Abstract
Insulin resistance is a well-known risk factor for obesity, metabolic syndrome (MetSyn) and associated cardiovascular diseases, but its mechanisms are undefined in the lymphatics. Mesenteric lymphatic vessels from MetSyn or LPS-injected rats exhibited impaired intrinsic contractile activity and associated inflammatory changes. Hence, we hypothesized that insulin resistance in lymphatic muscle cells (LMCs) affects cell bioenergetics and signaling pathways that consequently alter contractility. LMCs were treated with different concentrations of insulin or glucose or both at various time points to determine insulin resistance. Onset of insulin resistance significantly impaired glucose uptake, mitochondrial function, oxygen consumption rates, glycolysis, lactic acid, and ATP production in LMCs. Hyperglycemia and hyperinsulinemia also impaired the PI3K/Akt while enhancing the ERK/p38MAPK/JNK pathways in LMCs. Increased NF-κB nuclear translocation and macrophage chemoattractant protein-1 and VCAM-1 levels in insulin-resistant LMCs indicated activation of inflammatory mechanisms. In addition, increased phosphorylation of myosin light chain-20, a key regulator of lymphatic muscle contraction, was observed in insulin-resistant LMCs. Therefore, our data elucidate the mechanisms of insulin resistance in LMCs and provide the first evidence that hyperglycemia and hyperinsulinemia promote insulin resistance and impair lymphatic contractile status by reducing glucose uptake, altering cellular metabolic pathways, and activating inflammatory signaling cascades.—Lee, Y., Fluckey, J. D., Chakraborty, S., Muthuchamy, M. Hyperglycemia- and hyperinsulinemia-induced insulin resistance causes alterations in cellular bioenergetics and activation of inflammatory signaling in lymphatic muscle.
Keywords: metabolism, lymphatic contractility, insulin signaling, glycolysis, signal transduction
Insulin resistance is a hallmark of metabolic diseases and has recently emerged as the major link between non–insulin-dependent diabetes mellitus, metabolic syndrome (MetSyn), and cardiovascular disease (1, 2). Insulin resistance, defined as reduced sensitivity of tissues toward insulin, impairs insulin action, glucose tolerance, and metabolism of target cells or whole organisms. Direct causes of insulin resistance are still not fully identified, but it is known that prolonged hyperglycemia and hyperinsulinemia result in insulin resistance in both humans and animal models (3–7).
The lymphatic system is composed of a network of thin-walled capillaries, collecting vessels and nodes that run parallel to the blood vascular system. Excess extracellular fluid is absorbed from the interstitium by the initial lymphatics by passive drainage and then becomes lymph. The capillaries are composed of a single layer of endothelial cells without a continuous basement membrane and are tethered to the interstitium through anchoring filaments (8, 9). Unlike blood vessels, lymphatics typically lack pericytes; however, a subset of blind-ended lymphatic capillaries that lack the lymphatic marker have been shown to express the pericyte marker (10). From lymphatic capillaries, lymph flows through progressively larger precollecting and collecting lymphatic vessels, lymph nodes, and postnodal (efferent) lymphatic vessels. The collecting lymphatic vessels are also surrounded by muscle cells; the functional units within the muscular collecting lymphatic vessels, called lymphangions, are arranged in series and separated by highly competent valves that facilitate unidirectional lymph flow. The extrinsic lymph pump relies on compression and expansion of the lymphatics by external forces that arise in the surrounding tissue to generate the pressure gradients needed for flow. In the intrinsic lymph pump, flow through a lymphatic bed is generated by coordinated contractions of the lymphatic muscle cells (LMCs), and these contractions are initiated by pacemaker activity located within the muscle layer of the lymphatic wall. The LMCs are a unique combination of striated and smooth muscle components that enable the rapid tonic and phasic contractions of lymphatic vessels (11–13).
The lymphatic system, with its intrinsic physiologic functions in cellular homeostasis, immune cell trafficking, and lipid absorption (11–13), contributes significantly to the pathogenesis of metabolic disorders (14–17). We have demonstrated that lymphatic vessels from MetSyn rats exhibit impaired intrinsic contractile activity (18, 19). Several studies have also underscored this important link and demonstrated that obesity or related metabolic disorders impair lymphatic contractility in both mice and humans (20–22). In addition, LPS, a triggering factor for insulin resistance, has been shown to alter immune cell response in the vicinity of lymphatic vessels and to impair contractility (23). However, the underlying cellular mechanisms that may contribute to lymphatic contractile dysfunction during onset and progression of insulin resistance in lymphatic muscle remain unknown.
Insulin affects various physiologic processes, including cell survival, proliferation, differentiation, and metabolism, by stimulating key players, such as insulin receptor substrates (IRSs)-1, PI3K, and protein kinase B/Akt (24–26). Defects in insulin signaling pathways result in insulin resistance by 1) enhanced phosphorylation or degradation of IRS proteins (26–28), 2) suppression of PI3K (29, 30), 3) increased phosphatase activities [i.e., phosphate and tensin homolog (PTEN) (31, 32)], and 4) suppression of downstream signaling through Akt (5, 33). Consequently, they impair glucose transport into the cells by disrupting glucose transporter action, glycogen synthesis, and bioenergy production. Furthermore, Akt signaling is necessary for blood vessel dilation via myosin phosphatase–targeting protein and myosin light chain (MLC)-20, whereas impaired Akt phosphorylation in diabetic animal is associated with constricted vessel (34–37). In addition, insulin stimulates the ERKs p38MAPK and JNK, which promote cellular proliferation and inflammation (38–40). ERK phosphorylation is also associated with regulation of MLC20 phosphorylation, a key regulator of lymphatic contractility (41). Hence, we hypothesized that insulin resistance would impair glucose uptake and metabolism in LMCs and would modulate the PI3K/Akt and ERK/p38/JNK pathways, coupled with MLC20 phosphorylation.
In the present study, we determined the effects of insulin resistance induced by prolonged hyperglycemia and hyperinsulinemia in LMCs. We examined glucose uptake, and alterations in cellular bioenergetics (mitochondrial function, glycolysis, and intracellular bioenergy production) in LMCs. We also identified key changes in the insulin signaling pathways, such as PI3K/Akt and ERK/p38MAPK/JNK pathways, and identified critical inflammatory mediators in response to insulin resistance in LMCs. In our experiments, hyperglycemia and hyperinsulinemia induced insulin resistance in LMCs by decreasing glucose uptake, selectively impairing the PI3K/Akt pathway and activating the EKR/p38MAPK/JNK pathway. In addition, insulin resistance impaired cell metabolism, activated inflammatory signaling, and increased MLC20 phosphorylation.
MATERIALS AND METHODS
Materials
Phospho-MLC20 (Ser19), phospho-p38 MAPK, p-38MAPK, phospho-ERK1/2, ERK1/2, phospho-Akt (Thr308), phospho-Akt (Ser473), Akt, β-actin, phospho-glycogen synthase kinase (GSK)-3β (Ser9), phospho-IRS1 (Ser1101), IRS1, phospho-IκB, phosphor JNK, JNK, and PI3Kp85, were purchased from Cell Signaling Technology (Danvers, MA, USA). Glucose transporter (GLUT)-4, and VCAM-1 were obtained from Abcam (Cambridge, MA, USA). PTEN was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Total MLC20, p65NF-κB, insulin (I12643), carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP; C2920), oligomycin, rotenone (R8875), and glucose were purchased from Sigma-Aldrich (St. Louis, MO, USA). 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxyglucose (2-NBDG; 11046) and deoxyglucose were purchased from Cayman Chemical (Ann Arbor, MI, USA). DMEM, fetal bovine serum (FBS), trypsin, triple antibiotics (penicillin, streptomycin, and Amphotericin B), Prolong Gold antifade mounting medium with DAPI, dihydroethidium (DHE), MitoSox dye, and wheat germ agglutinin (WGA) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). All other chemicals and reagents were from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture and treatments
The primary rat mesenteric LMCs were obtained from mesenteric tissue explants of male Sprague-Dawley rats, as we have described in other publications (41, 42). Cells from passages 2–4 were used in all the experiments. LMCs were cultured in DMEM, containing 10% FBS, and 1% triple antibiotics and maintained at 37°C in a 10% CO2 incubator. LMCs were plated in 24-well culture plates and then grown to ∼70–80% confluence. The cells were serum starved for 24 h and treated with 5 mM glucose (control) or high glucose (HG; 25 mM), with or without insulin (50, 100, and 150 nM) at various time points (from 20 min to 72 h), to determine the onset of insulin resistance. After time and dosage of insulin were determined by assaying for glucose uptake, they were kept constant throughout the study. Possible effects of osmolality on glucose uptake and insulin signaling were tested by treating LMCs with mannose (25 mM), using the different treatment conditions described above.
Glucose transport assay
Glucose transport was assessed by measuring 2-NBDG uptake in LMCs, as modified from another study (43). For measuring glucose uptake, 3 separate LMC plates (in triplicate) were used in each experiment for each plate per sample. To determine the optimum duration for 2-NBDG incubation, we initially measured 2-NBDG uptake in LMCs at various time points, from 20 min to 6 h (n = 6–9/group). Results demonstrated that the maximum uptake of 2-NBDG in LMCs was at 4 h of incubation (data not shown). Hence, in our experiments, cells were incubated with 2-NBDG (150 μg/ml) in glucose/sodium-free DMEM for 4 h. At the end of the 2-NBDG treatment, the plate was centrifuged for 5 min at 400 g at room temperature. The supernatants were removed, and cell-based assay buffer was added to each well. After centrifugation for 5 min at 400 g at room temperature, the supernatants were removed, and cell-based assay buffer was added. The amount of 2-NBDG taken up by LMCs was measured spectrometrically at 485- and 535-nm wavelengths.
Mitochondrial bioenergetics
The XF24 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA, USA) was used to measure the rate changes in extracellular flux of dissolved O2 and protons (44, 45). In brief, the optimum number of LMCs per well was determined to be 20 × 103 (0.32 cm2) before the experiments began. The LMCs were seeded in XF 24-well microplates at 20 × 103 cells/well in 200 μl DMEM with 10% FBS at 37°C in a 10% CO2 incubator for 24 h. The LMCs were treated as described earlier (n = 5/group). The medium was replaced with assay medium with corresponding treatment (i.e., glucose or insulin) for 1 h before the beginning of the assay at 37°C. The oxygen consumption rates (OCRs) were measured with the following sequential reagents: oligomycin (5 μM, mitochondrial complex V inhibitor), FCCP (1 μM, an uncoupling agent), and rotenone (5 μM, mitochondrial complex I inhibitor). On completion of the assay, the number of cells was counted on an automated cell counter (Thermo Fisher Scientific), for normalization of the values. All the OCRs are expressed in picomoles per minute of oxygen consumed. Reserve capacity was calculated as the difference between basal OCR and that obtained in the presence of FCCP. Maximum respiratory capacity was calculated as the difference in OCR between rotenone and FCCP.
Cellular glycolysis analysis
Cellular glycolytic rates were estimated by measuring extracellular acidification rate (ECAR) as described earlier (46). LMCs were plated in XF 24-well microplates. LMCs were glucose-starved in assay medium in a CO2-free XF prep station at 37°C for 1 h followed by treatment, as described above (n = 5/group). Differences in basal, maximum, and spare glycolytic capacity were measured by sequential injection of glucose (100 mM), oligomycin (1 μM), and 2DG (500 mM). Values were normalized by number of cells and were represented, as milli-pH units per minute. Spare glycolytic capacity was determined as the difference between basal ECAR and that obtained in the presence of oligomycin.
Intracellular ATP content measurement
Intracellular ATP content was measured to determine whether insulin resistance in LMCs has any effect on cellular energy production. After the treatments (n = 9/group), the LMCs were collected, and intracellular ATP contents were determined by using an ATP assay kit (Abcam), according to the manufacturer’s instructions. ATP was measured in triplicate and normalized according to the number of cells.
Lactate measurement
LMCs (0.8 × 105 cells) were plated in 60-mm dishes in DMEM with 10% FBS. The LMCs were treated as described earlier (n = 6/group). Cumulative lactate content was measured at the end of the 24 h incubation period directly from culture medium (5 μl), with a commercial Lactate Plus Analyzer (Nova Biomedical, Waltham, MA, USA) (47, 48). The equipment was initially calibrated using standard lactate concentration solution (1.6, 6.5, 10, 18, and 22 mM). The lactate concentration of the DMEM without cells was found to be less than <0.3 mM for all 4 conditions (control, control+insulin, HG, HG+insulin). At the end of the measurements, the number of cells was counted on an automated cell counter. The final lactate contents (millimolar per 105cells per hour) were used to determine the differences between groups.
Protein isolation and Western immunoblot analysis
Protein expression was quantified by Western analysis (23, 41). In brief, LMC protein lysates were prepared from LMCs and separated onto a 4–20% gradient SDS-polyacrylamide gel and transferred, and Western blot analysis was performed. The following dilutions of the primary antibodies were used: p-Akt1 (Ser473), p-Akt1 (Thr308), p-Akt2 (Thr474), p-IRS (Ser1101), IRS, p-GSK (Ser9), PTEN, GLUT4, PI3K (p85), p-MLC20, VCAM-1, β-actin (1:1000) and MLC20 (1:5000). After a Tris-buffered saline wash in triplicate, membranes were incubated with the appropriate horseradish peroxidase–conjugated secondary antibodies. Protein detection was conducted with an ultrasensitive enhanced chemiluminescence system and visualized via image processor Fuji LAS-4000 Mini (GE Healthcare Bio-Science, Pittsburgh, PA, USA). β-Actin expression was used as the loading control. Densitometry analyses on the resulting bands were determined with the ImageJ [National Institutes of Health (NIH), Bethesda, MD, USA]. For quantification, experiments were repeated 4 times for each sample, and the resulting means ± sem were calculated (n = 4/group).
Immunofluorescence
Immunofluorescence experiments were performed as described earlier, with some modifications (49), to determine GLUT4 and NF-κB localization. In brief, the treated LMCs were fixed with 2% paraformaldehyde. For cell membrane staining, WGA (1:200) dye was applied for 10 min and then rinsed with PBS 3 times. Cells were permeabilized, blocked, and incubated with the corresponding primary antibody GLUT4 or NF-κB (1:100). Normal rat serum was used in place of the corresponding primary antibody as a control. Cells were incubated with fluorescence-conjugated secondary antibody and mounted in ProLong antifade solution containing DAPI (Thermo Fisher Scientific). An LSM 780 NLO Multiphoton microscope (Zeiss, Oberkochen, Germany) Plan-Apo ×40 [numeric aperture (NA) = 1.4, oil] was used for imaging. Average projections of series sections with a step size of 0.5-μm thickness were imaged and quantified with the NIH ImageJ plug-in colocalization analysis program. In brief, the images were first converted into 8-bit gray-scale images and 2 points in the images were considered colocalized according to the following criteria: 1) respective pixel intensities (0–255) were greater than 50.0, the threshold of their channels, and 2) the ratio of intensity was higher than the setting ratio (i.e., 50%). The colocalization regions were highlighted with a white overlay mask on the original red, green, blue images. Then, the generated colocalized images were used to analyze area and pixel intensity.
Intracellular superoxide anion measurement
To evaluate reactive oxygen species (ROS) production in live cells, we used the redox-sensitive fluorescence dye DHE (50, 51). DHE (25 μM) was added to LMCs after treatments and incubated at 37°C for 5 min in the dark. After washing 3 times with PBS, DHE images were taken with a Olympus BX41 fluorescence microscope using UplanApo ×20 objective (NA = 0.7) (Olympus America, Melville, NY, USA) at an excitation peak of 545 nm with an emission spectral peak of 610 nm. Bright-field images were also taken in identical position where DHE-stained images were captured to visualize the cell’s orientation and outline.
Detection of mitochondrial superoxide anion
MitoSOX red, a mitochondrial superoxide indicator, was used in live LMCs. After treatments, cells were loaded with MitoSox (5 μM) for 10 min at 37°C. Cells were then washed 3 times with PBS and MitoSox images were taken with an Olympus BX41 fluorescence microscope, with a UPlanFLN ×40 objective (NA = 1.3) at an excitation peak of 545 nm with an emission spectral peak of 610 nm. Mitochondrial ROS signal was measured with ImageJ.
Cytokine analysis in LMCs by real-time PCR
LMCs were plated in 24-well culture plates and then grown to ∼70–80% confluence. The cells were serum starved for 24 h and treated as previously described. Total RNA was extracted as described earlier (49). The quality and quantity of RNAs were determined with a spectrophotometer (ND-1000; NanoDrop Technologies, Wilmington, DE, USA). From each sample, 100 ng of total RNAs were reverse transcribed with a Superscript III cDNA synthesis kit (Thermo Fisher Scientific). The cDNA was mixed with SYBR Green PCR master mix (Thermo Fisher Scientific). Primers specific to IL-1β, IL-6, iNOS, TNF-α, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-2, and IL-8 were designed as indicated in our previous studies (49, 52). RPL19 was used as an internal loading control. The differences in the relative expression of cytokines between groups were calculated by using the 2−ΔΔCt method.
Statistical analysis
Glucose uptake analyses for different time and dose were performed with 1-way ANOVA. Mean differences were compared with control LMCs by Fisher’s least-significant difference (LSD) post hoc test (SPSS19.0; IBM Corp., Armonk, New York, USA). All other data were analyzed by 2-way ANOVAs followed by the Bonferroni post hoc test. If there were any significant interactions (glucose × insulin) or main effects, mean difference between test groups were assessed using 1-way ANOVA with Fisher’s LSD post hoc test, where significant interaction was found among the 2 main effects, glucose, and insulin. All values are expressed as means ± se. The significance level was set at P = 0.05. All graphs were generated with Prism 5 (GraphPad Software, La Jolla, CA, USA).
RESULTS
Acute insulin treatment activated insulin signaling and increased glucose uptake and intracellular ATP content in LMCs
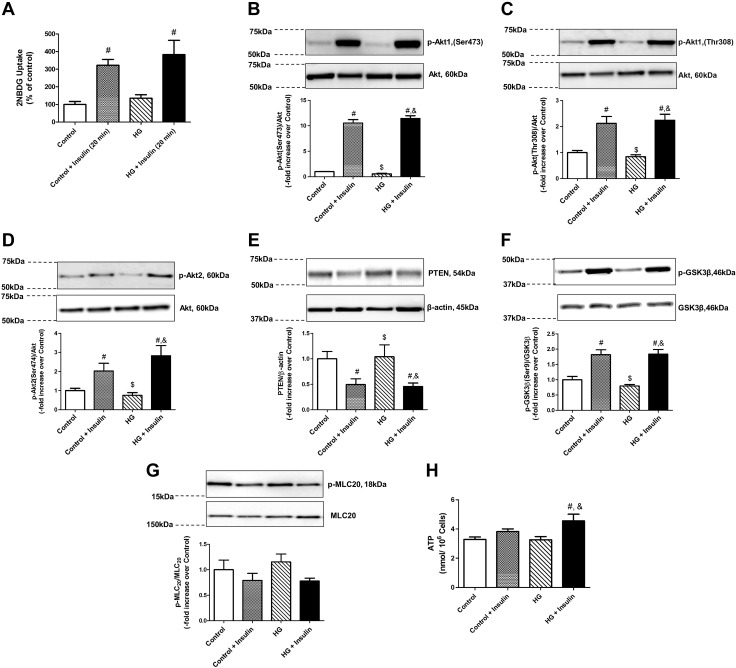
We first tested the effect of acute insulin on glucose uptake by the LMCs. We observed (Fig. 1) an increase in glucose uptake in LMCs grown under control or HG conditions after a 20-min insulin treatment (322.37 ± 32.7 and 382.5 ± 81.38%, control+INS and HG+INS, respectively, compared to control, 100 ± 17.05%; P < 0.005). Next, we examined the expression of key insulin signaling pathway molecules (i.e., PI3K, Akt1, and Akt2). Both Akt1 (Ser473 and Thr308) and Akt2 (Ser474) phosphorylations were significantly elevated in acute insulin-treated LMCs (Fig. 1B–D). Furthermore, PTEN, a negative regulator of Akt, showed a significant decrease in insulin-treated LMCs (Fig. 1E). Increased GSK3β phosphorylation, a key downstream effector molecule, mediating glycogen synthesis by targeting glycogen synthase, confirmed activation of the insulin signaling pathway in LMCs treated with insulin (Fig. 1F). We have also observed an increased Akt1 Ser473, Akt1 Thr208, and GSK3β phosphorylation at earlier time points (5, 10, and 15 min), indicating an early activation of insulin signaling pathway in LMCs, which was found to peak at 20 min (data not shown). We next wanted to test whether activation of insulin signaling modulates MLC20 phosphorylation. No significant effects of insulin or glucose treatments on MLC20 phosphorylation levels were observed (Fig. 1G). To examine the effects of activated insulin signaling on cellular metabolism, we measured intracellular ATP content. Our results clearly show that insulin treatment during hyperglycemia increased intracellular ATP content (+0.38-fold vs. control; P < 0.05) in comparison with control LMCs (Fig. 1H). However, there was no significant elevation of intracellular ATP content in acute HG- and insulin-treated LMCs when compared with control cells treated with insulin.
Figure 1.
Acute insulin treatment activated the insulin signaling pathway and increased glucose uptake and intracellular ATP production in LMCs. A) Glucose uptake was measured using 2-NBDG in cultured LMCs treated with HG (25 mM), insulin (100 nM), or both for 20 min (n = 4/group). B–G) LMCs were treated with insulin, and representative Western blots are shown for Akt1 (Ser473) phosphorylation (B), Akt1 (Thr308) phosphorylation (C), Akt2 phosphorylation (D), PTEN (E), GSK3β (Ser9) phosphorylation (F), and MLC20 phosphorylation (G). The relative expression of p-Akt1 (Ser473)/Akt, p-Akt1(Thr308)/Akt, p-Akt2/Akt, PTEN/β-actin, p-GSK3β/total GSK-3β, and p-MLC20/MLC20 were quantified and plotted (n = 4/group). H) Intracellular ATP production was determined in LMCs after 20 min treatment with HG, insulin, or both in LMCs (n = 6–9/group). Data are means ± se. #P < 0.05 vs. control; $P < 0.05 vs. control+insulin; &P < 0.05 vs. HG.
Prolonged hyperglycemia and hyperinsulinemia developed insulin resistance in LMCs
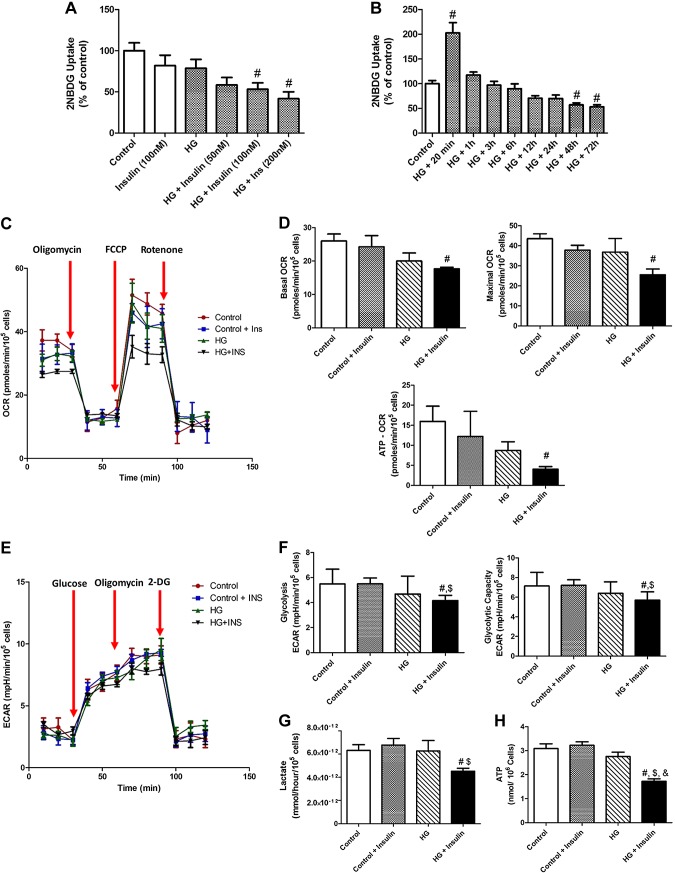
One of the hallmarks of insulin resistance is impairment of insulin sensitivity that reduces glucose uptake into peripheral tissues or cells (3). Thus, we directly measured glucose uptake level using fluorescence-labeled 2-NBDG to determine whether prolonged hyperinsulinemia or hyperglycemia induces insulin resistance in LMCs. Results demonstrate that glucose uptake was significantly decreased by insulin in a dose-dependent manner in the presence of HG for 48 h in LMCs (Fig. 2A). Next, we examined various time points (20 min and 1, 3, 6, 12, 24, 28, and 72 h) to identify when elevated glucose uptake by acute insulin treatment (Fig. 1A) was abolished or further decreased. As shown in Fig. 2B, an increased glucose uptake by acute insulin treatment was decreased to the basal level at 1 h after insulin treatment (202.8 ± 20.57 vs.117.3 ± 6.28%; P < 0.001, respectively). At 48 h or longer HG and insulin treatment, the cells showed a significant reduction (57.1 ± 4.08%; P < 0.04) of glucose uptake (Fig. 2B). This result provides critical information as to when LMCs start developing effective insulin resistance during chronic hyperglycemia and hyperinsulinemia. Hence, we selected 100 nM insulin for 48 h for generating insulin-resistant LMCs. To further rule out any possible effects of osmolarity on glucose uptake, we first determined the osmolarity changes caused by glucose concentrations in the DMEM and compared with 25 mM mannose-containing medium, with or without insulin. Following is the osmolarity of each solution (mOsmol/kg): control, 325 ± 15; control+insulin, 325.1 ± 15; HG, 335 ± 15; HG+insulin, 335.1 ± 12; mannose, 345 ± 20; and mannose+insulin, 345.1 ± 20. There was no significant difference in glucose uptake in 48 h mannose-treated LMCs (in the absence or presence of insulin) when compared to control LMCs (control, 100 ± 12.6 and 87.7 ± 15.1; mannose, 110.8 ± 22.2 and 118.0 ± 9.2, respectively), suggesting that osmolarity changes related to 20 mM glucose concentration did not affect glucose uptake.
Figure 2.
Prolonged hyperglycemia and hyperinsulinemia developed insulin resistance and impaired cell metabolisms in LMCs. A, B) Glucose uptake was measured using 2-NBDG in LMCs in different insulin concentrations (50–200 nM) in low-glucose or HG conditions for 48 h (n = 9/group) (A) and various time points (from 20 min to 72 h) in HG (25 mM)– and insulin (100 nM)–treated LMCs (n = 6–9/group) (B). C, D) Mitochondrial function in LMCs is assessed by detecting OCR. C) Graphic representation showing real-time analysis of mitochondrial OCRs in HG- or insulin-treated LMCs (n = 5/group). Arrows: points where oligomycin, FCCP, or rotenone are applied that represent basal oxygen OCR, maximum OCR, spare respiratory capacity, and ATP linked. D) Basal, maximum, and ATP-linked OCR were quantified and plotted. E, F) Glycolysis and maximum glycolytic capacity were assessed by measuring ECAR. E) Real-time analysis of ECARs in LMCs after 48 h HG and insulin treatments (n = 5/group). Arrows: glucose, oligomycin, and 2DG application F) Glycolysis and maximum glycolytic capacity were quantified and plotted. G) Lactate level was measured in HG- and/or insulin-treated LMCs (n = 6/group). H) Intracellular ATP content was measured in HG- and/or insulin-treated LMCs. Data are means ± se. #P < 0.05 vs. control; $P < 0.05 vs. control+insulin; &P < 0.05 vs. HG.
Decreased mitochondrial respiration, glycolysis, and intracellular ATP content in insulin-resistant LMCs
As shown in Fig. 2C, D, there was a significant decrease in basal (−0.32-fold vs. control; P < 0.027) and maximum (−0.41-fold vs. control; P < 0.01) OCRs in LMCs in hyperglycemia- and hyperinsulinemia-induced insulin resistance; whereas, LMCs grown under HG or hyperinsulinemic conditions showed similar levels of basal and maximum respiration (Fig. 2D). The difference between basal OCR and the OCR after oligomycin exposure was the rate that was responsible for mitochondrial ATP production. There were no significant differences in control group LMCs and in HG LMCs, but there was a significant decrease in ATP-coupled OCRs in insulin-resistant LMCs (Fig. 2D; −0.75-fold vs. control; P < 0.049). We further determined the glycolytic capacity by monitoring ECARs in LMCs (Fig. 2E). Both glycolysis and glycolytic capacity were significantly reduced in insulin-resistant LMCs when compared to the control groups (Fig. 2F; −0.24-fold vs. control, P < 0.05 and −0.20-fold vs. control; P < 0.05, respectively). However, there were no significant differences between HG- and HG+insulin-treated LMCs. To further test glycolytic flux, we measured lactate excretion (Fig. 2G). In HG+insulin-treated LMCs, there was a significant decrease in lactate level (4.52E-12 mM/h/105 cells) compared to control (6.31E-12 mM/h/105 cells; P < 0.05) and control+insulin (6.77E-12 mM/h/105 cells; P < 0.016) groups. There was no significant difference between HG- and HG+insulin-treated groups. In addition, as shown in Fig. 2H, intracellular ATP content was significantly decreased in insulin-resistant LMCs (−0.56-fold vs. control; P < 0.001) compared to all other groups.
Hyperglycemia- and hyperinsulinemia-induced insulin resistance increased oxidative stress
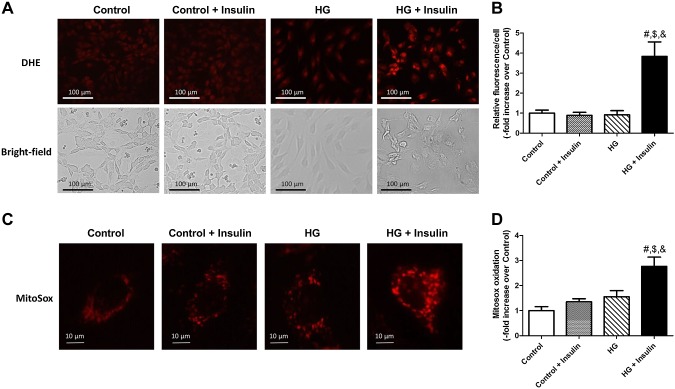
Because increased oxidative stress is strongly associated with insulin resistance in various tissues, we examined both intracellular superoxide anion (O2·−) using DHE and mitochondrial ROS using MitoSox. DHE oxidation was significantly increased in HG+insulin-treated LMCs (+2.8-fold vs. control; P < 0.001) when compared to all other groups (Fig. 3A, B). Insulin-resistant LMCs displayed a higher level of mitochondrial O2·− (+1.7-fold vs. control; P < 0.0001) compared to all other groups (Figs. 3C, D). There was no glucose or insulin effect on mitochondrial ROS.
Figure 3.
Insulin resistance increased intracellular and mitochondrial O2·−. A) Representative DHE and corresponding bright-field images in LMCs treated for 48 h with glucose, insulin, or both. Images were obtained with a ×20 objective (NA = 0.7) on a fluorescence microscope. B) Fluorescence intensity of individual cells was measured and quantified as relative fluorescence. Data from 7 images per group were analyzed (n = 7/group). C) Representative MitoSox oxidation images in LMCs treated for 48 h with glucose, insulin, or both. Images were obtained with ×40 objective (NA = 1.3) on a fluorescence microscope. D) Relative fluorescence of individual cells was quantified. Each cell exhibited 8–100 red fluorescence signal in the mitochondria or adjacent region where indicated by oxidized MitoSox dye. Each signal particle was analyzed and averaged per cell. A total of 15 cells were quantified in 5 different experiments (n = 15/group).
Hyperglycemia- and hyperinsulinemia-induced insulin resistance selectively impaired insulin dependent PI3K and Akt signaling and activated ERK/p38MAPK/JNK signaling pathways
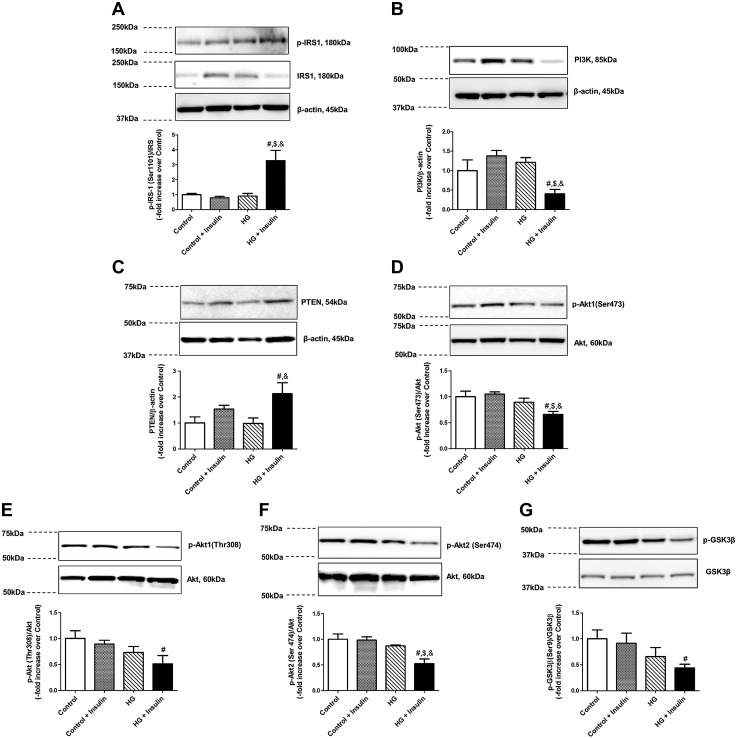
To further investigate the detailed molecular mechanisms underlying insulin resistance–induced impairment of LMC metabolism, we analyzed the effect of key insulin-signaling molecules, PI3K/Akt and ERK/MAPK in HG- and/or insulin-treated LMCs. IRS1 phosphorylation (Ser1101) was significantly increased by HG and insulin treatments (+2.27-fold vs. control, P < 0.01; Fig. 4A). PI3K was found to be significantly decreased in insulin-resistant LMCs (−0.6-fold vs. control; P < 0.033) compared with all other groups (Fig. 4B). PTEN, a negative regulator of Akt was significantly increased only in the HG+insulin-treated group compared with the control (+1.12-fold vs. control; P < 0.013) or HG-treated LMCs (+1.14-fold vs. HG; P < 0.012), but not in control and insulin-treated LMCs (Fig. 4C). Akt1 phosphorylation (Ser473) was down-regulated in HG+insulin-treated LMCs (−0.34-fold vs. control; P < 0.007) when compared to all other groups (Fig. 4D). Akt1 phosphorylation (Thr473) was only significantly decreased in insulin-resistant LMCs compared to control LMCs (−0.49-fold vs. control; P < 0.021; Fig. 4E). As shown in Fig. 4F, Akt2 phosphorylation was also significantly down-regulated in HG+insulin-treated LMCs when compared to all other groups. GSK3β phosphorylation, one of the key downstream effectors of Akt, was significantly reduced in HG+insulin-treated LMCs (−0.56-fold vs. control; P < 0.029) compared to the control groups (Fig. 4G). To evaluate any possible osmolarity effects on insulin signaling, levels of Akt and GSK3β phosphorylation were measured in 25 mM mannose (345 ± 15 mOsml/kg)-treated LMCs; no significant difference was found between control and mannose-treated LMCs (data not shown).
Figure 4.
Insulin resistance selectively impaired insulin-dependent PI3K and Akt signaling in LMCs. Representative Western blots show IRS (Ser1101) phosphorylation (A), PI3K (B), PTEN (C), Akt (Ser473) phosphorylation (D), Akt (Thr308) phosphorylation (E), Akt2 (Ser474) phosphorylation (F), and GSK3β (Ser9) phosphorylation response (G) of LMCs treated for 48 h with HG, insulin, or both. The relative expression of p-IRS/total-IRS, PI3K/β-actin, PTEN/β-actin, p-Akt (Ser473)/total Akt, p-Akt (Thr308)/Akt, p-Akt2 (Ser474)/total Akt, and p-GSK3β (Ser9)/total GSK3β were quantified and plotted (n = 4/group). Data are means ± se. #P < 0.05 vs. control; $P < 0.05 vs. control+insulin; &P < 0.05 vs. HG.
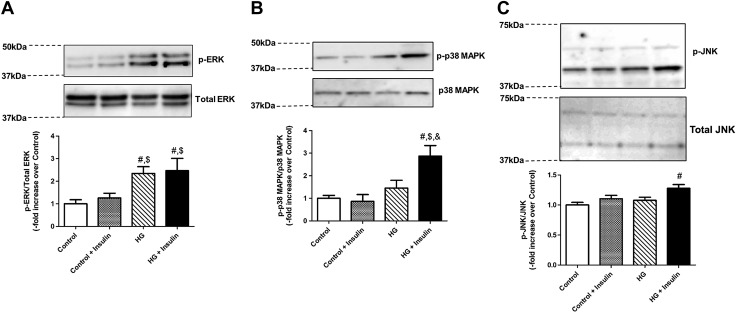
Because insulin resistance has been shown to modulate ERK/p38MAPK/JNK pathways in various cell types (53–55), we further examined the phosphorylation of ERK/p38MPAK/JNK in LMCs. As shown in Fig. 5A, a significant increase in p42/44 ERK phosphorylation levels was found in HG-treated LMCs compared to control-treated LMCs regardless of insulin treatment. In addition, p38MAPK and JNK phosphorylations were up-regulated only in HG+insulin-treated LMCs (+1.87 vs. control; P < 0.004 and +0.27-fold vs. control; P < 0.003, respectively) when compared to all other groups (Fig. 5B, C).
Figure 5.
Insulin resistance enhanced the ERK/p38MAPK/JNK pathway in LMCs. Representative Western blots showing ERK (A), p38MAPK (B), and JNK phosphorylation response (C) of LMCs treated for 48 h with HG, insulin, or both. The relative expression of p-ERK/ERK, p-p38MAPK/p38MAPK, and p-JNK/JNK was quantified and plotted (n = 4/group). Data are means ± se. #P < 0.05 vs. control; $P < 0.05 vs. control+insulin; &P < 0.05 vs. HG.
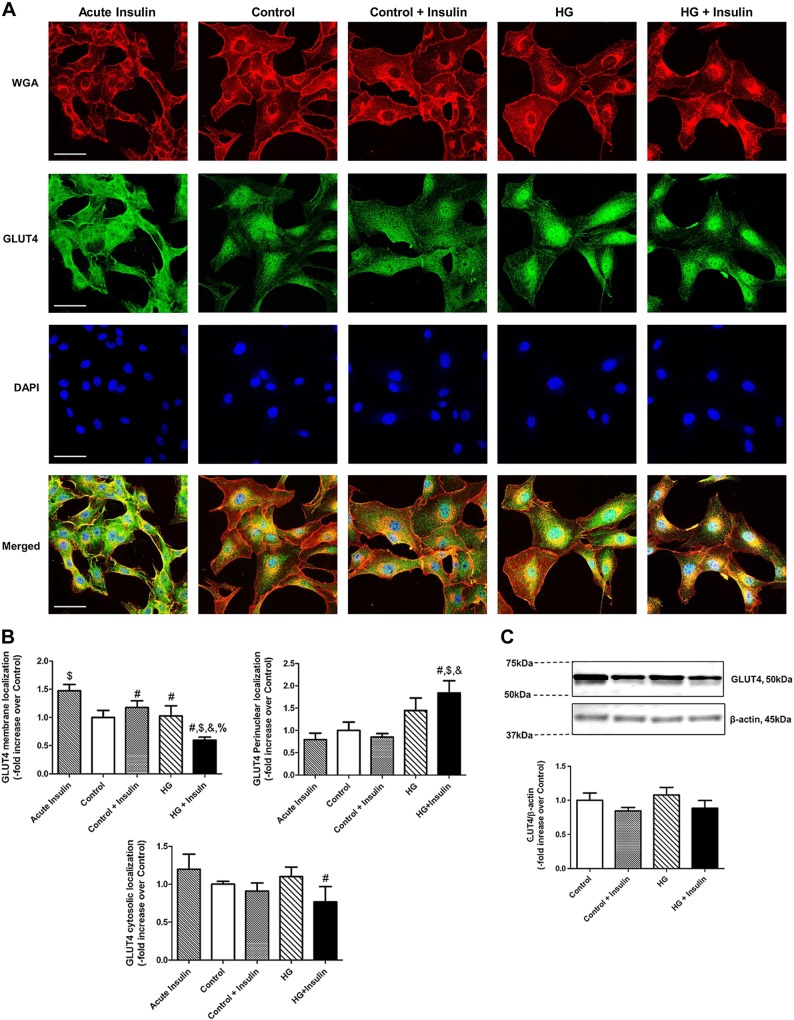
Hyperglycemia and hyperinsulinemia impaired GLUT4 membrane translocation and increased its subcellular localization in the perinuclear region of LMCs
We further examined the localization of GLUT4 in LMCs to determine its regulation by insulin. Acute insulin treatment induced robust GLUT4 membrane translocation (+0.47-fold vs. control; P < 0.015; Fig. 6A, B) in LMCs regardless of glucose concentration. In the control condition, GLUT4 mostly remained in the subcellular area, whereas HG treatment in LMCs induced more perinuclear GLUT4 localization. However, insulin treatment did not induce GLUT4 membrane translocation, as observed in response to acute insulin. In addition, insulin-resistant LMCs showed a significant decrease in membrane GLUT4 presence (−0.4-fold vs. control; P < 0.023) and an increase in perinuclear localization (+3.61-fold vs. control; P < 0.001; Figs. 6A, B). We also analyzed GLUT4 levels by Western blot; however, as expected, no significant difference was observed in the total GLUT4 protein expression between the HG and insulin treatment groups (Fig. 6C).
Figure 6.
Insulin resistance impaired GLUT4 membrane translocation in LMCs. A) Representative images of GLUT4, WGA, and DAPI in 20 min and 48 h HG- or insulin-treated LMCs. Images were obtained with a ×40 objective (NA = 1.4) on a multiphoton microscope. Scale bars, 50 μm. A minimum of 9 fields were quantified and averaged (n = 3/group). B) Quantification of membranous, perinuclear, and cytosolic GLUT4 in are plotted. C) GLUT4 protein expression was measured by Western blot analysis. Representative Western blot shows GLUT4 response of LMCs treated with HG, insulin, or both for 48 h. The relative expression of GLUT4/β-actin was quantified and plotted (n = 4/group). Data are means ± se. #P < 0.05 vs. acute insulin (20 min); $P < 0.05 vs. control; &P < 0.05 vs. control+insulin; %P < 0.05 vs. HG.
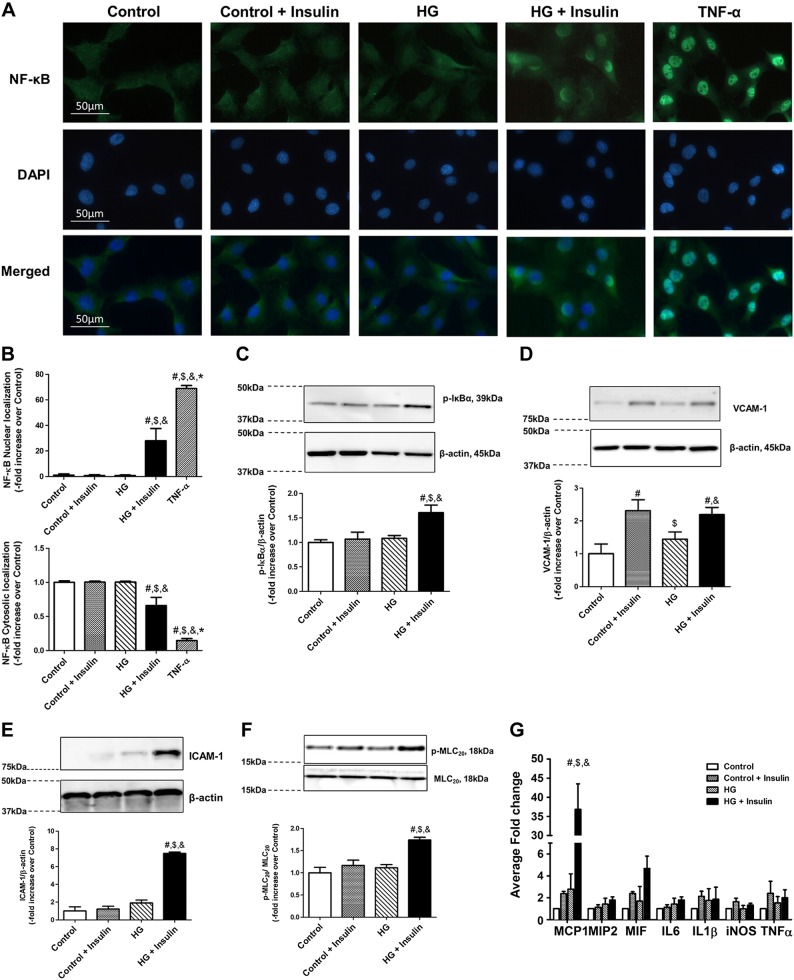
Insulin resistance activated NF-κB, inflammatory cell adhesion molecules, and cytokines, coupled with increased MLC20 phosphorylation in LMCs
Insulin resistance increased NF-κB nuclear translocation in LMCs (Fig. 7A, B). Nuclear translocation of NF-κB in TNF-α-treated LMCs is known to be a control for inflammatory stimulus; as expected, TNF-α-treated LMCs showed robust NF-κB nuclear translocation. Hyperglycemia and hyperinsulinemia did not induce strong NF-κB activation, as we observed in TNF-α-treated LMCs. However, we found significant elevation of NF-κB in insulin-resistant LMCs (+27.0-fold vs. control; P < 0.027), which may correspond with mild chronic inflammation in insulin-resistance disorders. This finding was also associated with increased p-IκBα in insulin-resistant LMCs (+0.6-fold vs. control; P < 0.002) as shown in Fig. 7C. VCAM-1, which is one of the key attractants for inflammatory leukocytes, was significantly up-regulated in insulin-treated LMCs (Fig. 7D), whereas the ICAM-1 level was dramatically up-regulated only in HG+insulin-treated LMCs (+6.4-fold vs. control; P < 0.0001; Fig. 7E). We also analyzed the inflammatory cytokines MCP1, IL6, TNF-α, iNOS, MIF, and MIP in the LMCs under the different treatment conditions, as described above. As shown in Fig. 7G, MCP1 was significantly induced in the HG+insulin conditions (+35.8-fold vs. control, P < 0.0001) compared to all other treated groups, whereas no significant difference was observed in the other cytokines between the different treatment conditions.
Figure 7.
Insulin resistance increased inflammatory signaling and MLC20 phosphorylation. A) Immunofluorescence images of activated NF-κB translocation into nucleus (DAPI) in LMCs treated with HG and insulin for 48 h using ×40 objective (NA = 1.3). TNF-α (20 ng/ml) was treated for 24 h as a positive control. B) Relative nuclear and cytoplasmic signal intensity in each cell was quantified. Six fields of view were used for data quantification from triplicate experiments (n = 6/group). C–F) Representative Western blots show IκB phosphorylation (C) VCAM-1 (D), ICAM-1 (E), and MLC20 phosphorylation response (F) of LMCs treated with HG, insulin, or both for 48 h. The relative expression of p-IκB/β-actin, VCAM-1/ β-actin, ICAM-1/β-actin, and p-MLC20/MLC20 were quantified and plotted (n = 4/group). G) Induction of inflammatory cytokine mRNA in insulin-resistant LMCs. Expression of MCP1, MIP2, MIF, IL6, IL1β, iNOS, and TNF-α was analyzed. Real-time quantification was performed and fold change relative to low-glucose control was calculated (n = 3/group). Data are means ± se. #P < 0.05 vs. control; $P < 0.05 vs. control+insulin; &P < 0.05 vs. HG.
Because MLC20 phosphorylation status is one of the critical determinants of lymphatic contractility (56, 57), we examined how hyperglycemia or hyperinsulinemia influence MLC20 phosphorylation in LMCs. MLC20 phosphorylation was significantly increased in HG+insulin conditions compared to the control (+0.74-fold vs. control; P < 0.001; Fig. 7F). However, neither HG nor insulin treatment alone showed any significant change in MLC20 phosphorylation in LMCs compared to the control (Fig. 7F).
DISCUSSION
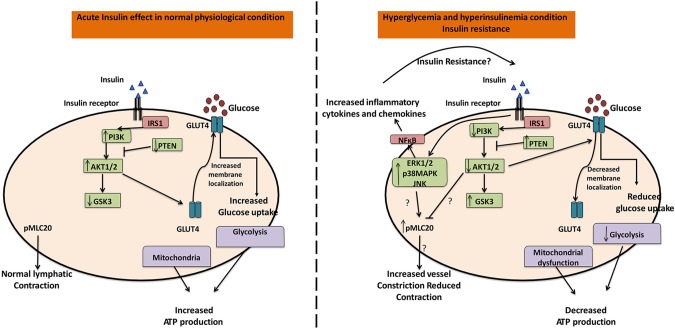
Insulin resistance mechanisms have been studied in several muscle types but remain undefined in the lymphatic muscle (58–61). In ventricular cardiomyocytes, hyperglycemia alone have been shown to induce insulin resistance by significantly decreasing glucose uptake and promoting myocyte dysfunction, even in low-insulin conditions (62, 63). In vascular smooth muscle cells, HG or insulin alone is sufficient to affect the glucose uptake and glucose transport activities, and differentially regulates the PI3K/Akt and MAPK/ERK pathways, respectively (64, 65). The results presented in this study provide the first evidence that both hyperglycemia and hyperinsulinemia are necessary for the onset of insulin resistance mechanisms in LMCs, and only HG or insulin conditions produce no significant effect. Our data also provide the first identification of alterations in cellular bioenergetics in insulin-resistant LMCs via mitochondrial dysfunction, decreased glycolytic capacity, and ATP content coupled with increased oxidative stress. Furthermore, hyperglycemia or hyperinsulinemia in LMCs significantly affect the insulin signaling pathway and its downstream effectors to modulate inflammation and contractile mechanisms (Fig. 8).
Figure 8.
Insulin resistance in LMC metabolism, inflammation, and contractility. In the normal physiologic condition, insulin stimulates glucose uptake via GLUT4 translocation downstream of activated PI3K/Akt signaling. The cellular energy required for LMC contractility is produced via glycolysis and mitochondria that maintain proper lymphatic vessel contraction with lymphatic vascular tone. However, insulin resistance inhibits the PI3K/Akt pathway and impairs GLUT4 translocation to the cellular membrane. Therefore, less glucose is transported into the LMCs. Insulin resistance further impairs glycolysis and mitochondrial function, thus reducing bioenergy (i.e., ATP). Impaired LMC metabolism and increased MLC20 phosphorylation by insulin resistance decreases lymphatic contraction. On the other hand, insulin resistance selectively activates the ERK/p38MAPK/JNK pathway, consequently activating proinflammatory signaling.
Acute insulin treatment indicated that activation of the insulin signaling pathway was necessary for regulating cellular glucose transport and bioenergy production. However, prolonged hyperglycemia and hyperinsulinemia decreased glucose uptake, indicating decreased insulin sensitivity in LMCs. Our data clearly demonstrate that both hyperglycemia (25 mM) and hyperinsulinemia (>100 nM) are necessary for generating insulin resistance in LMCs during 48 h treatment (Fig. 2A, B), which is consistent with the insulin resistance responses across different cell lines (59, 60). Although a direct causal relationship between oxidative stress and insulin resistance has not been clearly established, there is a significant evidence of a link between these 2 mechanisms in several metabolic diseases (66–68). Our data (Fig. 3) show that in HG+insulin-treated LMCs, there was an increase in both intracellular ROS and mitochondrial ROS that are consistent with a decrease in insulin sensitivity.
Muscle contraction, a crucial function of lymphatic muscle, is a highly energy-dependent process that relies on the production of ATP by mitochondria or by glycolysis (69, 70). Decreased basal, maximum, and ATP-linked OCRs in insulin-resistant LMCs (Fig. 2C, D) indicate severe mitochondrial dysfunction. The increased mitochondrial ROS in insulin-resistant LMCs further supports this notion. In addition, insulin-resistant LMCs exhibited a decrease in glycolysis, maximum glycolytic capacity, and ATP content (Fig. 2E–H), indicating that insulin resistance not only reduces intracellular glucose levels but also affects glycolytic function and cellular bioenergetics. Our results corroborate well with the findings from various insulin-resistant tissues or cell types, including human samples. HG-treated human mesenchymal stem cells from adipose tissue exhibited a decreased OCR in a time-dependent manner (71). Insulin-resistant cerebral arteries from obese rats exhibited impairments in mitochondrial functions, coupled with a decrease in mitochondrial proteins (72). Indeed, mitochondrial dysfunction is one of the key features in insulin-resistant human skeletal muscle (73, 74). Furthermore, it has been reported that impaired mitochondria in pancreatic β cells results in insulin resistance (75). In the present study, we found mitochondrial dysfunction coupled with an increase in mitochondrial oxidative stress. This result suggests that mitochondrial ROS is the major source of the ROS in insulin-resistant LMCs that further contribute to the pathology in insulin resistance. Further studies are warranted to test the direct causal relationship between mitochondrial dysfunction and insulin resistance in LMCs. Our data suggest that reduced intracellular energy in insulin-resistant LMCs can be attributed to the combination of reduced intracellular glucose availability and disrupted mitochondrial and glycolytic functions. We have previously demonstrated a decrease in the contractile frequency and force production of mesenteric lymphatic vessels from high-fructose diet–induced MetSyn rats (18), which showed an elevated fasting insulin level. Hence, we can speculate that an impaired metabolism and decreased intracellular energy production of LMCs contributes to the impairment of intrinsic lymphatic contractile function observed in MetSyn, which is a highly energy-dependent process.
Our data from insulin-resistant LMCs showed that increased IRS phosphorylation and consequently reduced PI3K activation, followed by dysregulation of Akt isoforms and signaling, are consistent with the insulin-PI3K-Akt pathways in various cell types (24–26, 30, 76, 77). In vascular smooth muscle cells, impaired Akt was associated with insulin resistance and decreased glucose synthesis and contractility that promote vascular disease in metabolic disorders (34). Furthermore, impaired Akt signaling in insulin resistance cardiomyocytes promotes cardiomyopathy via enhanced apoptosis and decreased myocyte contractility (78), whereas skeletal muscle showed protein synthesis impaired by decreased Akt in insulin resistance, contributing to pathologic muscle loss and sarcopenic obesity (77). Akt ablation also has been shown to directly lead to insulin resistance, independent of hyperglycemia or hyperinsulinemia (79–81). Given the unique characteristics of LMCs (42), we can speculate that impaired Akt signaling also induces contractile dysfunction and impaired glycogen synthesis and cellular energetics.
Akt signaling is also central to glucose uptake from the extracellular area to the cytosol via direct regulation of GLUT expression (82). In LMCs, acute insulin treatment showed more GLUT4 localized on the membrane, but in insulin-resistant LMCs, more GLUT4 translocated to the perinuclear regions (Fig. 6A, B), as reported in other cell types (83–85). GLUT4 protein levels were not affected by the different treatments in this study, and the decreased glucose uptake in insulin resistance could therefore be attributable to alterations in GLUT4 translocation that is mediated by an impaired insulin signaling pathway.
During insulin resistance, selective activation of the ERK/p-38MAPK/JNK pathway has also been described in vascular smooth muscle cells and other tissues (53, 54, 76, 86). Both hyperglycemia and insulin-resistant LMCs showed up-regulation of ERK (Fig. 5A), whereas p38MAPK and JNK were activated only in insulin-resistant LMCs (Fig. 5B, C). We have shown that inhibition of ERK by a specific ERK inhibitor, PD98059, directly affects MLC20 phosphorylation and regulation of contractile pathway in LMCs (23, 41). Hence, we presume that an increase in MLC20 phosphorylation (Fig. 7F) in response to insulin resistance in LMCs is related to an increase in ERK activation in these cells (Fig. 8). We have demonstrated a significant reduction in the intrinsic lymphatic pump capability of the mesenteric lymphatic vessel in MetSyn rats, as a result of significantly reduced phasic contraction frequency (18). The lymphatic vessels from MetSyn rats were smaller in diameter (constricted), but with no change in tone, and the lymphatic contractility indexes (frequency, lymphatic pump flow, and fractional pump flow) were significantly reduced. Our data in this present study have demonstrated an increase in MLC20 phosphorylation in insulin-resistant LMCs. Hence, we surmise that the constricted vessel we observed in MetSyn animal could be caused by an increase in MLC20 phosphorylation in LMCs. At this condition, one would expect that lymph filling would be decreased, which consequently decreases the phasic contractile frequency and the lymphatic pump flow in the MetSyn condition. In addition, we have shown that resident and innate immune cells, NO production, and expression of cytokines and chemokines modulate lymphatic function during MetSyn and lymphatic inflammation (23, 52). Our current studies on the complete profile of contractile and regulatory proteins of lymphatic vessels from MetSyn and the isolated vessels cultured in HG and insulin conditions and with inflammatory cytokines/chemokines would provide additional insights into the remodeling and function of lymphatics under these conditions.
Obesity and insulin resistance are associated with low-grade chronic systemic inflammation (87). We observed activation of NF-κB nuclear translocation and up-regulation of p-IκB levels in LMCs during hyperglycemia- and hyperinsulinemia-induced insulin resistance (Fig. 7A–C), suggesting an activation of downstream inflammatory response in insulin-resistant LMCs. In LPS-mediated lymphatic inflammation, we have observed a more severe impairment of lymphatic pumping activity (23) compared to vessels from MetSyn animals (18, 52). As lymphatic vessels are directly involved in both innate and adaptive immune responses, the lower inflammatory activation may explain the mild inflammation with impaired immunity in MetSyn relevant disease. Increase in the adhesion molecules VCAM-1 and ICAM-1, key attractants for inflammatory leukocytes, suggests that hyperinsulinemia and hyperglycemia set the stage for a more widespread inflammatory reaction. Data showing a significant induction of MCP1 in insulin-resistant LMCs (Fig. 7G) are an important finding, because elevated MCP1 has been shown to induce adipocyte differentiation and contribute to pathologies associated with hyperinsulinemia and obesity, including type II diabetes (88). Studies have also shown that insulin resistance is associated with increased macrophage infiltration that is mediated by MCP1 (89). We have shown that MCP1 is induced in LPS-treated LMCs and is a significant chemoattractant for M1 macrophage polarization in lymphatic vessels and adjacent areas in MetSyn animals (52). Therefore, MCP1 induction is a crucial precursor for more widespread inflammation during insulin resistance, through active recruitment of inflammatory macrophages. We also have shown that dietary endotoxin LPS-induced inflammation impairs lymphatic contractility and promotes innate immune cell modulation (i.e., neutrophils and macrophages) and related cytokines and chemokines in mesentery lymphatics (23). Hence, we propose that a chronic insulin resistance mechanism in lymphatic muscle would also promote chronic inflammatory stress via activation of the NF-κB pathway and inflammatory cytokines, altering cellular metabolism and an increase in pMLC20 via up-regulation of ERK would cause vessel constriction and impaired lymphatic contractility in MetSyn animals. However, further studies are warranted to carefully evaluate the role of insulin resistance in inflammation and specific chemokines and cytokines that may perpetuate the inflammatory signaling and contractile pathways in LMCs.
In summary, novel findings from the current study demonstrate that both hyperglycemia and hyperinsulinemia are necessary to promote insulin resistance in LMCs and this concision leads to impairment of cell metabolism via reduced glucose uptake, mitochondrial dysfunction, and decreased glycolytic capacity. Onset of insulin resistance blunts PI3K/Akt signaling and selectively enhances ERK/p38MAPK/JNK signaling pathway in LMCs. It also activates the MLC20 phosphorylation and inflammatory signaling in LMCs (Fig. 8). Impaired bioenergetics, contractile dysfunction, and inflammatory signaling activation could act in tandem to provide mechanisms for impaired lymphatic pumping activity and immune cell regulations in metabolic disorders or related diseases.
ACKNOWLEDGMENTS
The authors thank the Texas A&M Health Science Center Integrated Microscopy and Imaging Laboratory, Texas A&M Image Analysis Laboratory, and Texas A&M Qualitative Biology Facility Core for their technical support. This work was supported by U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 DK99221 (to M.M.) and Department of Medical Physiology, Lymphatic Graduate Student Fellowship (to Y.L.). The authors declare no conflicts of interest.
Glossary
- 2-NBDG
2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxyglucose
- DHE
dihydroethidium
- ECAR
extracellular acidification rate
- FBS
fetal bovine serum
- FCCP
carbonyl cyanide-p-trifluoromethoxyphenylhydrazone
- GLUT
glucose transporter
- GSK3β
glycogen synthase kinase-3β
- HG
high glucose
- ICAM
intercellular adhesion molecule
- IRS
insulin receptor substrate
- LMC
lymphatic muscle cell
- MCP
monocyte chemotactic protein
- MetSyn
metabolic syndrome
- MIP
macrophage inflammatory protein
- MLC
myosin light chain
- NA
numeric aperture
- O2·−
superoxide anion
- OCR
oxygen consumption rate
- PI3K
phosphatidyl-inositol-3 kinase
- PTEN
phosphate and tensin homolog
- ROS
reactive oxygen species
- WGA
wheat germ agglutinin
AUTHOR CONTRIBUTIONS
Y. Lee, S. Chakraborty and M. Muthuchamy designed the study; Y. Lee and S. Chakraborty performed the experiments; Y. Lee, S. Chakraborty, J. D. Fluckey, and M. Muthuchamy analyzed data; J. D. Fluckey and M. Muthuchamy contributed new reagents or analytic tools necessary to perform experiments; and Y. Lee, S. Chakraborty, and M. Muthuchamy wrote the paper.
REFERENCES
- 1.International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645 [DOI] [PubMed] [Google Scholar]
- 2.Huang P. L. (2009) A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2, 231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry R. R., Gumbiner B., Flynn T., Thorburn A. W. (1990) Metabolic effects of hyperglycemia and hyperinsulinemia on fate of intracellular glucose in NIDDM. Diabetes 39, 149–156 [DOI] [PubMed] [Google Scholar]
- 4.Vaidyula V. R., Rao A. K., Mozzoli M., Homko C., Cheung P., Boden G. (2006) Effects of hyperglycemia and hyperinsulinemia on circulating tissue factor procoagulant activity and platelet CD40 ligand. Diabetes 55, 202–208 [PubMed] [Google Scholar]
- 5.Martínez-Hervás S., Vinué A., Núñez L., Andrés-Blasco I., Piqueras L., Real J. T., Ascaso J. F., Burks D. J., Sanz M. J., González-Navarro H. (2014) Insulin resistance aggravates atherosclerosis by reducing vascular smooth muscle cell survival and increasing CX3CL1/CX3CR1 axis. Cardiovasc. Res. 103, 324–336 [DOI] [PubMed] [Google Scholar]
- 6.Pandey G., Makhija E., George N., Chakravarti B., Godbole M. M., Ecelbarger C. M., Tiwari S. (2015) Insulin regulates nitric oxide production in the kidney collecting duct cells. J. Biol. Chem. 290, 5582–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomás E., Lin Y. S., Dagher Z., Saha A., Luo Z., Ido Y., Ruderman N. B. (2002) Hyperglycemia and insulin resistance: possible mechanisms. Ann. N. Y. Acad. Sci. 967, 43–51 [DOI] [PubMed] [Google Scholar]
- 8.Baluk P., Fuxe J., Hashizume H., Romano T., Lashnits E., Butz S., Vestweber D., Corada M., Molendini C., Dejana E., McDonald D. M. (2007) Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204, 2349–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norrmén C., Tammela T., Petrova T. V., Alitalo K. (2011) Biological basis of therapeutic lymphangiogenesis. Circulation 123, 1335–1351 [DOI] [PubMed] [Google Scholar]
- 10.Robichaux J. L., Tanno E., Rappleye J. W., Ceballos M., Stallcup W. B., Schmid-Schonbein G. W., Murfee W. L. (2010) Lymphatic/blood endothelial cell connections at the capillary level in adult rat mesentery. Anat. Rec. (Hoboken) 293, 1629–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakraborty S., Davis M. J., Muthuchamy M. (2015) Emerging trends in the pathophysiology of lymphatic contractile function. Semin. Cell Dev. Biol. 38, 55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muthuchamy M., Zawieja D. (2008) Molecular regulation of lymphatic contractility. Ann. N. Y. Acad. Sci. 1131, 89–99 [DOI] [PubMed] [Google Scholar]
- 13.Von der Weid P. Y., Muthuchamy M. (2010) Regulatory mechanisms in lymphatic vessel contraction under normal and inflammatory conditions. Pathophysiology 17, 263–276 [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen J. C., Herbst K. L., Aldrich M. B., Darne C. D., Tan I. C., Zhu B., Guilliod R., Fife C. E., Maus E. A., Sevick-Muraca E. M. (2014) An abnormal lymphatic phenotype is associated with subcutaneous adipose tissue deposits in Dercum’s disease. Obesity (Silver Spring) 22, 2186–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vuorio T., Nurmi H., Moulton K., Kurkipuro J., Robciuc M. R., Ohman M., Heinonen S. E., Samaranayake H., Heikura T., Alitalo K., Ylä-Herttuala S. (2014) Lymphatic vessel insufficiency in hypercholesterolemic mice alters lipoprotein levels and promotes atherogenesis. Arterioscler. Thromb. Vasc. Biol. 34, 1162–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nurmi H., Saharinen P., Zarkada G., Zheng W., Robciuc M. R., Alitalo K. (2015) VEGF-C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol. Med. 7, 1418–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey N. L., Srinivasan R. S., Dillard M. E., Johnson N. C., Witte M. H., Boyd K., Sleeman M. W., Oliver G. (2005) Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet. 37, 1072–1081 [DOI] [PubMed] [Google Scholar]
- 18.Zawieja S. D., Wang W., Wu X., Nepiyushchikh Z. V., Zawieja D. C., Muthuchamy M. (2012) Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am. J. Physiol.. 302, H643–H653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zawieja S. D., Gasheva O., Zawieja D. C., Muthuchamy M. (2016) Blunted flow-mediated responses and diminished nitric oxide synthase expression in lymphatic thoracic ducts of a rat model of metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 310, H385–H393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arngrim N., Simonsen L., Holst J. J., Bulow J. (2013) Reduced adipose tissue lymphatic drainage of macromolecules in obese subjects: a possible link between obesity and local tissue inflammation? Int. J. Obes. 37, 748–750 [DOI] [PubMed] [Google Scholar]
- 21.Savetsky I. L., Torrisi J. S., Cuzzone D. A., Ghanta S., Albano N. J., Gardenier J. C., Joseph W. J., Mehrara B. J. (2014) Obesity increases inflammation and impairs lymphatic function in a mouse model of lymphedema. Am. J. Physiol. Heart Circ. Physiol. 307, H165–H172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weitman E. S., Aschen S. Z., Farias-Eisner G., Albano N., Cuzzone D. A., Ghanta S., Zampell J. C., Thorek D., Mehrara B. J. (2013) Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One 8, e70703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakraborty S., Zawieja S. D., Wang W., Lee Y., Wang Y. J., von der Weid P. Y., Zawieja D. C., Muthuchamy M. (2015) Lipopolysaccharide modulates neutrophil recruitment and macrophage polarization on lymphatic vessels and impairs lymphatic function in rat mesentery. Am. J. Physiol. 309, H2042–H2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermann C., Assmus B., Urbich C., Zeiher A. M., Dimmeler S. (2000) Insulin-mediated stimulation of protein kinase Akt: a potent survival signaling cascade for endothelial cells. Arterioscler. Thromb. Vasc. Biol. 20, 402–409 [DOI] [PubMed] [Google Scholar]
- 25.Saltiel A. R. (1996) Diverse signaling pathways in the cellular actions of insulin. Am. J. Physiol. 270, E375–E385 [DOI] [PubMed] [Google Scholar]
- 26.Schmitz-Peiffer C., Whitehead J. P. (2003) IRS-1 regulation in health and disease. IUBMB Life 55, 367–374 [DOI] [PubMed] [Google Scholar]
- 27.Zick Y. (2005) Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci. STKE 2005, pe4. [DOI] [PubMed] [Google Scholar]
- 28.Li Y., Soos T. J., Li X., Wu J., Degennaro M., Sun X., Littman D. R., Birnbaum M. J., Polakiewicz R. D. (2004) Protein kinase C Theta inhibits insulin signaling by phosphorylating IRS1 at Ser(1101). J. Biol. Chem. 279, 45304–45307 [DOI] [PubMed] [Google Scholar]
- 29.Fulton D. J. (2009) Mechanisms of vascular insulin resistance: a substitute Akt? Circ. Res. 104, 1035–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nadler S. T., Stoehr J. P., Rabaglia M. E., Schueler K. L., Birnbaum M. J., Attie A. D. (2001) Normal Akt/PKB with reduced PI3K activation in insulin-resistant mice. Am. J. Physiol. Endocrinol. Metab. 281, E1249–E1254 [DOI] [PubMed] [Google Scholar]
- 31.Egawa K., Maegawa H., Shimizu S., Morino K., Nishio Y., Bryer-Ash M., Cheung A. T., Kolls J. K., Kikkawa R., Kashiwagi A. (2001) Protein-tyrosine phosphatase-1B negatively regulates insulin signaling in l6 myocytes and Fao hepatoma cells. J. Biol. Chem. 276, 10207–10211 [DOI] [PubMed] [Google Scholar]
- 32.Vinciguerra M., Foti M. (2006) PTEN and SHIP2 phosphoinositide phosphatases as negative regulators of insulin signalling. Arch. Physiol. Biochem. 112, 89–104 [DOI] [PubMed] [Google Scholar]
- 33.Stratford S., Hoehn K. L., Liu F., Summers S. A. (2004) Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 279, 36608–36615 [DOI] [PubMed] [Google Scholar]
- 34.Lee J. H., Palaia T., Ragolia L. (2009) Impaired insulin-mediated vasorelaxation in diabetic Goto-Kakizaki rats is caused by impaired Akt phosphorylation. Am. J. Physiol. Cell Physiol. 296, C327–C338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J. H., Palaia T., Ragolia L. (2012) Impaired insulin-stimulated myosin phosphatase Rho-interacting protein signaling in diabetic Goto-Kakizaki vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 302, C1371–C1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J. H., Ragolia L. (2006) AKT phosphorylation is essential for insulin-induced relaxation of rat vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 291, C1355–C1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandu O. A., Ragolia L., Begum N. (2000) Diabetes in the Goto-Kakizaki rat is accompanied by impaired insulin-mediated myosin-bound phosphatase activation and vascular smooth muscle cell relaxation. Diabetes 49, 2178–2189 [DOI] [PubMed] [Google Scholar]
- 38.Li H., Wang Y. P., Zhang L. N., Tian G. (2014) Perivascular adipose tissue-derived leptin promotes vascular smooth muscle cell phenotypic switching via p38 mitogen-activated protein kinase in metabolic syndrome rats. Exp. Biol. Med. (Maywood) 239, 954–965 [DOI] [PubMed] [Google Scholar]
- 39.Izawa Y., Yoshizumi M., Fujita Y., Ali N., Kanematsu Y., Ishizawa K., Tsuchiya K., Obata T., Ebina Y., Tomita S., Tamaki T. (2005) ERK1/2 activation by angiotensin II inhibits insulin-induced glucose uptake in vascular smooth muscle cells. Exp. Cell Res. 308, 291–299 [DOI] [PubMed] [Google Scholar]
- 40.Tang X., Shen H., Chen J., Wang X., Zhang Y., Chen L. L., Rukachaisirikul V., Jiang H. L., Shen X. (2011) Activating transcription factor 6 protects insulin receptor from ER stress-stimulated desensitization via p42/44 ERK pathway. Acta Pharmacol. Sin. 32, 1138–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chakraborty S., Nepiyushchikh Z., Davis M. J., Zawieja D. C., Muthuchamy M. (2011) Substance P activates both contractile and inflammatory pathways in lymphatics through the neurokinin receptors NK1R and NK3R. Microcirculation 18, 24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muthuchamy M., Gashev A., Boswell N., Dawson N., Zawieja D. (2003) Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J. 17, 920–922 [DOI] [PubMed] [Google Scholar]
- 43.Lloyd P. G., Hardin C. D., Sturek M. (1999) Examining glucose transport in single vascular smooth muscle cells with a fluorescent glucose analog. Physiol. Res. 48, 401–410 [PubMed] [Google Scholar]
- 44.Gardner S. E., Humphry M., Bennett M. R., Clarke M. C. (2015) Senescent vascular smooth muscle cells drive inflammation through an interleukin-1α-dependent senescence-associated secretory phenotype. Arterioscler. Thromb. Vasc. Biol. 35, 1963–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perez J., Hill B. G., Benavides G. A., Dranka B. P., Darley-Usmar V. M. (2010) Role of cellular bioenergetics in smooth muscle cell proliferation induced by platelet-derived growth factor. Biochem. J. 428, 255–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.TeSlaa T., Teitell M. A. (2014) Techniques to monitor glycolysis. Methods Enzymol. 542, 91–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suhane S., Kanzaki H., Arumugaswami V., Murali R., Ramanujan V. K. (2013) Mitochondrial NDUFS3 regulates the ROS-mediated onset of metabolic switch in transformed cells. Biol. Open 2, 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimojo N., Naka K., Uenoyama H., Hamamoto K., Yoshioka K., Okuda K. (1993) Electrochemical assay system with single-use electrode strip for measuring lactate in whole blood. Clin. Chem. 39, 2312–2314 [PubMed] [Google Scholar]
- 49.Chakraborty S., Zawieja D. C., Davis M. J., Muthuchamy M. (2015) MicroRNA signature of inflamed lymphatic endothelium and role of miR-9 in lymphangiogenesis and inflammation. Am. J. Physiol. Cell Physiol. 309, C680–C692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zielonka J., Vasquez-Vivar J., Kalyanaraman B. (2008) Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat. Protoc. 3, 8–21 [DOI] [PubMed] [Google Scholar]
- 51.Dikalov S. I., Harrison D. G. (2014) Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid. Redox Signal. 20, 372–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zawieja S. D., Wang W., Chakraborty S., Zawieja D. C., Muthuchamy M. (2016) Macrophage alterations within the mesenteric lymphatic tissue are associated with impairment of lymphatic pump in metabolic syndrome. Microcirculation 23, 558–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hwang S. L., Jeong Y. T., Li X., Kim Y. D., Lu Y., Chang Y. C., Lee I. K., Chang H. W. (2013) Inhibitory cross-talk between the AMPK and ERK pathways mediates endoplasmic reticulum stress-induced insulin resistance in skeletal muscle. Br. J. Pharmacol. 169, 69–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan Y., Ichikawa T., Li J., Si Q., Yang H., Chen X., Goldblatt C. S., Meyer C. J., Li X., Cai L., Cui T. (2011) Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes 60, 625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng Y., Zhang W., Pendleton E., Leng S., Wu J., Chen R., Sun X. J. (2009) Improved insulin sensitivity by calorie restriction is associated with reduction of ERK and p70S6K activities in the liver of obese Zucker rats. J. Endocrinol. 203, 337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang W., Nepiyushchikh Z., Zawieja D. C., Chakraborty S., Zawieja S. D., Gashev A. A., Davis M. J., Muthuchamy M. (2009) Inhibition of myosin light chain phosphorylation decreases rat mesenteric lymphatic contractile activity. Am. J. Physiol. Heart Circ. Physiol. 297, H726–H734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nepiyushchikh Z. V., Chakraborty S., Wang W., Davis M. J., Zawieja D. C., Muthuchamy M. (2011) Differential effects of myosin light chain kinase inhibition on contractility, force development and myosin light chain 20 phosphorylation of rat cervical and thoracic duct lymphatics. J. Physiol. 589, 5415–5429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chakraborty S., Zawieja S., Wang W., Zawieja D. C., Muthuchamy M. (2010) Lymphatic system: a vital link between metabolic syndrome and inflammation. Ann. N. Y. Acad. Sci. 1207(Suppl 1), E94–E102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen R., Peng X., Du W., Wu Y., Huang B., Xue L., Wu Q., Qiu H., Jiang Q. (2015) Curcumin attenuates cardiomyocyte hypertrophy induced by high glucose and insulin via the PPARγ/Akt/NO signaling pathway. Diabetes Res. Clin. Pract. 108, 235–242 [DOI] [PubMed] [Google Scholar]
- 60.Niu P., Zhang Y., Shi D., Chen Y., Deng J. (2013) Cardamonin ameliorates insulin resistance induced by high insulin and high glucose through the mTOR and signal pathway. Planta Med. 79, 452–458 [DOI] [PubMed] [Google Scholar]
- 61.Souto Padron de Figueiredo A., Salmon A. B., Bruno F., Jimenez F., Martinez H. G., Halade G. V., Ahuja S. S., Clark R. A., DeFronzo R. A., Abboud H. E., El Jamali A. (2015) Nox2 mediates skeletal muscle insulin resistance induced by a high fat diet. J. Biol. Chem. 290, 13427–13439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davidoff A. J., Ren J. (1997) Low insulin and high glucose induce abnormal relaxation in cultured adult rat ventricular myocytes. Am. J. Physiol. 272, H159–H167 [DOI] [PubMed] [Google Scholar]
- 63.Davidoff A. J., Davidson M. B., Carmody M. W., Davis M. E., Ren J. (2004) Diabetic cardiomyocyte dysfunction and myocyte insulin resistance: role of glucose-induced PKC activity. Mol. Cell. Biochem. 262, 155–163 [DOI] [PubMed] [Google Scholar]
- 64.Wang C. C., Gurevich I., Draznin B. (2003) Insulin affects vascular smooth muscle cell phenotype and migration via distinct signaling pathways. Diabetes 52, 2562–2569 [DOI] [PubMed] [Google Scholar]
- 65.Kaiser N., Sasson S., Feener E. P., Boukobza-Vardi N., Higashi S., Moller D. E., Davidheiser S., Przybylski R. J., King G. L. (1993) Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes 42, 80–89 [DOI] [PubMed] [Google Scholar]
- 66.Styskal J., Van Remmen H., Richardson A., Salmon A. B. (2012) Oxidative stress and diabetes: what can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic. Biol. Med. 52, 46–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y., Qi W., Richardson A., Van Remmen H., Ikeno Y., Salmon A. B. (2013) Oxidative damage associated with obesity is prevented by overexpression of CuZn- or Mn-superoxide dismutase. Biochem. Biophys. Res. Commun. 438, 78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aroor A. R., DeMarco V. G. (2014) Oxidative stress and obesity: the chicken or the egg? Diabetes 63, 2216–2218 [DOI] [PubMed] [Google Scholar]
- 69.Paul R. J. (1983) Functional compartmentalization of oxidative and glycolytic metabolism in vascular smooth muscle. Am. J. Physiol. 244, C399–C409 [DOI] [PubMed] [Google Scholar]
- 70.Paul R. J., Bauer M., Pease W. (1979) Vascular smooth muscle: aerobic glycolysis linked to sodium and potassium transport processes. Science 206, 1414–1416 [DOI] [PubMed] [Google Scholar]
- 71.Sen S., Domingues C. C., Rouphael C., Chou C., Kim C., Yadava N. (2015) Genetic modification of human mesenchymal stem cells helps to reduce adiposity and improve glucose tolerance in an obese diabetic mouse model. Stem Cell Res. Ther. 6, 242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Merdzo I., Rutkai I., Tokes T., Sure V. N., Katakam P. V., Busija D. W. (2016) The mitochondrial function of the cerebral vasculature in insulin-resistant Zucker obese rats. Am. J. Physiol. Heart Circ. Physiol. 310, H830–H838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ritov V. B., Menshikova E. V., He J., Ferrell R. E., Goodpaster B. H., Kelley D. E. (2005) Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54, 8–14 [DOI] [PubMed] [Google Scholar]
- 74.Kelley D. E., He J., Menshikova E. V., Ritov V. B. (2002) Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51, 2944–2950 [DOI] [PubMed] [Google Scholar]
- 75.Lowell B. B., Shulman G. I. (2005) Mitochondrial dysfunction and type 2 diabetes. Science 307, 384–387 [DOI] [PubMed] [Google Scholar]
- 76.Jiang Z. Y., Lin Y. W., Clemont A., Feener E. P., Hein K. D., Igarashi M., Yamauchi T., White M. F., King G. L. (1999) Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J. Clin. Invest. 104, 447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karlsson H. K., Zierath J. R., Kane S., Krook A., Lienhard G. E., Wallberg-Henriksson H. (2005) Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes 54, 1692–1697 [DOI] [PubMed] [Google Scholar]
- 78.Qi Y., Zhu Q., Zhang K., Thomas C., Wu Y., Kumar R., Baker K. M., Xu Z., Chen S., Guo S. (2015) Activation of Foxo1 by insulin resistance promotes cardiac dysfunction and β-myosin heavy chain gene expression. Circ Heart Fail 8, 198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cho H., Thorvaldsen J. L., Chu Q., Feng F., Birnbaum M. J. (2001) Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276, 38349–38352 [DOI] [PubMed] [Google Scholar]
- 80.Garofalo R. S., Orena S. J., Rafidi K., Torchia A. J., Stock J. L., Hildebrandt A. L., Coskran T., Black S. C., Brees D. J., Wicks J. R., McNeish J. D., Coleman K. G. (2003) Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J. Clin. Invest. 112, 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cho H., Mu J., Kim J. K., Thorvaldsen J. L., Chu Q., Crenshaw E. B. III, Kaestner K. H., Bartolomei M. S., Shulman G. I., Birnbaum M. J. (2001) Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292, 1728–1731 [DOI] [PubMed] [Google Scholar]
- 82.Summers S. A., Garza L. A., Zhou H., Birnbaum M. J. (1998) Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol. 18, 5457–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martinez-Arca S., Lalioti V. S., Sandoval I. V. (2000) Intracellular targeting and retention of the glucose transporter GLUT4 by the perinuclear storage compartment involves distinct carboxyl-tail motifs. J. Cell Sci. 113, 1705–1715 [DOI] [PubMed] [Google Scholar]
- 84.Palacios S., Lalioti V., Martinez-Arca S., Chattopadhyay S., Sandoval I. V. (2001) Recycling of the insulin-sensitive glucose transporter GLUT4. Access of surface internalized GLUT4 molecules to the perinuclear storage compartment is mediated by the Phe5-Gln6-Gln7-Ile8 motif. J. Biol. Chem. 276, 3371–3383 [DOI] [PubMed] [Google Scholar]
- 85.Ploug T., van Deurs B., Ai H., Cushman S. W., Ralston E. (1998) Analysis of GLUT4 distribution in whole skeletal muscle fibers: identification of distinct storage compartments that are recruited by insulin and muscle contractions. J. Cell Biol. 142, 1429–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Avogaro A., de Kreutzenberg S. V., Fadini G. P. (2008) Oxidative stress and vascular disease in diabetes: is the dichotomization of insulin signaling still valid? Free Radic. Biol. Med. 44, 1209–1215 [DOI] [PubMed] [Google Scholar]
- 87.Wellen K. E., Hotamisligil G. S. (2005) Inflammation, stress, and diabetes. J. Clin. Invest. 115, 1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Patsouris D., Cao J. J., Vial G., Bravard A., Lefai E., Durand A., Durand C., Chauvin M. A., Laugerette F., Debard C., Michalski M. C., Laville M., Vidal H., Rieusset J. (2014) Insulin resistance is associated with MCP1-mediated macrophage accumulation in skeletal muscle in mice and humans. PLoS One 9, e110653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sartipy P., Loskutoff D. J. (2003) Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 100, 7265–7270 [DOI] [PMC free article] [PubMed] [Google Scholar]