant relationship (P < 0.05). [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
This is the first study to compare LV mechanics between males and females, matched for LV lengthd, during altered adrenergic stimulation. In support of our hypothesis, females had greater LV twist and faster untwisting velocity than males during β1‐AR blockade. However, in contrast, no sex differences in LV twist mechanics were observed with increased adrenergic stimulation during PEI.
Effects of post‐exercise ischaemia on sex differences in LV mechanics
In the current study, PEI was used to activate the muscle metaboreflex, and effectively increase adrenergic stimulation independently of increases to HR (O'Leary, 1993; Nishiyasu et al. 1994). During PEI, LVSV was increased in both males and females. However, contrary to our hypothesis, LV twist was not different from baseline or between the sexes. This occurred despite a small but significant increase to basal rotation in males during PEI. The increases to LVSV in our study are in agreement with Crisafulli et al. (2003, 2006) who have demonstrated that SV increases to ∼130% of baseline during PEI in an all‐male cohort. In females, Shoemaker et al. (2007) have also reported a trend of elevated SV during PEI. In the current study, the elevations to LVSV resulted from increases to EDV, while ESV was unchanged, suggesting that the increases to LV contractility were enough to offset the pronounced increases in afterload as indicated by the elevated systolic wall stress. Both groups had increases to A filling velocity, suggesting that increased atrial contraction and filling potentially contributed to increasing EDV. Increases to central venous pressure also occur during PEI (Shoemaker et al. 2007; Marongiu et al. 2013) and are likely to have increased venous return, thus explaining the higher LVEDV in the current study.
Given that increases to adrenergic stimulation and LV preload can each independently increase LV twist, the concomitant increases to LVEDV and SV during PEI would be expected to accompany increases to LV twist. While LVEDV and SV were increased in this study, neither males nor females had alterations to LV apical rotation or twist during PEI. Given that increases to afterload reduce LV twist, especially at the apex (Gibbons Kroeker et al. 1995; Dong et al. 1999; Weiner et al. 2012), the increased end‐systolic wall stress during PEI may have countered any potential increases to apical rotation and thus LV twist in both groups. Finally, although increases to LV preload are reported to augment LV twist mechanics (Weiner et al. 2010b), the increases to LVEDV of ∼5–7 ml in the current study probably were not enough to increase LV twist. This is supported by prior investigations from our group (Williams et al. 2016) and others (Burns et al. 2010) in which small increases (∼10 ml) to LVEDV and LVSV did not produce significant alterations to LV twist.
While twist was not significantly altered in either sex during PEI, males did have a small but significant increase to basal rotation, but this did not result in significant sex differences in LV rotation or twist. Nonetheless, the increased basal rotation in males could provide some evidence that the responses of LV mechanics may differ between the sexes with increased adrenergic stimulation. The increases to basal rotation but not apical rotation in males may reflect greater receptor sensitivity at the base. However, this seems unlikely as greater receptor densities and augmented responsiveness to adrenergic stimulation have been demonstrated at the apex compared to the base (Mori et al. 1993; Akagawa et al. 2007). Additionally, while we theorized that males would have a larger increase in apical rotation and thus twist than females, it is possible that this effect was countered by the significantly greater LV afterload (as determined by end‐systolic wall stress) observed in males during PEI.
Recently, Balmain et al. (2016) used PEI in an attempt to discriminate between the contributions of increased afterload and chronotropy that occur during static handgrip exercise. In contrast to our findings, they observed reductions to LV apical rotation, twist and untwisting velocity during PEI, without changes to LVEDV, ESV and SV. While the authors proposed that large increases to LV afterload attenuated LV twist, the haemodynamic data are not entirely consistent with increased afterload, given that increases to LVESV and reductions to SV would be expected to occur when EDV is unchanged. The reduction to LV twist is thus surprising given that increases to sympathetic activation and LV contractility occur during PEI (Victor et al. 1988; Crisafulli et al. 2006). In the current study, the increase to EF in males and the lack of change to ESV in both sexes suggests that an increase in LV contractility maintained LV twist and offset the increased LV afterload.
Effects of β1‐AR blockade on sex differences in LV mechanics
The reduced LV twist mechanics in males compared to females during β1‐AR blockade predominantly resulted from reductions to LV apical rotation in males, whereas LV rotation and twist were unchanged in females. The lower LV apical rotation, twist and untwisting velocity in males during β1‐AR blockade provide preliminary evidence for sex‐related differences in LV adrenergic control of LV twist mechanics, specifically during reductions to adrenergic stimulation. Studies using heart rate variability consistently report greater low frequency power and low‐ to high‐frequency ratios in males, compared to females (Ryan et al. 1994; Gregoire et al. 1996; Kuo et al. 1999; Barantke et al. 2008) suggesting that males are more sympathetically mediated than their female counterparts. Our data support the contention that males are more sympathetically mediated as reductions to adrenergic stimulation during β1‐AR blockade resulted in significant reductions to LV twist in males but not in females. The finding that torsional shear (which controls for LV length and radius) is also reduced in males compared to females suggests that sex differences in LV twist mechanics are mediated, in part, by mechanisms independent of LV geometry. It is plausible that differences in the adrenergic control of myocardial contractility might exist, whereby males might have greater β1‐AR densities at the apex compared to females, resulting in greater reductions to myofibre shortening in comparison to females.
It has been proposed that changes to HR coincide with similar alterations to contractility and LV twist mechanics (Hodt et al. 2011). However, our data do not support this mechanistic link between HR and twist in females, as they experienced a greater reduction to HR without a significant reduction to LV twist. As both HR and twist were reduced in males, this suggests that altered adrenergic stimulation may affect chronotropy and twist differently between the sexes. This postulate is partially supported by previous work that demonstrated a greater increase in HR with a β1‐AR agonist in females, but a greater increase to an index of contractility in males (Convertino, 1998; Turner et al. 1999). Likewise, data from Evans et al. (2001) reported potentially greater reductions to HR in females than males during β1‐AR blockade with propranolol. Collectively, these data suggest that females have greater chronotropic responses to alterations in adrenergic stimulation whereas males may have greater inotropic responses. Thus, in the current study, it is possible that β1‐AR blockade reduced LV contractility in males and contributed to the attenuated LV twist mechanics compared to females, whereas females had greater reductions to HR but no alterations to LV twist.
Relationships between LV twist mechanics and chamber geometry
To our knowledge, this is the first study to match LV lengthd between the sexes, rather than scaling or indexing to LV dimensions or body size. First, we have demonstrated that for the same LV lengthd, females have a smaller LVIDd than males, resulting in a greater sphericity index, or a greater LV ellipsoid geometry compared to males. As a result, males have greater LV volumes than females for the same LV lengthd. Second, we did not observe any associations between LV lengthd, EDV or SV with apical rotation, basal rotation or twist in either sex. This confirms that sex differences in LV twist mechanics are probably not fundamentally determined by differences in LV size or volume. However, there was a negative relationship between LVIDd and twist (r = −0.35, P = 0.013), as well as a positive relationship for sphericity index with LV apical rotation (r = 0.32, P = 0.019) and twist (r = 0.35, P = 0.012) in females. In contrast, there were no relationships observed for LV structure or geometry with LV twist mechanics in males. Combined with the observed sex differences in LV twist during β1‐AR blockade, these data suggest that LV twist may be more sensitive to LV structure and geometry in females, but more sensitive to altered adrenergic stimulation in males.
We have previously demonstrated that females have greater LV twist and sphericity index than males during significant reductions to preload utilizing LBNP, despite similar relative reductions to LV volumes in both sexes (Williams et al. 2016). In connection with the current findings, these sex differences may reflect a greater influence of LV geometry on twist in females. Given that LV deformation is primarily determined by interactions between myofibre layers (Rademakers et al. 1994), alterations to LV shape and thus myofibre alignment can directly alter fibre mechanics and twist in various regions of the LV wall (Choi et al. 2011). Compared to a spherical ventricle, a more ellipsoid shape favours increased active fibre shortening and ejection performance (Choi et al. 2011). To that effect, LV sphericity index has been identified as a strong independent predictor of LV rotation and twist (Dalen et al. 2010). Therefore, the observed sex differences in LV sphericity index in this study and our previous work (Williams et al. 2016) suggest that sex differences in LV fibre alignment may occur for a given LV lengthd. This is supported by correlative data from this study that suggest LV twist mechanics may be more influenced by LV geometry in females compared to males. Intrinsic sex‐related differences in myocardial structure and geometry could potentially contribute to sex differences in the dynamic responses of LV twist mechanics to acute stress.
Limitations
An important limitation to this study was that we observed no increase in LV twist in either sex during PEI and were subsequently unable to investigate whether sex differences in LV twist occur with increased adrenergic stimulation. As blood pressure, LVSV and EF increased with PEI, we are confident that this intervention augmented adrenergic stimulation and the unaltered LV twist was likely to be due to the concomitant increases to LV afterload. Future studies should consider administrating pharmacological β1‐AR agonists (i.e. isoproterenol, dobutamine) to effectively augment LV twist (Moon et al. 1994; Akagawa et al. 2007) and to further examine whether sex differences in LV mechanics exist with increased adrenergic stimulation.
A limitation of measuring torsional shear using echocardiography is that the distance between measurement sites for basal and apical rotation cannot be accurately determined. As such we have measured LV length as the distance between the mitral valve leaflets and apical endocardium. While this may underestimate torsional shear, this would be consistent for males and females, so we believe the significant sex differences observed in the present study are real.
Conclusion
In males and females matched for LV lengthd, differences in LV twist mechanics occur during reductions to adrenergic stimulation. Females have greater LV apical rotation, twist, untwisting velocity and torsional shear than males during β1‐AR blockade. The reductions to apical rotation and twist in males are suggestive of greater sympathetically related adrenergic control of LV twist mechanics compared to females. Although sex differences in LV twist were not observed during increases to adrenergic stimulation with PEI, potentially greater increases to LV twist in males may have been countered by larger increases to afterload. In addition, the matching of LV lengthd has revealed marked sex differences in LV chamber geometry, which may contribute to differences in the responses of LV twist to altered loading and adrenergic stimulation. Altogether, our data provide preliminary evidence that LV twist may be more sensitive to alterations in adrenergic stimulation in males, but influenced to a greater extent by LV geometry in females.
Additional information
Competing interests
None declared.
Author contributions
All data collection and analysis were completed at the Centre of Heart, Lung and Vascular Health, at The University of British Columbia's Okanagan Campus. A.M.W. contributed to the conception and design of the study, data collection, analysis, interpretation of the data and drafting of the manuscript. N.D.E. contributed to the conception and design of the study, analysis and interpretation of the data and drafting of the manuscript. R.E.S. contributed to the conception and design of the study, interpretation of the data and critical revision of the manuscript. W.S.C. contributed to the analysis and interpretation of the data and drafting of the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was funded by the Natural Sciences and Engineering Research Council of Canada (371950). A.M.W. is supported by the Natural Sciences and Engineering Research Council of Canada (Application CGSD2‐460367‐2014). N.D.E. is supported by the Michael Smith Foundation for Health Research (Grant 7085).
Acknowledgements
The authors would like to thank Megan Harper for contributing her time to data collection.
References
- Aelen FW, Arts T, Sanders DG, Thelissen GR, Muijtjens AM, Prinzen FW & Reneman RS (1997). Relation between torsion and cross‐sectional area change in the human left ventricle. J Biomech 30, 207–212. [DOI] [PubMed] [Google Scholar]
- Akagawa E, Murata K, Tanaka N, Yamada H, Miura T, Kunichika H, Wada Y, Hadano Y, Tanaka T, Nose Y, Yasumoto K, Kono M & Matsuzaki M (2007). Augmentation of left ventricular apical endocardial rotation with inotropic stimulation contributes to increased left ventricular torsion and radial strain in normal subjects: quantitative assessment utilizing a novel automated tissue tracking technique. Circ J 71, 661–668. [DOI] [PubMed] [Google Scholar]
- Arbab‐Zadeh A, Perhonen M, Howden E, Peshock RM, Zhang R, Adams‐Huet B, Haykowsky MJ & Levine BD (2014). Cardiac remodeling in response to 1 year of intensive endurance training. Circulation 130, 2152–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine D, Lewandowski AJ, Lazdam M, Rai A, Francis J, Myerson S, Noble A, Becher H, Neubauer S, Petersen SE & Leeson P (2013). Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: comparison with tagging and relevance of gender. J Cardiovasc Magn Reson 15, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggish AL, Yared K, Wang F, Weiner RB, Hutter AM, Picard MH & Wood MJ (2008). The impact of endurance exercise training on left ventricular systolic mechanics. Am J Physiol Heart Circ Physiol 295, H1109–H1116. [DOI] [PubMed] [Google Scholar]
- Balmain B, Stewart GM, Yamada A, Chan J, Haseler LJ & Sabapathy S (2016). The impact of an experimentally induced increase in arterial blood pressure on left ventricular twist mechanics. Exp Physiol 101, 124–134. [DOI] [PubMed] [Google Scholar]
- Barantke M, Krauss TK & Ortak J (2008). Effects of gender and aging on differential autonomic responses to orthostatic maneuvers. J Cardiovasc Electrophysiol 19, 1296–1303. [DOI] [PubMed] [Google Scholar]
- Batterham AM, George KP & Mullineaux DR (1997). Allometric scaling of left ventricular mass by body dimensions in males and females. Med Sci Sports Exerc 29, 181–186. [DOI] [PubMed] [Google Scholar]
- Burns AT, La Gerche A, Prior DL & MacIsaac AI (2010). Left ventricular torsion parameters are affected by acute changes in load. Echocardiography 27, 407–414. [DOI] [PubMed] [Google Scholar]
- Choi HF, Rademakers FE & Claus P (2011). Left‐ventricular shape determines intramyocardial mechanical heterogeneity. Am J Physiol Heart Circ Physiol 301, H2351–H2361. [DOI] [PubMed] [Google Scholar]
- Convertino VA (1998). Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 275, R1909–R1920. [DOI] [PubMed] [Google Scholar]
- Crisafulli A, Salis E, Pittau G, Lorrai L, Tocco F, Melis F, Pagliaro P & Concu A (2006). Modulation of cardiac contractility by muscle metaboreflex following efforts of different intensities in humans. Am J Physiol Heart Circ Physiol 291, H3035–H3042. [DOI] [PubMed] [Google Scholar]
- Crisafulli A, Scott AC, Wensel R, Davos CH, Francis DP, Pagliaro P, Coats AJS, Concu A & Piepoli MF (2003). Muscle metaboreflex‐induced increases in stroke volume. Med Sci Sports Exerc 35, 221–228. [DOI] [PubMed] [Google Scholar]
- Dalen BMV, Soliman OII, Kauer F, Vletter WB, Zwaan HBVD, Cate FJT & Geleijnse ML (2010). Alterations in left ventricular untwisting with ageing. Circ J 74, 101–108. [DOI] [PubMed] [Google Scholar]
- Dedobbeleer C, Hadefi A, Naeije R & Unger P (2013). Left ventricular adaptation to acute hypoxia: a speckle‐tracking echocardiography study. J Am Soc Echocardiogr 26, 736–745. [DOI] [PubMed] [Google Scholar]
- Dong SJ, Hees PS, Huang WM, Buffer SA, Weiss JL & Shapiro EP (1999). Independent effects of preload, afterload, and contractility on left ventricular torsion. Am J Physiol Heart Circ Physiol 277, H1053–H1060. [DOI] [PubMed] [Google Scholar]
- Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS, Leonelli FM & Knapp CF (2001). Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J Appl Physiol 91, 2611–2618. [DOI] [PubMed] [Google Scholar]
- Fu Q, Arbab‐Zadeh A, Perhonen MA, Zhang R, Zuckerman JH & Levine BD (2004). Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol Heart Circ Physiol 286, H449–H457. [DOI] [PubMed] [Google Scholar]
- Gibbons Kroeker CA, Tyberg JV & Beyar R (1995). Effects of load manipulations, heart rate, and contractility on left ventricular apical rotation. An experimental study in anesthetized dogs. Circulation 92, 130–141. [DOI] [PubMed] [Google Scholar]
- Gregoire J, Tuck S, Yamamoto Y & Hughson RL (1996). Heart rate variability at rest and exercise: influence of age, gender, and physical training. Can J Appl Physiol 21, 455–470. [DOI] [PubMed] [Google Scholar]
- Haykowsky M, Taylor D, Teo K, Quinney A & Humen D (2001). Left ventricular wall stress during leg‐press exercise performed with a brief Valsalva maneuver. Chest 119, 150–154. [DOI] [PubMed] [Google Scholar]
- Hodt A, Hisdal J, Stugaard M, Stranden E, Atar D & Steine K (2011). Reduced preload elicits increased LV twist in healthy humans. Clin Physiol Funct Imaging 31, 382–389. [DOI] [PubMed] [Google Scholar]
- Kuo TB, Lin T, Yang CC, Li CL, Chen CF & Chou P (1999). Effect of aging on gender differences in neural control of heart rate. Am J Physiol Heart Circ Physiol 277, H2233–H2239. [DOI] [PubMed] [Google Scholar]
- Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W & Voigt J‐U (2015). Recommendations for cardiac chamber quantification by echocardiography in Adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28, 1–39.e14. [DOI] [PubMed] [Google Scholar]
- Lawton JS, Cupps BP, Knutsen AK, Ma N, Brady BD, Reynolds LM & Pasque MK (2011). Magnetic resonance imaging detects significant sex differences in human myocardial strain. BioMed Eng OnLine 10, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold G (1986). Balanced pharmacokinetics and metabolism of bisoprolol. J Cardiovasc Pharmacol 8, S16–S20. [DOI] [PubMed] [Google Scholar]
- Lyon AR, Rees PS, Prasad S, Poole‐Wilson PA & Harding SE (2008). Stress (Takotsubo) cardiomyopathy—a novel pathophysiological hypothesis to explain catecholamine‐induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med 5, 22–29. [DOI] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C & Wallin BG (1985). Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 57, 461–469. [DOI] [PubMed] [Google Scholar]
- Marongiu E, Piepoli M, Milia R, Angius L, Pinna M, Bassareo P, Roberto S, Tocco F, Concu A & Crisafulli A (2013). Effects of acute vasodilation on the hemodynamic response to muscle metaboreflex. Am J Physiol Heart Circ Physiol 305, H1387–H1396. [DOI] [PubMed] [Google Scholar]
- Martin WH, Spina RJ, Korte E & Ogawa T (1991). Effects of chronic and acute exercise on cardiovascular beta‐adrenergic responses. J Appl Physiol 71, 1523–1528. [DOI] [PubMed] [Google Scholar]
- Moon MR, Ingels NB, Daughters GT, Stinson EB, Hansen DE & Miller DC (1994). Alterations in left ventricular twist mechanics with inotropic stimulation and volume loading in human subjects. Circulation 89, 142–150. [DOI] [PubMed] [Google Scholar]
- Mori H, Ishikawa S, Kojima S, Hayashi J, Watanabe Y, Hoffman JI & Okino H (1993). Increased responsiveness of left ventricular apical myocardium to adrenergic stimuli. Cardiovasc Res 27, 192–198. [DOI] [PubMed] [Google Scholar]
- Nishiyasu T, Tan N, Morimoto K, Nishiyasu M, Yamaguchi Y & Murakami N (1994). Enhancement of parasympathetic cardiac activity during activation of muscle metaboreflex in humans. J Appl Physiol 77, 2778–2783. [DOI] [PubMed] [Google Scholar]
- Notomi Y, Popovic ZB, Yamada H, Wallick DW, Martin MG, Oryszak SJ, Shiota T, Greenberg NL & Thomas JD (2007). Ventricular untwisting: a temporal link between left ventricular relaxation and suction. Am J Physiol Heart Circ Physiol 294, H505–H513. [DOI] [PubMed] [Google Scholar]
- O'Leary DS (1993). Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol 74, 1748–1754. [DOI] [PubMed] [Google Scholar]
- Rademakers FE, Buchalter MB, Rogers WJ, Zerhouni EA, Weisfeldt ML, Weiss JL & Shapiro EP (1992). Dissociation between left ventricular untwisting and filling. Accentuation by catecholamines. Circulation 85, 1572–1581. [DOI] [PubMed] [Google Scholar]
- Rademakers FE, Rogers WJ, Guier WH, Hutchins GM, Siu CO, Weisfeldt ML, Weiss JL & Shapiro EP (1994). Relation of regional cross‐fiber shortening to wall thickening in the intact heart. Three‐dimensional strain analysis by NMR tagging. Circulation 89, 1174–1182. [DOI] [PubMed] [Google Scholar]
- Ramaekers D, Ector H, Aubert AE, Rubens A & Van de Werf F (1998). Heart rate variability and heart rate in healthy volunteers. Is the female autonomic nervous system cardioprotective? Eur Heart J 19, 1334–1341. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Goldberger AL, Pincus SM, Mietus J & Lipsitz LA (1994). Gender‐ and age‐related differences in heart rate dynamics: are women more complex than men? J Am Coll Cardiol 24, 1700–1707. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS & Sinoway LI (2001). Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol 281, H2028–H2035. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Mattar L, Kerbeci P, Trotter S, Arbeille P & Hughson RL (2007). WISE 2005: stroke volume changes contribute to the pressor response during ischemic handgrip exercise in women. J Appl Physiol 103, 228–233. [DOI] [PubMed] [Google Scholar]
- Stembridge M, Ainslie PN, Hughes MG, Stohr EJ, Cotter JD, Nio AQX & Shave R (2014). Ventricular structure, function, and mechanics at high altitude: chronic remodeling in Sherpa vs. short‐term lowlander adaptation. J Appl Physiol 117, 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stembridge M, Ainslie PN, Hughes MG, Stöhr EJ, Cotter JD, Tymko MM, Day TA, Bakker A & Shave RE (2015). Impaired myocardial function does not explain reduced left ventricular filling and stroke volume at rest or during exercise at high altitude. J Appl Physiol 19, 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohr EJ, Gonzalez‐Alonso J & Shave R (2011). Left ventricular mechanical limitations to stroke volume in healthy humans during incremental exercise. Am J Physiol Heart Circ Physiol 301, H478–H487. [DOI] [PubMed] [Google Scholar]
- Stöhr EJ, McDonnell B, Thompson J, Stone K, Bull T, Houston R, Cockcroft J & Shave R (2012). Left ventricular mechanics in humans with high aerobic fitness: adaptation independent of structural remodelling, arterial haemodynamics and heart rate. J Physiol 590, 2107–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorstensen A, Dalen H, Amundsen BH & Stoylen A (2011). Peak systolic velocity indices are more sensitive than end‐systolic indices in detecting contraction changes assessed by echocardiography in young healthy humans. Eur J Echocardiogr 12, 924–930. [DOI] [PubMed] [Google Scholar]
- Turner MJ, Mier CM, Spina RJ, Schechtman KB & Ehsani AA (1999). Effects of age and gender on the cardiovascular responses to isoproterenol. J Gerontol A Biol Sci Med Sci 54, B393–400, discussion B401–403. [DOI] [PubMed] [Google Scholar]
- van Dalen BM, Vletter WB, Soliman OII, Cate ten FJ & Geleijnse ML (2008). Importance of transducer position in the assessment of apical rotation by speckle tracking echocardiography. J Am Soc Echocardiogr 21, 895–898. [DOI] [PubMed] [Google Scholar]
- Victor RG, Bertocci LA, Pryor SL & Nunnally RL (1988). Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest 82, 1301–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner RB, DeLuca JR, Wang F, Lin J, Wasfy MM, Berkstresser B, Stöhr E, Shave R, Lewis GD, Hutter AM, Picard MH & Baggish AL (2015). Exercise‐induced left ventricular remodeling among competitive athletes: a phasic phenomenon. Circ Cardiovasc Imaging 8, e003651. [DOI] [PubMed] [Google Scholar]
- Weiner RB, Hutter AM, Wang F, Kim J, Weyman AE, Wood MJ, Picard MH & Baggish AL (2010a). The impact of endurance exercise training on left ventricular torsion. JACC Cardiovasc Imaging 3, 1001–1009. [DOI] [PubMed] [Google Scholar]
- Weiner RB, Weyman AE, Khan AM, Reingold JS, Chen‐Tournoux AA, Scherrer‐Crosbie M, Picard MH, Wang TJ & Baggish AL (2010b). Preload dependency of left ventricular torsion: the impact of normal saline infusion. Circ Cardiovasc Imaging 3, 672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner RB, Weyman AE, Kim JH, Wang TJ, Picard MH & Baggish AL (2012). The impact of isometric handgrip testing on left ventricular twist mechanics. J Physiol 590, 5141–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, Shave RE, Stembridge M & Eves ND (2016). Females have greater left ventricular twist mechanics than males during acute reductions to preload. Am J Physiol Heart Circ Physiol 311, H76–H84. [DOI] [PubMed] [Google Scholar]


