Abstract
Sepsis—typically caused by an uncontrolled and amplified host systemic inflammatory response to microbial infection—is a life-threatening complex clinical disorder and remains a major cause of infection-related deaths in the intensive care unit. Emerging evidence suggests that neuropilin 1 (Nrp1), an originally defined coreceptor for class 3 semaphorins and VEGF, plays important roles in the immune system; however, the function and regulation of macrophage Nrp1 in host immune defense against bacterial infection remain unknown. To address this problem, we generated myeloid cell–specific Nrp1-knockout (Nrp1myel-KO) mice and applied 2 stringent animal models of sepsis: cecal ligation and puncture as well as intraperitoneal injection of LPS. Here, we reported that myeloid cell–specific Nrp1-deficient mice exhibited enhanced susceptibility to cecal ligation and puncture– and LPS-induced sepsis, which correlated with significantly decreased survival rates and heightened levels of proinflammatory cytokines in both peritoneal lavage and serum. Mechanistically, LPS specifically attenuated the expression of Nrp1 in macrophages, which was mediated by TLR4–NF-κB p50 and -65 pathways. By using isolated primary macrophages, loss of Nrp1 consistently resulted in increased production of proinflammatory cytokines, including iNOS, TNF-α, and IL-6. Together, these findings demonstrate a novel role of macrophage Nrp1 in sepsis.—Dai, X. Okon, I., Liu, Z., Wu, Y., Zhu, H., Song, P., Zou, M.-H. A novel role for myeloid cell–specific neuropilin 1 in mitigating sepsis.
Keywords: macrophage, LPS, inflammation
Sepsis is characterized by systemically exaggerated inflammatory response to microbial infection and is often associated with high mortality rates caused by multiple organ injury and failure (1). For example, in the United States alone, incidence of sepsis approximates 500,000 patients, resulting in many deaths (2). Despite the severity of the condition, effective strategies to treat sepsis are still lacking. To date, more than 40 clinical trials of various agents that block cytokines, pathogen recognition, or inflammation signaling pathways have failed to yield successful outcomes (3, 4); therefore, it is imperative to deepen our understanding of the mechanisms that are involved in sepsis to develop novel therapeutic strategies.
Neuropilin 1 (Nrp1) was originally identified as a coreceptor for neuronal guidance cue class 3 semaphorins (Sema3A) and VEGF, with essential roles in the development of the CNS and angiogenesis (5–7). Emerging evidence suggests Nrp1 is highly expressed in immune cells and plays important roles in inflammation/immune response (8). In T cells, Nrp1 was identified as a surface marker of regulatory T (Treg) cells (9) and maintains the stability and function of Treg cells (10). By promoting CD4+Foxp3+ Treg infiltration into tumor sites, Nrp1 inhibits antitumor immune response and accelerates tumor progression (11). Sema3A, a ligand for Nrp1, increases the ability of CD4+Nrp1+ T cells to suppress alloresponses (12) and acts as a paracrine negative regulator of primary T-cell activation (13). Nrp1 also regulates adhesion and clustering between dendritic cells and resting T cells (14). Nrp1 has been identified as a marker for proangiogenic and proarteriogenic macrophages in physiologic and pathologic conditions (15–18). Gene deletion of Nrp1 in macrophages promotes entrapment of tumor-associated macrophages in normoxic tumor regions, which abates proangiogenic and immunosuppressive functions of Nrp1 and inhibits tumor growth and metastasis (19). In human monocyte-derived macrophages, Nrp1 is significantly up-regulated in response to IL-4 treatment, which promotes the M2 alternatively activated macrophage phenotype (20). This suggests that Nrp1 may play a role in the regulation of macrophage polarization; however, whether macrophage Nrp1 is directly involved in sepsis or whether the condition affects expression and function of Nrp1 remains unknown.
In the present study, we generated myeloid cell–specific Nrp1-knockout (Nrp1myel-KO) mice and applied 2 classic animal models of sepsis to test potential roles of macrophage Nrp1 in sepsis. Our results demonstrated that mice that lacked Nrp1 in myeloid cells exhibited enhanced susceptibility to cecal ligation and puncture (CLP)– and LPS-induced sepsis, which is accompanied by heightened and accentuated proinflammatory cytokine levels. Mechanistically, LPS promotes specific down-regulation of Nrp1 in macrophages, which was mediated by the TLR4–NF-κB pathway. Loss of Nrp1 resulted in hyper-responsiveness to LPS treatment and promoted classic activation of macrophages. Taken together, our findings uncover a direct link between macrophage Nrp1 and sepsis, providing potential avenues for therapeutic application.
MATERIALS AND METHODS
Mice
Nrp1flox/flox mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and backcrossed to C57BL/6J for at least 10 generations. These mice were crossed with lysozyme M (LysM)-Cre mice on a C57BL/6J background to generate Nrp1myel-KO mice. Nrp1flox/flox littermates without the LysM-Cre transgene served as wild-type (WT) controls. All mice were housed in temperature-controlled cages with a 12-h light/dark cycle and given free access to water and food. Animal protocol was reviewed and approved by Institutional Animal Care and Use Committee at Georgia State University.
Reagents
Ultrapure LPS (tlrl-smlps) from Salmonella minnesota R595 was from InvivoGen (San Diego, CA, USA). NF-κB activation inhibitor (481406) was from EMD Millipore (Billerica, MA, USA). Wortmannin (W1628), SB203580 (S8307), PD98059 (P215), and SP600125 (S5567) were purchased from Sigma-Aldrich (St. Louis, MO, USA). In immunoblotting experiments, primary Abs specific for Nrp1 (ab81321) and iNOS (ab15323) were obtained from Abcam (Cambridge, MA, USA). Abs against COX2 (sc-23983), arginase I (sc-20150), β-actin (sc-47778), and glyceraldehyde 3-phosphate dehydrogenase (sc-137179) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Ab against Ym1 (01404) was from StemCell Technologies (Vancouver, BC, Canada).
Cell culture
Peritoneal macrophages (PMs) were isolated as previously described (21). In brief, Mice were intraperitoneally injected with 1.5 ml 4% thioglycollate broth (BD Biosciences, Brea, CA, USA). Mice were euthanized 3 d later. Cells were collected by peritoneal lavage with 8 ml ice-cold PBS with 10 mM EDTA and 10% fetal bovine serum (FBS). Macrophages were plated at 1.0 × 106 per ml RPMI-1640 with 10% FBS. After incubation for 3 h at 37°C, nonadherent cells were washed away and adherent cells were collected for experiments.
Primary bone marrow–derived macrophages (BMDMs) were prepared by flushing bone marrow of the femur and tibia of mice. Cells were cultured with complete RPMI-1640 medium that was supplemented with mouse M-CSF recombinant protein (50 ng/ml; eBioscience, San Diego, CA, USA) for 7 d (22).
RAW264.7 cells were from American Type Culture Collection (Manassas, VA, USA). Cells were cultured at 1.0 × 106 per ml DMEM with 10% FBS under 95% air and 5% CO2 at 37.0°C.
Experimental sepsis induced by CLP
CLP surgery was performed on mice as previously described with minor modifications (23). In brief, mice were anesthetized with intraperitoneal injection of 100 mg/kg ketamine-HCl and 10 mg/kg xylazine-HCl. Under sterile surgical conditions, a 1-cm midline incision was made to the ventral surface of the abdomen, and the cecum was exposed. The cecum was partially ligated at its base with a 3.0 silk suture and punctured 2 times with a 21-gauge needle. Sham-surgery mice were subjected to a similar laparotomy without ligation and puncture. Serum and cell-free peritoneal lavage fluid were collected for cytokine protein analyses.
Endotoxic shock model induced by LPS
Mice were intraperitoneally injected with LPS (40 mg/kg body weight; Escherichia coli 0111:B4, L2630; Sigma-Aldrich).
Immunofluorescence staining
Cells were plated on glass coverslips and fixed by using 4% paraformaldehyde. Cells were permeabilized with 0.1% Triton X-100 and blocked in protein block buffer (Dako, Carpinteria, CA, USA) at room temperature for 1 h, then incubated with Ab against Nrp1 (ab81321) or iNOS (ab15323) overnight at 4°C. Slides were rinsed with washing buffer and incubated with goat anti-rabbit IgG conjugated to Alexa Fluor 488 (A11008) at 1:400 dilution. Nuclei were stained with DAPI. Slides were mounted and visualized by using an Olympus BX53 microscope (Olympus, Tokyo, Japan).
Immunohistochemistry staining
Consecutive frozen sections were incubated in endogenous peroxidase and protein block buffer (Dalp) and then with primary Abs, including iNOS (ab15323) or COX2 (sc-23983), overnight at 4°C. Slides were rinsed with washing buffer and incubated with labeled polymer–horseradish peroxidase–anti-mouse/anti-rabbit Abs followed by DAB+ chromogen detection (Dako). All positive staining was confirmed by ensuring that no staining occurred under the same conditions with the use of nonimmune rabbit or mouse control IgG. Sections were mounted and visualized by using an Olympus BX53 microscope.
RNA extraction and quantitative RT-PCR
Frozen tissue (kept at −80°C) or cell total RNA was extracted by using the Tri Reagent Solution (AM9738; Thermo Fisher Scientific, Waltham, MA, USA) and 1 µg of RNA was reverse-transcribed into cDNA using iScript cDNA Synthesis Kits (Bio-Rad, Hercules, CA, USA). PCR amplification was performed by using the SYBR PCR mix (Bio-Rad). Calculations were performed by a comparative method (2−∆∆Ct) using 18s RNA as internal control. Primer sets used in quantitative RT-PCR analyses are described in Supplemental Table 1.
Immunoblot analysis
Cell lysates or tissue homogenates were subjected to Western blot analysis, as described previously (24). In brief, protein content was assayed by BCA protein assay reagent (Pierce, Rockford, IL, USA). Protein (50 μg) was loaded onto SDS-PAGE and transferred to PVDF membrane. Membrane was incubated with a 1:1000 dilution of primary Ab, followed by a 1:2000 dilution of horseradish peroxidase–conjugated secondary Ab. Protein bands were visualized by ECL (GE Healthcare, Pittsburgh, PA, USA). Intensity (area × density) of individual bands on Western blots was measured by densitometry (GS-700 Imaging Densitometer; Bio-Rad). Background was subtracted from the calculated area. We used control as 100%.
Cell transfections
Small interfering RNAs (siRNAs) were transfected into RAW264.7 cells by using Lipofectamine RNAiMax reagent (Thermo Fisher Scientific) according to the manufacturer’s guidelines. In brief, 100 µl Opti-MEM I Reduced-Serum Medium (1×; Thermo Fisher Scientific) that contained 5 µl siRNA stock solution was gently mixed with 100 µl Opti-MEM I Reduced-Serum Medium (1×) that containing 5 µl RNAiMax (Thermo Fisher Scientific). After a 30 min of incubation at room temperature, siRNA-lipid complexes were added to cells in 800 µl Opti-MEM I Reduced-Serum Medium (1×), and cells were incubated with this mixture for 6 h at 37°C. Transfection medium was then replaced with normal medium and cells were cultured for 48 h. For transfection of plasmids, cells were transfected by using X-tremeGene HP DNA Transfection Reagent (Roche, Basel, Switzerland).
ELISA
Cytokines in supernatant of cultured BMDMs, peritoneal lavage, and serum were quantified by using the ELISA set for mouse TNF-α (430904; BioLegend, San Diego, CA, USA), IL-6 (431304; BioLegend), or IL-1β (MLB00C; R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Statistical analysis
Quantitative data are expressed as means ± sem of at least 3 independent experiments. The difference between 2 groups was analyzed by Student’s t test. The difference among more than 2 groups was analyzed by 1-way ANOVA followed by Newman-Keuls multiple comparison test, and comparisons of different parameters between each group were made by 2-way ANOVA analysis followed by Bonferroni post-tests. Survival statistics were analyzed with log-rank (Mantel-Cox) test. Statistical significance was evaluated with GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). Values of P < 0.05 were considered statistically significant.
RESULTS
Nrp1 deficiency in myeloid cells correlates with enhanced susceptibility to CLP-induced sepsis
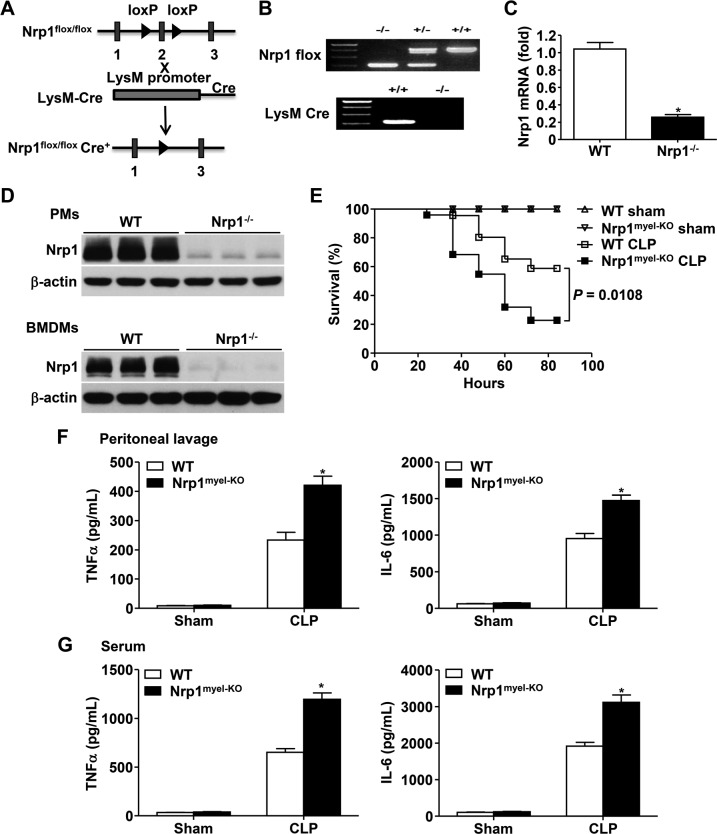
To evaluate the role of macrophage-specific Nrp1 in host defense against bacterial infection, Nrp1 expression in myeloid cells was depleted by crossing Nrp1flox/flox mice with LysM-Cre-recombinase–expressing mice (Fig. 1A). As depicted in Fig. 1B, Nrp1 flox+/+LysM-Cre+/+ mice were the Nrp1myel-KO mice, whereas Nrp1 flox+/+LysM-Cre−/− littermates served as controls (WT). To verify efficient disruption of Nrp1 in macrophages of these mice, we isolated PMs and BMDMs from WT and Nrp1myel-KO mice. Quantitative RT-PCR data demonstrated that Nrp1 mRNA was significantly lower in Nrp1−/− BMDMs compared with WT BMDMs (Fig. 1C). Nrp1 deletion in PMs and BMDMs was further confirmed by immunoblot analysis (Fig. 1D).
Figure 1.
Nrp1myel-KO mice demonstrate increased CLP-induced lethality of sepsis. A) Scheme for generating Nrp1myel-KO mice. B) Genotyping analysis of 4-wk-old mice. C) Nrp1 mRNA expression in BMDMs. D) Immunoblot analysis of Nrp1 in PMs and BMDMs. E) Survival rates of WT and Nrp1myel-KO mice (n = 13–24 per group) after sham surgery or CLP procedure. F, G) Inflammatory cytokines in peritoneal lavage (F) and serum (G) 24 h after sham surgery or CLP (n = 7–8 per group per experiment) were measured by ELISA. Data are expressed as means ± sem. *P < 0.05 vs. WT or as indicated.
Next, WT and Nrp1myel-KO mice were subjected to a well-defined CLP-induced polymicrobial peritonitis model that closely replicated the nature and course of clinical sepsis (25). As demonstrated in Fig. 1E, Nrp1myel-KO mice exhibited increased CLP-induced lethality compared with WT mice, which suggested that the absence of Nrp1 in myeloid cells decreases survival during microbial-induced sepsis. Production of a plethora of proinflammatory cytokines—referred to as a cytokine storm—accompanies sepsis and is frequently detrimental to animals (26); therefore, we next examined inflammatory cytokine levels in the peritoneal lavage and serum before and after CLP-induced peritonitis. At 24 h after CLP administration, Nrp1myel-KO mice produced significantly elevated levels of inflammatory cytokines, such as TNF-α and IL-6, in both peritoneal lavage fluid and serum compared with WT mice (Fig. 1F, G). Together, these data suggest that myeloid cell–specific Nrp1 deletion enhances susceptibility to CLP-induced sepsis, accompanied by exaggerated cytokine production.
Nrp1myel-KO mice exhibit higher mortality rates of LPS-induced sepsis
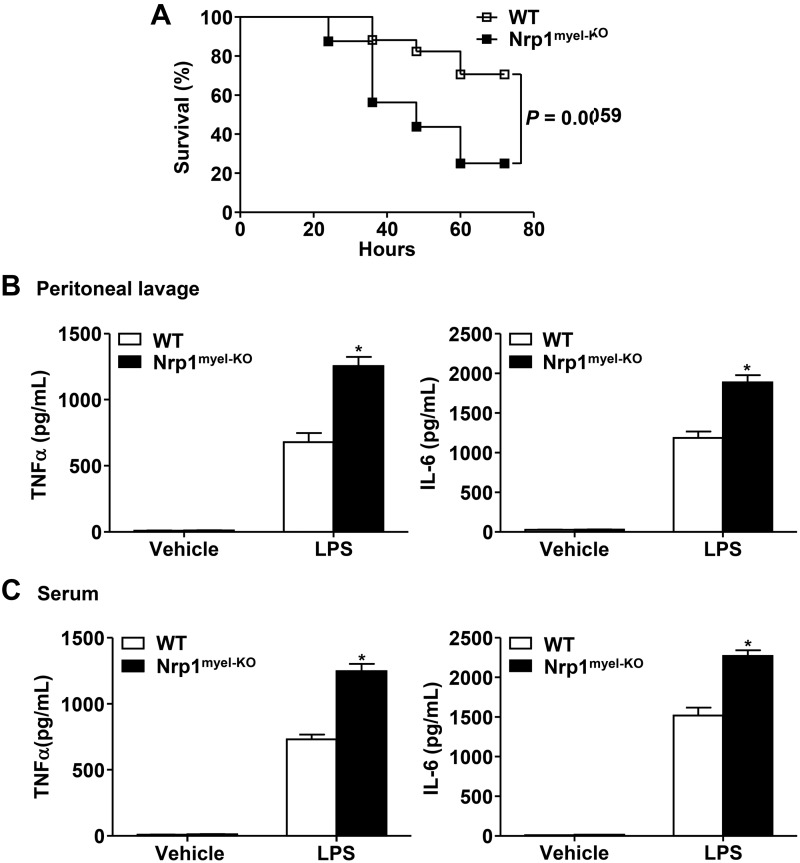
To further validate the role of Nrp1 in sepsis, we employed another mouse model of sepsis that involved i.p. injection of a lethal dose of LPS into WT and Nrp1myel-KO mice. Consistently, LPS-induced lethality within Nrp1myel-KO mice was significantly augmented compared with the WT group (Fig. 2A). In addition, at 4 h after LPS challenge, both localized (Fig. 2B) and systemic (Fig. 2C) levels of inflammatory cytokines, including TNF-α and IL-6, were elevated in Nrp1myel-KO mice compared with WT mice. These data support the notion that myeloid cell–specific Nrp1 deficiency results in heightened proinflammatory cytokines and increased mortality rates from sepsis.
Figure 2.
Nrp1myel-KO mice exhibit increased LPS-induced lethality of sepsis. Male 8-wk-old mice were intraperitoneally injected with LPS (40 mg/kg body weight), and mouse death was monitored. A) Survival rates of WT and Nrp1myel-KO mice (n = 16–17 per group). B, C) Inflammatory cytokines in peritoneal lavage (B) and serum (C) 2 h after administration of LPS (5 mg/kg body weight) or saline control (vehicle; n = 8 per group) were measured by ELISA. Data are expressed as means ± sem. *P < 0.05 vs. WT or as indicated.
LPS specifically down-regulates Nrp1 expression in macrophages
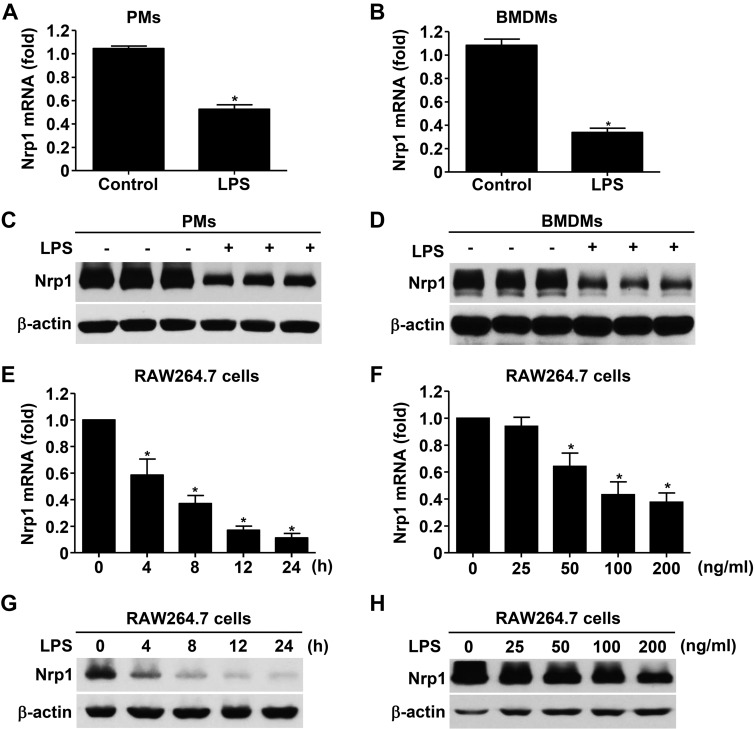
To obtain insight into the mechanism by which Nrp1 ablation contributes to hyper-responsiveness to inflammatory stimuli and enhanced susceptibility to sepsis, we investigated whether Nrp1 is a target of LPS-triggered TLR4 signaling. We first examined the effect of LPS on Nrp1 expression in primary macrophages in vitro. Surprisingly, Nrp1 mRNA level was dramatically decreased (∼56% in PMs and ∼32% in BMDMs) in the presence of LPS (Fig. 3A, B). Furthermore, Western blot analysis confirmed a significant reduction of Nrp1 protein by LPS (Fig. 3C, D).
Figure 3.
LPS attenuates Nrp1 in macrophages. A, B) Quantitative RT-PCR analysis of Nrp1 mRNA in PMs (A) and BMDMs (B) treated with or without LPS (100 ng/ml) for 12 h. C, D) Immunoblot analysis of Nrp1 in PMs (C) and BMDMs (D) treated with or without LPS (100 ng/ml) for 12 h. E) Quantitative RT-PCR analysis of Nrp1 mRNA in RAW264.7 cells treated with LPS (100 ng/ml) for 0–24 h. F) Quantitative RT-PCR analysis of Nrp1 mRNA in RAW264.7 cells treated for 24 h with LPS (0–200 ng/ml). G) Immunoblot analysis of Nrp1 in RAW264.7 cells treated with LPS (100 ng/ml) for 0–24 h. H) Immunoblot analysis of Nrp1 in RAW264.7 cells treated for 24 h with LPS (0–200 ng/ml). β-Actin serves as loading control. Data are expressed as means ± sem. *P < 0.05 vs. control, 0 h, 0 ng/ml, or vehicle.
To further observe the phasic change of Nrp1 after LPS treatment, RAW264.7 cells—a mouse monocyte/macrophage cell line—were treated with LPS as indicated. Nrp1 mRNA and protein were markedly down-regulated in a time (∼4–24 h; Fig. 3E, G)– and dose (∼25–200 ng/ml; Fig. 3F, H)–dependent manner in the presence of LPS.
We subsequently assessed whether LPS-mediated attenuation of Nrp1 occurred in other cell types. Unexpectedly, as shown in Supplemental Fig. 1, LPS failed to alter Nrp1 expression in mouse embryonic fibroblasts, rat fibroblasts, HUVECs, and human vascular smooth muscle cells, which indicated that LPS specifically down-regulates Nrp1 in macrophages. Taken together, LPS induces specific and negative regulation of Nrp1 expression in macrophages.
LPS-dependent TLR4–NF-κB pathway contributes to Nrp1 reduction
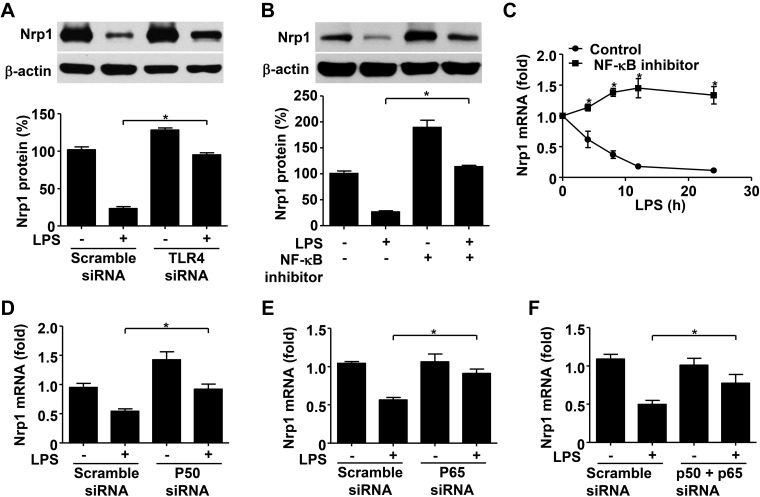
To explain the decreased Nrp1 expression caused by LPS, we first sought to clarify whether LPS-mediated attenuation of Nrp1 was dependent on TLR4, a known receptor for LPS. Immunoblot analysis showed that TLR4-specific knockdown reversed Nrp1 reduction by LPS, which suggested that TLR4 is required for LPS-mediated Nrp1 attenuation (Fig. 4A).
Figure 4.
TLR4-NF-κB p65/p50 pathway contributes to reduction of Nrp1 by LPS. A) Immunoblot analysis and quantification of Nrp1 in RAW264.7 cells transfected with scramble or TLR4 siRNA for 48 h and treated with 100 ng/ml LPS for 12 h. B) Immunoblot analysis and quantification of Nrp1 in RAW264.7 cells stimulated or unstimulated with LPS (100 ng/ml) for 12 h with or without incubation of NF-κB activation inhibitor (10 μM). Immunoblot analysis of Nrp1 in RAW264.7. β-Actin serves as loading control. C) Quantitative RT-PCR analysis of Nrp1 mRNA in RAW264.7 cells treated with LPS (100 ng/ml) alone or LPS (100 ng/ml) plus NF-κB activation inhibitor (10 μM) for indicated time. D–F) Quantitative RT-PCR analysis of Nrp1 mRNA in RAW264.7 cells transfected with scramble or NF-κB p50 (D), p65 (E), or p50/p65 (F) siRNA for 48 h and then treated with 100 ng/ml LPS for 12 h. Data are expressed as means ± sem. *P < 0.05 vs. control or indicated.
As it has been reported that LPS triggers activation of the NF-κB, MAPK, and PI3K/AKT pathways upon binding to TLR4 (27, 28), we next examined possible signaling pathways that could be involved in LPS/TLR4-mediated loss of Nrp1. RAW264 cells were treated with specific inhibitors of several signaling pathways, including NF-κB inhibitor (CAS 545380-34-5), PI3K inhibitor (Wortmannin), p38 inhibitor (SB203580), ERK inhibitor (0PD98059), or JNK inhibitor (SP600125) by the presence or absence of LPS. These data showed that NF-κB inhibition, rather than other inhibitors, abolished LPS-induced Nrp1 down-regulation (Fig. 4B and Supplemental Fig. 2). On the basis of these observations, the TLR4–NF-κB pathway seems to play an essential role in the down-regulation of Nrp1 by LPS in macrophages.
NF-κB p50 and p65 mediate reduction of Nrp1 mRNA by LPS in macrophages
Given a previous report that states that NF-κB p50 and p65 are suppressors of Nrp1 promoter (29), we reasoned that LPS may promote activation of NF-κB p50 and p65, which consequently inhibit Nrp1 mRNA transcription. As expected, inhibition of NF-κB activity abolished LPS-induced Nrp1 mRNA down-regulation (Fig. 4C). Specific knockdown of NF-κB p50 reversed the down-regulation of Nrp1 mRNA by LPS (Fig. 4D). Consistently, blockade of NF-κB p65 activity resulted in ablation of LPS-mediated Nrp1 reduction (Fig. 4E). Finally, knockdown of both NF-κB p50 and p65 showed similar results (Fig. 4F). Taken together, these results demonstrate that LPS promotes activation of NF-κB p50 and p65, which, in turn, act as transcriptional suppressors of Nrp1 in macrophages.
Nrp1 deficiency exaggerates LPS-induced inflammatory response in macrophages in vitro
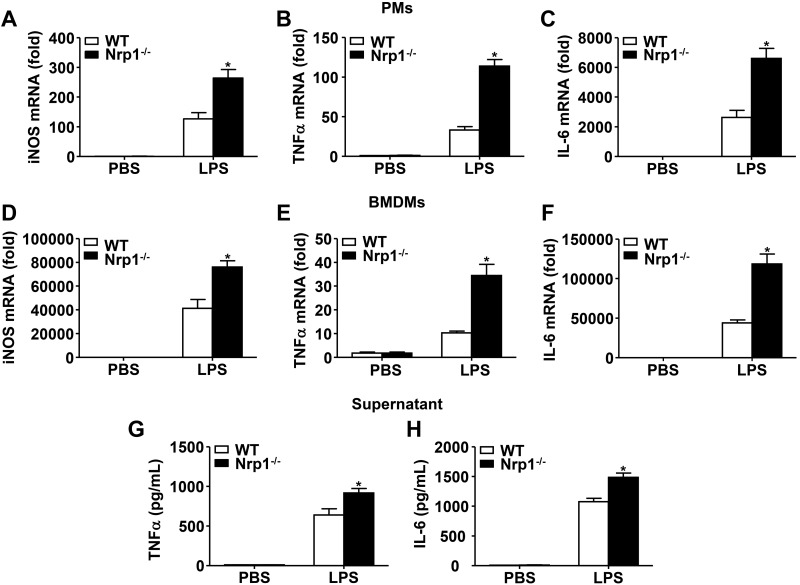
To understand the consequence of Nrp1 insufficiency in macrophages, we investigated the role of Nrp1 in cytokine production in response to TLR4 activation in macrophages. During TLR4 stimulation with LPS, Nrp1−/− PMs generated significantly higher mRNA levels of iNOS, TNF-α, and IL-6 mRNA compared with similarly treated WT controls (Fig. 5A–C). In addition to PMs, Nrp1−/− BMDMs exhibited significantly increased mRNA levels of iNOS, TNF-α, and IL-6 upon LPS stimulation compared with WT counterparts (Fig. 5D–F).
Figure 5.
Nrp1 deficiency exaggerates LPS-induced iNOS, TNF-α, and IL-6 levels in macrophages. A–F) PMs (A–C) or BMDMs (D–F) were unstimulated or stimulated with 100 ng/ml LPS for 4 h. iNOS (A, D), TNF-α (B, E), and IL-6 (C, F) mRNA levels were measured by quantitative RT-PCR. G, H) TNF-α (G) and IL-6 (H) levels in macrophage supernatants were determined by ELISA. Data are expressed as means ± sem. *P < 0.05 vs. WT.
Furthermore, we examined proinflammatory protein levels in macrophage supernatant by ELISA. As shown in Fig. 5G, H, Nrp1−/− BMDM supernatant exhibited increased TNF-α and IL-6 protein levels upon LPS stimulation compared with WT counterparts. These findings indicate that Nrp1 plays an inhibitory role in the production of LPS-induced proinflammatory cytokines in macrophages in vitro.
Nrp1 deficiency induces M1 macrophage polarization in vitro and in vivo
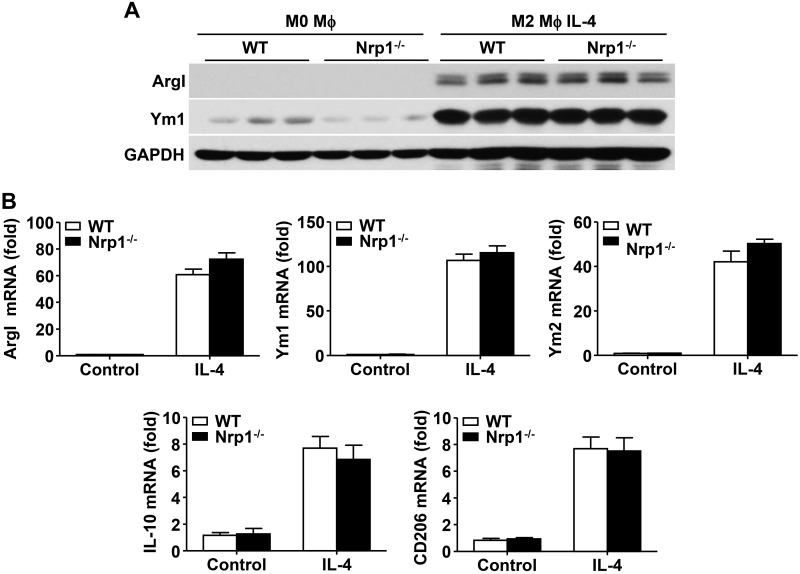
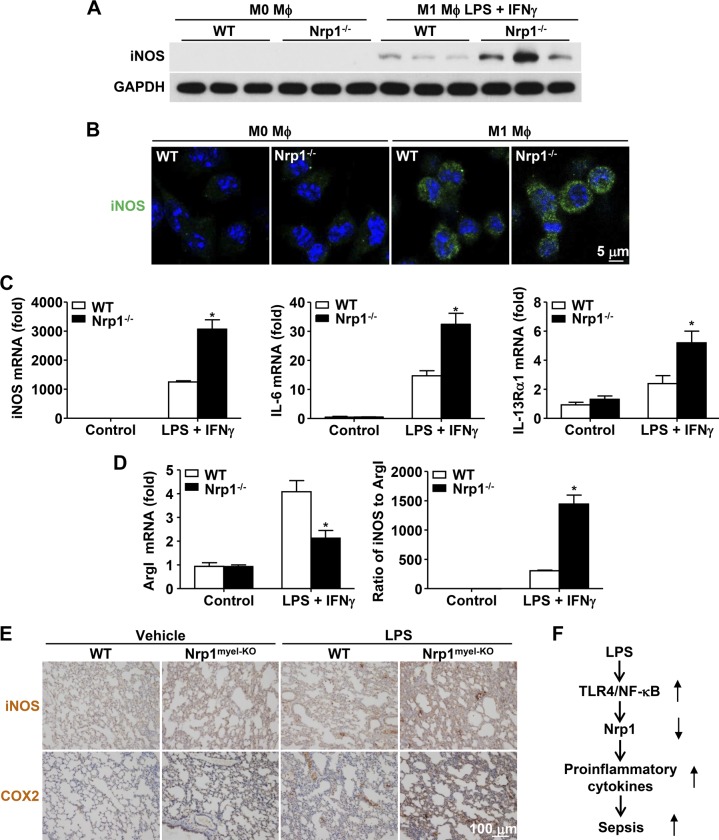
As a result of the elevated proinflammatory cytokine production in Nrp1−/− macrophages, we speculated that Nrp1 may regulate inflammatory response by affecting macrophage polarization; however, as detected by Western blot, no significant change in M2 polarization markers, namely arginase I (ArgI) and Ym1, was observed between WT and Nrp1−/− BMDMs (Fig. 6A). At the mRNA level, quantitative RT-PCR data showed that Nrp1 failed to alter several M2 polarization markers, including ArgI, Ym1, Ym2, IL-10, and CD206 (Fig. 6B). In contrast, the canonical M1 polarization marker, iNOS, was significantly increased in Nrp1−/− BMDMs compared with WT BMDMs (Fig. 7A). Consistent with Western blot data, immunofluorescence staining demonstrated a similar profile of increased iNOS expression in Nrp1−/− BMDMs compared with WT BMDMs (Fig. 7B). Furthermore, mRNA levels of M1 polarization markers (iNOS, IL-6, and IL-13Rα1) were up-regulated in Nrp1−/− BMDMs compared with WT BMDMs (Fig. 7C). In contrast, ArgI was significantly decreased in Nrp1−/− BMDMs compared with WT BMDMs (Fig. 7D). Consistently, the ratio of iNOS to ArgI was significantly up-regulated in Nrp1−/− BMDMs compared with WT BMDMs, which suggested that Nrp1 deficiency skews macrophages toward an M1 phenotype (Fig. 7D).
Figure 6.
Effect of Nrp1 deficiency on M2 polarization in BMDMs. Purified monocytes were incubated for 5 d with M-CSF (50 ng/ml) to differentiate into mature macrophages (M0-phenotype). M0-macrophages were then polarized for an additional 2 d into M2-macrophages by stimulation with IL-4 (20 ng/ml). A) Immunoblot analysis of ArgI and Ym1 in WT and Nrp1−/− BMDMs. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) serves as loading control. B) Quantitative RT-PCR analysis of ArgI, Ym1, Ym2, IL-10, and CD206 mRNA levels in WT and Nrp1−/− BMDMs. Data are expressed as means ± sem.
Figure 7.
Nrp1 deficiency induces M1 macrophage polarization. Purified monocytes were incubated for 5 d with M-CSF (50 ng/ml) to differentiate into mature macrophages (M0-phenotype). M0-macrophages were then polarized for an additional 2 d into M1-macrophages by substituting M-CSF for a mixture of LPS (100 ng/ml) and IFN-γ (20 ng/ml). A) Immunoblot analysis of iNOS in WT and Nrp1−/− macrophages. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) serves as loading control. B) Immunofluorescence staining of iNOS (green) in WT and Nrp1−/− BMDMs. Nuclei were stained with DAPI (blue). Scale bar, 5 μm. C) Quantitative RT-PCR analysis of iNOS, IL-6, and IL-13Rα1 mRNA levels. D) ArgI mRNA levels were measured by quantitative RT-PCR, and the ratio of iNOS to ArgI was calculated. E) Immunohistochemical staining of iNOS and COX2 in lung tissue from WT and Nrp1myel-KO mice 20 h after intraperitoneal injection of saline or LPS (20 mg/kg body weight; n = 5 per group). F) Schematic model for LPS-induced Nrp1 loss-exaggerated sepsis. LPS specifically down-regulates Nrp1 mRNA via activation of TLR4–NF-κB pathway in macrophages. Loss or deficiency of Nrp1 augments leads to increased proinflammatory cytokines and causes sepsis. Data are expressed as means ± sem. *P < 0.05 vs. WT.
Eventually, we checked macrophage polarization status in vivo. M1 macrophage markers, such as iNOS and COX2, were also significantly increased in the lung tissue of Nrp1myel-KO mice compared with those in the lung tissue of WT mice in response to sublethal dose of LPS (Fig. 7E). Taken together, Nrp1 deficiency promotes macrophage polarization from M0 to M1 in vitro and in vivo but demonstrates no significant effect on M2 polarization.
DISCUSSION
Sepsis is a systemically exaggerated inflammatory syndrome marked by multiple organ injury and failure, which contributes to high mortality rates in patients (30). The absence of effective therapeutic strategies has prompted investigations of the mechanisms that are involved in the pathophysiology of sepsis. In the present study, we demonstrate that loss of macrophage Nrp1 via gene deletion or attenuation by LPS augments inflammatory response, which leads to increased proinflammatory cytokines and sepsis (Fig. 7F). Our findings establish a novel role for macrophage Nrp1 in mitigating sepsis, which suggests important roles for Nrp1 as an endogenous modulator of innate immunity.
Emerging studies indicate that macrophage Nrp1 plays important roles in various pathologic conditions. For example, macrophage-specific Nrp1 deletion inhibits tumor growth and metastasis via inhibition of proangiogenic and immunosuppressive functions of Nrp1 (19). Sema3A carries out an osteoprotecting effect via its receptor, Nrp1 (29). In addition, a potential role of Nrp1 in aging has been indicated by age-related hypomethylation in human purified monocytes at CpG sites within the regions of the Nrp1 gene (31). By employing Nrp1myel-KO mice using 2 stringent models of sepsis—CLP and peritoneal LPS injection—we demonstrated that absence of Nrp1 in myeloid cells correlated with markedly enhanced susceptibility to sepsis, which was accompanied by higher levels of proinflammatory cytokines, such as TNF-α and IL-6. Together, we demonstrate the novel and inhibitory roles of macrophage Nrp1 in sepsis; however, identifying the specific Nrp1 ligand in our LPS-induced sepsis model is a challenge as numerous class 3 semaphorins (Sema3A–F) and growth factors, including VEGF, TGF-β, hepatocyte growth factor, and platelet-derived growth factor, have been shown to function as ligands for Nrp1 (8). Furthermore, Nrp1 can bind with heparin, integrins, fibronectin, and Sema4A (8). These ligands and binding partners are all potential ligands for Nrp1 in sepsis.
It has been previously reported that deficiency or inhibition of TLRs is associated with an enhanced survival rate of sepsis (32–35); therefore, negative regulation of TLR signaling might be beneficial for treating sepsis. We showed here that targeted deletion of Nrp1 in macrophages exaggerated LPS-activated TLR4 pathway, which resulted in increased levels of iNOS, TNF-α, and IL-6 in macrophages and/or in peritoneal lavage and serum. Our results strongly support the notion that Nrp1 functions as an endogenous negative modulator of TLR4–NF-κB pathway. In addition to the essential roles of TLR4-mediated inflammation in sepsis, TLR4 has been implicated in obesity, insulin resistance, diabetes, and vascular inflammation. Futuin-A, an endogenous activator of TLR4 (36), has been linked to innate immunity and lipid-induced insulin resistance (37). Kim et al. (38) reported further that TLR4 signaling was required for palmitate-mediated endothelial dysfunction and vascular inflammation; therefore, the inhibitory effect of Nrp1 on TLR4 signaling requires further investigation, especially within the context of metabolic diseases, such as obesity and type 2 diabetes.
Miyauchi et al. (39) reported that ablation of Nrp1 in glioma-associated microglia and macrophages inhibits glioma growth by promoting M1 macrophage polarization in gliomas. In addition, Sema3A restricts tumor growth by suppressing proliferation of protumoral M2 macrophages but promoting proliferation of antitumoral M1, which is mediated by its receptor Nrp1 (40). In this study, we showed that Nrp1 deficiency exhibited a strong effect on M1 macrophage polarization, as depicted by increased iNOS protein and up-regulated M1 polarization markers, iNOS, IL-6, and IL-13 mRNA in Nrp1−/− BMDMs compared with WT BMDMs. Furthermore, we confirmed increased M1 polarization in lung in vivo by immunohistochemical staining of iNOS and COX2; however, our data indicated that Nrp1 deficiency did not affect protein or mRNA of M2 polarization markers, including ArgI, Ym1, Ym2, IL-10, and CD206, in BMDMs.
In seeking the mechanism by which Nrp1 deficiency contributes to increased susceptibility to sepsis and exaggerated inflammatory response, we discovered that LPS dramatically down-regulates Nrp1 at transcriptional level in macrophages, which suggests Nrp1 to be a direct target of LPS-TLR4 signaling. Surprisingly, this effect seems to be specific to macrophages, rather than other cell types, as we did not observe LPS-mediated Nrp1 suppression in mouse embryonic fibroblasts, rat fibroblasts, HUVECs, or human vascular smooth muscle cells. TLR4 is ubiquitously expressed in the tested cells (data not shown); however, other tested cells showed less sensitivity to LPS than macrophages (data not shown). Therefore, we speculated that the difference in regulation of Nrp1 by LPS in a variety of cell types is likely a result of the different response to LPS administration but not TLR4 expression. LPS is the major structural component of gram-negative bacteria outer membrane and plays an important role in bacteria-induced sepsis (41). LPS has also been implicated in the regulation of the expression of certain proteins. For example, LPS negatively regulates single Ig IL-1 receptor–related molecule by inhibiting transcription factor specificity protein 1 (Sp1) binding to the promoter of single Ig IL-1 receptor–related molecule (42). Ye et al. (43) demonstrated that LPS increases Sp1-degrading enzyme (LISPDE, a unique trypsin-like serine protease) activity to degrade Sp1 protein. LPS induces degradation of programmed cell death protein 4 (PDCD4) in a mammalian target of rapamycin signaling–dependent manner, which leads to an increased IL-10 level (44). Liu et al. (45) demonstrated that LPS accelerates liver kinase B1 decay by S-nitrosylation–dependent proteasome pathways. Our results support the transcriptional control of Nrp1 by LPS in macrophages.
Hayashi et al. (29) reported that the osteoclast differentiation factor, RANKL, activates transcription factors NF-κB p50 and p65, which function as suppressors in Nrp1 promoter, leading to down-regulation of Nrp1. Involvement of p50/p5 heterodimers in attenuation of Nrp1 in response to hypoxia via hypoxia-inducible factor-2–mediated IKK induction has also been reported (19); however, whether NF-κB p50/p65 mediates LPS-induced Nrp1 suppression remains undefined. By using p50- and/or p65-specific siRNA, we clearly demonstrated that NF-κB p50 and p65 are required for LPS-mediated Nrp1 attenuation in macrophages.
Exact and elaborate regulation of innate immune response is of great importance to determine the outcome of sepsis. As a negative feedback regulation, LPS decreases proinflammatory PDCD4 via miR-21, which leads to limited inflammation and decreased susceptibility to sepsis (46). In contrast, we found that Nrp1 abundance negatively correlates with the proinflammatory effect of LPS signaling, which suggests that the Nrp1 down-regulation via the LPS-TLR4 pathway is important for exaggerating the proinflammatory effect of LPS. Our observations are in agreement with the function and regulation of signal regulatory protein α in the presence of LPS. Namely, LPS down-regulates the anti-inflammatory signal regulatory protein α, which results in enhanced susceptibility to LPS-induced sepsis (47). Therefore, control of Nrp1 level is a vital step in the regulation of the inflammatory response to LPS.
In summary, we demonstrate that mice that lack Nrp1 in myeloid cells exhibit enhanced susceptibility to CLP- and LPS-induced sepsis. Mechanistically, LPS specifically down-regulates Nrp1 in macrophages, which is mediated by the TLR4–NF-κB pathway. Loss of Nrp1 causes hyper-responsiveness to LPS treatment and promotes classic macrophage activation. These findings unveil novel anti-inflammatory roles for macrophage Nrp1 in sepsis and identify LPS as a negative regulator of Nrp1 in macrophages. Nrp1 may represent a potential therapeutic target for treating human sepsis.
ACKNOWLEDGMENTS
This study was supported by funding from the following agencies: U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (Grants HL079584 and HL080499), NIH National Cancer Institute (Grant CA213022), and NIH National Institute on Aging (Grant AG047776). M.-H.Z. is the Eminent Scholar in Molecular and Translational Medicine of Georgia Research Alliance.
Glossary
- ArgI
arginase I
- BMDM
bone marrow–derived macrophage
- CLP
cecal ligation and puncture
- FBS
fetal bovine serum
- LysM
lysozyme M
- Nrp1
neuropilin 1
- Nrp1myel-KO
myeloid cell–specific Nrp1 knockout
- PDCD4
programmed cell death protein 4
- PM
peritoneal macrophage
- Sema3A
class 3 semaphorin
- siRNA
small interfering RNA
- Sp1
specificity protein 1
- Treg
regulatory T
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
X. Dai, I. Okon, P. Song, and M.-H. Zou designed experiments; X. Dai, Z. Liu, Y. Wu, and H. Zhu performed experiments and analyzed data; and X. Dai, I. Okon, and M.-H. Zou wrote the paper.
REFERENCES
- 1.Deutschman C. S., Tracey K. J. (2014) Sepsis: current dogma and new perspectives. Immunity 40, 463–475 [DOI] [PubMed] [Google Scholar]
- 2.Ruf W. (2010) New players in the sepsis-protective activated protein C pathway. J. Clin. Invest. 120, 3084–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen J., Opal S., Calandra T. (2012) Sepsis studies need new direction. Lancet Infect. Dis. 12, 503–505 [DOI] [PubMed] [Google Scholar]
- 4.Angus D. C. (2011) The search for effective therapy for sepsis: back to the drawing board? JAMA 306, 2614–2615 [DOI] [PubMed] [Google Scholar]
- 5.Kolodkin A. L., Levengood D. V., Rowe E. G., Tai Y. T., Giger R. J., Ginty D. D. (1997) Neuropilin is a semaphorin III receptor. Cell 90, 753–762 [DOI] [PubMed] [Google Scholar]
- 6.He Z., Tessier-Lavigne M. (1997) Neuropilin is a receptor for the axonal chemorepellent semaphorin III. Cell 90, 739–751 [DOI] [PubMed] [Google Scholar]
- 7.Soker S., Takashima S., Miao H. Q., Neufeld G., Klagsbrun M. (1998) Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92, 735–745 [DOI] [PubMed] [Google Scholar]
- 8.Kumanogoh A., Kikutani H. (2013) Immunological functions of the neuropilins and plexins as receptors for semaphorins. Nat. Rev. Immunol. 13, 802–814 [DOI] [PubMed] [Google Scholar]
- 9.Bruder D., Probst-Kepper M., Westendorf A. M., Geffers R., Beissert S., Loser K., von Boehmer H., Buer J., Hansen W. (2004) Neuropilin-1: a surface marker of regulatory T cells. Eur. J. Immunol. 34, 623–630 [DOI] [PubMed] [Google Scholar]
- 10.Delgoffe G. M., Woo S. R., Turnis M. E., Gravano D. M., Guy C., Overacre A. E., Bettini M. L., Vogel P., Finkelstein D., Bonnevier J., Workman C. J., Vignali D. A. (2013) Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 501, 252–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen W., Hutzler M., Abel S., Alter C., Stockmann C., Kliche S., Albert J., Sparwasser T., Sakaguchi S., Westendorf A. M., Schadendorf D., Buer J., Helfrich I. (2012) Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J. Exp. Med. 209, 2001–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catalano A. (2010) The neuroimmune semaphorin-3A reduces inflammation and progression of experimental autoimmune arthritis. J. Immunol. 185, 6373–6383 [DOI] [PubMed] [Google Scholar]
- 13.Catalano A., Caprari P., Moretti S., Faronato M., Tamagnone L., Procopio A. (2006) Semaphorin-3A is expressed by tumor cells and alters T-cell signal transduction and function. Blood 107, 3321–3329 [DOI] [PubMed] [Google Scholar]
- 14.Tordjman R., Lepelletier Y., Lemarchandel V., Cambot M., Gaulard P., Hermine O., Roméo P. H. (2002) A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat. Immunol. 3, 477–482 [DOI] [PubMed] [Google Scholar]
- 15.Fantin A., Vieira J. M., Gestri G., Denti L., Schwarz Q., Prykhozhij S., Peri F., Wilson S. W., Ruhrberg C. (2010) Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116, 829–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pucci F., Venneri M. A., Biziato D., Nonis A., Moi D., Sica A., Di Serio C., Naldini L., De Palma M. (2009) A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood 114, 901–914 [DOI] [PubMed] [Google Scholar]
- 17.Rolny C., Mazzone M., Tugues S., Laoui D., Johansson I., Coulon C., Squadrito M. L., Segura I., Li X., Knevels E., Costa S., Vinckier S., Dresselaer T., Åkerud P., De Mol M., Salomäki H., Phillipson M., Wyns S., Larsson E., Buysschaert I., Botling J., Himmelreich U., Van Ginderachter J. A., De Palma M., Dewerchin M., Claesson-Welsh L., Carmeliet P. (2011) HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 19, 31–44 [DOI] [PubMed] [Google Scholar]
- 18.Takeda Y., Costa S., Delamarre E., Roncal C., Leite de Oliveira R., Squadrito M. L., Finisguerra V., Deschoemaeker S., Bruyère F., Wenes M., Hamm A., Serneels J., Magat J., Bhattacharyya T., Anisimov A., Jordan B. F., Alitalo K., Maxwell P., Gallez B., Zhuang Z. W., Saito Y., Simons M., De Palma M., Mazzone M. (2011) Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature 479, 122–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casazza A., Laoui D., Wenes M., Rizzolio S., Bassani N., Mambretti M., Deschoemaeker S., Van Ginderachter J. A., Tamagnone L., Mazzone M. (2013) Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell 24, 695–709 [DOI] [PubMed] [Google Scholar]
- 20.Ji J. D., Park-Min K. H., Ivashkiv L. B. (2009) Expression and function of semaphorin 3A and its receptors in human monocyte-derived macrophages. Hum. Immunol. 70, 211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi N., Kawada T., Goto T., Kim C. S., Taimatsu A., Egawa K., Yamamoto T., Jisaka M., Nishimura K., Yokota K., Yu R., Fushiki T. (2003) Abietic acid activates peroxisome proliferator-activated receptor-gamma (PPARgamma) in RAW264.7 macrophages and 3T3-L1 adipocytes to regulate gene expression involved in inflammation and lipid metabolism. FEBS Lett. 550, 190–194 [DOI] [PubMed] [Google Scholar]
- 22.Bruscia E. M., Zhang P. X., Satoh A., Caputo C., Medzhitov R., Shenoy A., Egan M. E., Krause D. S. (2011) Abnormal trafficking and degradation of TLR4 underlie the elevated inflammatory response in cystic fibrosis. J. Immunol. 186, 6990–6998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen H., Dou Y., Hogaboam C. M., Kunkel S. L. (2008) Epigenetic regulation of dendritic cell-derived interleukin-12 facilitates immunosuppression after a severe innate immune response. Blood 111, 1797–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S., Xu J., Song P., Viollet B., Zou M. H. (2009) In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase I. Diabetes 58, 1893–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hubbard W. J., Choudhry M., Schwacha M. G., Kerby J. D., Rue L. W. III, Bland K. I., Chaudry I. H. (2005) Cecal ligation and puncture. Shock 24(Suppl 1), 52–57 [DOI] [PubMed] [Google Scholar]
- 26.Wen H., Lei Y., Eun S. Y., Ting J. P. (2010) Plexin-A4-semaphorin 3A signaling is required for Toll-like receptor- and sepsis-induced cytokine storm. J. Exp. Med. 207, 2943–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawai T., Akira S. (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 [DOI] [PubMed] [Google Scholar]
- 28.Liew F. Y., Xu D., Brint E. K., O’Neill L. A. (2005) Negative regulation of Toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 5, 446–458 [DOI] [PubMed] [Google Scholar]
- 29.Hayashi M., Nakashima T., Taniguchi M., Kodama T., Kumanogoh A., Takayanagi H. (2012) Osteoprotection by semaphorin 3A. Nature 485, 69–74 [DOI] [PubMed] [Google Scholar]
- 30.Hotchkiss R. S., Karl I. E. (2003) The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348, 138–150 [DOI] [PubMed] [Google Scholar]
- 31.Tserel L., Limbach M., Saare M., Kisand K., Metspalu A., Milani L., Peterson P. (2014) CpG sites associated with NRP1, NRXN2 and miR-29b-2 are hypomethylated in monocytes during ageing. Immun. Ageing 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roger T., Froidevaux C., Le Roy D., Reymond M. K., Chanson A. L., Mauri D., Burns K., Riederer B. M., Akira S., Calandra T. (2009) Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc. Natl. Acad. Sci. USA 106, 2348–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plitas G., Burt B. M., Nguyen H. M., Bamboat Z. M., DeMatteo R. P. (2008) Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J. Exp. Med. 205, 1277–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiller S., Elson G., Ferstl R., Dreher S., Mueller T., Freudenberg M., Daubeuf B., Wagner H., Kirschning C. J. (2008) TLR4-induced IFN-gamma production increases TLR2 sensitivity and drives gram-negative sepsis in mice. J. Exp. Med. 205, 1747–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alves-Filho J. C., Freitas A., Souto F. O., Spiller F., Paula-Neto H., Silva J. S., Gazzinelli R. T., Teixeira M. M., Ferreira S. H., Cunha F. Q. (2009) Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc. Natl. Acad. Sci. USA 106, 4018–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pal D., Dasgupta S., Kundu R., Maitra S., Das G., Mukhopadhyay S., Ray S., Majumdar S. S., Bhattacharya S. (2012) Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 18, 1279–1285 [DOI] [PubMed] [Google Scholar]
- 37.Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., Flier J. S. (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim F., Pham M., Luttrell I., Bannerman D. D., Tupper J., Thaler J., Hawn T. R., Raines E. W., Schwartz M. W. (2007) Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ. Res. 100, 1589–1596 [DOI] [PubMed] [Google Scholar]
- 39.Miyauchi J. T., Chen D., Choi M., Nissen J. C., Shroyer K. R., Djordevic S., Zachary I. C., Selwood D., Tsirka S. E. (2016) Ablation of neuropilin 1 from glioma-associated microglia and macrophages slows tumor progression. Oncotarget 7, 9801–9814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallerius M., Wallmann T., Bartish M., Östling J., Mezheyeuski A., Tobin N. P., Nygren E., Pangigadde P., Pellegrini P., Squadrito M. L., Pontén F., Hartman J., Bergh J., De Milito A., De Palma M., Östman A., Andersson J., Rolny C. (2016) Guidance molecule SEMA3A restricts tumor growth by differentially regulating the proliferation of tumor-associated macrophages. Cancer Res. 76, 3166–3178 [DOI] [PubMed] [Google Scholar]
- 41.Beutler B., Rietschel E. T. (2003) Innate immune sensing and its roots: the story of endotoxin. Nat. Rev. Immunol. 3, 169–176 [DOI] [PubMed] [Google Scholar]
- 42.Ueno-Shuto K., Kato K., Tasaki Y., Sato M., Sato K., Uchida Y., Sakai H., Ono T., Suico M. A., Mitsutake K., Tokutomi N., Kai H., Shuto T. (2014) Lipopolysaccharide decreases single immunoglobulin interleukin-1 receptor-related molecule (SIGIRR) expression by suppressing specificity protein 1 (Sp1) via the Toll-like receptor 4 (TLR4)-p38 pathway in monocytes and neutrophils. J. Biol. Chem. 289, 18097–18109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye X., Liu S. F. (2007) Lipopolysaccharide causes Sp1 protein degradation by inducing a unique trypsin-like serine protease in rat lungs. Biochim. Biophys. Acta 1773, 243–253 [DOI] [PubMed] [Google Scholar]
- 44.Van den Bosch M. W., Palsson-Mcdermott E., Johnson D. S., O’Neill L. A. (2014) LPS induces the degradation of programmed cell death protein 4 (PDCD4) to release Twist2, activating c-Maf transcription to promote interleukin-10 production. J. Biol. Chem. 289, 22980–22990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Z., Dai X., Zhu H., Zhang M., Zou M. H. (2015) Lipopolysaccharides promote S-nitrosylation and proteasomal degradation of liver kinase B1 (LKB1) in macrophages in vivo. J. Biol. Chem. 290, 19011–19017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheedy F. J., Palsson-McDermott E., Hennessy E. J., Martin C., O’Leary J. J., Ruan Q., Johnson D. S., Chen Y., O’Neill L. A. (2010) Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat. Immunol. 11, 141–147 [DOI] [PubMed] [Google Scholar]
- 47.Kong X. N., Yan H. X., Chen L., Dong L. W., Yang W., Liu Q., Yu L. X., Huang D. D., Liu S. Q., Liu H., Wu M. C., Wang H. Y. (2007) LPS-induced down-regulation of signal regulatory protein alpha contributes to innate immune activation in macrophages. J. Exp. Med. 204, 2719–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]