Abstract
Myocardial remuscularization can be achieved by cardiomyocyte implantation. Electromechanical integration and long‐term survival of cardiomyocyte grafts are essential for maximal therapeutic impact. Cardiomyocyte application with support material has been instrumental in enhancing cell retention. Co‐administration of pro‐survival factors and immunological matching are additional strategies for increased cell graft survival. Finally, larger cardiomyocyte grafts, although therapeutically desirable, will increase the risk for arrhythmias and, if pluripotent stem cells are used to derive cardiomyocytes, tumour formation. This review introduces major challenges pertaining to myocardial remuscularization (cardiomyocyte retention, arrhythmogenicity and tumourigenicity), discusses studies addressing these challenges, and suggests strategies to overcome remaining challenges for the translation of myocardial remuscularization.
Keywords: cardiomyocyte, heart failure, regeneration, stem cell, tissue engineering
Introduction
In the United States and the European Union more than 10 million individuals live with heart failure (Nichols et al. 2014; Mozaffarian et al. 2015). High mortality and no therapeutic options for myocardial remuscularization create an unmet need for advanced regenerative therapies. Strategies to directly address the underlying cause of heart failure, namely cardiomyocyte loss, include the activation of hibernating endogenous repair mechanisms and the reinforcement of the failing myocardium by cardiomyocyte implantation (Fig. 1). Despite exciting experimental results, targeted activation of endogenous progenitor cells or (myo)fibroblast‐to‐cardiomyocyte conversion in situ and controlled reintroduction of cell cycle activity in post‐mitotic adult cardiomyocytes have not yet been realized. A better understanding of the fundamental mechanisms underlying the loss of regenerative potential as well as cell cycle withdrawal in cardiomyocytes shortly after birth will be pivotal to targeting endogenous repair mechanisms in a cell type‐specific manner.
Figure 1. Modes of heart repair.
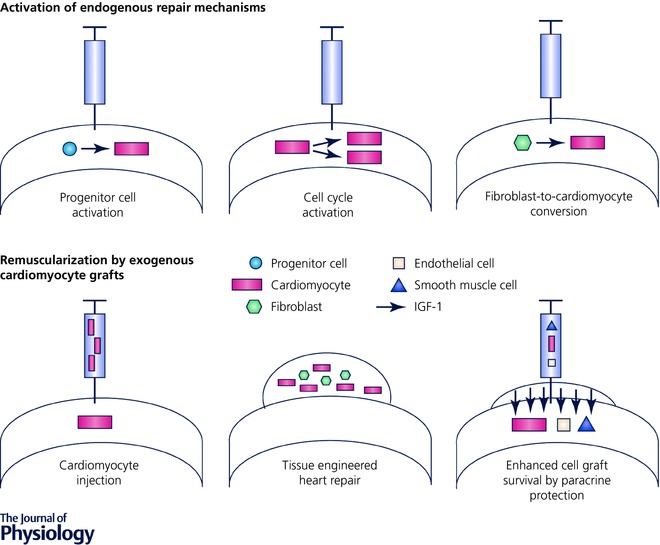
Schematic overview of strategies aiming at the remuscularization of the failing heart either by activation of endogenous mechanisms or by cardiomyocyte supplementation.
Remuscularization of the heart by cardiomyocyte implantation follows a simple strategy – cardiomyocyte loss is addressed by implantation of an equivalent amount of exogenously produced cardiomyocytes (Fig. 1). A major milestone towards this goal, particularly with respect to the production of human cardiomyocytes, was met by the introduction of human pluripotent stem cells (Thomson et al. 1998; Takahashi et al. 2007) and the means to direct their differentiation towards bona fide cardiomyocytes (Burridge et al. 2012). Subsequently, scalability to address medical needs was demonstrated (Chen et al. 2015; Riegler et al. 2015). Remaining challenges pertain to (1) suboptimal cell retention and functional integration, (2) the risk of arrhythmia induction, and (3) tumour formation. Whether a certain degree of maturity of cell grafts needs to be reached remains a matter of contention. Despite several open questions, there is compelling evidence for the principle feasibility of myocardial remuscularization by the implantation of immature cardiomyocytes, stemming either from rodent allograft models (Klug et al. 1996; Shimizu et al. 2006; Zimmermann et al. 2006; Didié et al. 2013) or from human xenografts in rat (Riegler et al. 2015), guinea pig (Shiba et al. 2012; Weinberger et al. 2016), and macaque (Chong et al. 2014) models. Collectively these data allow for guarded optimism regarding the translatability of myocardial remuscularizartion either by intramyocardial cardiomyocyte injection or by epicardial implantation of tissue‐engineered heart muscle patches.
Experimental approaches to enhanced remuscularization of the heart
The assumption is that 1 billion cardiomyocytes are lost in patients experiencing a haemodynamically relevant myocardial infarction (Gepstein, 2002). Consequently, it has been reasoned that for optimal therapeutic impact matching numbers of cardiomyocytes need to be engrafted and electromechanically integrated (Chong et al. 2014). This has not been achieved to date. The use of survival cocktails comprising extracellular matrix produced by the Engelbreth‐Holm‐Swarm tumour, also known as Matrigel™, apoptosis inhibitors ZVAD (benzyloxycarbonyl‐Val‐Ala‐Asp(O‐methyl)‐fluoromethyl ketone) and Bcl‐XL BH4, cyclosporine A, insulin‐like growth factor‐1 (IGF‐1) and pinacidil (Laflamme et al. 2007) as well as tissue engineering technologies have improved long‐term cardiomyocyte retention (Riegler et al. 2015). Matrix–cell combinations appear particularly advantageous with demonstrated long‐term cardiomyocyte retention of up to 25% (average 10%) of the engrafted cells for as long as 12 weeks by application via collagen‐based engineered heart muscle (Riegler et al. 2015). Similar cell retention has been observed with the co‐injection of cardiomyocytes, endothelial cells, and smooth muscle cells derived from induced pluripotent stem cells together with the co‐administration of IGF‐1 via an overlying epicardial patch (Ye et al. 2014). Regardless of these advances, further improvements are needed for optimal therapeutic efficacy according to the proposed mechanism of action, i.e. contractile support by electromechanically integrated cardiomyocyte grafts. Of note, the data collectively suggest that cardiomyocyte survival rates of as little as 10% may already have a therapeutic effect on the failing heart (Ye et al. 2014; Riegler et al. 2015; Qin et al. 2016; Shiba et al. 2016; Weinberger et al. 2016), which may be the result of paracrine effects, mechanical stabilization (according to the law of Laplace), and functional remuscularization or a combination of any or all of these mechanisms.
It seems plausible that increasing the engrafted muscle mass will not only improve efficacy, but also increase the risk for arrhythmia development and tumour, namely teratoma or teratocarcinoma, formation. Studies in clinically highly relevant macaque models confirmed that 1 billion pluripotent stem cell‐derived cells comprising 70–80% cardiomyocytes can cause ventricular tachycardia (Chong et al. 2014; Shiba et al. 2016). Importantly, tumour formation has not been reported, even in long‐term studies (> 200 days in nude rats) with tissue‐engineered grafts comprising 5 million cardiomyocytes (Riegler et al. 2015). It can be anticipated that stringent quality control measures to exclude tumourigenic cells in cardiomyocyte grafts will further reduce the potential risk of tumour formation.
Finally, terminal differentiation of cardiomyocyte grafts appears to be supported by both engineered and recipient heart muscle environments. Reported differences in cell cycle activity in cardiomyocyte grafts in situ (12% in Weinberger et al. 2016 and < 1% in Riegler et al. 2015) may stem from the use or omission of Matrigel, which is commonly used in oncology research to support tumour cell engraftment and growth in immunocompromised rodent models (Benton et al. 2011). Matrigel‐supplemented collagen type I was first introduced in the tissue engineering of heart muscle from neonatal rat ventricular cardiomyocytes (Zimmermann et al. 2000). This protocol was subsequently adapted for the use in human heart muscle engineering (Tulloch et al. 2011). More than 40% of the human cardiomyocytes in this mixed collagen type I/Matrigel hydrogel model displayed evidence of ongoing DNA replication by BrdU incorporation (Tulloch et al. 2011); whether this can be related to bona fide cardiomyocyte proliferation (cytokinesis) or multi‐nucleation by karyokinesis without cell division requires further analyses. In a recently optimized defined Matrigel‐ and serum‐free collagen formulation, DNA synthesis was observed in only ∼5% of the human cardiomyocyte and non‐myocyte populations by Ki67 labelling (Tiburcy et al. 2017). The rate of active DNA replication was further reduced to ∼1% 1 month after implantation (Riegler et al. 2015). Low DNA replication (< 1%/year), as reported for adult human heart (Bergmann et al. 2015), appears advantageous from a safety perspective.
Future strategies for enhanced therapeutic efficacy of cardiomyocyte grafts
Despite some evidence for efficacy of tissue‐engineered and directly injected cardiomyocyte grafts in rat (Zimmermann et al. 2006, Riegler et al. 2015; Qin et al. 2016), mouse (Didié et al. 2013), guinea pig (Weinberger et al. 2016) and macaque (Shiba et al. 2016) models, there is a clear need for an improvement of graft size. In fact, recent studies demonstrated consistently the dependency of therapeutic impact on cardiomyocyte dosing in rat (Maass et al. 2017) and graft size in macaque (Shiba et al. 2016) allograft models. The latter study highlights the need for the assessment of therapeutic efficacy in same‐species graft‐recipient (allograft) models if functional remuscularization by electromechanical integration is considered the primary mechanism of action. Xenograft studies are, despite evidence for electrical coupling in vitro and in vivo (Kehat et al. 2004), limited by the mismatch of cardiovascular physiology and immunological responses. For example, it appears unlikely that a mismatch of heart rate and the genetically encoded differences in excitation–contraction coupling will enable sustained electro‐mechanical integration in xenograft models under clinically acceptable immune suppression (calcineurin inhibition by cyclosporine or tacrolimus, antiproliferative agents mycophenolat mofetil or azathioprine, and corticoids). This is exemplified by two recent studies with the demonstration of anti‐arrhythmic and pro‐arrhythmic effects of human cardiomyocyte grafts in guinea pig (Shiba et al. 2012) and macaque (Chong et al. 2014) models, respectively. Of note, the anticipated pro‐arrhythmic effects of cardiomyocyte grafts was recently confirmed in a macaque model (Shiba et al. 2016). Untameable immune responses have so far been insurmountable obstacles to the xenografting of living organs (heart, kidney, lung, liver) and it appears unlikely that immune suppression beyond the current clinical state‐of‐the‐art or, for example, genome editing, at its present state of development, to reduce immunogenicity will be acceptable for the clinical translation of myocardial remuscularization. Conversely, paracrine or passive mechanical effects may contribute to the therapeutic effects of tissue grafts. Paracrine effects could be studied well in xenograft models if the paracrine mechanisms were evolutionarily conserved. In line with a paracrine or mechanical contribution of tissue‐engineered constructs to heart regeneration, we have observed small therapeutic effects by non‐contractile (Zimmermann et al. 2006) or human xenografts (Riegler et al. 2015) in rats. These effects were, however, clearly less pronounced compared to the therapeutic effects observed in recipients of cardiomyocyte‐containing allografts (Zimmermann et al. 2006; Didié et al. 2013).
Major histocompatibility complex (MHC)‐matching of allografts was demonstrated to be instrumental for cardiomyocyte graft survival even under immune suppression with tacrolimus and methylprednisolone (Shiba et al. 2016). In patients, MHC‐matching may be achievable via access to the Bone Marrow Donors Worldwide (BMDW) registry that includes > 17 million human leukocyte antigen (HLA)‐typed volunteer stem cell donors. The utility of the BMDW registry for HLA‐matching within the UK has recently been interrogated (Taylor et al. 2012). In this registry, 236 matching HLA‐haploidentical donors were identified that provided a zero mismatch to 93% of the UK population; 50 matching HLA‐haploidentical donors provided a zero mismatch to 79% of the putative recipients. An even simpler approach to obtain HLA‐haploidentical cells may be by the use of parthenogenetic stem cells (Revazova et al. 2008). If derived from unfertilized meiosis II oocytes it appears that ∼75% of the parthenogenetic stem cells exhibit HLA‐haploidentity, making the prospective sourcing of haploidentical pluripotent stem cells a relatively easy task (e.g. 60 000 unfertilized oocytes are discarded annually in Germany). The utility of parthenogenetic stem cells in myocardial remuscularization without the need for immune suppression (only methylprednisolone was administered to control foreign body reactions) was demonstrated recently in mice (Didié et al. 2013). Given the demonstrated pluripotency in human parthenotes it should be straightforward to generate cardiomyocytes and tissue‐engineered human myocardium thereof using established protocols.
Finally, control of arrhythmias will be equally important for optimal efficacy and safety. This may be achieved by the development of electromechanically quiescent grafts composed mainly of ventricular cardiomyocytes, simultaneous applications of antiarrhythmic drugs or the use of pacemaker/defibrillation devices for graft–host synchronization. Large animal and patient studies are needed to fully determine, and if needed develop strategies to reduce, a potential arrhythmogenic burden associated with myocardial remuscularization. In addition, assessment of optimal dosing and timing of human epicardial tissue graft implantation will be important endpoints in first‐in‐patient studies.
Conclusion
Myocardial remuscularization has been realized in small and large animal models. Therapeutic efficacy will depend on cardiomyocyte retention and proper electromechanical integration. Presently, maximal retention rates of 10–25% of the cardiomyocyte grafts have been reported with evidence for safety and efficacy. Enhanced cardiomyocyte retention can be realized by making use of a co‐administration of pro‐survival factors and tissue engineering approaches. Combinations of both may be useful to further increase cardiomyocyte survival and enhance efficacy. Electrical integration will occur as long as donor–recipient contact can be firmly established. The process of electrical integration will likely be accompanied by an increased risk of ventricular arrhythmias as confirmed in the recent pioneering xeno‐ and allograft studies in the macaque model (Chong et al. 2014; Shiba et al. 2016). Accordingly, means to control rate and rhythm of the cardiomyocyte grafts will have to be established for clinical translation. As to this end, concomitant device therapy appears more suitable than pharmacological control of rate and rhythm, which may, especially in immature cardiomyocytes, enhance rather than reduce arrhythmic burden.
Tumour formation appears to be less of a concern if cardiomyocyte grafts can be defined well and produced according to stringent quality control criteria. Whether cardiomyocyte graft immaturity is good or bad cannot be answered at the present time. Immature cardiomyocytes appear to be metabolically less demanding, but also electrically less stable. Several studies have demonstrated consistently that cardiomyocytes mature upon implantation and become indistinguishable morphologically and functionally from the recipient heart's cardiomyocytes (Rubart et al. 2003; Didié et al. 2013). Low DNA replication appears advantageous from a safety point of view.
A first clinical study investigates the application of cardiac progenitor cells derived from human embryonic stem cells (Menasche et al. 2015). These progenitor cells are defined by their SSEA1 and Isl1 expression. Enhanced retention and localization of the progenitor cell graft is achieved by making use of a fibrin patch. In this study, the aim is not to directly remuscularize the heart, but to exploit paracrine support to stabilize disease progression and potentially activate endogenous repair mechanisms. In future studies, based on existing preclinical evidence, remuscularization will be attempted clinically by intramyocardial injection of cardiomyocytes and tissue‐engineered heart repair (Fig. 1). As in any type of pharmacological therapy, it will be essential to define safe and efficacious doses and an optimal timing (acute or chronic, early or advanced disease condition) for cardiomyocyte‐based remuscularization. This will finally require carefully designed clinical studies. Late preclinical animal studies will have to focus primarily on safety to inform the design of the anticipated first‐in‐patient studies.
Additional information
Competing interests
W.H.Z. is co‐founder and advisor of Repairon GmbH.
Funding
W.H.Z. is supported by the DZHK (German Center for Cardiovascular Research), the German Federal Ministry for Science and Education (BMBF FKZ 13GW0007A [CIRM‐ET3]), the German Research Foundation (DFG ZI 708/10‐1; SFB 937 TP18, SFB 1002 TPs C04, S1; IRTG 1618 RP12), the European Union FP7 CARE‐MI, the Foundation Leducq, and the NIH (U01 HL099997).
Biography
Wolfram‐Hubertus Zimmermann, MD, is the Director of the Institute of Pharmacology and Toxicology at the University Medical Center of the Georg‐August‐University in Göttingen, Germany. He studied Medicine and Molecular Biology at the University Medical Center Hamburg‐Eppendorf with clinical rotations in Cardiology and Cardio‐Thoracic Sugery at Duke University Medical School, Harvard Medical School and the University of Cape Town. He trained at the Institutes of Pharmacology and Toxicology at the Friedrich‐Alexander University in Erlangen and the University Medical Center Hamburg‐Eppendorf. His research interests include tissue engineered heart repair and stem cell‐based models for drug development as well as disease modelling with a focus on heart failure.

References
- Benton G, Kleinman HK, George J & Arnaoutova I (2011). Multiple uses of basement membrane‐like matrix (BME/Matrigel) in vitro and in vivo with cancer cells. Int J Cancer 128, 1751–1757. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andra M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H & Frisen J (2015). Dynamics of cell generation and turnover in the human heart. Cell 161, 1566–1575. [DOI] [PubMed] [Google Scholar]
- Burridge PW, Keller G, Gold JD & Wu JC (2012). Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 10, 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen VC, Ye J, Shukla P, Hua G, Chen D, Lin Z, Liu JC, Chai J, Gold J, Wu J, Hsu D & Couture LA (2015). Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res 15, 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA & Murry CE (2014). Human embryonic‐stem‐cell‐derived cardiomyocytes regenerate non‐human primate hearts. Nature 510, 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didié M, Christalla P, Rubart M, Muppala V, Döker S, Unsöld B, El‐Armouche A, Rau T, Eschenhagen T, Schwoerer AP, Ehmke H, Schumacher U, Fuchs S, Lange C, Becker A, Tao W, Scherschel JA, Soonpaa MH, Yang T, Lin Q, Zenke M, Han D‐W, Schöler HR, Rudolph C, Steinemann D, Schlegelberger B, Kattman S, Witty A, Keller G, Field LJ & Zimmermann W‐H (2013). Parthenogenetic stem cells for tissue‐engineered heart repair. J Clin Invest 123, 1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepstein L (2002). Derivation and potential applications of human embryonic stem cells. Circ Res 91, 866–876. [DOI] [PubMed] [Google Scholar]
- Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz‐Eldor J & Gepstein L (2004). Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol 22, 1282–1289. [DOI] [PubMed] [Google Scholar]
- Klug MG, Soonpaa MH, Koh GY & Field LJ (1996). Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest 98, 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J & Murry CE (2007). Cardiomyocytes derived from human embryonic stem cells in pro‐survival factors enhance function of infarcted rat hearts. Nat Biotechnol 25, 1015–1024. [DOI] [PubMed] [Google Scholar]
- Maass M, Krausgrill B, Eschrig S, Kaluschke T, Urban K, Peinkofer G, Plenge TG, Oeckenpohler S, Raths M, Ladage D, Halbach M, Hescheler J & Muller‐Ehmsen J (2017). Intramyocardially transplanted neonatal cardiomyocytes show structural and electrophysiological maturation and integration and dose‐dependently stabilize function of infarcted rat hearts. Cell Transplant 26, 157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Guillemain R, Suberbielle Boissel C, Tartour E, Desnos M & Larghero J (2015). Human embryonic stem cell‐derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J 36, 2011–2017. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee (2015). Heart disease and stroke statistics–2015 update: a report from the American heart association. Circulation 131, e29–e322. [DOI] [PubMed] [Google Scholar]
- Nichols M, Townsend N, Scarborough P & Rayner M (2014). Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J 35, 2950–2959. [DOI] [PubMed] [Google Scholar]
- Qin X, Riegler J, Tiburcy M, Zhao X, Chour T, Ndoye B, Nguyen M, Adams J, Ameen M, Denney TS Jr, Yang PC, Nguyen P, Zimmermann WH & Wu JC (2016). Magnetic resonance imaging of cardiac strain pattern following transplantation of human tissue engineered heart muscles. Circ Cardiovasc Imaging 9, e004731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revazova ES, Turovets NA, Kochetkova OD, Agapova LS, Sebastian JL, Pryzhkova MV, Smolnikova VI, Kuzmichev LN & Janus JD (2008). HLA homozygous stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells 10, 11–24. [DOI] [PubMed] [Google Scholar]
- Riegler J, Tiburcy M, Ebert A, Tzatzalos E, Raaz U, Abilez OJ, Shen Q, Kooreman NG, Neofytou E, Chen VC, Wang M, Meyer T, Tsao PS, Connolly AJ, Couture LA, Gold JD, Zimmermann WH & Wu JC (2015). Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ Res 117, 720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubart M, Pasumarthi KB, Nakajima H, Soonpaa MH, Nakajima HO & Field LJ (2003). Physiological coupling of donor and host cardiomyocytes after cellular transplantation. Circ Res 92, 1217–1224. [DOI] [PubMed] [Google Scholar]
- Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE & Laflamme MA (2012). Human ES‐cell‐derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489, 322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, Ido D, Shiina T, Ohkura M, Nakai J, Uno N, Kazuki Y, Oshimura M, Minami I & Ikeda U (2016). Allogeneic transplantation of iPS cell‐derived cardiomyocytes regenerates primate hearts. Nature 538, 388–391. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, Kikuchi A, Kobayashi E & Okano T (2006). Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J 20, 708–710. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K & Yamanaka S (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. [DOI] [PubMed] [Google Scholar]
- Taylor CJ, Peacock S, Chaudhry AN, Bradley JA & Bolton EM (2012). Generating an iPSC bank for HLA‐matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell 11, 147–152. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz‐Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS & Jones JM (1998). Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. [DOI] [PubMed] [Google Scholar]
- Tiburcy M, Hudson JE, Balfanz R, Schlick S, Meyer T, Chang Liao ML, Levent E, Raad F, Zeidler S, Wingender E, Riegler J, Wang M, Gold JD, Kehat I, Wettwer E, Ravens U, Dierickx P, van Laake LW, Goumans MJ, Khadjeh S, Toischer K, Hasenfuss G, Couture LA, Unger A, Linke WA, Araki T, Neel B, Keller K, Gepstein L, Wu JC & Zimmermann WH (2017). Defined engineered human myocardium with advanced maturation for applications in heart failure modelling and repair. Circulation 135, 1832–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H & Murry CE (2011). Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res 109, 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger F, Breckwoldt K, Pecha S, Kelly A, Geertz B, Starbatty J, Yorgan T, Cheng KH, Lessmann K, Stolen T, Scherrer‐Crosbie M, Smith G, Reichenspurner H, Hansen A & Eschenhagen T (2016). Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci Transl Med 8, 363ra148. [DOI] [PubMed] [Google Scholar]
- Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS, Guo J, Jang A, Rosenbush D, Greder L, Dutton JR, Zhang J, Kamp TJ, Kaufman DS, Ge Y & Zhang J (2014). Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell‐derived cardiovascular cells. Cell Stem Cell 15, 750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann WH, Fink C, Kralisch D, Remmers U, Weil J & Eschenhagen T (2000). Three‐dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol Bioeng 68, 106–114. [PubMed] [Google Scholar]
- Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H & Eschenhagen T (2006). Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med 12, 452–458. [DOI] [PubMed] [Google Scholar]


